Abstract
OBJECTIVE
To examine the association between diabetes, glycemic control, and risk of fracture-related hospitalization in the Atherosclerosis Risk in Communities (ARIC) Study.
RESEARCH DESIGN AND METHODS
Fracture-related hospitalization was defined using International Classification of Diseases, 9th revision, codes (733.1–733.19, 733.93–733.98, or 800–829). We calculated the incidence rate of fracture-related hospitalization by age and used Cox proportional hazards models to investigate the association of diabetes with risk of fracture after adjustment for demographic, lifestyle, and behavioral risk factors.
RESULTS
There were 1,078 incident fracture-related hospitalizations among 15,140 participants during a median of 20 years of follow-up. The overall incidence rate was 4.0 per 1,000 person-years (95% confidence interval [CI], 3.8–4.3). Diagnosed diabetes was significantly and independently associated with an increased risk of fracture (adjusted hazard ratio [HR], 1.74; 95% CI, 1.42–2.14). There also was a significantly increased risk of fracture among persons with diagnosed diabetes who were treated with insulin (HR, 1.87; 95% CI, 1.15–3.05) and among persons with diagnosed diabetes with hemoglobin A1c (HbA1c) ≥8% (1.63; 1.09–2.44) compared with those with HbA1c <8%. Undiagnosed diabetes was not significantly associated with risk of fracture (HR, 1.12; 95% CI, 0.82–1.53).
CONCLUSIONS
This study supports recommendations from the American Diabetes Association for assessment of fracture risk and implementation of prevention strategies in persons with type 2 diabetes, particularly those persons with poor glucose control.
Bone fractures represent a significant burden of morbidity, especially among women and persons older than 65 years of age (1). Fractures that result in inpatient hospitalization are particularly associated with significant health care costs, reductions in quality of life, and increased mortality (2,3). Evidence suggests that persons with type 2 diabetes may be at increased risk for bone fractures, despite having higher bone mineral density (4). However, the association of diabetes with fracture risk has differed depending on the location of fracture being investigated (5,6). Furthermore, few large, community-based studies of fracture risk have included both genders and nonwhite participants. Additionally, few studies have sufficiently controlled for potential confounders or investigated the possible association of undiagnosed diabetes with risk of fracture.
The aim of this study was to investigate the association of diabetes with fracture-related hospitalization in the Atherosclerosis Risk in Communities (ARIC) Study. We compared the risk of fracture hospitalization in persons with no diabetes, undiagnosed diabetes, or diagnosed diabetes in this community-based cohort. A secondary aim was to examine the associations of diabetes medication use (among persons with diagnosed diabetes) and chronic hyperglycemia (as assessed by hemoglobin A1c [HbA1c]) with fracture risk. We hypothesized that diabetes, particularly poorly controlled diabetes, would be associated with an increased risk of fracture.
RESEARCH DESIGN AND METHODS
Study population
Participants in the ARIC Study were recruited from four United States communities: Washington County, Maryland; suburbs of Minneapolis, Minnesota; Forsyth County, North Carolina; and Jackson, Mississippi. The first examination of 15,792 participants took place from 1987 to 1989 (visit 1), when participants were aged 45–64 years. Subsequent in-person visits were conducted in 1990–1992, 1993–1995, and 1996–1998; a fifth visit is ongoing (2011–2013) (7,8). We excluded participants from the primary analysis if they were missing any baseline covariates used in our statistical models, resulting in a final sample of 15,140 participants.
Outcome: fracture hospitalization
The ARIC Study obtains hospitalization information from annual telephone contact with study participants and through active surveillance of all hospitalizations in the study communities; inpatient hospitalization records to 1 January 2009 were available for this analysis. Incident fracture hospitalization was defined as any hospitalization after visit 1 (1987–1989) that included the International Classification of Diseases, 9th revision (ICD-9), discharge codes of 733.1–733.19, 733.93–733.98, or 800–829.
Exposure: diabetes definition
Participants were considered to have diagnosed diabetes if they self-reported being told by a physician that they had diabetes or were using diabetes medications at visit 1. Participants were considered to have undiagnosed diabetes if they failed to meet the criteria for diagnosed diabetes but had a fasting serum glucose ≥126 mg/dL or a nonfasting glucose ≥200 mg/dL at visit 1. Among persons with diagnosed diabetes, we categorized diabetes medication use as none, oral diabetes medications only, and insulin only or both oral diabetes medications and insulin based on self-reported information and information from medication containers brought to the visit. In a separate analysis, we examined the relationship of chronic hyperglycemia and fracture, based on HbA1c values available from participants who completed ARIC visit 2 in 1990–1992 (n = 13,508), the only visit for which whole blood samples were available for the measurement of HbA1c. HbA1c was measured using high-performance liquid chromatography methods (Tosoh 2.2 and Tosoh G7) (9).
Covariates
All covariates and medication usage data were measured at visit 1. BMI was measured according to standard methods and categorized as underweight (<18.5), normal (18.5–<25.0), overweight (25.0–<30.0), or obese (≥30.0). Physical activity level was assessed using the Baecke questionnaire (10), and scores were divided into tertiles. We examined glucocorticoids and antidepressants, which are categories of medications that are associated with increased fracture risk (11,12). We additionally examined thiazide diuretics, which are associated with lower fracture risk (13). Thiazolidinediones also have been shown to increase fracture risk, but this drug category had not been brought to the market at baseline and was rarely reported during follow-up (2.4% reported use during follow-up) (14,15). Similarly, although hormone replacement therapy is thought to reduce fracture risk (16), very few participants were using hormone replacement therapy at baseline (<0.1% of women in the study population); therefore, we were not able to assess the impact of thiazolidinediones or hormone replacement therapy on fracture risk.
Statistical analysis
Using age at the time of fracture as the time axis, we calculated the incidence rate (per 1,000 person-years of follow-up) and 95% confidence interval (CI) of fracture hospitalization overall and by 10-year age-groups (50–younger than 60, 60–younger than 70, 70–younger than 80, and 80 years or older) using Poisson regression.
With years of follow-up since visit 1 as the time axis, we used Cox proportional hazards models to compare risk of fracture hospitalization among persons without diabetes with those with undiagnosed diabetes or with diagnosed diabetes. Model 1 was adjusted for age, sex, and race/study center. Model 2 was adjusted for variables in model 1 plus BMI, sports activity tertile, alcohol consumption, cigarette smoking, glucocorticoid or antidepressant use, and thiazide diuretic use. We performed a sensitivity analysis additionally adjusting for history of coronary heart disease, history of stroke, and estimated glomerular filtration rate calculated from serum creatinine using the Modification of Diet in Renal Disease equation (17). We also conducted an analysis restricted to participants with diagnosed diabetes (n = 1,195) to assess the impact of diabetes medications on fracture risk. In this subpopulation, we conducted a fully adjusted model with a categorical term for diabetes medication. Additionally, we conducted a competing risks analysis (fracture vs. death) to assess the impact of survival bias, because persons with diabetes may be more likely to die at a younger age and therefore are more likely than persons without diabetes to die before a fracture could occur because older age is associated with fracture incidence (18). We also conducted analyses stratified by age (younger than 54 years vs. 54 years or older at baseline), race (black or white), and gender, and a sensitivity analysis excluding hospitalizations for pathological fractures (ICD codes 733.1–733.19; n = 133). We also performed fully adjusted analyses to examine the association between diabetes status and specific subtypes of fracture (torso, upper limb, lower limb, vertebral, hip, skull or face).
Among those participants who also attended ARIC visit 2 and who had data available for HbA1c (n = 13,508), we examined the association of clinical categories of HbA1c with fracture risk. Participants were considered to have diagnosed diabetes if they self-reported being told by a physician that they had diabetes or were using diabetes medications at visit 1 or visit 2. Participants were considered to have undiagnosed diabetes if they failed to meet the criteria for diagnosed diabetes but had HbA1c ≥6.5% at visit 2. Categories of HbA1c were defined as <5.7%, 5.7–<6.5%, and ≥6.5% for participants without a self-reported history of diabetes, and as <8% and ≥8% for participants with a self-reported history of diabetes (19).
All reported P values are two-sided and P < 0.05 was considered statistically significant. Statistical analyses were conducted using Stata version 11 (StataCorp, College Station, TX).
RESULTS
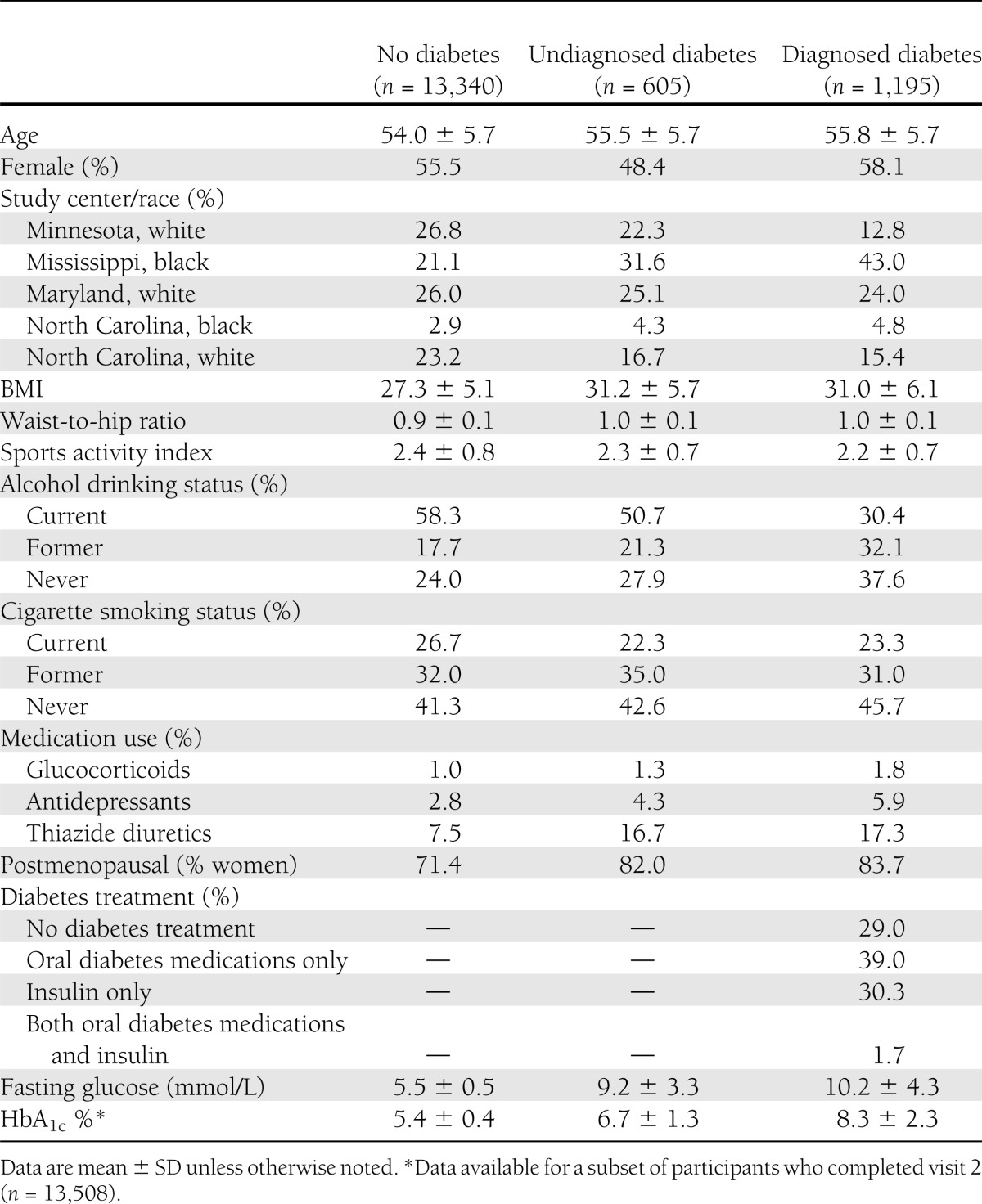
Compared with participants without diabetes, those with diagnosed diabetes were more likely to be black (48% vs. 24%), to be never drinkers of alcohol (38% vs. 24%), and to use either glucocorticoid or antidepressant medications, which are associated with an increased fracture risk (8% vs. 4%) (Table 1). Participants with undiagnosed diabetes were comparable with those with diagnosed diabetes in terms of race and BMI, but they were more similar to those without diabetes in terms of alcohol use.
Table 1.
Baseline characteristics of study participants by diabetes status during visit 1, 1987–1989
Among the 15,140 participants included in our main analysis, there were 1,078 incident cases of fracture hospitalization over a median of 20 years of follow-up. The overall incidence rate of fracture hospitalization was 4.0 (95% CI, 3.8–4.3) per 1,000 person-years. The types of fracture included skull or face (n = 50), spine (n = 104), ribs (n = 129), hip (n = 50), shoulder (n = 23), arm (n = 138), wrist (n = 12), hand (n = 30), leg (n = 325), ankle (n = 167), and foot (n = 50). The unadjusted incidence rate for all fracture hospitalizations was 2.5 (95% CI, 2.0–2.8) per 1,000 person-years of follow-up for those 50–younger than 60 years old, 3.6 (3.2–3.9) for those 60–younger than 70 years old, 6.7 (6.0–7.4) for those 70–younger than 80 years old, and 14.2 (11.3–17.7) for those 80 years or older. Overall, persons with diagnosed diabetes had significantly higher incidence rates of fracture compared with persons without diabetes (6.6 [95% CI, 5.4–7.9] vs. 3.9 [3.6–4.1] per 1,000 person-years of follow-up). Figure 1 shows the unadjusted incidence rate (per 1,000 person-years of follow-up) according to age and diabetes status. For all ages, the incidence rate for persons with diagnosed diabetes was higher than that for persons without diabetes. Persons with undiagnosed diabetes did not have significantly different risk of fracture compared with those without diabetes for any age-group (data not shown).
Figure 1.
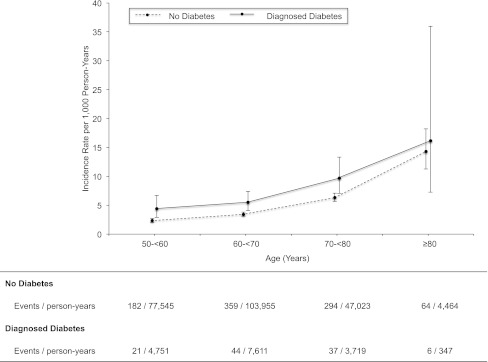
Incidence rate (per 1,000 person-years) of hospitalization for fracture according to age and diabetes status. Point estimates are incidence rates per 1,000 person-years for 10-year age intervals. Vertical bars are 95% CIs. Fractures were defined as an ICD-9 discharge code of 733.1–733.19, 733.93–733.98, or 800–829.
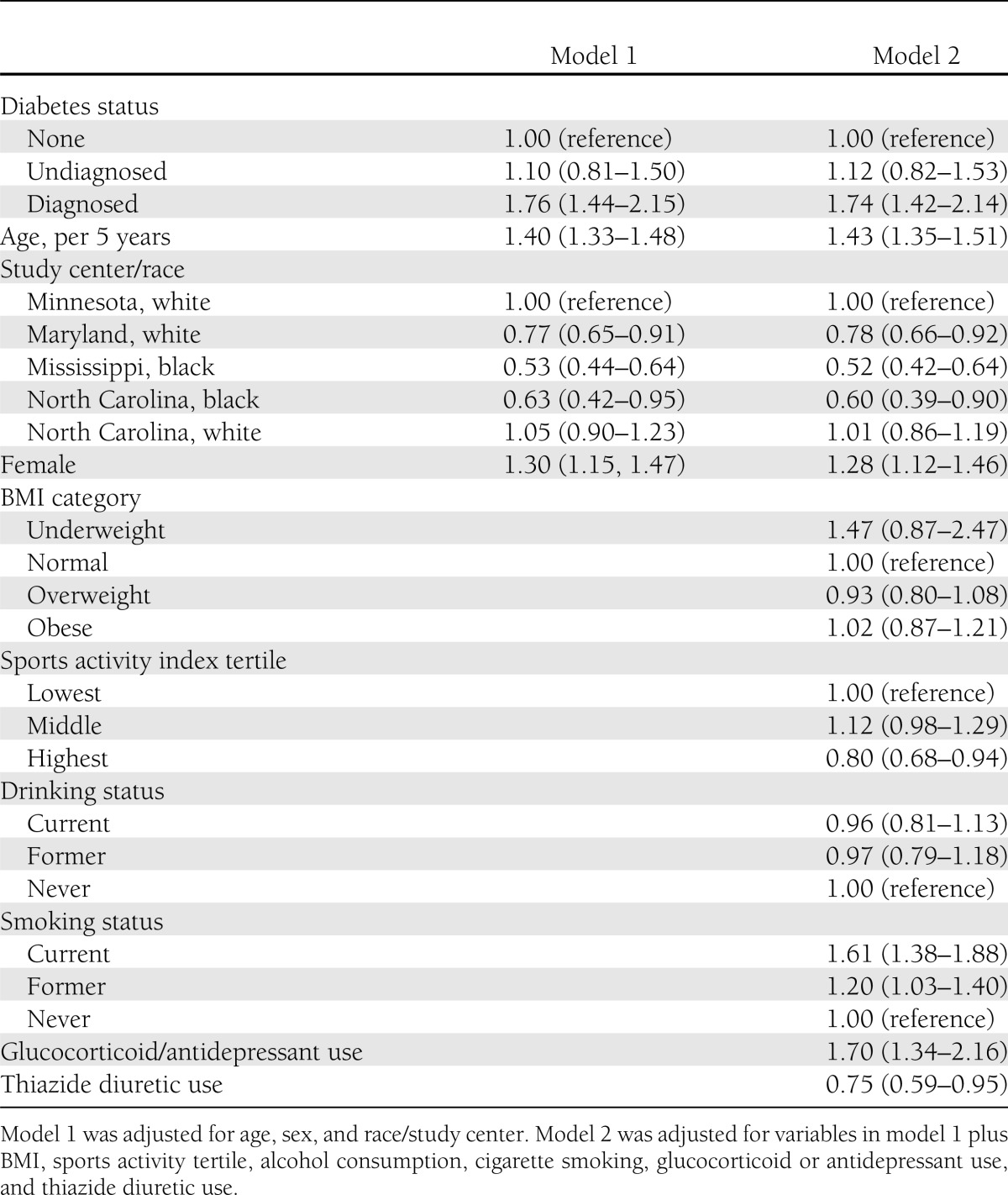
Table 2 shows the adjusted hazard ratios (HRs) for incident fracture hospitalization. Comparing model 1 and model 2, we see that progressive adjustment somewhat attenuates the associations with fracture risk. Diagnosed diabetes was associated with a significant increase in fracture risk after adjustment for all covariates (model 2) (HR, 1.74; 95% CI, 1.42–2.14). In fully adjusted analyses, however, persons with undiagnosed diabetes had a similar risk of fracture as those without diabetes (HR, 1.12; 95% CI, 0.82–1.53). Further adjustment for history of coronary heart disease, history of stroke, and estimated glomerular filtration rate did not appreciably alter the results. In analyses restricted to persons with diagnosed diabetes, oral medication use was not associated with an increased risk for fracture compared with no medication use (HR, 0.97; 95% CI, 0.60–1.55), but the use of insulin (alone or in combination with oral medication) was associated with an increased risk of fracture (1.87; 1.15–3.05). Results were not appreciably altered after excluding fractures coded as pathological (ICD codes 733.1–733.19; n = 133). In the analysis accounting for competing mortality, HRs were attenuated, but the association of diagnosed diabetes with fracture risk remained statistically significant (HR, 1.42; 95% CI, 1.15–1.76).
Table 2.
Adjusted hazard ratios for incident fracture hospitalization among study participants from baseline at visit 1 during 1987–1989 to 1 January 2009
The association of diagnosed diabetes with fracture risk did not differ by age (individuals younger than 54 years at baseline: HR, 2.02; 95% CI, 1.40–2.91 vs. individuals 54 years or older at baseline: 1.74; 1.36–2.24; P for interaction = 0.50). In models stratified by race, the association of diagnosed diabetes with risk of fracture also was not significantly different in blacks (HR, 1.97; 95% CI, 1.34–2.84) compared with in whites (1.66; 1.30–2.13; P for interaction = 0.89). Similarly, the association of diagnosed diabetes with fracture risk was not significantly different in women (HR, 1.90; 95% CI, 1.47–2.47) compared with in men (1.52; 1.08–2.15; P for interaction = 0.56). Among women, menopausal status was not independently associated with fracture risk in the fully adjusted model (HR, 0.99; 95% CI, 0.79–1.24).
In analyses examining the association between diabetes status and specific subtypes of fracture, diagnosed diabetes was significantly associated with increased risk for upper limb (HR, 2.16; 95% CI, 1.31–3.57), lower limb (2.22; 1.69–2.91), vertebral (2.03; 1.05–3.89), and skull or face fracture (2.60; 1.17–5.76) compared with those without diabetes (Supplementary Table 1). The only fracture subtype that occurred at a significantly higher rate among persons with undiagnosed diabetes was hip fracture (HR, 2.99; 95% CI, 1.24–7.21).
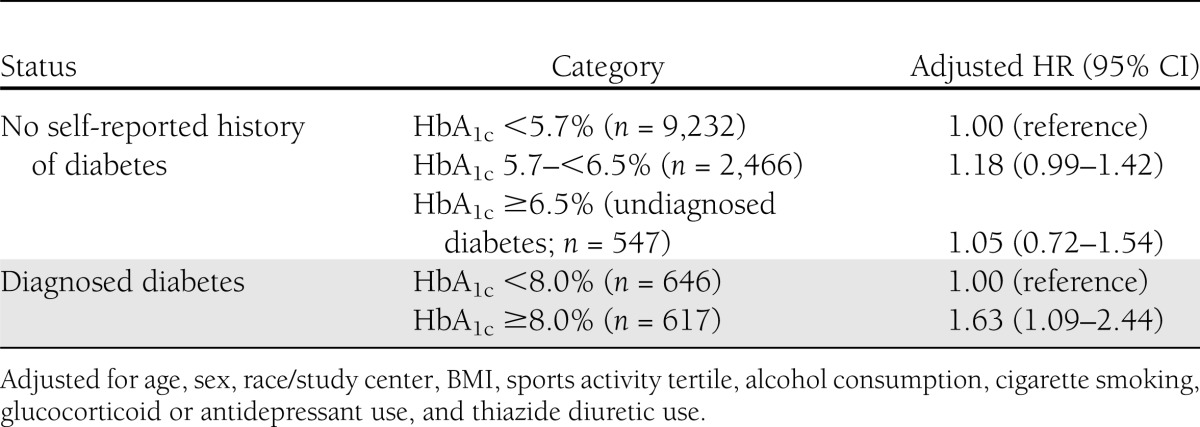
In the sample of individuals who had HbA1c measured at visit 2 (n = 13,508), there were 976 incident fracture hospitalizations during a median of 17 years of follow-up (using visit 2 as baseline). In adjusted analyses (Table 3), clinical categories of HbA1c were not independently associated with fracture risk among persons without a self-reported history of diabetes. In persons with diagnosed diabetes, HbA1c ≥8% was independently associated with fracture risk (model 2 HR: 1.63; 95% CI, 1.09–2.44; compared with HbA1c <8%). The association of HbA1c ≥8% in persons with diagnosed diabetes with fracture risk was attenuated after further adjustment for diabetes medication use (HR, 1.50; 95% CI, 0.97–2.32). Among persons with diagnosed diabetes, the 10-year crude cumulative incidence of fracture for persons with HbA1c ≥8% was 4.9 (95% CI, 3.3–7.1) compared with 4.4 (3.0–6.5) for those with HbA1c <8%.
Table 3.
Adjusted hazard ratios for incident fracture hospitalization according to HbA1c category and diabetes status in 1990–1992 (n = 13,508)
CONCLUSIONS
Our results suggest that persons with diagnosed diabetes are at an increased risk for overall fracture-related hospitalization. Persons with diagnosed diabetes who had poor glucose control, as defined by HbA1c ≥8% or who used insulin were at particularly high risk for fracture-related hospitalization. However, we did not observe overall increase in risk of fracture-related hospitalization in persons with undiagnosed diabetes.
The association of diabetes with fracture risk is inconsistent in the literature. In some studies, diabetes was significantly associated with an increased risk for nonvertebral fractures (4,5), vertebral fractures (20), hip fractures (4,6), wrist fractures (6), and overall fractures (21). In contrast, other studies have found no association of diabetes with vertebral fractures (5) or overall fractures (6). Our study found an association between diagnosed diabetes and overall fractures, as well as upper and lower limb, vertebral, and skull or face fractures. Observed differences in the association of diabetes with fracture risk could partly be attributable to differences in case definition. Most previous studies relied on self-report to define cases of both diabetes and fracture, although some studies confirmed fracture cases by obtaining medical records (5,6). In addition to self-reported information, our study also incorporated medication use and blood glucose levels to define diabetes, and we defined fracture using ICD-9 hospital discharge codes. Differences in results across studies also may be attributable to differences in demographic characteristics of the study population, particularly age. Competing risks, such as death, may be an important analytical issue among older persons with diabetes in analyses of fracture risk.
Our finding that diagnosed diabetes, particularly poorly controlled diabetes and more severe diabetes (HbA1c ≥8% or use of insulin) was associated with an increased risk of fracture is consistent with some previous studies. Two previous studies found that longer duration of diabetes was associated with greater fracture risk (16,22), and another study found that the use of insulin was associated with increased fracture risk (16). Similarly, an analysis of persons with and without diabetes from the Rotterdam Study found that the risk of nonvertebral fracture was only significant among persons with treated diabetes (23). In contrast, results from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial suggested no increased risk of fracture among those randomized to intensive glycemic control compared with the standard care (24). Taken as a whole, these results suggest that mechanisms linking diabetes and fracture risk may relate to diabetes severity, glycemic control, or the use of insulin. It has been hypothesized that physiological changes resulting from chronic hyperglycemia could degrade bone quality through inhibition of osteocalcin, increased reactive oxygen species, bone accumulation of advanced glycation end products, or inhibition of insulin-like growth factor 1 (25). It also has been hypothesized that the complications of diabetes (peripheral neuropathy, peripheral vascular disease), diabetes treatment (insulin), or both could increase the risk of falls and fractures (14,26). Lower-limb fractures, in particular, primarily may be caused by diabetic neuropathy, peripheral vascular disease, or acute hypoglycemia, rather than by osteoporosis (21).
Certain limitations of this study should be considered when interpreting our results. Our study differs from previous investigations on this topic in that it includes only fractures that resulted in inpatient hospitalization, because these were the only data available on incident fracture in the ARIC Study. We were unable to formally validate the cases identified in this study. As a result, more mild (outpatient cases) and asymptomatic fractures, particularly vertebral fractures, were likely missed by our case definition. Our main analysis also included fractures coded as pathological (13% of all fractures) based on evidence that nonpathologic fractures may be coded using an ICD-9 code for pathologic fractures (27). We conducted a sensitivity analysis excluding pathologic fracture ICD-9 codes and found similar results. We performed sensitivity analyses stratified by fracture subtype (torso, upper limb, lower limb, vertebral, hip, skull or face), but because of small numbers of events, these estimates are rather imprecise and should be interpreted cautiously. Additionally, survival bias is an important concern because persons with diabetes are more likely to die at a younger age, whereas older age is associated with fracture incidence. To address this, we performed a competing risks analysis and found similar results. Additionally, we did not have information on duration of diabetes or on episodes of hypoglycemia, and we only had a single measurement of HbA1c, an inherently time-varying measure. We also were not able to evaluate the possible impact of thiazolidinediones or hormone replacement therapy because very few participants used these medications in our population.
Our study also has a number of important strengths, including the large prospective design and the geographically diverse, biracial population, which included both men and women. We had substantial follow-up (median of 20 years), and our study was strengthened by the comprehensive assessment of diabetes, glucose control, and medication use. The availability of glucose and HbA1c measurements allowed us to examine the association with undiagnosed diabetes. We also were able to control for important confounders, including BMI and waist-to-hip ratio, which were rigorously measured in the ARIC Study.
In conclusion, we found that diagnosed, but not undiagnosed, diabetes was associated with an increased risk of fracture. Our results support recommendations from the American Diabetes Association for assessment of fracture risk and implementation of primary and secondary prevention strategies in appropriate patient populations (28). Our study also suggests that persons with poor glycemic control (defined by HbA1c ≥8% or the use of insulin) may particularly benefit from aggressive prevention efforts, regardless of age. A Cochrane review found that falls can be prevented through exercise programs that include a combination of at least two of the following elements: strength, balance, flexibility, or endurance (26). Evidence from randomized controlled trials also suggests that a combination of strength and aerobic exercise training in persons with diabetes may reduce fracture risk (29). Further studies are needed to understand if exercise interventions or strategies to improve glycemic control while minimizing hypoglycemic episodes may prevent fractures among persons with diabetes.
Acknowledgments
The Atherosclerosis Risk in Communities Study was performed as a collaborative study and was supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This research was also supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R21 DK080294. E.S. also was supported by NIH/NIDDK grant K01 DK076595. A.L.C.S. was supported by NIH/NIDDK training grant T32 DK062707.
No potential conflicts of interest relevant to this article were reported.
A.L.C.S. and E.K.W. drafted the manuscript and performed statistical analyses. A.L.C.S., E.K.W., F.L.B., S.B., J.C., and E.S. reviewed and edited the manuscript and contributed to discussion. A.L.C.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the staff and participants of the ARIC Study for their important contributions.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1168/-/DC1.
References
- 1.Armstrong ME, Cairns BJ, Banks E, Green J, Reeves GK, Beral V, Million Women Study Collaborators Different effects of age, adiposity and physical activity on the risk of ankle, wrist and hip fractures in postmenopausal women. Bone 2012;50:1394–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prieto-Alhambra D, Aviles FF, Judge A, Van Staa T, Nogues X, Arden NK, et al. Burden of pelvis fracture: a population-based study of incidence, hospitalisation and mortality. Osteoporos Int 2012;23:2797–2803 [DOI] [PubMed] [Google Scholar]
- 3.Hepgüler S, Cetin A, Değer C, Erkent U. Osteoporotic hip fracture costs in the elderly Turkish population. Acta Orthop Traumatol Turc 2011;45:316–325 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz AV, Vittinghoff E, Bauer DC, et al. Study of Osteoporotic Fractures (SOF) Research Group. Osteoporotic Fractures in Men (MrOS) Research Group. Health, Aging, and Body Composition (Health ABC) Research Group Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 2011;305:2184–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007;166:495–505 [DOI] [PubMed] [Google Scholar]
- 6.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int 2007;18:427–444 [DOI] [PubMed]
- 7.Jackson R, Chambless LE, Yang K, et al. The Atherosclerosis Risk in Communities (ARIC) Study Investigators Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. J Clin Epidemiol 1996;49:1441–1446 [DOI] [PubMed] [Google Scholar]
- 8.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 9.Selvin E, Coresh J, Zhu H, Folsom AR, Steffes MW. Measurement of HbA1c from stored whole blood samples in the Atherosclerosis Risk in Communities study. J Diabetes 2010;2:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942 [DOI] [PubMed] [Google Scholar]
- 11.Khazai NB, Beck GR, Jr, Umpierrez GE. Diabetes and fractures: an overshadowed association. Curr Opin Endocrinol Diabetes Obes 2009;16:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 2005;48:1292–1299 [DOI] [PubMed] [Google Scholar]
- 13.Aung K, Htay T. Thiazide diuretics and the risk of hip fracture. Cochrane Database Syst Rev 2011;10:CD005185. [DOI] [PubMed] [Google Scholar]
- 14.Montagnani A, Gonnelli S, Alessandri M, Nuti R. Osteoporosis and risk of fracture in patients with diabetes: an update. Aging Clin Exp Res 2011;23:84–90 [DOI] [PubMed] [Google Scholar]
- 15.Riche DM, King ST. Bone loss and fracture risk associated with thiazolidinedione therapy. Pharmacotherapy 2010;30:716–727 [DOI] [PubMed] [Google Scholar]
- 16.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ, Blue Mountains Eye Study Diabetes and risk of fracture: the Blue Mountains Eye Study. Diabetes Care 2001;24:1198–1203 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 18.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 19.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl. 1):S64–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 2009;24:702–709 [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ, 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res 2008;23:1334–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med 2005;165:1612–1617 [DOI] [PubMed] [Google Scholar]
- 23.de Liefde, II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int 2005;16:1713–1720 [DOI] [PubMed]
- 24.Schwartz AV, Margolis KL, Sellmeyer DE, et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care 2012;35:1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moseley KF. Type 2 diabetes and bone fractures. Curr Opin Endocrinol Diabetes Obes 2012;19:128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2009;2:CD007146. [DOI] [PubMed] [Google Scholar]
- 27.Curtis JR, Taylor AJ, Matthews RS, Ray MN, Becker DJ, Gary LC, et al. “Pathologic” fractures: should these be included in epidemiologic studies of osteoporotic fractures? Osteoporos Int 2009;20:1969–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon BA, Benson AC, Bird SR, Fraser SF. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res Clin Pract 2009;83:157–175 [DOI] [PubMed] [Google Scholar]


