Abstract
OBJECTIVE
To quantitatively assess the strength and shape of the association between blood 25-hydroxy vitamin D [25(OH)D] levels and incident risk of type 2 diabetes.
RESEARCH DESIGN AND METHODS
A systematic search of the MEDLINE and Embase databases and a hand search of references from original reports were conducted up to 31 October 2012. Prospective observational studies that assessed the association between blood levels of 25(OH)D and risk of incident type 2 diabetes were included for meta-analysis. DerSimonian and Laird’s random-effects model was used. A quadratic spline regression analysis was used to examine the shape of the association with a generalized least-squares trend test performed for the dose-response relation.
RESULTS
A total of 21 prospective studies involving 76,220 participants and 4,996 incident type 2 diabetes cases were included for meta-analysis. Comparing the highest to the lowest category of 25(OH)D levels, the summary relative risk for type 2 diabetes was 0.62 (95% CI 0.54–0.70). A spline regression model showed that higher 25(OH)D levels were monotonically associated with a lower diabetes risk. This inverse association did not differ by sex, duration of follow-up, study sample size, diabetes diagnostic criteria, or 25(OH)D assay method. A linear trend analysis showed that each 10 nmol/L increment in 25(OH)D levels was associated with a 4% lower risk of type 2 diabetes (95% CI 3–6; P for linear trend < 0.0001).
CONCLUSIONS
Our meta-analysis showed an inverse and significant association between circulating 25(OH)D levels and risk of type 2 diabetes across a broad range of blood 25(OH)D levels in diverse populations.
Low vitamin D status is prevalent in many populations and has become a common public health problem worldwide (1,2). Vitamin D is well-known for its essential role in calcium homeostasis and bone health (2). Emerging evidence from both in vitro and in vivo studies has suggested extraskeletal effects of vitamin D, including on insulin action and secretion (2,3). Population studies have provided further support to the hypothesis that low vitamin D status, as assessed by circulating 25-hydroxy vitamin D [25(OH)D] levels, is associated with impaired β-cell function, insulin resistance, and impaired glucose intolerance and thereby may be associated with higher risk of type 2 diabetes (4–7). An inverse association between 25(OH)D levels and prevalent type 2 diabetes has been shown (4,8–10); however, a temporal relationship cannot be established from such cross-sectional studies that are subject to bias due to the possibility of reverse causation. Recently, several prospective observational studies have reported a significant association between high circulating levels of 25(OH)D and lower incidence of type 2 diabetes (11–17). However, no association was observed in other studies (18–21). In addition, individual studies have been underpowered to examine this relation across a broad range of circulating 25(OH)D levels. As highlighted by the recent report on vitamin D from the Institute of Medicine (IOM) (1,22), available evidence on the relation between vitamin D status and type 2 diabetes remains inconsistent and inconclusive, and there is a need for further research to clarify the optimal levels of 25(OH)D for nonskeletal outcomes, including type 2 diabetes, and to assess whether there is a nonlinear relationship between 25(OH)D and diabetes risk. Assessment of possible threshold levels of 25(OH)D will greatly advance our understanding of the magnitude and shape of the association of vitamin D with incidence of type 2 diabetes.
Available evidence from prospective studies with circulating 25(OH)D levels measured in baseline blood samples collected before the onset of disease allow us to address the temporal relation of vitamin D status with risk of type 2 diabetes. We therefore conducted a meta-analysis of prospective studies from various populations to quantify the association between circulating 25(OH)D levels and subsequent risk of type 2 diabetes and examine possible threshold effect of 25(OH)D levels.
RESEARCH DESIGN AND METHODS
Data source and searches
Relevant studies were identified by searching MEDLINE and Embase databases for all published articles up to 31 October 2012, using the search terms “vitamin D,” “25-hydroxy vitamin D,” “25(OH)D,” “1,25-dihydroxy vitamin D,” “1,25(OH)2D,” “calcidiol,” “calcitriol,” “insulin resistance,” “insulin secretion,” “insulin sensitivity,” “glucose intolerance,” “glucose metabolism,” “beta-cell function,” “type 2 diabetes,” and “diabetes.” The search was further restricted to English-language articles, human studies, and adult subjects aged ≥19 years. Additional studies were retrieved through a hand search of references from original reports. All prospective studies on this topic were considered eligible if they provided data on the relationship between baseline circulating levels of 25(OH)D and risk of type 2 diabetes.
In the first round of screening (n = 542), 470 articles were excluded for at least one of the following reasons: studies that did not study circulating 25(OH)D as an exposure or type 2 diabetes as an outcome (200 articles), studies among children and adolescence populations (4 articles), nonhuman studies (15 articles, including chemistry, animal, cell line, and isolated tissue studies), reviews/editorials/guidelines/letters/commentaries (185 articles), cross-sectional or retrospective studies (61 articles), and case reports (5 articles) (Fig. 1). In the second round of screening, full-text articles were retrieved (n = 72). Sixty-three articles were further excluded for at least one of the following reasons: updated data available from other studies in the same population or duplicate publications (3 articles), diabetes complications as major outcomes (33 articles), association measures for diabetes-related risk factors (19 articles), and lack of directly measured 25(OH)D levels (2 articles using predicted score). Our search strategy and inclusion/exclusion criteria resulted in a total of 21 independent prospective studies (extracting from 15 articles) being included in the current meta-analysis.
Figure 1.
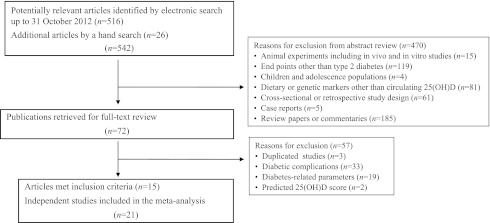
Flow chart of study selection.
Data extraction
Three investigators (Y.S., L.W., and L.C.D.G.) independently reviewed each eligible article and extracted relevant data examining the prospective associations of circulating levels of 25(OH)D with type 2 diabetes risk. Differences, if any, were reconciled through group discussion. Data extracted include population source, study design, follow-up period, sample size, subject characteristics (age, sex, and comorbid conditions), 25(OH)D assay methods, diabetes end points, and main study findings. When results were available only on different subpopulations in the same study (13,14,23), we separately extracted data for each subpopulation (study characteristics described in Supplementary Table 1).
Statistical analysis
To provide quantitative evidence from all studies and maximize statistical power for hypothesis testing, we performed meta-analyses using DerSimonian and Laird’s random-effects model with inverse-variance (SE) weighting of individual study results (24) when data could be combined. The summary relative risks (RR) with 95% CI were calculated for the highest versus the lowest category of 25(OH)D levels. We also performed subgroup meta-analyses to explore potential effect modification by prespecified factors, including sex, follow-up duration, sample size of cases, adjustment for BMI, adjustment for BMI and other metabolic parameters (including hypertension, lipids, or inflammatory biomarkers), and 25(OH)D assay methods. Heterogeneity across studies was assessed by Cochran Q statistic. Because the Cochran Q statistic has low statistical power (25), we also calculated the I2 and H statistics to reflect between-study heterogeneity. The percentage of I2 ∼25 (I2 = 25), 50 (I2 = 50), and 75 (I2 = 75) indicates low, medium, and high heterogeneity, respectively (26). The H statistic is a complement to the I2 statistic in assessing study heterogeneity; an H <1.2 indicates little heterogeneity, and an H >1.5 raises caution regarding notable heterogeneity (26). We visually assessed publication bias using Begg modified funnel plots, in which the RR was plotted on a logarithmic scale (log[RR]) against its SE from each study. Publication bias was also assessed by two formal tests: Begg adjusted rank correlation test and Egger regression asymmetry test (27,28). The former was used to examine if there was significant correlation between the effect estimates and their variances, and the latter uses an inverse-variance weighted regression of the effect sizes on their precision (the inverse of SE) to test whether the intercept deviates significantly from zero.
We tested for possible nonlinear association between circulating 25(OH)D levels and type 2 diabetes using a quadratic polynomial spline regression analysis, considering a possibility of data perturbation at an extreme end of the exposure range (29). We also used the method described by Greenland and Longnecker (30) for the dose-response analysis to compute the linear trend from the correlated RRs and 95% CIs across categories of 25(OH)D. The median level of 25(OH)D in each category was assigned to the corresponding RR when reported in the study. If not reported, the values assigned were the means or the midpoint of the lower and upper bound in each category. For extreme open-ended categories, half the width of the adjacent category was subtracted (for the lowest category) or added (for the uppermost category) to obtain the midpoint. The numbers of case and control subjects or person-years by category were also collected if available.
All analyses were performed using the STATA statistical software (Version 10.1; STATA Corp., College Station, TX). Statistical significance was defined as two-tailed α < 0.05.
RESULTS
We included 15 publications (11–16,18,19,23,31–33) that met our inclusion criteria in our meta-analysis, which provided data from 21 independent studies [11 cohort studies (11–13,17,18,20,21,31–33), 8 nested case-control studies (14,15,19,23), and 2 case-cohort studies (16,33)] (Fig. 1). These prospective studies comprised a total of 76,220 participants and 4,996 incident diabetes cases. As summarized in Supplementary Table 1, 13 studies were population-based (12–14,16,17,20,21,23,32,33), and 2 studies included postmenopausal women only (18,19). Nineteen studies included largely whites (11–18,20,21,23,32,33), and 2 studies included multiple racial/ethnic populations (19,31). The outcome of diabetes was ascertained using a combination of criteria, including diabetes-specific pharmacotherapy, diabetes-related hospitalization, self-report, and glycemic status information (11–16,18,19). Five studies used the American Diabetes Association glycemic criteria for diagnosis (20,21,23,31,33). The assay method for 25(OH)D varied across studies. Radioimmunoassay was used in 8 studies (14,15,18,21,33), chemiluminescent immunoassay was used in 10 studies (11–13,16,17,19,23,32), and only 3 studies used liquid chromatography–tandem mass spectrometry, which is the gold standard to measure 25(OH)D (20,31,33).
Hypothesis testing
Fig. 2 shows the summary RR of type 2 diabetes comparing the highest to lowest category of 25(OH)D levels for hypothesis testing. The summary RR was 0.62 (95% CI 0.54–0.70), indicating a significant inverse association between baseline 25(OH)D levels and risk of type 2 diabetes. The P value for the Cochran Q test was 0.21, the H statistic was 1.1 (1.0–1.5), and the I2 statistic was 19% (0–53), indicating no evidence of significant between-study heterogeneity. The Begg funnel plot for the visual assessment of publication bias showed that smaller RRs with large SEs tended to be above the horizontal line, indicating a possibility of publication bias in favor of small studies with null findings (data not shown). However, the Egger test (P = 0.39) and the Begg test (P = 0.99) did not show evidence of publication bias.
Figure 2.
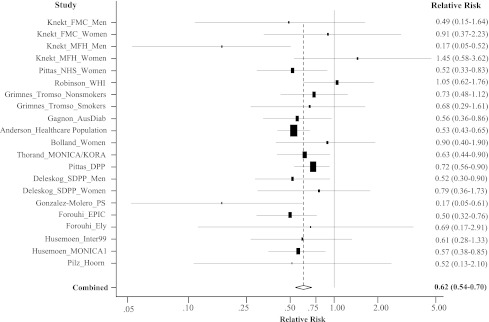
A random-effects meta-analysis of 21 independent prospective studies with adjusted RR and 95% CI of type 2 diabetes in relation to serum 25(OH)D levels (the highest category versus the lowest category). AusDiab, Australian Diabetes, Obesity and Lifestyle Study; DPP, Diabetes Prevention Program; Ely, Medical Research Council Ely Study; EPIC, European Prospective Investigation into Cancer-Norfolk Study; FMC, Finnish Mobile Clinic Health Examination Survey; Hoorn, Hoorn Study; Inter99, Inter99 Study; MFH, Mini-Finland Health Survey; MONICA1, Danish MONICA1 survey; MONICA/KORA, Monitoring of Trends and Determinants in Cardiovascular Disease/Cooperative Health Research in the Region of Augsburg Study; NHS, Nurses’ Health Study; PS, Pizarra Study; SDPP, Stockholm Diabetes Prevention Program.
Sensitivity analysis
We conducted a sensitivity analysis to assess the extent to which individual studies with extremely large RRs influenced the summary RR. The exclusion of the study by Anderson et al. (11) that included the largest sample size and RR estimate reduced between-study heterogeneity but did not appreciably change the summary RR (0.64; 95% CI 0.56–0.73; P for Q statistic = 0.27, H = 1.1 [1.0–1.4], and I2 = 15% [0–50]). Omitting four studies from the earliest publication by Knekt et al. (14), which included small sample size and heterogeneous results, also did not substantially influence the summary RR (0.61; 95% CI 0.55–0.68; P for Q statistic = 0.46, H = 1.0 [1.0–1.4], and I2 = 0% [0–51]). The most recent study with the smallest number of cases (n = 37) yielded large variance in the effect estimate; removing this study led to almost the same summary RR (0.62; 95% CI 0.56–0.70; P for Q statistic = 0.35, H = 1.0 [1.0–1.3], and I2 = 9% [0–44]).
Sources of heterogeneity
We evaluated potential effect modifiers in stratified analyses (Table 1). The inverse association between 25(OH)D and diabetes risk was consistently observed in men, women, and a mixed population of men and women. Associations tended to be stronger for men than for women alone, but the difference did not reach statistical significance. Nor did duration of follow-up, study sample size, diabetes diagnostic criteria, and 25(OH)D assay methods influence the summary RRs. Several studies presented RRs for 25(OH)D levels and type 2 diabetes with and without adjustment for metabolic variables, including BMI, hypertension, markers of glycemia and insulin sensitivity, plasma lipid levels, or inflammatory markers. The associations between 25(OH)D levels and diabetes risk were attenuated but remained statistically significant after adjustment for BMI and hypertension and/or other biomarkers: the RRs were 0.52 (95% CI 0.46–0.58) and 0.63 (0.56–0.72) in the models without and with adjustment for these covariates, respectively (P for interaction = 0.02). Likewise, adjustment for BMI alone slightly attenuated the association (P for interaction = 0.06).
Table 1.
Subgroup analyses for the relation between circulating 25(OH)D and type 2 diabetes

Dose-response analysis
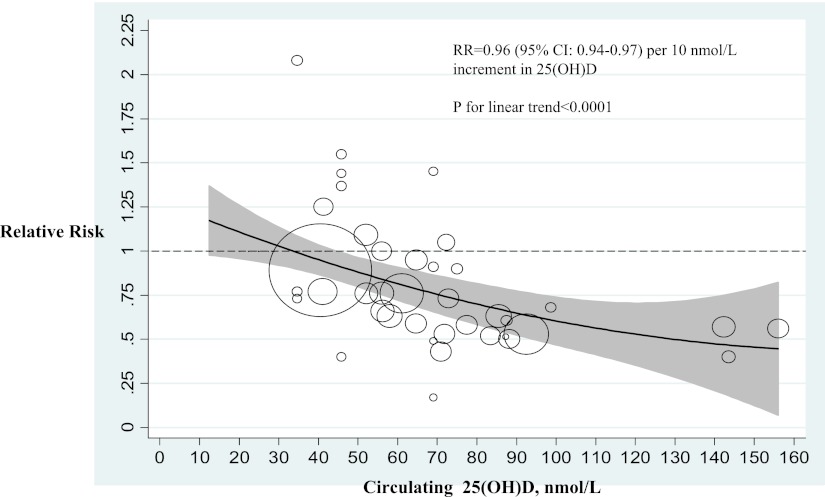
Fig. 3 shows the dose-response relation between circulating 25(OH)D and risk of type 2 diabetes. Overall, the test for a linear relation across the range of 25(OH)D from 20 up to 160 nmol/L was significant (P < 0.0001). The RR for type 2 diabetes was 0.96 (95% CI 0.94–0.97) per 10 nmol/L increment in 25(OH)D. A significantly lower risk of type 2 diabetes became evident when 25(OH)D approximated 50 nmol/L. Because most studies had 25(OH)D ranges <100 nmol/L, current evidence for the relation of 25(OH)D of >100 nmol/L with type 2 diabetes was weak.
Figure 3.
Relation between the risk of type 2 diabetes and baseline levels of 25(OH)D in 18 independent prospective studies included in the meta-analysis. The relation is modeled by the quadratic spline regression. Circles indicate RR in each study. The circle size is proportional to the precision of the RR (inverse of variance). The gray-shaded region shows the 95% CIs around the regression line.
CONCLUSIONS
On the basis of 21 prospective studies including 4,996 incident cases of type 2 diabetes and 76,220 nondiabetic controls, our meta-analysis showed a significantly inverse association between 25(OH)D levels and incidence of type 2 diabetes. The association was consistent and did not differ appreciably by sex, study size, follow-up duration, diabetes diagnosis criteria, or 25(OH)D assay methods. The association was attenuated but remained significant after adjustment for BMI and/or intermediate biomarkers.
Blood 25(OH)D levels, which reflect all sources of vitamin D exposure and have a half-life of 2 to 3 weeks, have been widely used as a surrogate of vitamin D status (2,3). Due to differences in assay methods and population characteristics, there is no consensus on the cutoff values defining vitamin D insufficiency or deficiency. Vitamin D insufficiency has been previously reported to range from levels of 40–75 nmol/L (16–30 ng/mL), and vitamin D deficiency is generally defined as levels of <50 nmol/L (20 ng/mL) (2,34). Using 25(OH)D levels ≤50 nmol/L (20 ng/mL) to define vitamin D deficiency, data from 4,495 adults in the National Health and Nutrition Examination Survey 2005–2006 showed that the overall prevalence of vitamin D deficiency was 41.6%, with the highest rate found in blacks (82.1%), followed by Hispanics (62.9%), others (57.6%), and whites (30.9%) (35). The IOM’s new guidelines for vitamin D intake and desirable blood levels, which were based on a systematic scientific review of available evidence at the time (1,22), state that a level of 50 nmol/L (20 ng/mL) of serum 25(OH)D is needed to maintain bone health for most individuals. The IOM report called for additional research to clarify the relation of vitamin D levels with nonskeletal outcomes (1,22).
The optimal 25(OH)D levels for type 2 diabetes prevention remain unknown. Epidemiological evidence relating lower circulating vitamin D levels to hyperglycemia, insulin resistance, or type 2 diabetes primarily derives from cross-sectional reports (4,8–10). Prospective data are limited and have been inconclusive (36). Our dose-response curve showed an inverse and significant relation between 25(OH)D and type 2 diabetes across a broad range of 25(OH)D levels in diverse populations. Our results also confirmed that baseline 25(OH)D levels ≥50 nmol/L were significantly associated with a lower risk of type 2 diabetes, although further studies with higher power are required to provide more stable estimates of this association.
The observed inverse association did not differ by sex, study sample size, duration of follow-up, diabetes diagnosis criteria, and 25(OH)D assay methods. An early pooled analysis of 2 nested case-control studies of 412 incident cases of type 2 diabetes and 986 control subjects found a strong inverse association in men but not in women (14). However, this finding of sex-specific relation of 25(OH)D with type 2 diabetes was not confirmed by subsequently published studies nor by our meta-analysis. It is likely that such sex difference resulted from residual confounding or statistical fluctuation due to small sample size. Adiposity is another important covariate in vitamin D–diabetes relation. The interrelationship between vitamin D status and adiposity is complex and possibly bidirectional. Increased storage of 25(OH)D in adipose tissue and less sun exposure due to limited mobility and/or excessive subcutaneous fat deposits in obese individuals will lead to low circulating levels of 25(OH)D (2,3). Conversely, vitamin D may directly affect adiposity and other metabolic parameters such as dyslipidemia, hypertension, and systemic inflammation that mediate the pathway from vitamin D status to type 2 diabetes (36,37). In this sense, adiposity could be considered as a confounder or an intermediate variable. Adjustment for adiposity and other obesity-related metabolic parameters may be an overadjustment, possibly underestimating the true association. Finally, with the exception of one study (31), all studies used a single measurement of 25(OH)D at baseline, which is not a time-integrated measure of vitamin D status. 25(OH)D was also assayed using different methods in previous studies. There was substantial intra- and interassay variation in 25(OH)D levels (38). However, the summary RR of type 2 diabetes in relation to 25(OH)D did not differ by assay methods.
Our findings of an inverse relation between 25(OH)D and type 2 diabetes are supported by biological evidence that vitamin D may be implicated in the pathogenesis of diabetes and its complications (36,37). A large body of literature has suggested that optimal vitamin D homeostasis is essential for insulin action and secretion (2,3), two fundamental features in the pathogenesis of type 2 diabetes. Clearly, direct evidence from ongoing (39) and future clinical trials of higher-dose vitamin D supplementation is warranted to clarify any beneficial effects of vitamin D on primary prevention of type 2 diabetes.
Findings from our meta-analysis of observational studies will augment and complement findings from randomized trials of the effect of vitamin D supplements on type 2 diabetes. Although randomized trials are critical for establishing cause-and-effect relationships between vitamin D supplementation and health outcomes, they will not address all the potential questions because of their fixed dose (or at most, a few doses) of vitamin D and narrow range of 25(OH)D levels achieved by supplementation. Prospective observational studies allow us to assess 25(OH)D thresholds for diabetes risk across a broad spectrum of 25(OH)D levels. The largest randomized trial of vitamin D supplement to date, the Women’s Health Initiative (WHI) Clinical Trial, has evaluated vitamin D plus calcium supplementation for fracture prevention in >36,000 postmenopausal women (40). Secondary analysis of the WHI trial with 33,951 initially nondiabetic women did not observe any effect from daily intake of 1,000 mg elemental calcium plus 400 IU vitamin D3 on incident diabetes over 7 years of follow-up (40). Of note, a dose of 400 IU/day vitamin D in the WHI raised median levels of serum 25(OH)D from 42.3 to only 54.1 nmol/L (∼12 nmol/L), which is below the optimal value of 75 nmol/L or more for skeletal and nonskeletal health including type 2 diabetes (41). Similarly, secondary analysis of another large randomized trial, the Randomized Evaluation of Calcium Or vitamin D (RECORD) trial, did not observe any effect from daily intake of 800 IU vitamin D3 on incident diabetes over 2–5 years, although such a daily dose raised serum 25(OH)D from 38 to 62 nmol/L on average (42).
Our meta-analysis has some limitations. First, the observational nature of prospective studies included in our analysis cannot rule out residual confounding, although the consistency of our results across multiple strata and sensitivity analyses minimizes the likelihood that residual confounding explains the findings. Second, as in any meta-analysis, publication bias is possible, although our visual examination and formal tests did not suggest the presence of substantial publication bias. Finally, limited data from existing prospective studies lacked sufficient power to detect potential sex- or ethnicity-specific 25(OH)D thresholds or dose-response relations.
In conclusion, our meta-analysis of 21 prospective studies demonstrates a significant inverse association between circulating 25(OH)D levels and risk of incident type 2 diabetes in a dose-response manner across a wide spectrum of 25(OH)D levels. Direct evidence from future randomized trials is warranted to clarify a cause-and-effect relationship between vitamin D and type 2 diabetes.
Acknowledgments
This work was supported by grants R01-DK-58845 and R01-DK-088078 (to Y.S.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Institutes of Health (NIH). Y.S. is the Principal Investigator of an ancillary study (R01-DK-088078) investigating diabetes prevention in the VITamin D and OmegA-3 TriaL (VITAL). A.G.P. was supported by grants R01-DK-76092 and U34-DK-91958 from the NIDDK, the Office of the Director-NIH, and the NIH Office of Dietary Supplements. C.Z. was supported by the Intramural Research Program of the NIH/Eunice Kennedy Shriver National Institute of Child Health & Human Development. J.E.M. is the Principal Investigator of the parent VITAL study, a large-scale randomized trial of vitamin D and omega-3 fatty acids for the prevention of cancer and cardiovascular disease and funded by the NIH.
No potential conflicts of interest relevant to this article were reported.
Y.S. did the literature search and data extraction, selected the studies, carried out the statistical analyses, wrote the first draft of the original manuscript, and contributed to discussion. L.W. did the literature search and data extraction, selected the studies, and contributed to discussion. A.G.P., C.Z., J.E.M., and F.B.H. contributed to discussion. L.C.D.G. did the literature search and data extraction, selected the studies, and contributed to discussion.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0962/-/DC1.
The National Institutes of Health had no role in the study design and conduct or the decision to write this review.
References
- 1.The Institute of Medicine (IOM) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC, The National Academies Press, 2011 [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 3.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 2008;88:491S–499S [DOI] [PubMed] [Google Scholar]
- 4.Need AG, O’Loughlin PD, Horowitz M, Nordin BE. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol (Oxf) 2005;62:738–741 [DOI] [PubMed] [Google Scholar]
- 5.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract 1995;27:181–188 [DOI] [PubMed] [Google Scholar]
- 6.Snijder MB, van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 2005;90:4119–4123 [DOI] [PubMed] [Google Scholar]
- 7.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004;79:820–825 [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 2005;28:1228–1230 [DOI] [PubMed] [Google Scholar]
- 9.Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes 2008;57:298–305 [DOI] [PubMed] [Google Scholar]
- 10.Scragg R, Sowers M, Bell C, Third National Health and Nutrition Examination Survey Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–2818 [DOI] [PubMed] [Google Scholar]
- 11.Anderson JL, May HT, Horne BD, et al. Intermountain Heart Collaborative (IHC) Study Group Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol 2010;106:963–968 [DOI] [PubMed] [Google Scholar]
- 12.Gagnon C, Magliano DJ, Ebeling PR, et al. Association between hyperglycaemia and fracture risk in non-diabetic middle-aged and older Australians: a national, population-based prospective study (AusDiab). Osteoporos Int 2010;21:2067–2074 [DOI] [PubMed] [Google Scholar]
- 13.Grimnes G, Emaus N, Joakimsen RM, et al. Baseline serum 25-hydroxyvitamin D concentrations in the Tromsø Study 1994-95 and risk of developing type 2 diabetes mellitus during 11 years of follow-up. Diabet Med 2010;27:1107–1115 [DOI] [PubMed] [Google Scholar]
- 14.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008;19:666–671 [DOI] [PubMed] [Google Scholar]
- 15.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care 2010;33:2021–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorand B, Zierer A, Huth C, et al. Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the MONICA/KORA Augsburg study. Diabetes Care 2011;34:2320–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husemoen LL, Skaaby T, Thuesen BH, Jørgensen T, Fenger RV, Linneberg A. Serum 25(OH)D and incident type 2 diabetes: a cohort study. Eur J Clin Nutr 2012;66:1309–1314DOI: 10.1038/ejcn.2012.134 [DOI] [PubMed] [Google Scholar]
- 18.Bolland MJ, Bacon CJ, Horne AM, et al. Vitamin D insufficiency and health outcomes over 5 y in older women. Am J Clin Nutr 2010;91:82–89 [DOI] [PubMed] [Google Scholar]
- 19.Robinson JG, Manson JE, Larson J, et al. Lack of association between 25(OH)D levels and incident type 2 diabetes in older women. Diabetes Care 2011;34:628–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husemoen LL, Thuesen BH, Fenger M, et al. Serum 25(OH)D and type 2 diabetes association in a general population: a prospective study. Diabetes Care 2012;35:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilz S, van den Hurk K, Nijpels G, et al. Vitamin D status, incident diabetes and prospective changes in glucose metabolism in older subjects: the Hoorn study. Nutr Metab Cardiovasc Dis 2012;22:883–889 [DOI] [PubMed] [Google Scholar]
- 22.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deleskog A, Hilding A, Brismar K, Hamsten A, Efendic S, Östenson CG. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia 2012;55:1668–1678 [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 25.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med 1998;17:841–856 [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Spiegelhalter DJ. Being sceptical about meta-analyses: a Bayesian perspective on magnesium trials in myocardial infarction. Int J Epidemiol 2002;31:96–104 [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101 [PubMed] [Google Scholar]
- 29.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol 2004;159:1077–1086 [DOI] [PubMed] [Google Scholar]
- 30.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–1309 [DOI] [PubMed] [Google Scholar]
- 31.Pittas AG, Nelson J, Mitri J, et al. Diabetes Prevention Program Research Group Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program. Diabetes Care 2012;35:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Molero I, Rojo-Martinez G, Morcillo S, et al. Vitamin D and incidence of diabetes: A prospective cohort study. Clin Nutr 2012;31:571–573 [DOI] [PubMed] [Google Scholar]
- 33.Forouhi NG, Ye Z, Rickard AP, et al. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia 2012;55:2173–2182 [DOI] [PubMed] [Google Scholar]
- 34.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28 [DOI] [PubMed] [Google Scholar]
- 35.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 2011;31:48–54 [DOI] [PubMed] [Google Scholar]
- 36.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia 2005;48:1247–1257 [DOI] [PubMed] [Google Scholar]
- 38.Hollis BW. Assessment of circulating 25(OH)D and 1,25(OH)2D: emergence as clinically important diagnostic tools. Nutr Rev 2007;65:S87–S90 [DOI] [PubMed] [Google Scholar]
- 39.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 2012;33:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Boer IH, Tinker LF, Connelly S, et al. Women’s Health Initiative Investigators Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008;31:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005;16:713–716 [DOI] [PubMed] [Google Scholar]
- 42.Avenell A, Cook JA, MacLennan GS, McPherson GC, RECORD trial group Vitamin D supplementation and type 2 diabetes: a substudy of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing 2009;38:606–609 [DOI] [PubMed] [Google Scholar]