Abstract
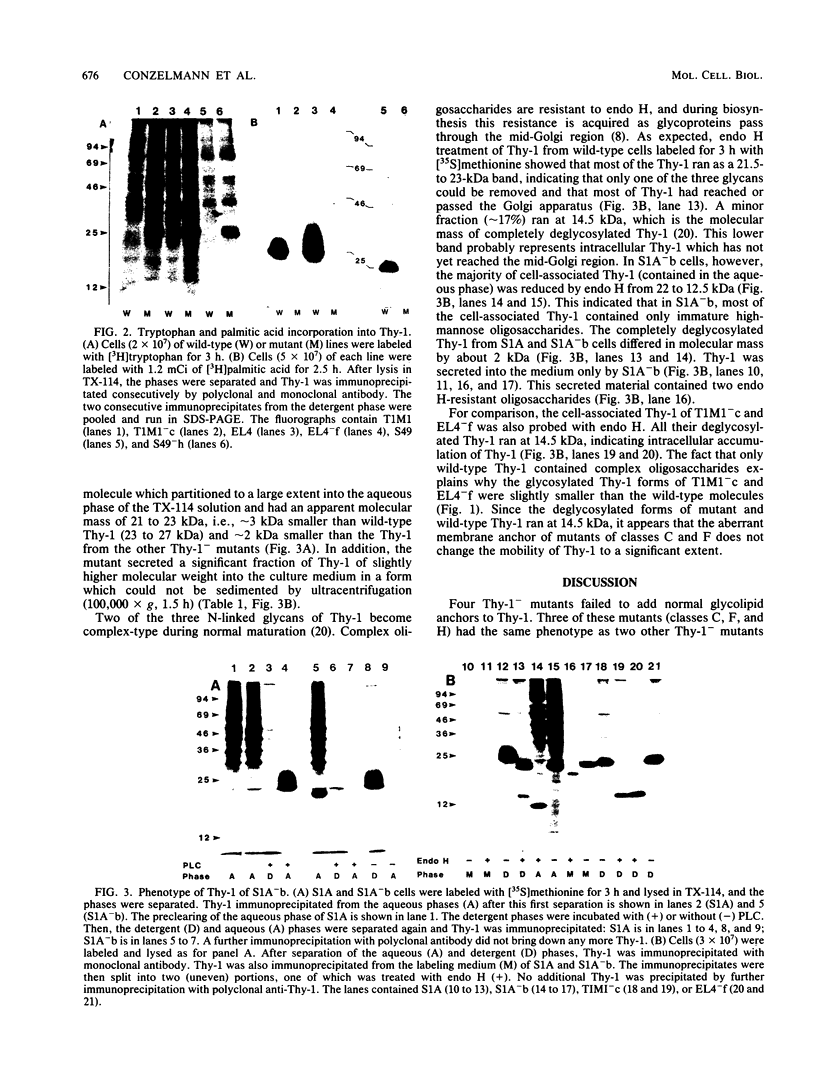
Recent evidence shows that the mature Thy-1 surface glycoprotein lacks the C-terminal amino acids 113 to 143 predicted from the cDNA sequence and is anchored in the plasma membrane by a complex, phosphatidylinositol-containing glycolipid attached to the alpha-carboxyl group of amino acid 112. Here we studied the biosynthesis of Thy-1 in two previously described and two newly isolated Thy-1-deficient mutant cell lines. Somatic cell hybridization indicated that their mutations affected some processing step rather than the Thy-1 structural gene. The Thy-1 made by mutants of classes C, F, and H bound detergent but, in contrast to wild-type Thy-1, their detergent-binding moieties could not be removed by phospholipase C. In addition, tryptophan, which only occurs in position 124, was incorporated into Thy-1 of these mutants but not of wild-type cells. Last, the Thy-1 of wild-type but not mutant cells could be radiolabeled with [3H]palmitic acid. Together, these findings strongly suggest that mutants of classes C, F, and H accumulate a biosynthetic intermediate of Thy-1 which retains at least part of the hydrophobic C-terminal peptide. The Thy-1 of these mutants remained endoglycosidase H sensitive, suggesting that it accumulated in the rough endoplasmic reticulum or the Cis-Golgi. A different Thy-1 intermediate was found in a class B mutant cell line: the Thy-1 of this mutant was 2 kilodaltons smaller than the Thy-1 of other cell lines, did not bind detergent, and was rapidly secreted via a normal secretory pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangs J. D., Hereld D., Krakow J. L., Hart G. W., Englund P. T. Rapid processing of the carboxyl terminus of a trypanosome variant surface glycoprotein. Proc Natl Acad Sci U S A. 1985 May;82(10):3207–3211. doi: 10.1073/pnas.82.10.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chapman A., Fujimoto K., Kornfeld S. The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J Biol Chem. 1980 May 25;255(10):4441–4446. [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Bron C. Glycolipid anchors are attached to Thy-1 glycoprotein rapidly after translation. Biochem J. 1987 Sep 15;246(3):605–610. doi: 10.1042/bj2460605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Hyman R., Bron C. Anchoring of membrane proteins via phosphatidylinositol is deficient in two classes of Thy-1 negative mutant lymphoma cells. EMBO J. 1986 Dec 1;5(12):3291–3296. doi: 10.1002/j.1460-2075.1986.tb04642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Structure of the variant glycoproteins and surface coat of Trypanosoma brucei. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):3–12. doi: 10.1098/rstb.1984.0104. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brands R., Rothman J. E. Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell. 1985 Feb;40(2):463–472. doi: 10.1016/0092-8674(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Fatemi S. H., Haas R., Jentoft N., Rosenberry T. L., Tartakoff A. M. The glycophospholipid anchor of Thy-1. Biosynthetic labeling experiments with wild-type and class E Thy-1 negative lymphomas. J Biol Chem. 1987 Apr 5;262(10):4728–4732. [PubMed] [Google Scholar]
- Fatemi S. H., Tartakoff A. M. Hydrophilic anchor-deficient Thy-1 is secreted by a class E mutant T lymphoma. Cell. 1986 Aug 29;46(5):653–657. doi: 10.1016/0092-8674(86)90340-5. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Haldar K., Cross G. A. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J Biol Chem. 1985 Apr 25;260(8):4963–4968. [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Holder A. A. Carbohydrate is linked through ethanolamine to the C-terminal amino acid of Trypanosoma brucei variant surface glycoprotein. Biochem J. 1983 Jan 1;209(1):261–262. doi: 10.1042/bj2090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Hyman R. Genetic basis for Ly-6- defect: complementation between Ly-6- and Thy-1- mutant cell lines. Immunogenetics. 1983;17(3):261–270. doi: 10.1007/BF00364410. [DOI] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Hyman R. Studies on surface antigen variants. Isolation of two complementary variants for Thy 1.2. J Natl Cancer Inst. 1973 Feb;50(2):415–422. doi: 10.1093/jnci/50.2.415. [DOI] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Luescher B., Bron C. Biosynthesis of mouse Thy-1 antigen. J Immunol. 1985 Feb;134(2):1084–1089. [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Reiser H., Oettgen H., Yeh E. T., Terhorst C., Low M. G., Benacerraf B., Rock K. L. Structural characterization of the TAP molecule: a phosphatidylinositol-linked glycoprotein distinct from the T cell receptor/T3 complex and Thy-1. Cell. 1986 Nov 7;47(3):365–370. doi: 10.1016/0092-8674(86)90593-3. [DOI] [PubMed] [Google Scholar]
- Seki T., Chang H. C., Moriuchi T., Denome R., Ploegh H., Silver J. A hydrophobic transmembrane segment at the carboxyl terminus of thy-1. Science. 1985 Feb 8;227(4687):649–651. doi: 10.1126/science.2857501. [DOI] [PubMed] [Google Scholar]
- Seki T., Moriuchi T., Chang H. C., Denome R., Silver J. Structural organization of the rat thy-1 gene. Nature. 1985 Feb 7;313(6002):485–487. doi: 10.1038/313485a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R., Mazauskas C. The synthesis and properties of T25 blycoprotein in Thy-1-negative mutant lymphoma cells. Cell. 1978 May;14(1):21–32. doi: 10.1016/0092-8674(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R. Thy-1 variants of mouse lymphomas: biochemical characterization of the genetic defect. Cell. 1975 Nov;6(3):279–287. doi: 10.1016/0092-8674(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]