Abstract
OBJECTIVE
Studies have shown that patients without a consistent primary care provider have inferior outcomes. However, little is known about the mechanisms for these effects. This study aims to determine whether primary care physicians (PCPs) provide more frequent medication intensification, lifestyle counseling, and patient encounters than other providers in the primary care setting.
RESEARCH DESIGN AND METHODS
This retrospective cohort study included 584,587 encounters for 27,225 patients with diabetes and elevated A1C, blood pressure, and/or LDL cholesterol monitored for at least 2 years. Encounters occurred at primary care practices affiliated with two teaching hospitals in eastern Massachusetts.
RESULTS
Of the encounters documented, 83% were with PCPs, 13% were with covering physicians, and 5% were with midlevel providers. In multivariable analysis, the odds of medication intensification were 49% (P < 0.0001) and 26% (P < 0.0001) higher for PCPs than for covering physicians and midlevel providers, respectively, whereas the odds of lifestyle counseling were 91% (P < 0.0001) and 21% (P = 0.0015) higher. During visits with acute complaints, covering physicians were even less likely, by a further 52% (P < 0.0001), to intensify medications, and midlevel providers were even less likely, by a further 41% (P < 0.0001), to provide lifestyle counseling. Compared with PCPs, the hazard ratios for time to the next encounter after a visit without acute complaints were 1.11 for covering physicians and 1.19 for midlevel providers (P < 0.0001 for both).
CONCLUSIONS
PCPs provide better care through higher rates of medication intensification and lifestyle counseling. Covering physicians and midlevel providers may enable more frequent encounters when PCP resources are constrained.
The disease burden from diabetes is increasing in the U.S. and worldwide (1,2). With this increased burden, efficient, quality care becomes even more important.
Many studies have shown that patients who see multiple providers have inferior outcomes (3–6). Continuity of care has further been associated with improved detection (7,8) and management of hypertension (8), greater adherence to diabetes preventive care and other guideline-consistent services (9–11), improved medication adherence (12), better glycemic control in patients with diabetes (13,14), lower rates of hospitalizations (15–17), and lower long-term mortality (18).
Having multiple providers of primary care was also associated with increased medical services expenditures (17) through increased office visits, prescriptions, and number of specialists seen for disease-specific populations (19). Continuity of care was especially important to patients who perceived their health as poor (20), but the mechanisms for these effects are not fully understood.
However, modern models of health care delivery, such as the patient-centered medical home, emphasize a team-based approach to patient care (21,22). These teams will need to deliver effective care even when the patient is not always seen by the same provider. Under these circumstances, it becomes critical to recognize the benefits and mechanisms of continuity of care so they can be replicated in the team setting.
Process measures tightly linked to outcomes may be an effective way to measure quality of care (23). During the last decade, several process measures tightly linked to patient outcomes in the treatment of diabetes have been identified (24), including medication intensification, lifestyle counseling (25), and encounter frequency (25–28). We therefore conducted a study to determine whether primary care physicians (PCPs) perform better on these measures of care than other providers.
RESEARCH DESIGN AND METHODS
Design
We designed this retrospective cohort study to determine if PCPs are more likely than covering providers to intensify medications, provide lifestyle counseling, and have shorter intervals to the next encounter for patients with diabetes and elevated A1C, LDL, or blood pressure (BP).
Study cohort
Adults with diabetes treated at primary care practices affiliated with Brigham and Women’s (BWH) and Massachusetts General (MGH) Hospitals for at least 2 years between 1 January 2000 and 1 January 2010 were studied. Primary care practices included internal medicine and family practice specialties. All of the practices in the study used Longitudinal Medical Record, an internally developed Office of the National Coordinator's Authorized Testing and Certification Body–certified electronic medical record (EMR) where all patient care documentation, including problem lists, electronic prescribing, and provider notes, was recorded. Patients were included in the study if they were at least 18 years old, had a documented diagnosis of diabetes or A1C ≥7.0%, and at least one instance of A1C, BP, or LDL above treatment target. Patients with missing zip codes were excluded to enable adjustment for median household income by zip code. We used treatment goals of <7.0% for A1C, <100 mg/dL for LDL, and <140/90 mmHg for BP.
This study was approved by the Partners HealthCare System institutional review board, and the requirement for written informed consent was waived.
Study measurements
An encounter with a health care provider in a primary care practice served as the unit of analysis. Encounters during uncontrolled periods were included in the analysis. An uncontrolled period started on the day when A1C, BP, or LDL was first noted above the treatment target (27). The period ended on the first subsequent date when all measures fell below the target. Encounters that fell within the uncontrolled period were included in the analysis, whether or not measurements were taken on that date.
The lowest measurement on a given date was used in the analysis. Lowest BP was defined as the BP measurement with the lowest mean arterial pressure. BP measurements were only included in the encounter analysis if they were measured on the same date as the encounter. If A1C and LDL measurements were unavailable on the encounter date, the most recent was carried forward if the measurement was within 6 months of the encounter date. Transient elevations were defined as isolated elevated measurements that subsequently normalized without any medication intensification and were excluded from the analysis. Periods without any medication information available in the EMR were excluded to enable inclusion of insulin treatment as a confounder variable in the analysis. Periods that contained multiple encounters with an endocrinologist were excluded to focus the analysis on the primary care setting where, nationwide, most of diabetes care takes place. Hyperglycemic and hyperlipidemic periods in which rates of A1C and LDL change, respectively, were greater than three standard deviations from the mean were excluded to eliminate likely measurement errors. Finally, encounters with a time to the next encounter exceeding 1 year were excluded to ensure continuous receipt of care at study practices.
A PCP was defined as the PCP with whom the patient had the majority of continuous encounters over a given interval and could change in the course of an uncontrolled period. A covering physician was any other physician in a primary care setting who treated a patient. These physicians were usually other PCPs in the same practice (similarly qualified with respect to specialty and board certification) who were assigned to urgent care or covering duty on a particular day. Encounters with nurse practitioners and physician assistants were assigned a midlevel provider category.
We identified face-to-face encounters based on availability of appropriate billing codes; all notes without corresponding billing codes were considered remote encounters. Acute encounters were defined by ICD-9 diagnosis codes for an acute complaint (e.g., acute pain and/or infection) as previously described (29).
Documentation of lifestyle counseling (diet, exercise, or weight loss) was computationally abstracted from the notes, including direct (eg, “strongly encouraged more walking”) and inferred (eg, “weight has gone up”) instances of lifestyle counseling, as previously described (27,30). We inferred lifestyle counseling if the subject was referred to in a way that indicated it was likely discussed with the patient (eg, not simply weight recorded in the vital signs section). When compared with human double-entry, the software had a sensitivity of between 91 and 97% and a specificity of between 88 and 94%. Weight loss counseling was only considered for encounters when a patient had a BMI ≥30 kg/m2. None of the practices studied during the study period had a program that encouraged a particular type of lifestyle counseling or monitored lifestyle counseling delivered by providers.
Medication intensification was defined as initiation of a new medication or an increase in the dose of an existing medication (29).
Demographic information, weight, height, BP measurements, and medication and laboratory data were obtained from the EMR at Partners HealthCare, an integrated health care delivery network in eastern Massachusetts that includes BWH and MGH.
Statistical analysis
Summary statistics were constructed by using frequencies and proportions for categorical data and using means, standard deviations, medians, and ranges for continuous variables.
The marginal Cox proportional hazards model for clustered data (31) was used to estimate the association between provider type and time to next encounter, and logistic regression models were used to calculate the odds of medication intensification and lifestyle counseling for different provider types. All models were adjusted for demographic confounders (age, sex, race, primary language, health insurance, and median income by zip code), as well as the patient’s Charlson Comorbidity Index (CCI) (32), treatment with insulin as a marker of severity of disease, presence of obesity, diagnosis codes for metastatic cancer within 1 year before the encounter date; measurements of A1C, systolic and diastolic BP, and LDL, face-to-face versus remote encounters, acute complaints, hospitalization before the next encounter (time to next encounter analysis only), and an interaction term between acute status and provider type. Two-sided P values were obtained using type III test and were adjusted for multiple hypothesis testing using the Simes-Hochberg method (33,34). All analyses were performed with SAS 9.3 software (SAS Institute Inc., Cary, NC).
RESULTS
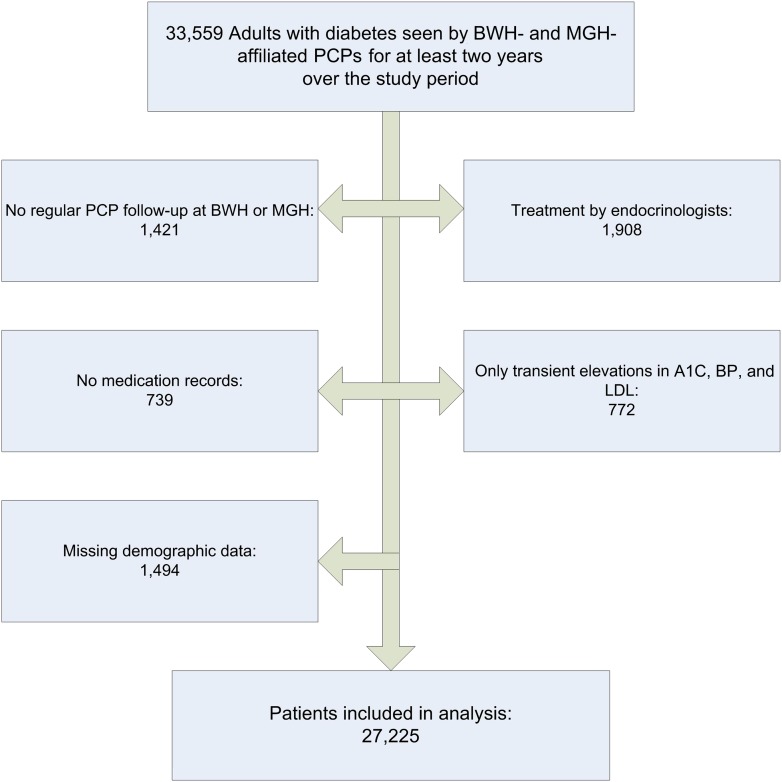
We identified 33,559 adults with diabetes who experienced at least one hyperglycemic, hypertensive, or hyperlipidemic period and were regularly seen in primary care practices associated with BWH or MGH (Fig. 1). After excluding patients regularly treated by endocrinologists, without medication records, only transient elevations in A1C, BP, and LDL, likely A1C or LDL measurement errors, and missing demographic information, the remaining 27,225 unique individuals with 584,587 primary care encounters were included in the study.
Figure 1.
Flow chart shows selection of study patients.
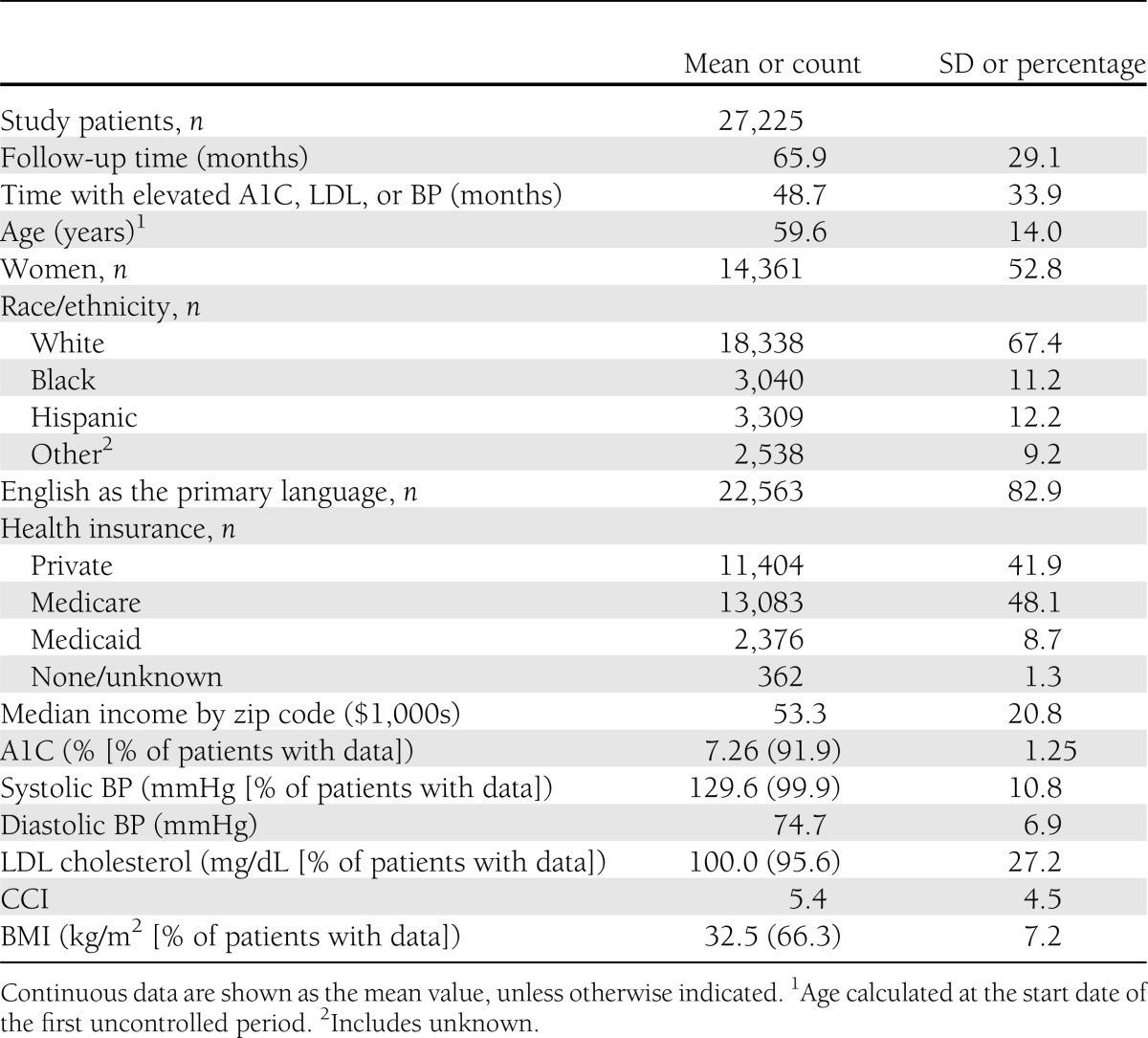
Study patients did not have at least one measure under control over a mean of 78% of total follow-up time (Table 1). During the study period, patients’ mean maximum A1C was 8.7%, BP was 157/90 mmHg, and LDL was 131 mg/dL. The percentage of patients with measurements available during the follow-up period ranged from 92.9% of the time for A1C to 99.9% of the time for BP.
Table 1.
Patient characteristics
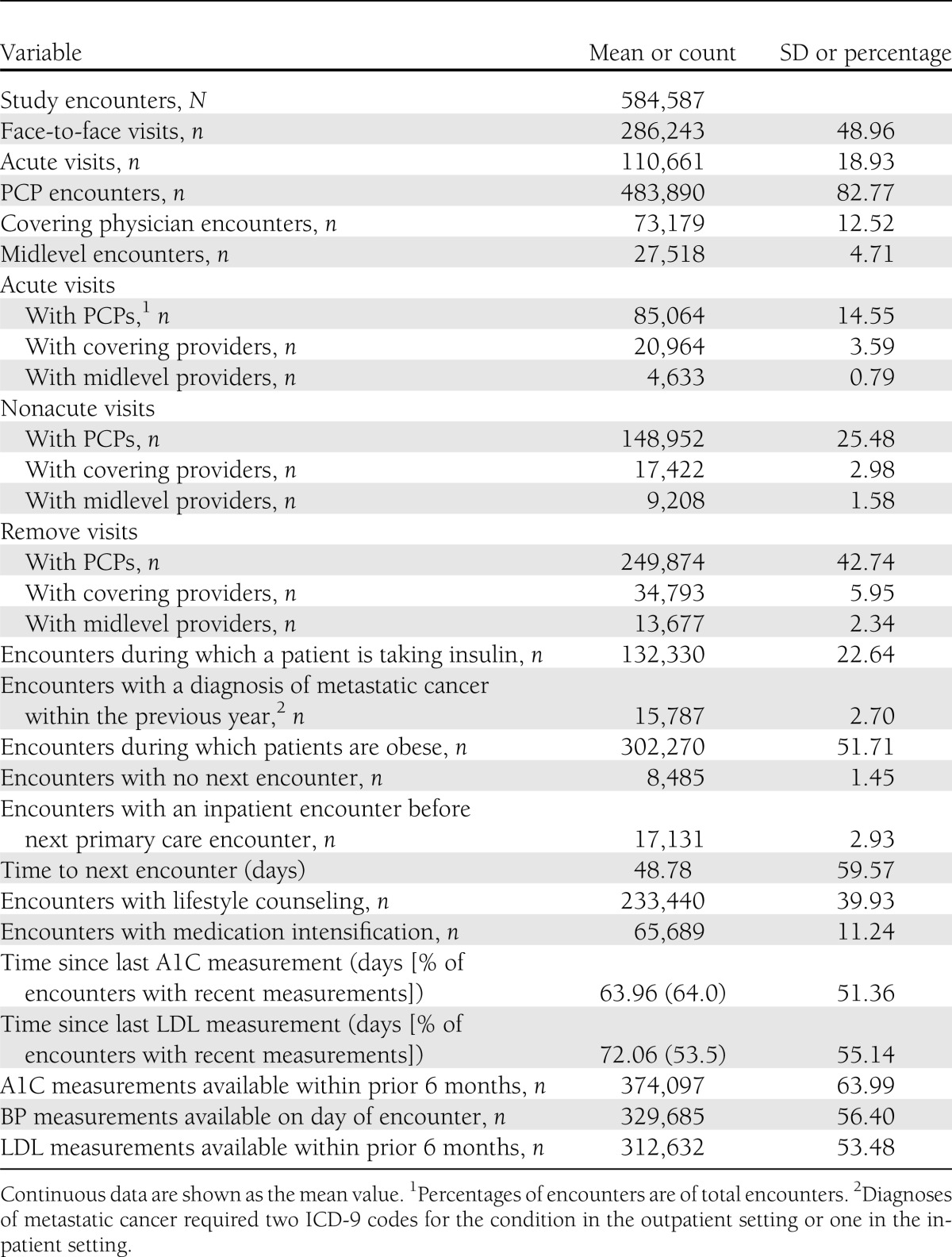
During uncontrolled periods, 83% of the encounters (Table 2) were with PCPs, 13% were with covering physicians, and 5% were with midlevel providers. Face-to-face visits constituted 49% and acute visits 19% of total encounters. PCP encounters constituted 84–85% of nonacute and remote encounters but only 77% of acute encounters. Covering physicians, however, had a higher proportion (19%) of acute encounters and 10–12% of nonacute and remote encounters. Midlevel providers consistently had 4–5% of acute, nonacute, and remote encounters. During all encounters, medication intensification occurred at 10% and lifestyle counseling occurred at 40% of encounters, whereas the mean time to the next encounter was 1.6 months. Mean times since last A1C and LDL measurements were 9 and 10 weeks, respectively. Providers had access to up-to-date A1C, BP, and LDL measures at 64.0, 56.4, and 53.5% of encounters, respectively.
Table 2.
Encounter characteristics
In a multivariable logistic regression model that controlled for patient demographics, CCI, obesity, A1C, BP, and LDL measurements, metastatic cancer diagnosis, insulin status, and an interaction term between acute encounter status and provider type, the odds of medication intensification during nonacute encounters were 49% (P < 0.0001) and 26% (P < 0.0001) higher for PCPs than for covering physicians and midlevel providers, respectively (Table 3). Odds of lifestyle counseling during nonacute encounters were 91% (P < 0.0001) higher for PCPs than covering physicians and 21% (P = 0.0015) higher than midlevel providers. During acute encounters, covering physicians were less likely to intensify medications by a further 52% (P < 0.0001), whereas midlevel providers were less likely to provide lifestyle counseling by a further 41% (P < 0.0001).
Table 3.
Encounter-level analysis estimates, comparing PCPs with other physicians and midlevel providers in multivariable analysis
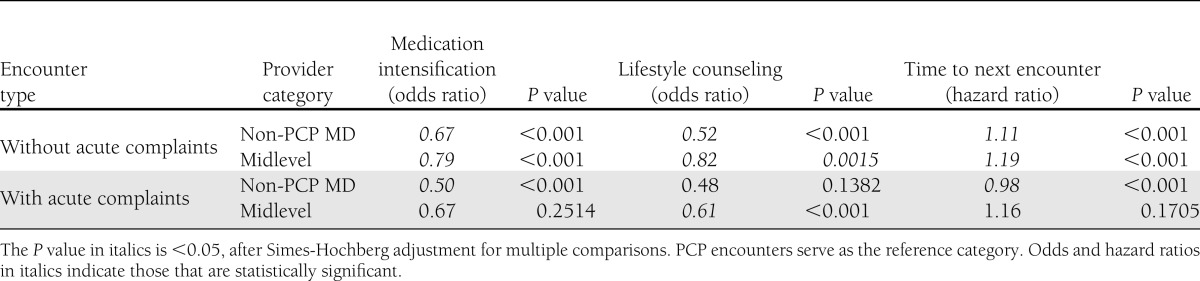
In a multivariable Cox proportional hazards model that adjusted for demographics, CCI, insulin status, obesity, metastatic cancer diagnosis, measurements of A1C, systolic and diastolic BP, and LDL, indicators for face-to-face and acute visits, hospitalization before the next encounter, and an interaction term between acute status and provider type, the hazard ratios for the time to the next encounter after a visit without acute complaints were 1.11 for covering physicians and 1.19 for midlevel providers (P < 0.0001 for both) compared with PCPs.
CONCLUSIONS
In this large retrospective study, we have demonstrated that PCPs were significantly more likely than other providers in the primary care setting to provide lifestyle counseling and medication intensification for patients with uncontrolled diabetes. This association was even stronger during visits in which the patient had an acute complaint. These results suggest that increased frequency of lifestyle counseling and medication intensification may be the mechanisms that underlie better outcomes seen in patients who have higher continuity of care.
Several other explanations for the effect of continuity of care have been proposed: increased time spent with one physician improved the patient’s trust of his or her physician (4), enhanced communication between patient and physician, and increased the physician’s knowledge of the patient (10), but the evidence for their direct effect on patient outcomes is limited. Our study, however, describes mechanisms that have been directly linked to A1C, BP, and LDL control (25–28,35,36). It is likely that multiple mechanisms contribute to the effects of better disease outcomes and that the importance of provider type may vary by mechanism.
Many studies have shown that midlevel providers can be more effective than PCPs in treatment of chronic diseases such as diabetes and hypertension (37–40), particularly with respect to medication intensification (41). The major difference between our study and these clinical trials is that the trials usually required midlevel providers to follow a structured algorithm, whereas midlevel providers in the practices we studied did not follow any particular algorithm. This current finding should be considered when designing new practice models, such as patient-centered medical homes.
Although PCPs are more effective than midlevel providers and covering physicians in providing lifestyle counseling and intensifying medication, a patient may be seen more frequently in practices with other providers available. This is corroborated by the shorter time to follow-up visits after encounters with covering physicians and midlevel providers found in our study. Therefore, practices with midlevel providers may provide more cost-efficient care because there are more opportunities for medication intensification and lifestyle counseling at a lower cost, even if they are not used as frequently. Midlevel providers could, therefore, be especially helpful in situations in which PCP resources are constrained, as they are almost universally across the country (42–44).
These findings have several implications for clinical practice. First, they suggest there should be less cross-covering by other physicians. If patients must be seen by a covering provider, better documentation of the PCP's treatment plan in the medical records may facilitate their decision making and lower the threshold for intervention. Finally, structured algorithms for treatment of chronic disease may be helpful in optimizing the care delivered by midlevel providers.
Lack of intervention for uncontrolled diabetes by a covering provider who does not know the patient well may be seen as appropriate. However, it results in additional delay in treatment; in our study, the average interval between encounters was at least 7 weeks. A proactive approach where the PCP documents a specific plan of action could improve coordination of care and allow covering providers to take timely action, accelerating achievement of diabetes control.
This study used natural language processing technology that permitted cost- and time-efficient computational analysis of thousands of patient encounters, including examination of hundreds of thousands of narrative provider notes in a matter of hours. In the future, similar technologies could also be used to monitor quality of patient care and/or supply feedback to providers. This feedback could help narrow the gap in care provided between PCPs and covering providers if feedback is used consistently.
Our study had a number of strengths. The analysis focused on process-of-care measures that are tightly linked to better patient outcomes, allowing us to identify likely mechanisms for the beneficial effects of continuity of care. This was a large study, conducted in an ethnically and gender-diverse population, and thus is likely to be generalizable to other settings.
This retrospective cohort study also has some limitations, beyond its inability to establish causality. We did not use standard performance measures to assess provider performance. Instead, we focused on measures that have been shown to be tightly linked to patient outcomes. We used the CCI as a measure of the patients' overall disease burden in multivariable analyses. The CCI was originally developed and validated for hospitalized patients and may therefore have skewed the results. However, the CCI has also been shown to correlate with mortality in multiple outpatient populations (45–47), and the conditions it includes have face validity as predictors of mortality in both outpatients and inpatients.
Some of the data pertinent to the analysis might have been missing; for example, physicians may not have recorded some of the BP measurements they made. If missing data were distributed unequally between different provider categories, it could have biased the study findings. To minimize this effect, we used BP information from structured EMR records and also from narrative provider notes (obtained using natural language processing) where clinicians are more likely to document their own BP measurements. We have previously shown that this approach results in a more complete data collection (48). Physicians also might have been more likely to round the BP measurements down if they were individually judged according to BP-based quality indicators. However, no quality indicators were implemented at the individual provider level in the practices studied during the study period.
The study was conducted at two teaching hospitals in eastern Massachusetts. The patients who seek care and the providers who work in such networks may be different from other populations. The practices we studied did not have a large number of midlevel providers, making it difficult to study the care they provide in more detail. Furthermore, because no treatment algorithms were in place in any of the practices studied, midlevel providers who followed an algorithm could not be compared with those who did not.
We did not have information on the patients' health-related behaviors that could have accounted for some of the observed effects if they were distributed unequally between PCP versus non-PCP encounters.
Finally, we were unable to distinguish patients with type 1 diabetes from those with type 2 diabetes. Because most of the patients studied likely had type 2 diabetes, our conclusions may not be applicable to patients with type 1 diabetes.
In conclusion, this large, long-term retrospective study showed that PCPs perform better on a number of critical process measures of diabetes care than covering physicians or midlevel providers. These findings suggest mechanisms for well-described improvements in quality of treatment seen with higher continuity of care. They should be taken into consideration in the design and evaluation of novel health care delivery models, such as patient-centered medical homes, and in quality improvement in traditional care settings.
Acknowledgments
This study was supported in part by grants from the Agency for Healthcare Research and Quality (5R18HS017030), the National Library of Medicine (5RC1LM010460), and the Diabetes Action Research and Education Foundation. The funding sources had no role in the design and conduct of the study, collection, management, analysis, or interpretation of the study or in the preparation, review, or approval of the manuscript.
No potential conflicts of interest relevant to the article were reported.
F.M. conducted data analysis and drafted the manuscript. M.S. assisted in study design, provided biostatistical support, and critically reviewed the manuscript. S.I.G. assisted in study design and analysis and critically reviewed the manuscript. A.T. designed the study, obtained funding, and critically reviewed the manuscript. F.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 2010;33:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 3.Sweeney KG, Gray DP. Patients who do not receive continuity of care from their general practitioner—are they a vulnerable group? Br J Gen Pract 1995;45:133–135 [PMC free article] [PubMed] [Google Scholar]
- 4.Wasson JH, Sauvigne AE, Mogielnicki RP, et al. Continuity of outpatient medical care in elderly men. A randomized trial. JAMA 1984;252:2413–2417 [PubMed] [Google Scholar]
- 5.Parchman ML, Burge SK, Residency Research Network of South Texas Investigators Continuity and quality of care in type 2 diabetes: a Residency Research Network of South Texas study. J Fam Pract 2002;51:619–624 [PubMed] [Google Scholar]
- 6.Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med 2005;3:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koopman RJ, Mainous AG, 3rd, Baker R, Gill JM, Gilbert GE. Continuity of care and recognition of diabetes, hypertension, and hypercholesterolemia. Arch Intern Med 2003;163:1357–1361 [DOI] [PubMed] [Google Scholar]
- 8.Konrad TR, Howard DL, Edwards LJ, Ivanova A, Carey TS. Physician-patient racial concordance, continuity of care, and patterns of care for hypertension. Am J Public Health 2005;95:2186–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor PJ, Desai J, Rush WA, Cherney LM, Solberg LI, Bishop DB. Is having a regular provider of diabetes care related to intensity of care and glycemic control? J Fam Pract 1998;47:290–297 [PubMed] [Google Scholar]
- 10.Parchman ML, Burge SK. The patient-physician relationship, primary care attributes, and preventive services. Fam Med 2004;36:22–27 [PubMed] [Google Scholar]
- 11.Atlas SJ, Grant RW, Ferris TG, Chang Y, Barry MJ. Patient-physician connectedness and quality of primary care. Ann Intern Med 2009;150:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brookhart MA, Patrick AR, Schneeweiss S, et al. Physician follow-up and provider continuity are associated with long-term medication adherence: a study of the dynamics of statin use. Arch Intern Med 2007;167:847–852 [DOI] [PubMed] [Google Scholar]
- 13.Mainous AG, 3rd, Koopman RJ, Gill JM, Baker R, Pearson WS. Relationship between continuity of care and diabetes control: evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health 2004;94:66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dearinger AT, Wilson JF, Griffith CH, Scutchfield FD. The effect of physician continuity on diabetic outcomes in a resident continuity clinic. J Gen Intern Med 2008;23:937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mainous AG, 3rd, Gill JM. The importance of continuity of care in the likelihood of future hospitalization: is site of care equivalent to a primary clinician? Am J Public Health 1998;88:1539–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight JC, Dowden JJ, Worrall GJ, Gadag VG, Murphy MM. Does higher continuity of family physician care reduce hospitalizations in elderly people with diabetes? Popul Health Manag 2009;12:81–86 [DOI] [PubMed] [Google Scholar]
- 17.Weiss LJ, Blustein J. Faithful patients: the effect of long-term physician-patient relationships on the costs and use of health care by older Americans. Am J Public Health 1996;86:1742–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolinsky FD, Bentler SE, Liu L, et al. Continuity of care with a primary care physician and mortality in older adults. J Gerontol A Biol Sci Med Sci 2010;65:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raddish M, Horn SD, Sharkey PD. Continuity of care: is it cost effective? Am J Manag Care 1999;5:727–734 [PubMed] [Google Scholar]
- 20.Rodriguez HP, Rogers WH, Marshall RE, Safran DG. The effects of primary care physician visit continuity on patients’ experiences with care. J Gen Intern Med 2007;22:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodenheimer T. Coordinating care—a perilous journey through the health care system. N Engl J Med 2008;358:1064–1071 [DOI] [PubMed] [Google Scholar]
- 22.Barr MS. The need to test the patient-centered medical home. JAMA 2008;300:834–835 [DOI] [PubMed] [Google Scholar]
- 23.Kerr EA, Krein SL, Vijan S, Hofer TP, Hayward RA. Avoiding pitfalls in chronic disease quality measurement: a case for the next generation of technical quality measures. Am J Manag Care 2001;7:1033–1043 [PubMed] [Google Scholar]
- 24.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care 2001;24:1821–1833 [DOI] [PubMed] [Google Scholar]
- 25.Morrison F, Shubina M, Turchin A. Lifestyle counseling in routine care and long-term glucose, blood pressure, and cholesterol control in patients with diabetes. Diabetes Care 2012;35:334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berlowitz DR, Ash AS, Glickman M, et al. Developing a quality measure for clinical inertia in diabetes care. Health Serv Res 2005;40:1836–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison F, Shubina M, Turchin A. Encounter frequency and serum glucose level, blood pressure, and cholesterol level control in patients with diabetes mellitus. Arch Intern Med 2011;171:1542–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selby JV, Uratsu CS, Fireman B, et al. Treatment intensification and risk factor control: toward more clinically relevant quality measures. Med Care 2009;47:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turchin A, Shubina M, Chodos AH, Einbinder JS, Pendergrass ML. Effect of board certification on antihypertensive treatment intensification in patients with diabetes mellitus. Circulation 2008;117:623–628 [DOI] [PubMed] [Google Scholar]
- 30.Turchin A, Goldberg SI, Breydo E, Shubina M, Einbinder JS. Copy/paste documentation of lifestyle counseling and glycemic control in patients with diabetes: true to form? Arch Intern Med 2011;171:1393–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med 1994;13:2233–2247 [DOI] [PubMed] [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 33.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988;75:800–802 [Google Scholar]
- 34.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika 1986;73:751–754 [Google Scholar]
- 35.Guthmann R, Davis N, Brown M, Elizondo J. Visit frequency and hypertension. J Clin Hypertens (Greenwich) 2005;7:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turchin A, Goldberg SI, Shubina M, Einbinder JS, Conlin PR. Encounter frequency and blood pressure in hypertensive patients with diabetes mellitus. Hypertension 2010;56:68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denver EA, Barnard M, Woolfson RG, Earle KA. Management of uncontrolled hypertension in a nurse-led clinic compared with conventional care for patients with type 2 diabetes. Diabetes Care 2003;26:2256–2260 [DOI] [PubMed] [Google Scholar]
- 38.Lee TH, Bodenheimer T, Goroll AH, Starfield B, Treadway K. Perspective roundtable: redesigning primary care. N Engl J Med 2008;359:e24. [DOI] [PubMed] [Google Scholar]
- 39.New JP, Mason JM, Freemantle N, et al. Specialist nurse-led intervention to treat and control hypertension and hyperlipidemia in diabetes (SPLINT): a randomized controlled trial. Diabetes Care 2003;26:2250–2255 [DOI] [PubMed] [Google Scholar]
- 40.Taylor CB, Miller NH, Reilly KR, et al. Evaluation of a nurse-care management system to improve outcomes in patients with complicated diabetes. Diabetes Care 2003;26:1058–1063 [DOI] [PubMed] [Google Scholar]
- 41.Vivian EM. Improving blood pressure control in a pharmacist-managed hypertension clinic. Pharmacotherapy 2002;22:1533–1540 [DOI] [PubMed] [Google Scholar]
- 42.Østbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med 2005;3:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodenheimer T. Primary care—will it survive? N Engl J Med 2006;355:861–864 [DOI] [PubMed] [Google Scholar]
- 44.Hauer KE, Durning SJ, Kernan WN, et al. Factors associated with medical students’ career choices regarding internal medicine. JAMA 2008;300:1154–1164 [DOI] [PubMed] [Google Scholar]
- 45.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med 2000;108:609–613 [DOI] [PubMed] [Google Scholar]
- 46.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–1267 [DOI] [PubMed] [Google Scholar]
- 47.Perkins AJ, Kroenke K, Unützer J, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol 2004;57:1040–1048 [DOI] [PubMed] [Google Scholar]
- 48.Turchin A, Shubina M, Breydo E, Pendergrass ML, Einbinder JS. Comparison of information content of structured and narrative text data sources on the example of medication intensification. J Am Med Inform Assoc 2009;16:362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]