Abstract
OBJECTIVE
This multicenter, open-label, parallel-arm study compared the efficacy and safety of exenatide once weekly (EQW) with titrated insulin detemir in patients with type 2 diabetes inadequately controlled with metformin (with or without sulfonylureas).
RESEARCH DESIGN AND METHODS
Patients were randomized to EQW (2 mg) or detemir (once or twice daily, titrated to achieve fasting plasma glucose ≤5.5 mmol/L) for 26 weeks. The primary outcome was proportion of patients achieving A1C ≤7.0% and weight loss ≥1.0 kg at end point, analyzed by means of logistic regression. Secondary outcomes included measures of glycemic control, cardiovascular risk factors, and safety and tolerability.
RESULTS
Of 216 patients (intent-to-treat population), 111 received EQW and 105 received detemir. Overall, 44.1% (95% CI, 34.7–53.9) of EQW-treated patients compared with 11.4% (6.0–19.1) of detemir-treated patients achieved the primary outcome (P < 0.0001). Treatment with EQW resulted in significantly greater reductions than detemir in A1C (least-square mean ± SE, −1.30 ± 0.08% vs. −0.88 ± 0.08%; P < 0.0001) and weight (−2.7 ± 0.3 kg vs. +0.8 ± 0.4 kg; P < 0.0001). Gastrointestinal-related and injection site–related adverse events occurred more frequently with EQW than with detemir. There was no major hypoglycemia in either group. Five (6%) patients in the EQW group and six (7%) patients in the detemir group experienced minor hypoglycemia; only one event occurred without concomitant sulfonylureas (detemir group).
CONCLUSIONS
Treatment with EQW resulted in a significantly greater proportion of patients achieving target A1C and weight loss than treatment with detemir, with a low risk of hypoglycemia. These results suggest that EQW is a viable alternative to insulin detemir treatment in patients with type 2 diabetes with inadequate glycemic control using oral antidiabetes drugs.
Type 2 diabetes is a chronic, progressive disease associated with obesity that requires multiple interventions to maintain long-term glycemic control (1). Thus, treatments that improve both hyperglycemia and body weight—with a low risk of hypoglycemia—may be more desirable in the treatment of type 2 diabetes than treatments associated with weight gain. Over time, many patients do not achieve adequate glycemic control with oral antidiabetes drugs (OADs) and additional therapies are required to achieve A1C targets of ≤6.5% (2) or ≤7% (1,3). After OADs (i.e., metformin) fail or if OADs are contraindicated, algorithms recommend the use of add-on therapies including insulin or glucagon-like peptide-1 (GLP-1) receptor agonists (1,2,4).
Exenatide once weekly (EQW), the extended-release formulation of exenatide, is a GLP-1 receptor agonist approved for use in the United States and Europe to improve glycemic control in adults with type 2 diabetes. EQW administered by subcutaneous injection has been shown to provide sustained glycemic control and does not have the undesirable effects of weight gain and hypoglycemia that are typically associated with insulin and some OADs (5–8). Further, weekly dosing may improve adherence to treatment, as suggested by improvements in patient-reported outcomes (9) and therefore may improve therapeutic outcomes (10).
At present, there have been no comparisons between EQW or other GLP-1 receptor agonists and insulin detemir, which is the most weight-neutral, long-acting insulin available (11). Compared with other basal insulin regimens (e.g., NPH insulin and insulin glargine), insulin detemir produced similar or greater improvements in glycemic control, was associated with less weight gain, and, in some trials, was associated with fewer events of hypoglycemia (12–15).
This study is the first to compare EQW and insulin detemir (administered once or twice daily) with respect to glycemic control, body weight, lipids, safety, tolerability, and patient-reported outcomes in patients with type 2 diabetes whose diabetes was inadequately controlled with OADs.
RESEARCH DESIGN AND METHODS
Study design
This 30-week (4-week screening and 26-week intervention), phase 3, multicenter, randomized, open-label, parallel-arm, active-comparator study (NCT01003184) was conducted in patients with type 2 diabetes not achieving adequate glycemic control using metformin or a combination of metformin and a sulfonylurea (SU). Patients were randomized in a 1:1 ratio to exenatide 2 mg subcutaneous injection once weekly or insulin detemir injection once daily or twice daily (BID) for 26 weeks, in addition to continuing current OAD therapy.
Detemir was initiated as once daily and titrated according to manufacturer labeling and a published “treat-to-target” titration algorithm described by Holman et al. (15,16), with an aim of target fasting plasma glucose (FPG) concentrations of <5.5 mmol/L (<99 mg/dL) before breakfast and before dinner. Patients initiated treatment using calculations described in the algorithm (15,16) and then titrated their doses weekly, as instructed by the investigator, based on daily self-monitored capillary fasting blood glucose measurements. Detemir dosages were reduced if hypoglycemia occurred or if the patient had a blood glucose measurement of <3.9 mmol/L (<70.2 mg/dL). Patients added an insulin injection before breakfast if glucose readings were at target before breakfast but not before the evening meal (and if nocturnal hypoglycemia limited an increase in dosage before bedtime).
Randomization was stratified according to baseline A1C level [≥7.0 to <8.5% (≥53 to 69 mmol/mol) or ≥8.5 to ≤10.0% (≥69 to ≤86 mmol/mol)] and SU use, and was centrally determined by a computer-generated random sequence using an interactive voice-response system. Oral metformin therapy was continued unchanged, whereas SU dosages were reduced by 50% at initiation of study treatment and further reduced or discontinued if hypoglycemia occurred or if A1C ≤7.0% (≤53 mmol/mol) was achieved.
This study was conducted in accordance with applicable laws and regulations, appropriate Ethical Review Board consents, and the International Conference on Harmonization guideline on good clinical practice and ethical principles established by the Declaration of Helsinki (17). All patients provided written informed consent.
Study population
Eligible patients were at least 18 years of age with type 2 diabetes and had A1C levels ≥7.1 to ≤10.0% (≥54 to ≤86 mmol/mol) despite use of OAD, BMI of 25 kg/m2 to 45 kg/m2, and stable weight (≤5% variability) for 3 months. Patients were required to be using a stable dose of metformin (≥1,000 mg/day) alone or in combination with a stable dose of SU (as a separate formulation) for at least 3 months before randomization.
Women of childbearing potential were ineligible unless using a reliable form of contraception throughout the study. Patients were excluded if they had a clinically significant medical condition that could preclude safe participation in this study, had more than three major hypoglycemic episodes in the past 6 months, or had been treated with a drug that promotes weight loss within 3 months of screening. Lipid-lowering and antihypertensive medications were allowed with appropriate dose adjustments (per investigator decision).
Interventions
Patients performed 7-point self-monitored blood glucose profiles at baseline and again between weeks 18 and 26. At baseline and week 26 (or the early termination visit for patients who discontinued before week 26), blood samples were taken to measure A1C, FPG, fasting plasma lipids, and other cardiovascular risk markers. Body weight, waist circumference, and vital signs were measured at each study visit.
Episodes of hypoglycemia, adverse events (AEs), and concomitant medications were recorded at each study visit and during telephone calls between visits. Events of nausea were recorded as spontaneous or solicited; only spontaneous reports were included in this analysis. A nausea evaluation questionnaire (e.g., nausea duration, timing, intensity, and others factors) was completed for patients who spontaneously reported nausea. Laboratory samples including clinical chemistry, hematology, and antiexenatide antibodies were collected at baseline and at week 26 (or the early discontinuation visit). Major hypoglycemia was defined as either any hypoglycemic episode with symptoms consistent with hypoglycemia that led to loss of consciousness or seizure, with prompt recovery in response to glucagon or glucose administration, or documented hypoglycemia [plasma glucose <3.0 mmol/L (<54 mg/dL)] requiring the assistance of another person because of severe impairment in consciousness or behavior (whether or not symptoms of hypoglycemia were detected by the patient). Minor hypoglycemia was defined as symptoms of hypoglycemia that were treated by the patient or resolved on their own, with documented plasma glucose <3.0 mmol/L (<54 mg/dL).
Patient-reported quality of life was assessed at all visits from baseline onwards using the Psychological General Well Being index (18) and Impact of Weight on Quality of Life-Lite (19). Patients who withdrew from the study completed these assessments at the discontinuation visit.
Outcomes
The primary objective of the study was to compare the effect of 26 weeks of EQW treatment to a titrated dosage of detemir once daily or BID with respect to the composite outcome of proportion of patients achieving A1C ≤7.0% (53 mmol/mol) and weight loss ≥1.0 kg at end point; the cut-off of ≥1.0 kg was chosen to reflect the variation in sequential measurements of weight and the margin of error for essentially no weight gain.
Secondary efficacy outcomes reported include the proportion of patients achieving the following: A1C ≤7.4% (≤57 mmol/mol) with weight loss ≥1.0 kg at end point; A1C ≤7.0% and ≤7.4% with any weight change ≤1 kg at end point; A1C ≤7.4%, ≤7.0%, and ≤6.5% (48 mmol/mol) at end point; changes in A1C and body weight from baseline to end point and from baseline to weeks 12, 18, and 26; changes in FPG and cardiovascular risk parameters [BMI, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, lipid profiles, plasminogen activator inhibitor-1 (PAI-1), high-sensitivity C-reactive protein (hs-CRP), and adiponectin] from baseline to end point; and the incidence of AEs. At the time the study was designed, A1C of ≤7.4% was the recommended target in the United Kingdom for reimbursement in general practice. A1C values also were reported in International Federation of Clinical Chemistry units (mmol/mol).
Statistical methods
A total of 170 patients (85 per treatment) were required to provide 90% power to detect a difference between treatments, with 5% significance, assuming the week 26 response rates (primary end point) were 50% in the EQW group and 25% in the detemir group. The study was not powered for a noninferiority analysis. Likely response rates were based on results from previous clinical trials (6–8). Assuming a drop-out rate of 20% resulted in a final overall sample size of 214 patients.
The last observation carried forward method was used for missing data for the intent-to-treat (ITT) analysis; end point was defined as the last study visit (between and including the baseline and week 26 visit). Study outcomes including final A1C concentration and change in weight used the last postbaseline measurement set of both nonmissing A1C and weight (measured at the same visit). All tests of treatment effects were conducted at a two-sided α level of 0.05. CIs were computed as two-tailed using 95% level, and differences were based on the EQW estimate minus the detemir estimate.
Reasons for discontinuation and drop-out rates were compared between treatment groups using Fisher exact test. For baseline characteristics, all categorical variables were summarized using frequencies and percentages, and continuous variables were summarized using descriptive statistics, including mean and SD.
The primary outcome was analyzed for the ITT population (defined as all randomized patients who received at least one dose of study drug) using a logistic regression model that included treatment group, use of SU, baseline A1C, and baseline weight as main factors. Hypothesis testing was based on the maximum likelihood estimate for the treatment main effect with corresponding 95% CI.
Secondary outcomes also were analyzed using the ITT population. Proportion of patients achieving A1C concentration ≤7.0% with weight loss ≥1.0 kg at 12 weeks, A1C ≤7.4% with weight loss ≥1.0 kg at end point, A1C ≤7.0% and ≤7.4% with weight change ≤1.0 kg at end point, and A1C ≤7.4%, ≤7.0%, and ≤6.5% were analyzed using the same methods as the primary outcome. The incidence of hypoglycemia was analyzed using logistic regression and categorized by use of concomitant SU. Exposure-adjusted incidence of hypoglycemia per 100 patient-years was reported.
Continuous variables with repeated measurements (A1C, body weight, BMI, waist circumference, SBP, and DBP) were evaluated using the likelihood-based mixed model repeated measures (MMRM) analysis that included the corresponding baseline value as covariate; study treatment, use of SU, baseline A1C, week of visit, and treatment-by-week interaction were fixed effects; patient and error were random effects. MMRM results are reported as least-square (LS) mean (± SE and 95% CI). An ANCOVA model was used for A1C, body weight, BMI, waist circumference, SBP, DBP, FPG, heart rate, lipid profiles, PAI-1, and adiponectin, in which the dependent variable was the last observation carried forward change from baseline to end point and independent variables were treatment group, corresponding baseline value, use of SU, and baseline A1C. LS means, SE, and 95% CI were reported for treatment group differences and differences from baseline to end point. Change in hs-CRP was analyzed using log transformation (ANCOVA model adjusted for treatment, baseline log value of A1C, use of SU, and corresponding baseline value); values ≥10 mg/L were excluded. Results are presented as geometric LS mean ratios of baseline to end point and EQW to detemir. The incidence of all treatment emergent AEs and serious AEs were summarized.
RESULTS
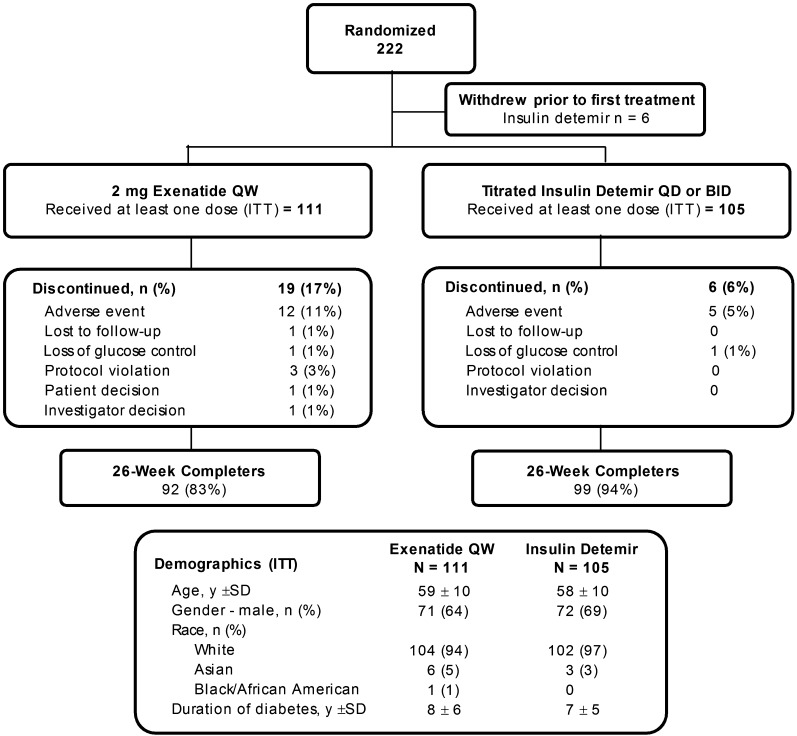
Of the 325 patients screened, 222 patients were randomized to treatment, 216 received at least one dose of study drug (ITT population), and 191 completed the study to week 26 (Fig. 1). Demographics (Fig. 1) and baseline characteristics (Table 1) were similar between groups. Because the majority of patients were Caucasian, assessments of efficacy related to ethnicity were not made. Mean ± SD exposure to treatment was 23.7 ± 6.5 weeks for EQW and 25.2 ± 5.6 weeks for detemir. The starting dose of insulin ranged from 2.0 IU/day to 62.0 IU/day. The mean ± SD daily dose of detemir was initially 0.21 ± 0.10 IU/kg per day (20.8 ± 10.8 IU/day); this increased to 0.51 ± 0.3 IU/kg per day (50.5 ± 28.1 IU/day) at end point. Most detemir-treated patients (89.5%) received one insulin injection daily; the remainder (10.5%) received two injections per day. The proportion of detemir-treated patients who achieved fasting blood glucose (self-monitored blood glucose measured before breakfast) of <5.5 mmol/L at end point was 17%.
Figure 1.
Study population, disposition, and demographics.
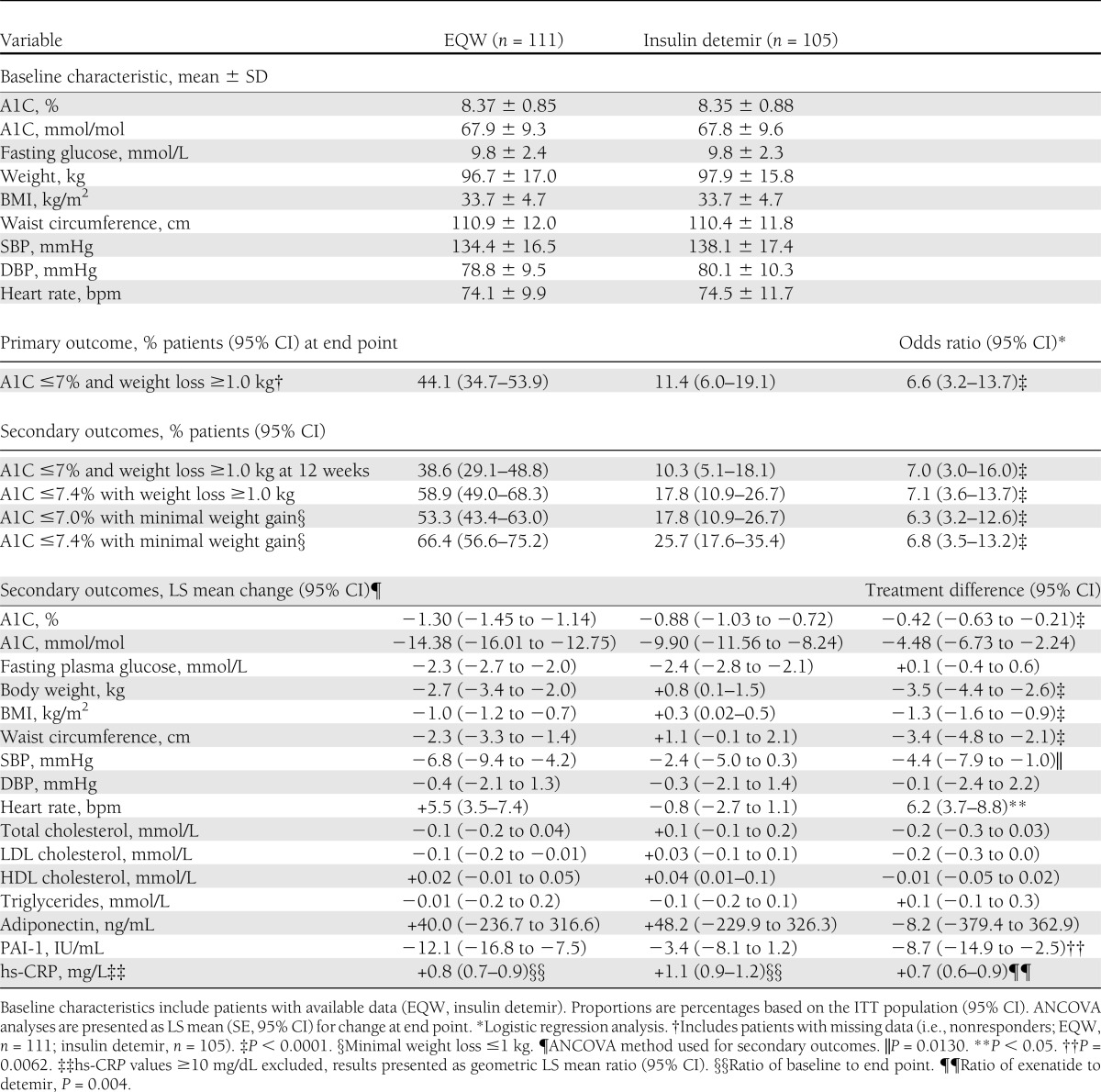
Table 1.
Baseline characteristics and treatment effects (baseline to end point) of EQW or insulin detemir
All patients received concomitant metformin (mean ± SD: 1,940.3 ± 550.9 mg) and 70% of EQW-treated patients and 72% of detemir-treated patients received a SU, including gliclazide (59 and 56%), glibenclamide (1 and 0%), glipizide (3 and 4%), glimepiride (6 and 10%), and sulphonamides/urea derivatives (1 and 3%). Of the EQW-treated patients, 80% of patients decreased SU therapy by end point, 4% discontinued, 15% had no change, and 1% increased SU therapy. Comparatively, in detemir-treated patients, 70% of patients decreased, none discontinued, 30% had no change, and none increased SU therapy by end point. Most decreases in SU dose occurred at the start of the study and involved decreasing the dose by half. Other nonantidiabetes concomitant therapy also was used by most patients, including antihypertensive medications (73%), fibrates (6%), and statins (83%).
Primary outcome
Forty-nine (44.1%; 95% CI, 34.7−53.9) patients in the EQW group and 12 (11.4%; 6.0–19.1) patients in the detemir group achieved A1C ≤7.0% with weight loss ≥1.0 kg at end point (Table 1). Logistic regression analysis showed that the odds ratio for patients in the EQW group achieving the primary outcome was 6.6 (3.2–13.7) compared with patients in the detemir group (P < 0.0001). Use of SU and baseline body weight did not influence a patient’s chance of achieving the primary outcome, but baseline A1C did have an effect, with higher baseline levels reducing the chance of achieving A1C ≤7.0% with weight loss ≥1.0 kg (P < 0.05).
Secondary outcomes
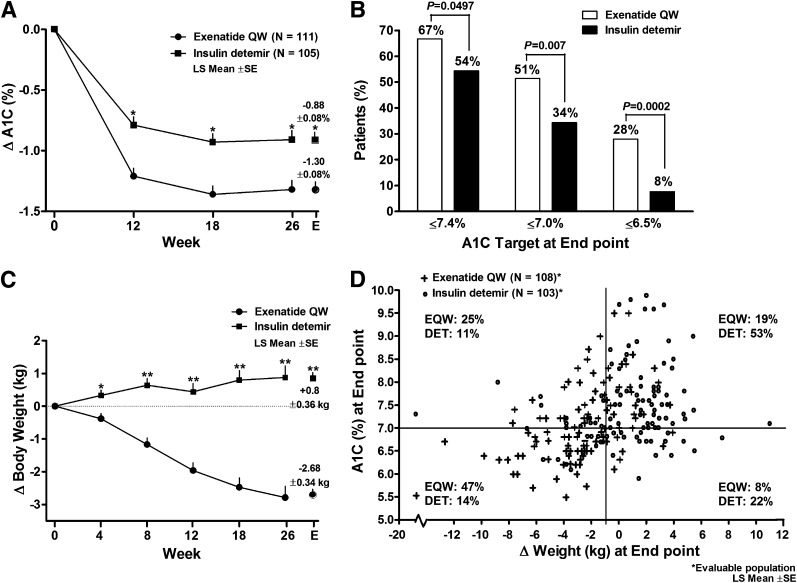
Glycemic control. Baseline A1C was 8.37 ± 0.85% in the EQW group and 8.35 ± 0.88% in the detemir group (Table 1). At study end point, A1C values [LS mean ± SD (95% CI)] were 7.07 ± 0.81% (6.91–7.22) in the EQW group and 7.50 ± 0.89% (7.32–7.67) in the detemir group [EQW, 53.7 ± 8.9 mmol/mol (52.0–55.4); detemir, 58.4 ± 9.8 mmol/mol (56.5–60.3)]. Mean change in A1C was significantly greater with EQW than with detemir as early as 12 weeks after treatment initiation (P < 0.0001), and the difference was maintained to week 26 (P = 0.0001; Fig. 2A). At end point, change in A1C [LS mean ± SE (95% CI)] in the ITT population was −1.30 ± 0.08% (−1.45 to 1.14) with EQW and −0.88 ± 0.08% (−1.03 to −0.72) with detemir [P < 0.0001; EQW, −14.38 ± 0.83 mmol/mol (−16.01 to −12.75); detemir, −9.90 ± 0.84 mmol/mol (−11.56 to −8.24)]. Results were similar for the ITT population and the 26-week completer population (change in A1C for ITT population shown in Fig. 2A).
Figure 2.
Change in glycemic control and body weight at end point and at specific time points over 26 weeks of treatment with EQW or insulin detemir (ITT population). A: LS mean ± SE change in A1C at 12, 18, and 26 weeks (MMRM used for analysis; data include patients with available data) and at end point (ANCOVA and last observation carried forward method used for analysis of change from baseline to end point). P indicated for treatment differences. *P < 0.0001. B: Proportion of patients who achieved target A1C of ≤7.4%, ≤7.0%, and ≤6.5%. C: LS mean ± SE change in body weight at weeks 4 to 26 and at end point (statistical methods same as those used for change in A1C); P indicated for treatment differences. *P = 0.0008 and **P < 0.0001. D: Scatter plot of A1C compared with change in weight at end point.
Other glycemic outcomes (target A1C ≤7.4%, ≤7.0%, ≤6.5%, composite target A1C and weight loss, composite target A1C and minimal weight gain) favored EQW over detemir, with many of the between-treatment differences showing statistical significance (Table 1 and Fig. 2B). There was a significant reduction in fasting glucose from baseline in both treatment groups [LS mean (95% CI): EQW −2.3 mmol/L (−2.7 to −2.0) vs. detemir −2.4 mmol/L (−2.8 to −2.1); Table 1], but there was no significant difference between groups.
Fasting glucose levels measured by 7-point self-monitored blood glucose decreased significantly from baseline to end point at all time points in both groups. The only significant differences between groups were in the prebreakfast (LS mean ± SE: EQW −1.9 ± 0.2 mmol/L vs. detemir −2.5 ± 0.2 mmol/L; P = 0.014), postdinner (EQW −2.7 ± 0.3 mmol/L vs. detemir −1.8 ± 0.3 mmol/L; P = 0.023), and bedtime (EQW −2.9 ± 0.3 mmol/L vs. detemir −1.8 ± 0.3 mmol/L; P = 0.005) measurements.
Cardiovascular risk factors. There was a progressive decrease in body weight from baseline at each visit in patients receiving EQW; in contrast, body weight increased in detemir-treated patients (Fig. 2C). At end point, body weight, BMI, and waist circumference were significantly reduced in the EQW group compared with the detemir group (P < 0.0001; Table 1). The change in body weight in EQW-treated patients appeared unrelated to the presence or absence of nausea, because patients without nausea lost (mean ± SD) 2.7 ± 3.3 kg compared with 2.8 ± 3.3 kg in patients who did experience nausea. Other specific measures of cardiovascular risk were improved in both treatment groups by study end (Table 1). Notably, EQW treatment resulted in significantly greater improvements compared with treatment with detemir in SBP (P < 0.01), PAI-1 (P < 0.006), and hs-CRP (P < 0.004) (Table 1). Individual patient A1C at end point versus changes in weight at end point are shown in Fig. 2D.
Patient-reported outcomes
Total scores on the Psychological General Well-Being questionnaire showed improvement [LS mean ± SE (95% CI)] from baseline to week 26 for the EQW group [+4.2 ± 1.1 (2.0–6.5)] but not the detemir group [+1.8 ± 1.2 (−0.6 to 4.1)], and there was no significant difference between treatment groups. The total scores for the Impact of Weight on Quality of Life-Lite questionnaire showed improvements from baseline to week 26 for both groups [LS mean: EQW +6.2 ± 1.0 (95% CI, 4.2–8.2) compared with detemir +2.8 ± 1.1 (0.7–4.9)], with significantly greater improvement in the EQW group versus the detemir group (0.7–6.1; P = 0.015).
Safety and tolerability
Overall, 103 (93%) EQW-treated patients and 86 (82%) detemir-treated patients experienced at least one treatment-emergent AE. Frequent AEs are shown in Table 2. After nasopharyngitis and headache, which were the most frequent AEs in both groups, the most common AEs in the EQW group were gastrointestinal-related, with spontaneously-reported nausea occurring in 18% of patients versus 2% in the detemir group (Table 2). Vomiting (17% vs. 11%) and diarrhea (14% vs. 9%) were frequently reported in both treatment groups (EQW vs. detemir). Evaluation of nausea events showed that nausea was mild to moderate in intensity and generally improved after eating a meal. Injection-site reactions, including injection-site pruritus (11%) and injection-site nodules (20%), were frequently reported in the EQW group compared with the detemir group (1 and 0%, respectively). Treatment-emergent AEs led to study discontinuation in 11% of EQW-treated patients and in 5% of detemir-treated patients. The most common AEs leading to withdrawal were injection site–related AEs (injection site–related erythema, nodule, pruritus, urticaria, or other reaction) in both groups (EQW 2% compared with detemir 4%) and gastrointestinal-related AEs (nausea) in the EQW group (2% vs. no discontinuations in the detemir group). Serious AEs were reported by 5% of EQW-treated patients and in 6% of detemir-treated patients. No patient died as a result of an AE in either group.
Table 2.
Safety and tolerability of 26 weeks of EQW or insulin detemir
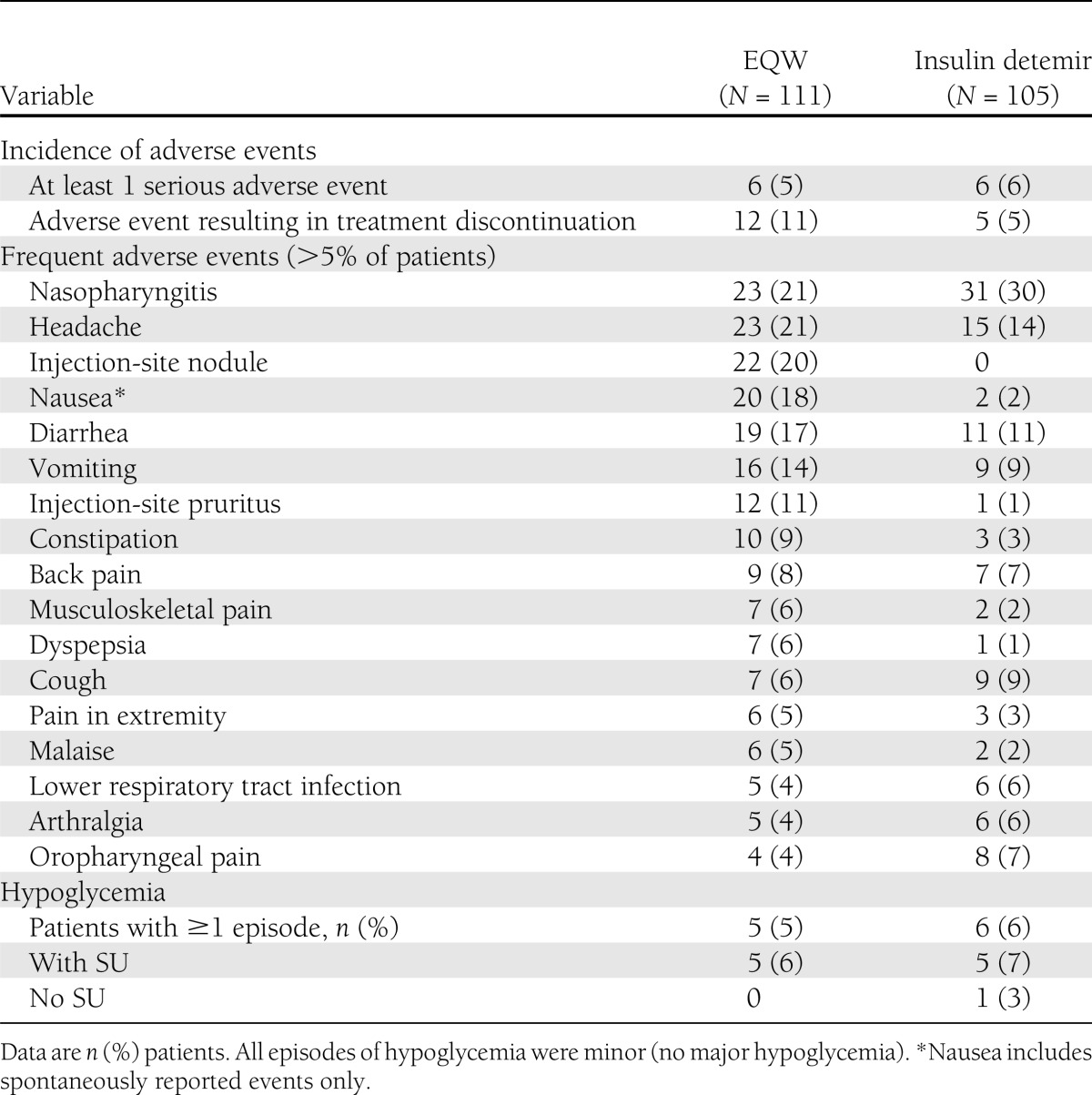
No patients experienced major hypoglycemia. Five patients (5% or 9.9 per 100 patient-years) in the EQW group and 6 patients (6% or 17.8 per 100 patient-years) in the detemir group experienced at least one episode of minor hypoglycemia (Table 2); there was no difference in incidence of hypoglycemia between groups. One case of hypoglycemia in each group was nocturnal. Only one patient experienced hypoglycemia without concomitant SU use (detemir group).
At baseline, two (2%) patients in the EQW group had antiexenatide antibodies (all low titers of <625). By end point, 64 (58%) patients in the EQW group were antibody-positive, of whom 52 (81.3%) patients had low titers and 12 (19%) had higher titers (≥625); 38 (42%) patients were antibody-negative. Although changes in A1C were variable among patients with positive titers (−3.5% to +1.2%), the change in A1C was lower in patients with higher titers (LS mean, −0.7%; 95% CI, −1.0 to 0) than in patients with low titers (−1.4%; −1.6 to −1.1) or who were antibody-negative (−1.3%; −1.6 to −1.1).
CONCLUSIONS
The results of this study comparing EQW with detemir in patients with type 2 diabetes who did not achieve adequate glycemic control with lifestyle modifications and OADs showed that additional therapy with EQW provided better glycemic and weight control compared with addition of detemir. For the primary outcome, patients who received EQW were 6.6-times more likely to achieve A1C ≤7.0% with weight loss ≥1.0 kg than patients who received detemir. Overall, 44.1% and 11.4% of patients in each treatment group, respectively, achieved the composite primary outcome. A greater proportion of patients treated with EQW also achieved A1C ≤7.4% with weight loss and A1C targets of ≤7.4%, ≤7.0%, and ≤6.5% compared with insulin detemir.
Consideration of treatment options for patients with type 2 diabetes not adequately controlled with metformin or metformin and SU should take into account the potential advantage of weight loss or weight neutrality in individual patients (1). Therefore, a combination of glycemic control with weight loss was selected as a clinically relevant primary end point, and insulin detemir was chosen as an appropriate comparator for EQW because it is a long-acting insulin analog that has been associated with less weight gain than NPH insulin or insulin glargine (12–15,20). EQW treatment resulted in significantly greater improvements compared with treatment with detemir in A1C (as early as 3 months) and body weight (as early as 4 weeks), and these improvements were maintained throughout the trial. Further, measures of psychological well-being and weight-related quality of life were improved in both groups, with greater improvements in weight-related assessments with EQW compared with detemir.
The present results build on previous experience comparing exenatide with insulins. Despite treatment with OADs and insulin, the majority of patients with type 2 diabetes have difficulty achieving or maintaining target glycemic control without associated weight gain (21,22). Initiating insulin therapy is often the next step, but many patients still do not achieve optimal glycemic control with insulin, partly because of weight gain (22). Exenatide BID has been shown to be more effective than insulin glargine for improving the composite outcomes of glycemic control with minimal weight gain when used as add-on therapy in overweight patients at high risk for cardiovascular disease and with type 2 diabetes inadequately controlled by two or three OADs (23). In another trial, exenatide BID and insulin glargine similarly reduced A1C levels and similar proportions of patients in each treatment group achieved A1C targets, but weight was significantly lower in exenatide-treated patients (24).
EQW also recently has been compared with insulin glargine in patients with type 2 diabetes suboptimally controlled using maximum tolerated doses of OADs (7). EQW produced significantly greater reductions in A1C than insulin glargine (LS mean, −1.5 vs. −1.3%) at 26 weeks and significantly more patients who received EQW achieved target A1C ≤6.5% (43% vs. 28%). Additionally, at 26 weeks, treatment with EQW resulted in mean reduction in body weight of −2.6 kg compared with an increase of 1.4 kg with insulin glargine (P < 0.001) (7). At 84 weeks, patients treated with EQW continued to have significantly greater reductions in A1C from baseline than those who received insulin glargine (LS mean, −1.16 vs. −0.98%) and the difference in body weight between groups was maintained (25).
The current study also measured the effects of EQW and detemir on a number of risk factors for cardiovascular disease. Both treatments were associated with improvements in several markers of cardiovascular risk from baseline, with EQW treatment resulting in significantly greater improvements in SBP, PAI-1, and hs-CRP than with detemir treatment. There was a significant improvement from baseline in LDL cholesterol in EQW-treated patients and in HDL cholesterol in detemir-treated patients, although the differences between treatments were not significant. Similarly, studies by Drucker et al. (6) and Davies et al. (23) have shown improved lipid profiles and SBP with EQW and exenatide BID; effects on lipids and SBP also were maintained after 3 years of follow-up in a study by MacConell et al. (26). The increase in heart rate observed in patients treated with EQW was consistent with increases seen in previous trials (25,27), although the significance of this increase is unknown. The underlying mechanisms of these effects have yet to be established, and potential cardiovascular effects of EQW are undergoing investigation in an ongoing prospective trial (EXSCEL).
The incidence of hypoglycemia was generally low with both treatments in the present trial, especially without concomitant SU use. The incidence of hypoglycemia was not different between the EQW and detemir groups, which differs from previous trials that compared insulin with GLP-1 receptor agonists (7,28,29). However, other trials showed inconsistent results, including a lower incidence of hypoglycemia with exenatide BID versus insulin aspart (28,30) or EQW (7) versus insulin glargine, and no difference between exenatide BID (23) or liraglutide (31) versus insulin glargine. Furthermore, the incidence of hypoglycemia with detemir (5.7% overall) was lower than the incidence reported in other trials of detemir (confirmed minor hypoglycemia, 16% to 56%) (12,32–35). However, the current study was not powered for an assessment of hypoglycemia, and the small number of events reported limits potential inferences that can be drawn from these data.
As has been reported previously, the most common adverse event experienced by EQW recipients was nausea. However, this event did not appear to be associated with additional weight loss and few patients discontinued the study because of nausea. The incidence of nausea with EQW in this study (18%) was similar to the incidence reported in other trials (11% to 26%) (5–8,27). Approximately half of the patients were antibody-positive for exenatide, most of whom had low titers. Overall, change in A1C was lower in patients with higher titers than in patients with low titers or those who were antibody-negative; however, antibody titers were not predictive of individual A1C changes, as previously reported (36).
A limitation of this study was that forced titration of detemir was not strictly enforced and patients reduced the dosage if hypoglycemia occurred, leading to a mean titrated dose of detemir at end point 0.51 IU/kg, which is at the lower end of the range of mean doses of detemir used in other trials of type 2 diabetes (0.4 to 0.9 IU/kg) (12,15,16,33,35,37–40). Consequently, lower than average insulin doses may have affected the potential magnitude in A1C change or masked treatment-related differences in hypoglycemia events.
This trial is the first to compare EQW and insulin detemir, which is associated with less weight gain than other insulins. The results showed that treatment with EQW resulted in a significantly greater proportion of patients achieving target A1C than with insulin detemir, with the added benefit of weight loss not seen with detemir. We conclude that EQW represents an alternative treatment option to insulin therapy in patients with type 2 diabetes using OADs with inadequate glycemic control.
Acknowledgments
This study was supported by Amylin Pharmaceuticals, LLC, and Eli Lilly and Company.
M.D. has acted as consultant, advisory board member, and speaker for Novartis, Novo Nordisk, sanofi-aventis, Eli Lilly and Company, Merck Sharp & Dohme, and Roche and as a speaker for Servier and has received grants from Novartis, Novo Nordisk, sanofi-aventis, Eli Lilly and Company, Pfizer, Merck Sharp & Dohme, GlaxoSmithKline, and Servier. S.H. has acted as a consultant, advisory board member, and speaker for Novo Nordisk and Eli Lilly and Company and as an advisory board member for Abbott Laboratories, Takeda Pharmaceuticals, AstraZeneca, and Johnson & Johnson. S.S. has acted as advisory board member and speaker for Novartis, Novo Nordisk, Eli Lilly and Company, Merck Sharp & Dohme, and Bristol-Myers Squibb and as a speaker for sanofi-aventis, GlaxoSmithKline, and AstraZeneca and has received grants from Novo Nordisk, Eli Lilly and Company, sanofi-aventis, AstraZeneca, and Bristol-Myers Squibb. A.T. and O.A. are employees of Eli Lilly and Company, LTD, U.K. H.S. is an employee of Lilly France. J.V. has acted as a consultant, advisory board member, and speaker for and has received research support from Novartis, Novo Nordisk, sanofi-aventis, Eli Lilly and Company, Merck Sharp & Dohme, Roche, and Abbott Laboratories. No other potential conflicts of interest relevant to this article were reported.
M.D., S.H., S.S., and J.V. were principal investigators in this trial. H.S., O.A., and A.T. were involved in the design of the trial. H.S. was primarily involved in the statistical analyses. All authors were involved in the interpretation of the data and writing of the manuscript. M.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors acknowledge Caroline Spencer (Rx Communications), Kate Gurney (Amylin Pharmaceuticals, LLC), and Carmelle V. Remillard (Amylin Pharmaceuticals, LLC) for writing and editorial assistance in the preparation of this article. The authors thank the investigators and patients who made this study possible.
Footnotes
Clinical Trial reg. no. NCT01003184, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1333/-/DC1.
A complete list of investigators can be found in the Supplementary Data online.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]
- 2.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation (IDF) Clinical Guidelines Task Force. Global guideline for type 2 diabetes. Brussels: International Diabetes Federation, 2005. Available from http://www.idf.org/webdata/docs/IDF%20GGT2D.pdf Accessed October 26, 2012
- 4.NICE clinical guideline 87. Type 2 diabetes: The management of type 2 diabetes. May 2009. Available from http://www.nice.org.uk/nicemedia/live/12165/44320/44320.pdf Accessed October 26, 2012
- 5.Bergenstal RM, Wysham C, MacConell L, et al. DURATION-2 Study Group Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431–439 [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ, Buse JB, Taylor K, et al. DURATION-1 Study Group Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 7.Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 2010;375:2234–2243 [DOI] [PubMed] [Google Scholar]
- 8.Russell-Jones D, Cuddihy RM, Hanefeld M, et al. DURATION-4 Study Group Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care 2012;35:252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Best JH, Boye KS, Rubin RR, Cao D, Kim TH, Peyrot M. Improved treatment satisfaction and weight-related quality of life with exenatide once weekly or twice daily. Diabet Med 2009;26:722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836–1841 [DOI] [PubMed] [Google Scholar]
- 11.Havelund S, Plum A, Ribel U, et al. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res 2004;21:1498–1504 [DOI] [PubMed] [Google Scholar]
- 12.Fajardo Montañana C, Hernández Herrero C, Rivas Fernández M. Less weight gain and hypoglycaemia with once-daily insulin detemir than NPH insulin in intensification of insulin therapy in overweight Type 2 diabetes patients: the PREDICTIVE BMI clinical trial. Diabet Med 2008;25:916–923 [DOI] [PubMed] [Google Scholar]
- 13.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269–1274 [DOI] [PubMed] [Google Scholar]
- 14.Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011. (7):CD006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holman RR, Farmer AJ, Davies MJ, et al. 4-T Study Group Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]
- 16.Holman RR, Thorne KI, Farmer AJ, et al. 4-T Study Group Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects [article online], 2008. Available from http://www.wma.net/en/30publications/10policies/b3/index.html Accessed October 26, 2012
- 18.Revicki DA, Leidy NK, Howland L. Evaluating the psychometric characteristics of the Psychological General Well-Being Index with a new response scale. Qual Life Res 1996;5:419–425 [DOI] [PubMed] [Google Scholar]
- 19.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–111 [DOI] [PubMed] [Google Scholar]
- 20.Zachariah S, Sheldon B, Shojaee-Moradie F, et al. Insulin detemir reduces weight gain as a result of reduced food intake in patients with type 1 diabetes. Diabetes Care 2011;34:1487–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubino A, McQuay LJ, Gough SC, Kvasz M, Tennis P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose-lowering agents in patients with Type 2 diabetes: a population-based analysis in the UK. Diabet Med 2007;24:1412–1418 [DOI] [PubMed] [Google Scholar]
- 22.Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract 2007;57:455–460 [PMC free article] [PubMed] [Google Scholar]
- 23.Davies MJ, Donnelly R, Barnett AH, Jones S, Nicolay C, Kilcoyne A. Exenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the Helping Evaluate Exenatide in patients with diabetes compared with Long-Acting insulin (HEELA) study. Diabetes Obes Metab 2009;11:1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, GWAA Study Group Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559–569 [DOI] [PubMed] [Google Scholar]
- 25.Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care 2012;35:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacConell L, Walsh B, Li Y, Pencek R, Maggs D. Exenatide once weekly: sustained improvement in glycemic control and weight loss through 3 years. Diabetes 2011;60(Suppl. 1):A265 [abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011;96:1301–1310 [DOI] [PubMed] [Google Scholar]
- 28.Gallwitz B, Böhmer M, Segiet T, et al. Exenatide twice daily versus premixed insulin aspart 70/30 in metformin-treated patients with type 2 diabetes: a randomized 26-week study on glycemic control and hypoglycemia. Diabetes Care 2011;34:604–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blevins T, Han J, Nicewarner D, Chen S, Oliveira JH, Aronoff S. Exenatide is non-inferior to insulin in reducing HbA1c: an integrated analysis of 1423 patients with type 2 diabetes. Postgrad Med 2010;122:118–128 [DOI] [PubMed] [Google Scholar]
- 30.Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ, 3rd, ; EGO Study Group The effects of LY2189265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab 2011;13:418–425 [DOI] [PubMed] [Google Scholar]
- 31.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009;52:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006;28:1569–1581 [DOI] [PubMed] [Google Scholar]
- 33.Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther 2008;30:1976–1987 [DOI] [PubMed] [Google Scholar]
- 34.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008;51:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swinnen SG, Dain MP, Aronson R, et al. A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care 2010;33:1176–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fineman MS, Cirincione BB, Maggs D, Diamant M. GLP-1 based therapies: differential effects on fasting and postprandial glucose. Diabetes Obes Metab 2012;14:675–688 [DOI] [PubMed] [Google Scholar]
- 37.Fogelfeld L, Dharmalingam M, Robling K, Jones C, Swanson D, Jacober S. A randomized, treat-to-target trial comparing insulin lispro protamine suspension and insulin detemir in insulin-naive patients with Type 2 diabetes. Diabet Med 2010;27:181–188 [DOI] [PubMed] [Google Scholar]
- 38.Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev 2009;25:542–548 [DOI] [PubMed] [Google Scholar]
- 39.Hompesch M, Ocheltree SM, Wondmagegnehu ET, et al. Pharmacokinetics and pharmacodynamics of insulin lispro protamine suspension compared with insulin glargine and insulin detemir in type 2 diabetes. Curr Med Res Opin 2009;25:2679–2687 [DOI] [PubMed] [Google Scholar]
- 40.Selam JL, Koenen C, Weng W, Meneghini L. Improving glycemic control with insulin detemir using the 303 Algorithm in insulin naïve patients with type 2 diabetes: a subgroup analysis of the US PREDICTIVE 303 study. Curr Med Res Opin 2008;24:11–20 [DOI] [PubMed] [Google Scholar]