Abstract
OBJECTIVE
We sought to investigate whether elevated levels of acute-phase serum amyloid A (A-SAA) protein precede the onset of type 2 diabetes independently of other risk factors, including parameters of glucose metabolism.
RESEARCH DESIGN AND METHODS
Within the population-based Cooperative Health Research in the Region of Augsburg (KORA) S4 study, we measured A-SAA concentrations in 836 initially nondiabetic subjects (55–74 years of age) without clinically overt inflammation who participated in a 7-year follow-up examination including an oral glucose tolerance test.
RESULTS
A-SAA concentrations were significantly associated with incident type 2 diabetes (odds ratio [OR] for a one-SD increase of A-SAA adjusted for age and sex = 1.28 [95% CI 1.08–1.53], P = 0.005), particularly in younger subjects (P value for interaction = 0.047). The association attenuated when adjusting for parameters of glucose metabolism (fasting glucose, fasting insulin, HbA1c, and 2-h glucose; OR 1.16 [0.95–1.42], P = 0.15). Similar analyses for high-sensitive C-reactive protein (hs-CRP) yielded the following ORs: 1.39 (1.10–1.68, P = 0.0006) and 1.13 (0.88–1.45, P = 0.34), respectively. In contrast, A-SAA concentrations were significantly associated with 2-h glucose levels at follow-up even after adjustment for parameters of glucose metabolism (P = 0.008, n = 803).
CONCLUSIONS
Our findings indicate similarly strong prospective associations with type 2 diabetes for A-SAA and hs-CRP and suggest a potential causal link via postchallenge hyperglycemia.
Type 2 diabetes is preceded by a differential activation of components of the innate immune system (1,2). Serum amyloid A (SAA) is a sensitive marker of the acute inflammatory state. Its acute-phase isoform (A-SAA) is up-regulated up to 1,000-fold in response to inflammatory stimuli such as trauma, infection, injury, and stress (3–5). The high inductive capacity, along with the fact that genes and proteins are highly conserved throughout the evolution of vertebrates and invertebrates, suggests that A-SAA plays a key role in pathogen defense mechanisms and probably functions as an effector molecule of the immune system (3).
Cross-sectional data have demonstrated an association between elevated systemic A-SAA concentrations and prevalent type 2 diabetes (6,7) as well as the related metabolic parameters homeostatic model assessment for insulin resistance, fasting insulin, and HbA1c (8,9). In a subgroup of the current study population, we have previously shown that circulating levels of A-SAA were increased not only in prevalent type 2 diabetes subjects but also in individuals with impaired glucose tolerance (7). To our knowledge, up until now, only one prospective study conducted in 492 Aboriginal Canadian subjects has investigated the association between baseline levels of SAA and incident type 2 diabetes and did not find evidence for a significant association (10).
We set out to investigate whether elevated levels of A-SAA precede the onset of type 2 diabetes during 7 years of follow-up in a large population-based study of initially nondiabetic, elderly Western European subjects without clinically overt inflammation. Moreover, we assessed whether this association was independent of other risk factors, including parameters of glucose metabolism (i.e., fasting glucose, fasting insulin, HbA1c, and 2-h glucose), which indicate early impairments of glucose homeostasis. Finally, the strength of the association between A-SAA and incident type 2 diabetes was compared with that of the most frequently used inflammatory marker, circulating high-sensitive C-reactive protein (hs-CRP).
RESEARCH DESIGN AND METHODS
Study population
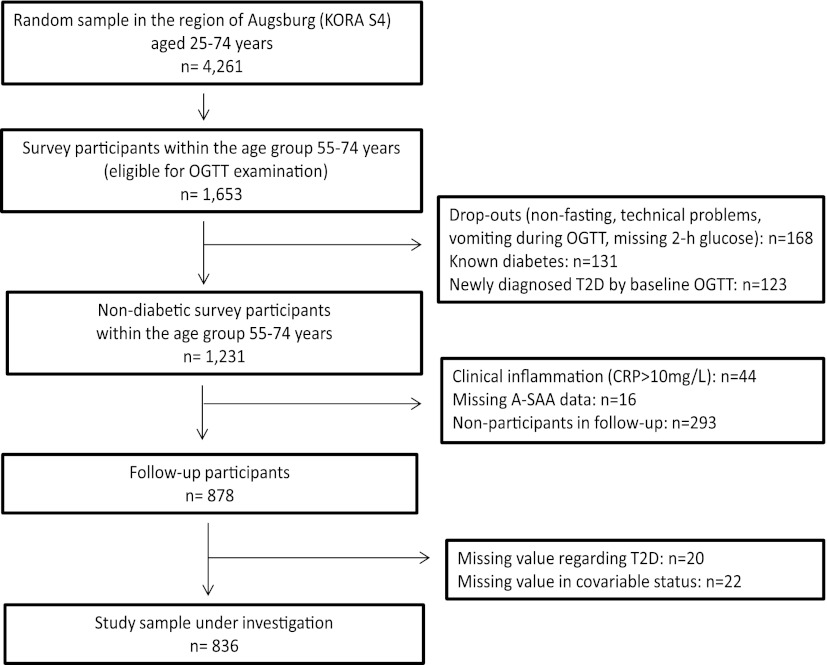
The current study included participants of the population-based Cooperative Health Research in the Region of Augsburg (KORA) survey S4 (1999–2001; N = 4,261) and its 7-year follow-up study F4 (2006–2008; N = 3,080) and was conducted in the region of Augsburg, Southern Germany. The study was approved by the ethics committee of the Bavarian Medical Association, and informed written consent was obtained from all participants. The selection process of study participants is displayed in Fig. 1. The sample included all KORA S4 participants for whom an oral glucose tolerance test (OGTT) was performed at baseline (i.e., only the age-group between 55 and 74 years) and who were nondiabetic according to the OGTT (n = 1,231). For the present analyses, subjects with elevated levels of hs-CRP at baseline, which indicated an acute proinflammatory state (hs-CRP >10 mg/L, n = 44), or with missing A-SAA data (n = 16) were excluded. Furthermore, participants in the follow-up examination (n = 878) with missing data in type 2 diabetes status (n = 20) or in one of the covariables used in the different models (n = 22) were also excluded. Thus, 836 subjects were included in the present analyses.
Figure 1.
Flowchart of the selection process of study participants for the present analyses. T2D, type 2 diabetes.
Ascertainment of type 2 diabetes and assessment of independent variables
Type 2 diabetes was defined on the basis of an OGTT according to the 1999 World Health Organization diagnostic criteria (11) or by a self-report, which was validated using data on glucose-lowering medication or by questioning the attending physician. At baseline, fasting EDTA plasma samples were collected and A-SAA was analyzed by immunonephelometry on a BN II analyzer using an N Latex–enhanced test (Siemens, Schwalbach, Germany). The interassay coefficient of variation was <7% (7,12).
Information regarding the collection of laboratory, anthropometric, and socioeconomic variables is provided in detail elsewhere (13–16).
Statistical analyses
A-SAA, hs-CRP, and covariables were tested for normal distribution. Furthermore, all numerical covariables were tested for correlations between each other using the Pearson coefficient of correlation for normally distributed variables and the Spearman coefficient of correlation for skewed distributed variables. Baseline characteristics of subjects who developed type 2 diabetes and those who remained diabetes free were compared using five different tests: 1) Pearson χ2 test to test for independence of categorical variables between groups, 2) Fisher exact test to test for independence between groups with small sample sizes in categories, 3) two-sample Student t test for normally distributed numerical variables with equal variances, 4) Welch t test for normally distributed numerical variables with unequal variances, and 5) the Mann-Whitney U test for skewed distributed numerical variables. Multivariable linear and logistic regression analyses with various degrees of adjustment were performed. Interactions between A-SAA and sex as well as A-SAA and age were assessed by adding an interaction term to the multivariable models. Two-sided P values that were <0.05 were considered to be statistically significant. All statistical analyses were conducted using SAS (version 9.2; SAS Institute, Cary, NC).
RESULTS
Baseline characteristics stratified by incident type 2 diabetes status are provided in Table 1.
Table 1.
Baseline characteristics of the study participants stratified by incident type 2 diabetes status
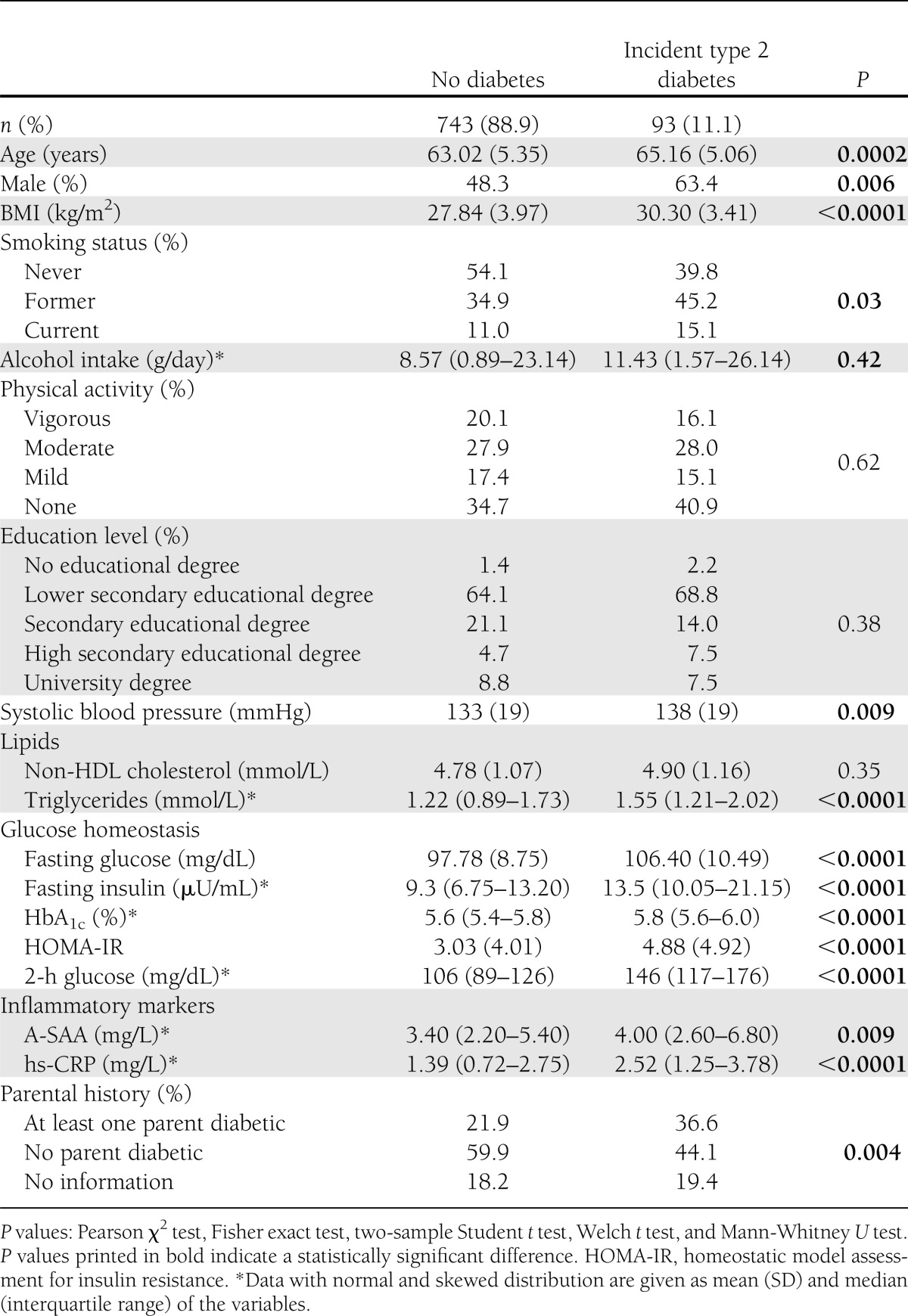
Seven-year cumulative incidence of type 2 diabetes was 11.1% (n = 93) in our study. Circulating systemic A-SAA levels were significantly higher in 1) diabetic subjects (median of A-SAA levels = 4.0 mg/L [IQR 2.6–6.8 mg/L]) than in nondiabetic subjects (3.4 mg/L [2.2–5.4 mg/L], P = 0.009), 2) women (median = 3.9 mg/L [2.6–6.1 mg/L]) than in men (2.9 mg/L [2.0–4.7 mg/L], P < 0.0001), and 3) subjects between 65 and 74 years of age (median = 3.6 mg/L [2.3–6.2 mg/L]) than in subjects between 55 and 64 years of age (3.3 mg/L [2.2–5.0 mg/L], P = 0.03). All covariables were correlated with each other with r < 0.5 except for hs-CRP and A-SAA (r = 0.50) (Supplementary Table 1).
Table 2 presents the association between baseline A-SAA levels and incident type 2 diabetes. A-SAA concentrations were significantly associated with the development of type 2 diabetes during the 7-year follow-up period. The odds ratio (OR) (adjusted for age and sex, model 1) for a one-SD (4.16 mg/L) increase in A-SAA was 1.28 (95% CI 1.08–1.53, P = 0.005). The association remained statistically significant after adjustment for multiple covariables, including BMI (model 2), and further traditional risk factors (smoking, alcohol intake, physical activity, education, parental history of diabetes, non-HDL cholesterol, fasting triglycerides, and systolic blood pressure; model 3). To further evaluate the impact of body fat and body composition on the association between A-SAA levels and incident type 2 diabetes, BMI was replaced by waist-to-hip ratio (WHR) or, alternatively, body fat mass index, lean BMI, and appendicular skeletal muscle mass index as proposed by Kyle et al. (17,18) in model 2 of the association analysis. These analyses yielded results similar to those displayed in Table 2 (Supplementary Table 2). The effect estimates decreased and the association was attenuated after additional adjustment for baseline parameters of glucose metabolism (fasting glucose, fasting insulin, HbA1c, and 2-h glucose; model 4). Sensitivity analyses demonstrated that the attenuation was attributable mostly to adjustment for 2-h glucose (model 3 + fasting glucose + fasting insulin: OR 1.25 [95% CI 1.03–1.52], P = 0.02; model 3 + HbA1c: 1.21 [1.01–1.46], P = 0.04; model 3 + 2-h glucose: 1.17 [0.97–1.42], P = 0.11). In multivariable linear regression analyses, the association between baseline levels of A-SAA and ln-transformed levels of 2-h glucose at follow-up was statistically significant in all four models [model 1: β = 0.01, se(β) = 0.003, P < 0.0001; model 2: β = 0.008, se(β) = 0.003, P = 0.0009; model 3: β = 0.007, se(β) = 0.002, P = 0.004; model 4: β = 0.006, se(β) = 0.002, P = 0.008; n = 803].
Table 2.
Results of the overall and age-stratified associations between circulating concentrations of A-SAA and incident type 2 diabetes according to four different models of covariable adjustment
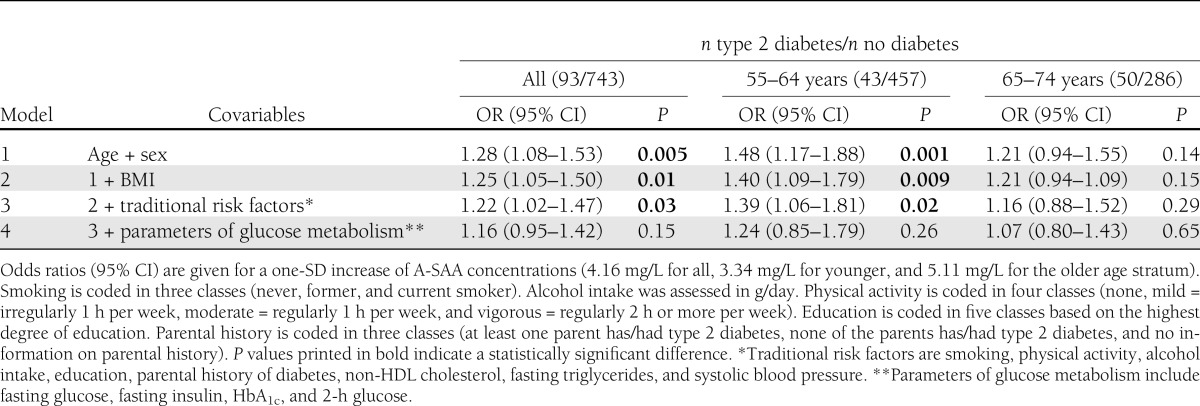
Stratification by sex showed that effect sizes in general were stronger in women than in men but the multiplicative interaction term between sex and A-SAA was not statistically significant (P > 0.1). Replacement of BMI by WHR, body fat mass index, lean fat mass index, or appendicular skeletal muscle mass index also did not indicate a statistically significant sex effect. However, the multiplicative interaction term between the metric age variable and A-SAA was borderline significant regarding the outcome of incident type 2 diabetes (P value for interaction adjusted for age and sex = 0.047). Therefore, we also calculated age-stratified ORs using 10-year age strata (Table 2). Stratified analyses showed that the A-SAA variability was higher in the age stratum 65–74 years (SD for age stratum 55–64 years = 3.34 mg/L; SD for age stratum 65–74 years = 5.11 mg/L). The association between A-SAA and incident type 2 diabetes was only significant in younger subjects (OR for the age-group 55–64 years adjusted for age and sex = 1.48 [95% CI 1.17–1.88], P = 0.001; OR for the age-group 65–74 years adjusted for age and sex = 1.21 [0.94–1.55], P = 0.14). Furthermore, sensitivity analyses demonstrated that for the age-group 55–64 years, the effect was mostly attenuated by adjustment for HbA1c and not 2-h glucose (model 3 + fasting glucose + fasting insulin: OR 1.44 [95% CI 1.07–1.92], P = 0.03; model 3 + HbA1c: 1.26 [0.93–1.70], P = 0.14; model 3 + 2-h glucose: 1.37 [1.00–1.86], P = 0.05), whereas for the age-group 65–74 years, it was mainly affected by adjustment for 2-h glucose (model 3 + fasting glucose + fasting insulin: 1.17 [0.89–1.55], P = 0.31; model 3 + HbA1c: 1.18 [0.90–1.56], P = 0.23; model 3 + 2-h glucose: 1.06 [0.80–1.41], P = 0.69).
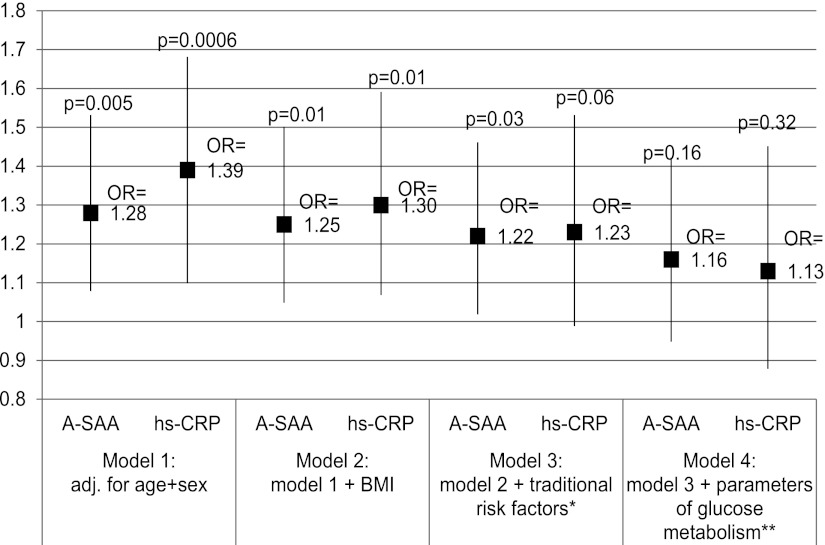
The association between baseline levels of hs-CRP and incident type 2 diabetes was analyzed similarly to the baseline levels of A-SAA (Fig. 2). The hs-CRP effect estimate was somewhat larger than the effect size for A-SAA when adjusted only for age and sex (OR 1.39 [95% CI 1.10–1.68], P = 0.0006), but similar after full adjustment for all covariables (1.13 [0.88–1.45], P = 0.34). No evidence for an age-specific effect was found for hs-CRP (P value for interaction >0.1).
Figure 2.
OR and 95% CI for the association between baseline levels of A-SAA and hs-CRP with incident type 2 diabetes. *, traditional risk factors include smoking, physical activity, alcohol intake, education, parental history of diabetes, non-HDL cholesterol, fasting triglycerides, and systolic blood pressure; **, parameters of glucose metabolism are baseline levels of fasting glucose and fasting insulin, HbA1c, and 2-h glucose. Smoking is coded in three classes (never, former, and current smoker). Physical activity is coded in four classes (none, mild = irregularly 1h per week, moderate = regularly 1 h per week, and vigorous = regularly 2 h or more per week). Education is coded in five classes based on the highest degree of education. Parental history is coded in three classes (at least one parent has/had type 2 diabetes, none of the parents has/had type 2 diabetes, and no information on parental history).
Sensitivity analyses were conducted to assess the impact of menopausal status, hormone replacement therapy, intake of aspirin, and liver disease on the association between levels of A-SAA and incident type 2 diabetes by adjusting for these variables, but all analyses yielded very similar results as those displayed in Table 2 (Supplementary Table 3).
CONCLUSIONS
We observed a statistically significant association between elevated systemic levels of A-SAA and the development of type 2 diabetes in a large population-based cohort with long-term follow-up. This finding extends observations from earlier cross-sectional studies that reported a positive association between systemic A-SAA concentrations and prevalent type 2 diabetes and related traits (6–9). Furthermore, we could show that the association between A-SAA and incident type 2 diabetes was independent of various established type 2 diabetes risk factors, most of which were correlated with A-SAA cross-sectionally in our study. These risk factors include age and sex and the traditional type 2 diabetes risk factors (smoking, physical activity, alcohol intake, education, parental history of diabetes, non-HDL cholesterol, fasting triglycerides, and systolic blood pressure). The association of A-SAA with incident type 2 diabetes was also independent of BMI or, alternatively, other measures of body fat and body composition, such as WHR, body fat mass index, and lean BMI. This is particularly notable since concentrations of A-SAA are known to be elevated in obese subjects (19) and since the proinflammatory cytokines and A-SAA inducers interleukin-6 and tumor necrosis factor-α, as well as A-SAA itself, were found to be partly expressed in adipose tissue (20–22). Additionally, the administration of recombinant A-SAA in mice adipocytes results in a downregulation of genes that are critical for insulin sensitivity in the treated cells (23). Our results suggest that apart from adipose tissue, other metabolically critical sites, such as the liver, seem to be involved in the course of the disease. Furthermore, there was no evidence for a role of appendicular skeletal muscle mass index in the association between A-SAA and incident type 2 diabetes, which makes confounding effects by myokines unlikely.
Relationship between A-SAA, postchallenge hyperglycemia, and incident type 2 diabetes
The difference in A-SAA levels between individuals with incident type 2 diabetes and study participants who remained diabetes free was attenuated after adjustment for baseline parameters of glucose metabolism, which indicates early impairments of glucose homeostasis. Sensitivity analyses showed that levels of 2-h glucose contributed most strongly to this attenuation. This suggests that elevated levels of A-SAA could be associated with early alterations in glucose control. Thus, elevated levels of circulating A-SAA might be a consequence of a prediabetes process, rather than a cause of it. Alternatively, postchallenge hyperglycemia might be an intermediate variable linking A-SAA to the development of type 2 diabetes. To further elucidate the question regarding the direction of causality, we analyzed baseline levels of A-SAA and levels of 2-h glucose at follow-up. These analyses yielded a significant association independent of baseline parameters of glucose metabolism. This suggests an independent impact of circulating levels of A-SAA on glucose homeostasis and thus supports the hypothesis of postchallenge hyperglycemia as an intermediate variable. However, our analyses do not allow inferences on reverse causality between levels of 2-h glucose and A-SAA. Consequently, we cannot rule out that the relationship between A-SAA and 2-h glucose might be bidirectional. Prospective analyses on levels of A-SAA or time series studies as performed for other markers of subclinical inflammation (24) might further clarify the relationship between subclinical low-grade inflammation, postchallenge hyperglycemia, and incident type 2 diabetes.
Comparison of our data with the literature
In accordance with a previous study (8), our study demonstrated that A-SAA concentrations were higher in women than in men but the estimates for A-SAA concentrations regarding risk of incident type 2 diabetes did not differ significantly between men and women, as has been suggested for hs-CRP (25).
The finding of the current study differed from those of the Sandy Lake Health and Diabetes Project (10). This study had an even broader age range (10–79 years), similar covariables, and a similar sex distribution but did not observe a significant association between SAA and incident type 2 diabetes, although effect estimates pointed into the same direction. The 10-year cumulative incidence was high (17.5%), but fewer subjects (n = 492) were analyzed. Thus, results may differ due to a lower statistical power of the Sandy Lake Health and Diabetes Project. In addition, the study was conducted in Aboriginal Canadians and ethnic factors might also be responsible for the different results.
The association between A-SAA and incident diabetes is possibly modulated by age
In the current study, the association between levels of A-SAA and incident type 2 diabetes seemed to be age dependent. Stratified analyses showed that the variability of A-SAA levels was higher in older subjects. Furthermore, the association of A-SAA with incident type 2 diabetes was only significant in younger subjects. In the age-group 55–64 years, the effect was attenuated mainly by adjustment for HbA1c but not by levels of 2-h glucose. This suggests that elevated levels of A-SAA may play a more important role in the development of type 2 diabetes in younger subjects and that the pathogenic mechanisms may differ in an age-dependent manner. Also, there was no evidence of an influence of hormonal changes during menopause or hormone replacement therapy in women on the observed age effect. Notably, the age-dependent differences were evident in our study population with elderly participants of a relatively small age range (55–74 years). It might be even more pronounced in study populations with younger participants and broader age ranges. However, we only found an effect modification by age for A-SAA but not for hs-CRP. Therefore, further research is required to confirm and clarify the role of age in the context of innate immunity and the development of type 2 diabetes.
A-SAA versus hs-CRP and incident type 2 diabetes
To compare the strength of the putative association between levels of A-SAA and incident type 2 diabetes with that of another acute-phase protein, we also analyzed levels of hs-CRP as the best established inflammatory marker. Effect estimates for hs-CRP were initially higher than for A-SAA but were attenuated more strongly when adjusting for the same covariables. After full adjustment for all covariables, effect sizes for A-SAA and hs-CRP were similar.
Limitations and strengths of the study
Several potential limitations of our study have to be mentioned. First, due to the restrictions in laboratory methods, our analyses were confined to the A-SAA isoform and did not capture the constitutively expressed C-SAA isoform, which, however, responds only moderately to inflammatory stimuli. Second, only one A-SAA measurement was available, although multiple measurements for the determination of inflammatory proteins are preferred (26). And third, this study did not assess the possible influence of energy intake or liver steatosis on the association between levels of A-SAA and incident type 2 diabetes. The strengths of this study are its prospective design, the well-defined study population sample, and the definition of type 2 diabetes based on validated diagnosis and OGTT at both time points. In addition, the current study accounted for baseline parameters of glucose metabolism and thus early impairments of glucose homeostasis.
Conclusion
In this prospective, population-based study in an elderly Western European population, we observed a statistically significant association between elevated systemic levels of A-SAA and the development of type 2 diabetes. The relevance of high circulating levels of A-SAA in the pathogenesis of type 2 diabetes may be modulated by age. The association with incident type 2 diabetes was independent of several established type 2 diabetes risk factors, including different measures of obesity and body composition. In the fully adjusted model, effect estimates for A-SAA were similar to those for hs-CRP. Adjustment for parameters of glucose metabolism, particularly 2-h glucose, attenuated the association between A-SAA and incident type 2 diabetes. In contrast, prospective analyses indicated a significant association between baseline levels of A-SAA and levels of 2-h glucose at follow-up, even after adjusting for baseline parameters of glucose metabolism. Prospective analyses on A-SAA or time series studies are warranted to clarify whether the relationship between circulating levels of A-SAA and 2-h glucose is bidirectional or if postchallenge hyperglycemia is the intermediate link between A-SAA and the development of type 2 diabetes.
Acknowledgments
The KORA research platform was initiated and financed by the Helmholtz Zentrum München, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria. Furthermore, this study was supported in part by a grant from the BMBF to the German Center for Diabetes Research (DZD) and through additional funds from the University of Ulm.
No potential conflicts of interest relevant to this article were reported.
C.Ma. conducted the data analyses and wrote the manuscript. C.Hu., C.He., and B.T. reviewed and edited the manuscript. J.B. contributed to data analyses. W.R., C.Me., H.-E.W., M.R., A.P., H.G., and T.I. were involved in the study organization, provided data, and reviewed the manuscript. W.K. provided data and reviewed the manuscript. W.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to all members of the Helmholtz Zentrum München and the field staff in Augsburg who were involved in the planning, organization, and conduct of the KORA studies. Furthermore, the authors thank the Augsburg registry team for the acquisition of the follow-up data. The authors also thank Gerlinde Trischler (University of Ulm Medical Center) for expert technical assistance. Finally, the authors express their appreciation to all study participants.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1514/-/DC1.
References
- 1.Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia 2005;48:1038–1050 [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27:813–823 [DOI] [PubMed] [Google Scholar]
- 3.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 1999;265:501–523 [DOI] [PubMed] [Google Scholar]
- 4.Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J 1998;334:489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol 2000;7:64–69 [DOI] [PubMed] [Google Scholar]
- 6.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997;40:1286–1292 [DOI] [PubMed] [Google Scholar]
- 7.Müller S, Martin S, Koenig W, et al. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia 2002;45:805–812 [DOI] [PubMed] [Google Scholar]
- 8.Sjöholm K, Lundgren M, Olsson M, Eriksson JW. Association of serum amyloid A levels with adipocyte size and serum levels of adipokines: differences between men and women. Cytokine 2009;48:260–266 [DOI] [PubMed] [Google Scholar]
- 9.Leinonen E, Hurt-Camejo E, Wiklund O, Hultén LM, Hiukka A, Taskinen MR. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis 2003;166:387–394 [DOI] [PubMed] [Google Scholar]
- 10.Ley SH, Harris SB, Connelly PW, et al. Adipokines and incident type 2 diabetes in an Aboriginal Canadian [corrected] population: the Sandy Lake Health and Diabetes Project. Diabetes Care 2008;31:1410–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Report of a WHO Consultation Geneva, World Health Org., 1999 [Google Scholar]
- 12.Marzi C, Albrecht E, Hysi PG, et al. Genome-wide association study identifies two novel regions at 11p15.5-p13 and 1p31 with major impact on acute-phase serum amyloid A. PLoS Genet 2010;6:e1001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathmann W, Haastert B, Icks A, et al. KORA Study Group Sex differences in the associations of socioeconomic status with undiagnosed diabetes mellitus and impaired glucose tolerance in the elderly population: the KORA Survey 2000. Eur J Public Health 2005;15:627–633 [DOI] [PubMed] [Google Scholar]
- 14.Rathmann W, Haastert B, Icks A, et al. High prevalence of undiagnosed diabetes mellitus in Southern Germany: target populations for efficient screening. The KORA survey 2000. Diabetologia 2003;46:182–189 [DOI] [PubMed] [Google Scholar]
- 15.Herder C, Kolb H, Koenig W, et al. Association of systemic concentrations of macrophage migration inhibitory factor with impaired glucose tolerance and type 2 diabetes: results from the Cooperative Health Research in the Region of Augsburg, Survey 4 (KORA S4). Diabetes Care 2006;29:368–371 [DOI] [PubMed] [Google Scholar]
- 16.Thorand B, Baumert J, Döring A, et al. KORA Group Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis 2006;184:216–224 [DOI] [PubMed] [Google Scholar]
- 17.Kyle UG, Genton L, Hans D, Pichard C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin Nutr 2003;22:537–543 [DOI] [PubMed] [Google Scholar]
- 18.Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C. Single prediction equation for bioelectrical impedance analysis in adults aged 20–94 years. Nutrition 2001;17:248–253 [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, He X, Shi X, et al. Association between serum amyloid A and obesity: a meta-analysis and systematic review. Inflamm Res 2010;59:323–334 [DOI] [PubMed] [Google Scholar]
- 20.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001;60:329–339 [DOI] [PubMed] [Google Scholar]
- 21.Sjöholm K, Palming J, Olofsson LE, et al. A microarray search for genes predominantly expressed in human omental adipocytes: adipose tissue as a major production site of serum amyloid A. J Clin Endocrinol Metab 2005;90:2233–2239 [DOI] [PubMed] [Google Scholar]
- 22.Yang RZ, Lee MJ, Hu H, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med 2006;3:e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheja L, Heese B, Zitzer H, et al. Acute-phase serum amyloid A as a marker of insulin resistance in mice. Exp Diabetes Res 2008;2008:230837 [DOI] [PMC free article] [PubMed]
- 24.Carstensen M, Herder C, Kivimäki M, et al. Accelerated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: Whitehall II prospective cohort study. Diabetes 2010;59:1222–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorand B, Baumert J, Kolb H, et al. Sex differences in the prediction of type 2 diabetes by inflammatory markers: results from the MONICA/KORA Augsburg case-cohort study, 1984-2002. Diabetes Care 2007;30:854–860 [DOI] [PubMed] [Google Scholar]
- 26.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med 2008;5:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]