Abstract
OBJECTIVE
The performance of glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) was compared in screening for diabetes by an oral glucose tolerance test (OGTT) in patients undergoing coronary angiography (CAG).
RESEARCH DESIGN AND METHODS
Patients without known diabetes admitted for CAG were eligible. OGTT and HbA1c were assessed 2–4 weeks after hospital discharge. The performance of HbA1c and FPG was evaluated by using receiver operating characteristic (ROC) analysis.
RESULTS
Diabetes was diagnosed in 83 of 400 patients (20.8%). The area under the ROC curve was higher for FPG than for HbA1c (0.81 vs. 0.73, P = 0.032). We proposed a screening algorithm and validated it in another 170 patients. Overall, this algorithm reduced the number of OGTTs by 71.4% (sensitivity 74.4%, specificity 100%).
CONCLUSIONS
FPG performed better than HbA1c in screening for diabetes in patients undergoing CAG. A screening algorithm might help to reduce the number of OGTTs.
Oral glucose tolerance test (OGTT) is recommended for abnormal glucose regulation screening in patients with coronary artery disease (CAD) (1). However, OGTT is not satisfactory as a routine test (2,3). Glycated hemoglobin (HbA1c) has been adopted as a diagnostic criterion for diabetes (4), and HbA1c testing has some advantages, such as requiring nonfasting samples and having less biological variability (3). On the other hand, the fasting plasma glucose (FPG) test is widely available and inexpensive (3). The performance of HbA1c and FPG in screening for diabetes has only been reported in a limited number of patients with acute coronary disease (5,6). Doerr et al. (7) reported that the sensitivity of HbA1c ≥6.5% for the detection of newly diagnosed diabetes (NDD) in patients undergoing coronary angiography (CAG) was only 16%. The present study aimed to compare the performance of HbA1c and FPG in screening for diabetes, as determined by an OGTT, and to develop a screening algorithm for patients undergoing CAG.
RESEARCH DESIGN AND METHODS
This study was approved by the institutional review board of Taichung Veterans General Hospital, Taichung, Taiwan and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before undergoing any study-related procedures. Adult patients without known diabetes were eligible if they were admitted for CAG for suspected or known CAD. Patients with serum creatinine ≥250 μmol/L, hemoglobin <10 g/dL, or history of blood transfusion within 3 months were excluded. CAD was defined as ≥50% stenosis of the lumen diameter in any coronary artery.
Two to four weeks after hospital discharge, a standard 75-g OGTT (8) was performed between 0800 and 1100 h after a 10–12-h overnight fast. Blood samples were collected at 0, 30, and 120 min for the measurements of HbA1c and plasma glucose and insulin concentrations. The methods of laboratory measurements are provided in the Supplementary Data.
Patient glucometabolic state was defined based on the results of the OGTT (4). Insulin resistance was calculated with the homeostasis model assessment of insulin resistance (HOMA-IR) (9). β-cell function was assessed with the homeostasis model assessment of β-cell function (HOMA-β) (9) and insulinogenic index (IGI) (10).
Statistical analyses were performed with SPSS version 10.0 (IBM, Chicago, IL) software. The performance of HbA1c and FPG for detecting NDD was evaluated by receiver operating characteristic (ROC) analysis, and diagnostic accuracy was assessed with the area under the curve (AUC) (11). P < 0.05 was considered statistically significant.
RESULTS
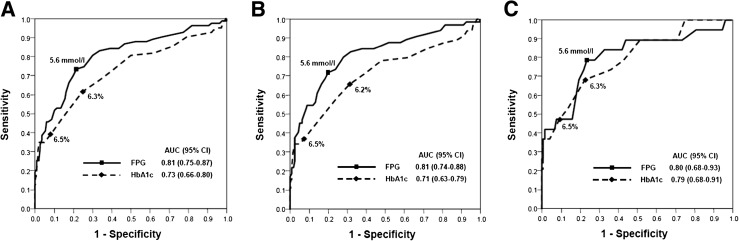
From December 2009–September 2011, OGTT was conducted in 400 of 780 eligible patients (mean age 65 ± 13 years, male 75.9%, CAD 67.8%) (Supplementary Table 1). Figure 1 shows the ROC curves for HbA1c and FPG to detect NDD. Overall, the AUC was higher for FPG than for HbA1c (0.81 vs. 0.73, P = 0.032). The optimal cutoff point was 5.6 mmol/L for FPG and 6.3% for HbA1c. In patients with CAD, the AUC was higher for FPG than for HbA1c (0.81 vs. 0.71, P = 0.017), whereas the difference was not significant in patients without CAD (0.80 vs. 0.79, P = 0.881).
Figure 1.
ROC curve for HbA1c and FPG to detect NDD in all patients (A), patients with CAD (B), and patients without CAD (C). The optimal cutoff points for HbA1c were 6.3 (A), 6.2 (B), and 6.3% (C). The optimal cutoff point for FPG was consistently 5.6 mmol/L in all three groups.
Patients were divided into different groups according to their FPG and HbA1c levels (Supplementary Table 2). Patients with FPG 5.6–6.9 mmol/L were more insulin resistant (HOMA-IR 2.4 ± 1.5 vs. 1.7 ± 1.2, P < 0.001) and had worse β-cell function (HOMA-β 74 ± 49 vs. 104 ± 70, P < 0.001; IGI 60 ± 57 vs. 104 ± 87, P < 0.001) than those with FPG <5.6 mmol/L. However, in patients with HbA1c 5.7–6.4%, the HOMA-IR, HOMA-β, and IGI were not significantly different from those in patients with HbA1c <5.7%.
On the basis of our findings, we proposed a screening algorithm (Supplementary Figure 1). Diabetes was diagnosed in patients with FPG ≥7.0 mmol/L. OGTT needs to be conducted in patients with FPG 5.6–6.9 mmol/L and may be waived in those with FPG <5.6 mmol/L. In this way, the number of OGTTs was reduced by 71.8%, and the sensitivity and specificity for detecting NDD was 73.5 and 100%, respectively.
This algorithm was tested in another 170 patients (mean age 62 ± 13 years, male 82.9%, CAD 67.1%) admitted for CAG between October 2011 and June 2012. Following this algorithm, an OGTT would be needed in 50 (29.4%) patients, and the sensitivity and specificity for detecting NDD was 76.5 and 100%, respectively.
CONCLUSIONS
We reported that the AUC was higher for FPG than for HbA1c in detecting NDD in patients undergoing CAG, especially in those with CAD. A recent study comparing the performance of HbA1c and fasting capillary glucose in screening for diabetes in a general Chinese population found a higher AUC for fasting capillary glucose than for HbA1c (men 0.77 vs. 0.67, P < 0.01; women 0.75 vs. 0.67, P < 0.01) (12). These findings were in line with the present results and suggest that FPG is a better test than HbA1c in screening for diabetes.
We observed that patients with FPG 5.6–6.9 mmol/L were more insulin resistant and had worse β-cell function than those with FPG <5.6 mmol/L. However, in patients with HbA1c 5.7–6.4%, the indexes of insulin resistance and β-cell function were not significantly different from those in patients with HbA1c <5.7%. Some studies reported that β-cell function progressively declined with the increase in either FPG or 2-h postchallenge glycemia (13,14). In contrast, the relationship between HbA1c and β-cell function was reported to be highly nonlinear (15). These results suggest that a higher-than-normal FPG might be a better index of insulin resistance and β-cell dysfunction than a higher-than-normal HbA1c.
There are some limitations in this study. First, only 51.3% of eligible patients participated. Second, we did not conduct a second OGTT to confirm the diagnosis, and the poor reproducibility of OGTT (3) may confound the results. Third, the efficacy of the screening algorithm has not been tested in another independent cohort.
In summary, we reported that the FPG test performed better than HbA1c in screening for diabetes in patients undergoing CAG. We proposed a screening algorithm, and its efficacy and practicability need further investigation.
Acknowledgments
This study was supported in part by research grants from the National Science Council, Taiwan (NSC 101-2314-B-075A-006-MY3) and Taichung Veterans General Hospital, Taichung, Taiwan (TCVGH-1013502B).
The funder played no role in the conduct of the study, collection of data, management of the study, analysis of data, interpretation of data, or preparation of the manuscript.
No potential conflicts of interest relevant to this article were reported.
J.-S.W. and W.H.-H.S. analyzed data and wrote the manuscript. I.-T.L. and W.-J.L. designed the study and critically revised the manuscript. S.-Y.L., C.-P.F., and K.-W.L. analyzed data and reviewed and edited the manuscript. C.-T.T. and W.-L.L. obtained and analyzed related clinical data and contributed to discussion. J.-S.W. and W.H.-H.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01198730, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1434/-/DC1.
References
- 1.Rydén L, Standl E, Bartnik M, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 2007;28:88–136 [DOI] [PubMed] [Google Scholar]
- 2.Bartnik M, Rydén L, Malmberg K, et al. Euro Heart Survey Investigators Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Heart 2007;93:72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care 2011;34:518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 2002;359:2140–2144 [DOI] [PubMed] [Google Scholar]
- 6.Del Olmo MI, Merino-Torres JF, Argente M, et al. Detection of glucose abnormalities in patients with acute coronary heart disease: study of reliable tools in clinical practice. J Endocrinol Invest 2012;35:71–76 [DOI] [PubMed] [Google Scholar]
- 7.Doerr R, Hoffmann U, Otter W, et al. Oral glucose tolerance test and HbA1c for diagnosis of diabetes in patients undergoing coronary angiography: the Silent Diabetes Study. Diabetologia 2011;54:2923–2930 [DOI] [PubMed]
- 8.World Health Organization Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part I: Diagnosis and Classification of Diabetes Mellitus. Geneva, WHO Department of Noncommunicable Disease Surveillance, 1999 [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 10.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008;16:1901–1907 [DOI] [PubMed] [Google Scholar]
- 11.Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 2007;96:644–647 [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Pang Z, Gao W, et al. Performance of an A1C and fasting capillary blood glucose test for screening newly diagnosed diabetes and pre-diabetes defined by an oral glucose tolerance test in Qingdao, China. Diabetes Care 2010;33:545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godsland IF, Jeffs JA, Johnston DG. Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia 2004;47:1157–1166 [DOI] [PubMed] [Google Scholar]
- 14.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA, San Antonio Metabolism Study Beta-cell dysfunction and glucose intolerance: results from the San Antonio Metabolism (SAM) study. Diabetologia 2004;47:31–39 [DOI] [PubMed] [Google Scholar]
- 15.Kanat M, Winnier D, Norton L, et al. The relationship between β-cell function and glycated hemoglobin: results from the Veterans Administration Genetic Epidemiology Study. Diabetes Care 2011;34:1006–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]