Abstract
OBJECTIVE
Recent evidence indicates that heat-enhanced food advanced glycation end products (AGEs) adversely affect vascular function. The aim of this study was to examine the acute effects of an oral load of heat-treated, AGE-modified β-lactoglobulins (AGE-BLG) compared with heat-treated, nonglycated BLG (C-BLG) on vascular function in patients with type 2 diabetes mellitus (T2DM).
RESEARCH DESIGN AND METHODS
In a double-blind, controlled, randomized, crossover study, 19 patients with T2DM received, on two different occasions, beverages containing either AGE-BLG or C-BLG. We measured macrovascular [brachial ultrasound of flow-mediated dilatation (FMD)] and microvascular (laser-Doppler measurements of reactive hyperemia in the hand) functions at baseline (T0), 90 (T90), and 180 (T180) min.
RESULTS
Following the AGE-BLG, FMD decreased at T90 by 80% from baseline and remained decreased by 42% at T180 (P < 0.05 vs. baseline, P < 0.05 vs. C-BLG at T90). By comparison, following C-BLG, FMD decreased by 27% at T90 and 51% at T180 (P < 0.05 vs. baseline at T180). A significant decrease in nitrite (T180) and nitrate (T90 and T180), as well as a significant increase in Nε-carboxymethyllisine, accompanied intake of AGE-BLG. There was no change in microvascular function caused by either beverage.
CONCLUSIONS
In patients with T2DM, acute oral administration of a single AGE-modified protein class significantly though transiently impaired macrovascular function in concert with decreased nitric oxide bioavailability. These AGE-related changes were independent of heat treatment.
Elevated levels of circulating advanced glycation end products (AGEs), a heterogeneous group of compounds formed by the nonezymatic glycation of proteins, lipids, or nucleic acids (1,2), are believed to play a major role in the pathogenesis of cardiovascular disease in diabetes (3–5). Although AGEs are better known as products of hyperglycemia, they also form in food during heat-enhanced cooking (6). Dietary AGEs, representing an important source for circulating AGEs in vivo (7–9), are partially absorbed (10,11) and either retained in the body or eliminated into the urine (11–13).
We have reported (14) that a single high-heat–processed, mixed meal with a high AGE content or the oral administration of an AGE-enriched cola beverage (15) induced acute impairment of arterial vasodilatation in response to ischemia, an early marker of endothelial dysfunction and atherosclerosis (16–18).
Since heat application for AGE generation (14) also potentially affected vitamin activity and triggered the formation of other non-AGE toxic substances and the effects (15) of cola-derived AGEs [small molecules such as caramelized products normally present in low concentration (19)] might not be representative for AGEs commonly found in foods, the present randomized, double-blind, controlled, crossover study was aimed at investigating the acute effects of dietary AGEs resulting from nonenzymatic, heat-enhanced glycation of a single protein class [β-lactoglobulins (BLG), common in food] and compare these effects to similarly heated but nonglycated BLG.
RESEARCH DESIGN AND METHODS
The study was conducted at the Diabetes Clinic of the Heart and Diabetes Center NRW (Bad Oeynhausen, Germany) following the requirements of the Declaration of Helsinki. Written informed consent was obtained from all patients, and the study protocol was approved by the ethics committee of the Ruhr-Universität Bochum. Throughout the course of the study, diet and all medications were kept unchanged.
This was a double-blind, controlled, crossover, randomized trial consisting of a screening visit, as well as two dosing visits when subjects attended at ∼7 a.m. the facility after an overnight fast without having taken any medication. Randomization of patients ensured that 10 subjects received first the AGE-rich beverage (AGE-BLG) and were then switched to receive the control beverage (C-BLG) after a washout period of at least 7 days (first sequence). The remaining nine subjects followed the inverse sequence of test beverages (second sequence).
On each visit, vascular studies of the macrocirculation [flow-mediated dilatation (FMD)] and microcirculation [laser Doppler measured at the hand (reactive hyperemia following forearm ischemia)], measurements of blood pressure, heart rate, and blood draws were performed at baseline (T0) as well as 90 (T90) and 180 min (T180) following the test beverage. Meanwhile, subjects remained fasting, and water intake was prohibited. All randomized subjects completed the study, and no adverse event occurred.
Patients’ characteristics
Nineteen subjects with type 2 diabetes mellitus (T2DM; 16 on oral therapy, 2 on basal insulin, and 1 on diet alone) without major cardiovascular events, surgical, or interventional history within the previous 6 months were enrolled into this study. Demographic characteristics were [mean (minimum–maximum)]: age, 54 (36–66) years; BMI, 35 (26–51) kg/m2; and diabetes duration, 6 (1–12) years. Baseline laboratory values (mean ± SEM): HbA1c, 8.6 ± 0.5%; creatinine, 0.9 ± 0.1 mg/dL; glomerular filtration rate, 89 ± 4 ml/min/1.73 m2; and albuminuria, 12 ± 1 mg/L. Comorbidities were diabetic peripheral neuropathy (n = 8), arterial hypertension (n = 17), and stable coronary heart disease (n = 1).
Preparation of the AGE-rich and control beverages
At the German Institute of Food Technologies (Quakenbrück, Germany), BLG (Davisco Foods International, Eden Prairie, MN; lot JE 003–6-922, certificate of analysis from 25 June 2009) and roferose dextrose monohydrate (Roquette Frères, Lestrem, France) were mixed in MilliQ H2O (German Institute of Food Technologies, Quakenbrück, Germany) to a molar ratio of 5 mol glucose/mol BLG. The solution was then lyophilized, passed through a 1-mm mesh sieve, put in a climate-regulated drying cupboard at 25°C and 44% humidity for 3 days, and then packaged into closed plastic bags. The product was afterward exposed to 50°C for 17 h, then dissolved in water (to achieve a concentration of 0.1 g/mL), and ultra-filtered against water to eliminate the remaining free sugars, small degradation products, and other contaminants. After lyophilization and sieving, portions bags (20 g) were prepared and stored in a refrigerator at 4°C until administration.
For the preparation of the control BLG, exactly the same procedure was applied, except for the fact that no dextrose was added. This resulted in a powder containing nonglycated, heat-treated BLG: C-BLG. Thus, the composition of beverages was matched.
Analyses of AGE content [Nε-carboxymethyllysine (CML) and methylglyoxal (MG)] in two samples showed 6 U CML/mg protein (120,000 U CML/serving) and 0.47 nmol MG/mg of protein (9,400 nmol MG/serving) for AGE-BLG and 0.97 U CML/mg protein (19,400 U CML/serving) and 0.04 nmol MG/mg of protein (800 nmol MG/serving) for C-BLG.
Beverages were prepared immediately previous to the administration by a person not involved in study procedures by dissolving each portion of 20 g powder in 100 mL tap water. Beverages were given to the subject in an opaque bottle, and they were asked to consume it within 5 min.
To enable comparisons, the same methods for the assessment of endothelial function were used as in the previous study (14).
FMD
Endothelium-dependent dilatation (FMD assessed at the right brachial artery by measuring the arterial diameter response to reactive hyperemia 60–80 s following a 4.5 min blood-pressure cuff–induced ischemia of the forearm) and endothelium-independent vasodilatation (GTN; 5 min after sublingual administration of 0.4 mg glycerotrinitrate spray) were measured at baseline, T90, and at T180 (only FMD), as previously described (14) and in accordance to the protocol first described by Celermajer and colleagues (20).
Laser-Doppler measurements of microcirculation
Blood flow (BF) was assessed using laser-Doppler flowmetry (O2C; LEA Medizintechnik, Giessen, Germany) with the probe on the dorsal thenar site of the right hand as previously described (14).
Laboratory measurements
Plasma or serum was obtained after centrifugation at 1,500 g for 20 min at 4°C. Aliquots of 750 µL were stored at −80°C.
CML and MG derivatives in serum were quantified by ELISAs using two non–cross-reacting monoclonal antibodies (4G9 and MG3D11) raised against synthetic standards, CML-BSA and MG-BSA, respectively.
The following laboratory methods were also used: serum glucose (glucose-oxidase method; Architect ci8200; Abbott Diagnostics, Wiesbaden, Germany), HbA1c (high-performance liquid chromatography; Menarini, Berlin, Germany), serum cholesterol, triglycerides, LDL and HDL cholesterol (Architect ci8200 analyzer; Abbott Diagnostics), thiobarbituric acid–reactive substances (OXI-TEK TBARS Assay Kit; ZeptoMetrix), human soluble E-selectin (CD62E Immunoassay; R&D Systems), nitrite and nitrate [total nitric oxide (NO) and nitrate/nitrite assay; R&D Systems], and human soluble vascular cell adhesion molecule-1 (VCAM-1; sVCAM-1 Immunoassay; R&D Systems).
Statistical analysis
Paired, two-tailed, t tests with Bonferroni correction for multiple testing were used to assess changes from baseline. Differences in changes (the 90- and 180-min value minus baseline value, respectively) were compared by a paired, two-tailed t test to assess differences between treatments. Univariate linear regression models were fitted to assess the relationship between changes in parameters. Three patients were excluded from the analysis of FMD due to poor image quality (eight subjects from each randomization sequence were analyzed). The level of significance was set at 0.05. Values are presented as mean ± SEM if not otherwise stated.
RESULTS
Values of all investigated parameters are listed in Table 1.
Table 1.
Study parameters
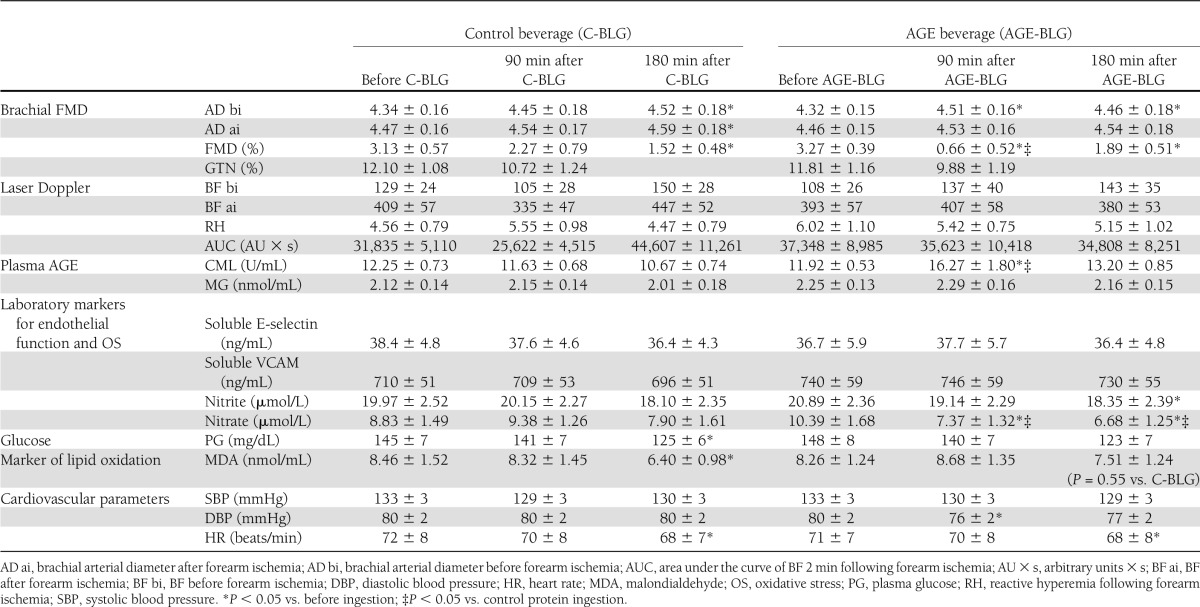
No significant differences in baseline values of any parameter were observed between the 2 study days.
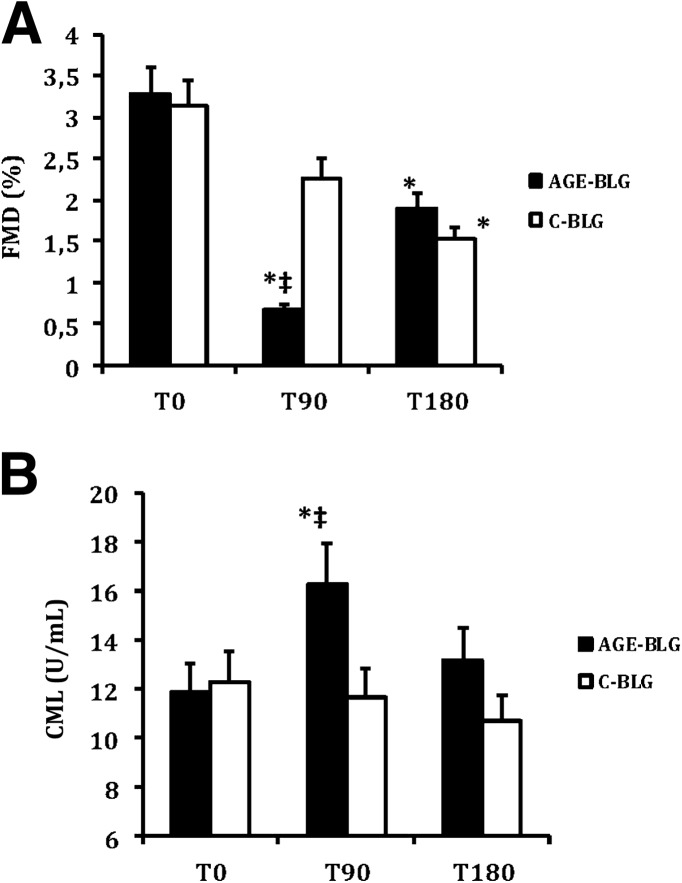
Following intake of the AGE-BLG preparation, FMD decreased significantly from baseline at T90 (by 80%) and T180 (by 42%) and significantly more than after the C-BLG at T90. Following the control beverage, a modest change from baseline was seen at T90 (by 27%), and a further decrease in FMD was noted at T180 (by 51%, similar to that of AGE-BLG, P < 0.05 vs. baseline) (Table 1 and Fig. 1).
Figure 1.
Changes in FMD (A) and CML (B) after administration of AGE-BLG and C-BLG (mean and SEM). *P < 0.05 vs. before ingestion; ‡P < 0.05 vs. control protein ingestion.
GTN did not significantly change on either occasion, nor did laser-Doppler measurements of BF (Table 1).
Serum carboxymethyllysine (sCML) levels increased significantly at T90 following the AGE-BLG and remained elevated at T180, although not significantly above baseline. A significant correlation (−0.39; P < 0.05) existed between the changes in FMD and sCML from T0 to T90. No significant correlation existed between nitrite, nitrate and sCML, or FMD. No change in sCML was seen following the C-BLG. Levels of serum MG remained unchanged following either beverage.
Total nitrite significantly decreased from baseline only at T180 after the AGE-BLG, whereas it remained unchanged after the C-BLG. Total nitrate significantly decreased from baseline at T90 and at T180 following the AGE-BLG. No changes were seen following the C-BLG.
No significant differences between study days existed for plasma glucose, systolic blood pressure, heart rate, circulating E-selectin, and VCAM-1.
CONCLUSIONS
The current study demonstrates that orally administered, AGE-modified BLG acutely impaired macrovascular function in people with T2DM and that this effect is significantly greater than the effect of nonglycated BLG. This effect was temporally associated with an increase in sCML supporting a detrimental effect of heat-derived dietary AGEs on vascular function in subjects with diabetes as previously suggested (14,15).
Generation of AGEs in food during the cooking process depends on the amount and type of substrates present (e.g., reducing sugars and proteins or lipids) and correlates positively with the temperature and duration of preparation and negatively with the humidity (6). We prepared our two test beverages using as substrate a single protein class (BLG) treated under controlled conditions of humidity and temperature, and, except for the protein glycation, the preparation of the two beverages was similar.
We have previously shown that a meal with a high AGE content (15,100 kU CML) (14) and a beverage obtained from a soft-drink extract with an AGE content of 1,800 kU CML (15) induced an impairment in macrovascular function in subjects with T2DM. In the current study, an even lower amount of AGEs (120 kU CML) impaired macrovascular function.
This study has several strengths: it is a randomized, double-blind, controlled study; we administered a well-defined AGE-modified protein class (BLG) obtained under accurately controlled conditions, thus ruling out the influence of other confounding factors like carbohydrates, lipids, inactivation of vitamins, or non–glycation-produced food toxins (8,14,21). We compared the effects of AGE-BLG to matched and identically temperature-prepared BLG and not to meals prepared under different temperature conditions (8,14,21), which enables us to identify effects of the AGE modification of proteins beyond the effect of heat (which might alter nutrients per se). We investigated the effects of whole AGE-modified proteins (BLG) rather than AGE adducts (15), which we consider a dietary-relevant aspect. In our opinion, this study confirms that AGE-modified proteins negatively impact on vascular function in humans, strengthening the findings of previous studies (8,14,21).
FMD was decreased by 80% of baseline value at 90 min after the administration of AGE-BLG, showing significant recovery at 180 min, though well below the baseline. Ninety minutes following C-BLG intake, FMD modestly decreased (by 27% of baseline) at T180, reaching 51% of baseline, a level comparable to that of AGE-BLG. This suggests that although glycated proteins exert a rapid vascular effect, heat-induced changes of BLG alone may also promote a delayed transient vascular dysfunction, the nature of which requires further study.
GTN did not significantly change on any occasion, therefore a change in NO sensitivity of the vascular smooth muscle cells can be ruled out. Circulating levels of NO metabolites nitrite [a surrogate of NO bioavailability under physiological and pathophysiological conditions (22)] and nitrate decreased by AGE-BLG, suggesting AGE-mediated reduced NO bioavailability as one mechanism of impaired FMD. The bioavailability of NO is dependent on endothelial production and also on its rate of inactivation by, for example, AGEs or by reactive oxygen species (23). Since we found neither a significant change in oxidative stress, nor a change in circulating levels of E-selectin and VCAM-1 as a measure of endothelial cell dysfunction (24,25), it is possible that ingested AGEs directly quenched NO, decreasing its bioavailability (23). But this hypothesis must be cautiously formulated, since we found no correlation between NO metabolites and CML change. Of note, we did not measure nitrotyrosine; therefore, this study gives limited information on nitrative stress.
In our previous study (14), thiobarbituric acid–reactive species as a measure of oxidative stress increased 2 h postprandially, decreasing afterward below the baseline value. A circadian variation in parameters regulating oxidative stress was reported, but remained controversial (26,27). In our study, at 90 min, malondialdehyde, a measure of oxidative stress, increased modestly after AGE-BLG and decreased compared with baseline at T180 following both beverages. The explanation could be a circadian variation in oxidative stress and/or an efficient activation of antioxidant mechanisms by oxidative stress (27).
In this study, we found no effect by AGE-BLG on microvascular function. These results appear somehow in contrast to those we reported following a high-heat, high-AGE meal (14), which, however, contained glucose, lipids, and other heat-generated substances not involved in the current setting.
In summary, we have demonstrated that even relatively low amounts of AGE-modified food proteins acutely impaired macrovascular function in patients with T2DM, independently of other effects imparted by thermal exposure of foods. Our study strengthens the evidence for a detrimental effect of food AGEs on vascular function in this population. Similar to postprandial hyperglycemia and hyperlipidemia (22,23), repeated postprandial alterations of vascular function due to oral glycotoxins might contribute to the development of vascular complications.
Limitations of the study
Since no healthy controls were investigated, the conclusions of the current study apply only to a population with T2DM.
We did not measure the endotoxin content of beverages. An interaction between endotoxins and the AGE receptor system exists (28) and endotoxin-like products might contaminate AGE preparations (29). While intravenous administration of endotoxins induces severe toxicity in mammals, oral administration of a high dose of lipopolysaccharides caused no toxicity or systemic inflammation (30), probably due to poor endotoxin absorption (31). We therefore do not believe that endotoxin effects influenced our results.
Acknowledgments
This study was supported by a grant from the Ruhr-University Bochum (FoRUM: AZ F631-2008).
Davisco Foods International provided the β-lactoglobulin. No other potential conflicts of interest relevant to this article were reported.
A.S. codesigned, prepared, and coordinated the performance of the study, performed statistics, and wrote the manuscript. P.K. performed the clinical investigations and reviewed the manuscript. K.F. and U.S. prepared the C-BLG and AGE-BLG and reviewed the manuscript. W.C. performed the AGE measurements. C.G. codesigned, prepared, and coordinated the performance of the study, contributed to discussion, and reviewed the manuscript. D.T. coordinated the performance of the study and reviewed the manuscript. A.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Prof. Helen Vlassara (Mount Sinai School of Medicine) for enabling AGE measurements in the laboratory and for performing together with Prof. Jaime Uribarri (Mount Sinai School of Medicine) a very thorough correction of the manuscript and for giving important input for the interpretation of data. The authors also thank Dr. Tim Heise (Profil Institute for Metabolic Research) for correcting the manuscript. This study was enriched by the excellent laboratory work of Marlen Ewald (Heart and Diabetes Center).
Footnotes
Clinical trial reg. no. NCT01456026, clinicaltrials.gov.
References
- 1.Vlassara H. Recent progress in advanced glycation end products and diabetic complications. Diabetes 1997;46(Suppl. 2):S19–S25 [DOI] [PubMed] [Google Scholar]
- 2.Vlassara H, Palace MR. Glycoxidation: the menace of diabetes and aging. Mt Sinai J Med 2003;70:232–241 [PubMed] [Google Scholar]
- 3.Nin JW, Jorsal A, Ferreira I, et al. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care 2011;34:442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 5.Tan KC, Chow WS, Ai VH, Metz C, Bucala R, Lam KS. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care 2002;25:1055–1059 [DOI] [PubMed] [Google Scholar]
- 6.Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 2010;110:911–916, e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlassara H, Cai W, Crandall J, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA 2002;99:15596–15601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uribarri J, Peppa M, Cai W, et al. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J Am Soc Nephrol 2003;14:728–731 [DOI] [PubMed] [Google Scholar]
- 9.Uribarri J, Peppa M, Cai W, et al. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am J Kidney Dis 2003;42:532–538 [DOI] [PubMed] [Google Scholar]
- 10.Förster A, Kühne Y, Henle T. Studies on absorption and elimination of dietary maillard reaction products. Ann N Y Acad Sci 2005;1043:474–481 [DOI] [PubMed] [Google Scholar]
- 11.Foerster A, Henle T. Glycation in food and metabolic transit of dietary AGEs (advanced glycation end-products): studies on the urinary excretion of pyrraline. Biochem Soc Trans 2003;31:1383–1385 [DOI] [PubMed] [Google Scholar]
- 12.Erbersdobler HF, Faist V. Metabolic transit of Amadori products. Nahrung 2001;45:177–181 [DOI] [PubMed] [Google Scholar]
- 13.Koschinsky T, He CJ, Mitsuhashi T, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA 1997;94:6474–6479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negrean M, Stirban A, Stratmann B, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr 2007;85:1236–1243 [DOI] [PubMed] [Google Scholar]
- 15.Uribarri J, Stirban A, Sander D, et al. Single oral challenge by advanced glycation end products acutely impairs endothelial function in diabetic and nondiabetic subjects. Diabetes Care 2007;30:2579–2582 [DOI] [PubMed] [Google Scholar]
- 16.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 1994;24:1468–1474 [DOI] [PubMed] [Google Scholar]
- 17.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 2005;1:183–198 [PMC free article] [PubMed] [Google Scholar]
- 18.Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol 1999;31:23–37 [DOI] [PubMed] [Google Scholar]
- 19.Ahmed N, Mirshekar-Syahkal B, Kennish L, Karachalias N, Babaei-Jadidi R, Thornalley PJ. Assay of advanced glycation endproducts in selected beverages and food by liquid chromatography with tandem mass spectrometric detection. Mol Nutr Food Res 2005;49:691–699 [DOI] [PubMed] [Google Scholar]
- 20.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992;340:1111–1115 [DOI] [PubMed] [Google Scholar]
- 21.Birlouez-Aragon I, Saavedra G, Tessier FJ, et al. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am J Clin Nutr 2010;91:1220–1226 [DOI] [PubMed] [Google Scholar]
- 22.Grau M, Hendgen-Cotta UB, Brouzos P, et al. Recent methodological advances in the analysis of nitrite in the human circulation: nitrite as a biochemical parameter of the L-arginine/NO pathway. J Chromatogr B Analyt Technol Biomed Life Sci 2007;851:106–123 [DOI] [PubMed] [Google Scholar]
- 23.Seftel AD, Vaziri ND, Ni Z, et al. Advanced glycation end products in human penis: elevation in diabetic tissue, site of deposition, and possible effect through iNOS or eNOS. Urology 1997;50:1016–1026 [DOI] [PubMed] [Google Scholar]
- 24.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004;291:1978–1986 [DOI] [PubMed] [Google Scholar]
- 25.Stirban A, Negrean M, Stratmann B, et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care 2006;29:2064–2071 [DOI] [PubMed] [Google Scholar]
- 26.Kanabrocki EL, Murray D, Hermida RC, et al. Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol Int 2002;19:423–439 [DOI] [PubMed] [Google Scholar]
- 27.Blanco RA, Ziegler TR, Carlson BA, et al. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr 2007;86:1016–1023 [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Tasaka S, Shiraishi Y, et al. Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury. Am J Respir Crit Care Med 2008;178:356–362 [DOI] [PubMed] [Google Scholar]
- 29.Buetler TM, Latado H, Leclerc E, et al. Glycolaldehyde-modified β-lactoglobulin AGEs are unable to stimulate inflammatory signaling pathways in RAGE-expressing human cell lines. Mol Nutr Food Res 2011;55:291–299 [DOI] [PubMed] [Google Scholar]
- 30.Inagawa H, Kohchi C, Soma G. Oral administration of lipopolysaccharides for the prevention of various diseases: benefit and usefulness. Anticancer Res 2011;31:2431–2436 [PubMed] [Google Scholar]
- 31.Sonaje K, Lin KJ, Tseng MT, et al. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011;32:8712–8721 [DOI] [PubMed] [Google Scholar]