Abstract
Polymethylmethacrylate (PMMA) bone cement technology has progressed from industrial Plexiglass administration in the 1950s to the recent advent of nanoparticle additives. Additives have been trialed to address problems with modern bone cements such as the loosening of prosthesis, high post-operative infection rates, and inflammatory reduction in interface integrity. This review aims to assess current additives used in PMMA bone cements and offer an insight regarding future directions for this biomaterial. Low index (< 15%) vitamin E and low index (< 5 g) antibiotic impregnated additives significantly address infection and inflammatory problems, with only modest reductions in mechanical strength. Chitosan (15% w/w PMMA) and silver (1% w/w PMMA) nanoparticles have strong antibacterial activity with no significant reduction in mechanical strength. Future work on PMMA bone cements should focus on trialing combinations of these additives as this may enhance favourable properties.
Keywords: Polymethylmethacrylate, Bone cement, Cement nanoparticle, Vitamin E additive, Arthroplasty, Artificial joint fixation, Post-operative infection, Mechanical weakness, Fat additive, Antibiotics
INTRODUCTION
Bone cement, or polymethylmethacrylate (PMMA), has been used in surgical fixation of artificial joints for over 50 years. The primary function of bone cement is to transfer forces from bone to prosthesis. This review explores the development of bone cements, the role of bone cement additives, identifies applications and discusses future directions.
HISTORICAL BACKGROUND
The pioneering work on PMMA technology is widely credited to German chemist Dr. Otto Rohm. He patented the PMMA product Plexiglass in 1933, which was used in submarine periscopes and airplane canopies[1], leading to an exponential increase in demand and interest during the pre-war and war era. Kulzer (1936) was at the forefront of mouldable cement technology after discovering that the dough formed by mixing ground PMMA powder and a liquid monomer hardens when benzoyl peroxide is added and the mixture heated to 100 °C in a stone mould[2]. The first clinical use of this PMMA mixture was in an attempt to close cranial defects in monkeys in 1938. Surgeons used the heat stable polymer Paladon 65 to close cranial defects in humans. The material was assembled in plates in the laboratory and later moulded in the surgical suite[2].
The era of modern PMMA bone cements stems from the patent by Degussa and Kulzer (1943), describing how MMA polymerizes at room temperature if a co-initiator, such as a tertiary aromatic amine, is added[2]. Dental surgeons were the first to use this technology for dental fixatives and fixtures.
The first bone cement use in orthopaedics is widely credited to English surgeon, Dr. John Charnley, who used “dental acrylic” in 1958 for total hip arthroplasty[3]. Initial clinical results were poor for mechanical and biological reasons, related to both cement and loading surface[2]. Dr. Charnley developed a new product called “bone cement” (Plexiglass) which had more adaptable biological characteristics[4] and which he marketed aggressively to the global orthopaedic community. American orthopaedic surgeons trained with Dr. Charnley at the Wrightington Hospital in the 1960’s and 1970’s to learn his pioneering technique[5]. When returning to America, these surgeons often took bags of bone cement with them, an illegal trade which was only eliminated in the mid-1970’s after the Food and Drug Administration approved the use of bone cement technology in the United States[5]. This material still had many shortcomings. Over the last two decades, additives have been developed to address these shortcomings[6].
PMMA PROPERTIES AND ADDITIVES
Mechanical weakness
A common complication of cemented arthroplasty is loosening of the cemented prosthesis. Mechanical weakness in the bone cement, primarily attributed to the addition of barium sulphate and zirconium oxides (for radiological detection), increases the risk of loosening[7]. Stabilisation of the bone cement matrix improves the transfer of load across the cement-prosthesis interface, reducing the likelihood of crack formation in the cement. Various additives such as steel fibres, glass fibres, carbon fibres and titanium fibres have been developed to improve mechanical strength[8-10]. Rubber toughened cement (PMMA matrix interspersed with rubber particles; Moeseley Rubber Co. Pvt. Ltd., United States) has 167% greater fracture toughness (the structural strength to withstand further cracking in fractured materials) than non-reinforced control (PMMA), although compressive strength and elasticity are compromised (raw data not available)[11]. PMMA reinforced with embedded continuous stainless steel coil (2.5 turns of coil; distal tip of prosthesis) significantly increases compressive stress 4.5-fold (control vs reinforced; 0.039 ± 0.001 MPa vs 0.009 ± 0.001 MPa) and tensile stress 4.5-fold (control vs reinforced; 4.272 ± 0.015 MPa vs 0.95 ± 0.005 MPa) on 3-dimensional finite element computational analysis[12]. This reinforcement increases mechanical strength, thus decreasing the likelihood of fracture formation. The use of additives with rubber toughened cements and stainless steel coils may improve other properties and needs to be investigated.
Interface integrity
The long-term stability of cemented hip arthroplasty is also dependent on the integrity of the bone-cement interface. Interface integrity is related to the strength of bonding and the degree of cement penetration (extent of interdigitation into bone). Increased migration behavior and micromotions of the prosthesis and bone cement are a result of abrasion. The production of wear particles from roughened metallic surfaces and from the PMMA cement promotes local inflammatory activity, resulting in chronic complications to hip replacements[13]. Lower bone cement viscosity affects the mechanical strength of the connection, giving an immediate limitation to the benefits of certain water-based additives, like antibiotics, in comparison to those in powder form[14]. The addition of an amphiphilic bonder, such as glutaraldehyde, may lead to significant improvements in the longevity of cemented metal stems[13,15]. Strength is maximized by increasing the amount of trabecular bone in the cement[16]. Interface integrity should be the optimal outcome of any additive trial. Powder based additives should generally be preferred to their water based counterparts, with greater importance placed on ensuring increased trabecular bone in cement matrix and/or amphiphilic bonders.
Osteoconduction
Osteoconduction refers to a process in which the three-dimensional structure of a substance is conducive to the on growth and/or ingrowth of newly formed bone. Bone growth on an implant surface depends on the action of differentiated bone cells; pre-existing pre-osteoblasts/osteoblasts activated by trauma or recruited from primitive mesenchymal cells by osteoinduction[17,18]. Bone conduction is dependent on the conditions for bone repair as well as the biomaterial used and its reactions[19]. More than 60% by weight of bioactive ceramic powders should be added to PMMA powders to achieve satisfactory osteoconductive properties after setting[20].
Thermal reduction
The polymerisation of bone cement is an exothermic process that can cause tissue necrosis. The high peak curing temperatures of acrylic bone cements is a major concern that needs to be addressed. The use of oxygen plasma increases the maximum curing temperature of bone cement. For example, 100 W of oxygen plasma applied to PMMA powdered polymer (Sigma-Aldrich Chemie, Germany) increases the maximum temperature from 83.48 ± 7.35 °C to 96.50 ± 4.52 °C (no reported significance)[21]. This is explained by the catalytic activity in polymerization, which results in more rapid heat release. A number of additives have also been tested for their potential effects on heat reduction. PMMA bone cement modification with 1-dodecyl mercaptan (DDM, Acros Organics, United States) lowers peak temperatures by 4-6 °C (no reported significance), possibly by acting as a chain stopping agent[21]. Endothermic reactions involving ammonium nitrate (Acros Organics United States) also help to reduce temperatures (73.64 vs 96.5 °C; no reported significance). Zeolites (ZSM-5, Acros Organics, United States) further improve the exothermic profile of bone cements, reducing temperature from 90.12 to 86.9 °C with DDM, and from 73.64 to 72.66 °C with ammonium nitrate (no reported significance)[21]. In addition to limiting PMMA toxicity, the antioxidant N-Acetylcysteine (NAc) has also been shown to significantly reduce heat release in a dose dependent manner[22]. The maximum polymerization temperature was 42.6 °C with 1.00% (w/w) NAc, compared to 57.0 °C in the absence of NAc.
Radio-opacifying additives
Ceramic particles, such as barium sulfate and zirconia (zirconium oxide), are incorporated into bone cement to allow visualization through X-ray imaging[23]. They have an adverse influence on the biocompatibility of PMMA, leading to mechanical weakness[23-25]. Barium sulfate (BaSO4; Horii Pharmaceutical, Osaka, Japan) at 10% w/w monomer has a compressive load test strength of 85( ± 5) MPa[26]. Increasing concentrations of BaSO4 (20%; 30%; 40% w/w monomer) reduce this strength (86 ± 4 MPa; 87 ± 8 MPa; 69 ± 10 MPa), although only the reduction between 30% and 40% is statistically significant (P < 0.02)[26]. The 10% w/w monomer has a fracture load of 88 ± 10 MPa in the three point bending load test, and this strength reduces in proportion to increasing barium concentration[26]. Furthermore, impact load testing of 10% w/w monomer reveals a strength of 3.1 ± 0.9 kJ/m2, which is the same as for the 20%, 30% and 40% w/w monomers (P < 0.01)[26]. Thus, increasing concentrations of barium sulfate (10%-40%) reduce mechanical strength of cement. Additionally, conventional barium sulfate (Reade Materials; Providence, RI, United States) promotes poor osteoblast (bone forming cells) function at the surface of PMMA, in human osteoblast cell culture lines (CRL-11372), as seen by scanning electron microscopy and atomic force microscopy[25]. Kobayashi et al[27] analysed the effect of barium concentrations in PMMA additives (10%, 30% wt and empty control; Simplex® and Spineplex®, Stryker Instruments) in animal models at 12 and 90 d. Higher concentrations of barium sulfate were associated with stronger foreign body reaction at 90 d, suggesting lower levels of biocompatibility at higher concentrations. Further work is needed weighing the benefit of higher cement visualization against the lower biocompatibility at higher BaSO4 concentrations in humans.
Iodine-containing acrylic bone cement has comparable biocompatibility to the barium sulfate-containing equivalent, while maintaining its useful radiopaque properties[28]. Analysis suggested that there was no significant difference in mechanical strength (fracture toughness and four-point loading test) between iodine and barium sulfate based cements.although further work needed to assess clinical application of iodine based cement[28].
The use of ceramic nanoparticles, such as magnesium oxide (MgO; 12.8 nm; Sigma Aldrich; St. Louis, MO, United States) and BaSO4 (80-500 nm; Reade Materials; Providence, RI, United States), improves osteoblast adhesion (PMMA + nanoMgO 3.25 cells/mm2; PMMA + nanoBaSO4 3.6 cells/mm2; cell density on adhesion assay and fluorescence microscopy) compared to conventional PMMA (2.6 cells/mm2), although this improvement is not statistically significant (P < 0.1)[25]. The addition of nanoBaSO4 (100 nm; Sachtleben, Duisburg, Germany) to PMMA (CMW1 bone cement; DePuy Orthopaedics Inc., Warsaw, IN, United States) at 10% w/w has no significant difference on uniaxial compression strength (P = 0.08) or uniaxial tensile strength (ultimate stress and elastic modulus; P = 0.3 and P = 0.4 respectively)[29]. The addition of nanoMgO (at 10% w/w per total PMMA cement) also reduces the exothermic nature of in vitro PMMA solidification (Table 1), thus minimizing tissue necrosis[25]. Overall, nanoMgO and nanoBaSO4 improve osteoblast adhesion, with nanoMgO minimizing tissue necrosis and nanoBaSO4 having no impact on mechanical strength. Further work is needed to fully assess the mechanical parameters of nanoMgO and the exothermic activity of nanoBaSO4.
Table 1.
Exothermic activity of polymethylmethacrylate mixed with nano-MgO (12.8 nm) vs polymethylmethacrylate control[25]
| 1 s | 1 min | 2 min | 10 min | 107 min | |
| PMMA (°C) | 44.98 | 45.82 | 50.10 | 52.5 | 47.85 |
| PMMA and nano-MgO (°C) | 39.65 | 40.36 | 46.99 | 48.85 | 44.10 |
PMMA: Polymethylmethacrylate.
Organobismuth compounds also have radio-opaque properties that have been tested in bone cement. One particular study found that 5%, 10%, 15% and 20% (w/w) bismuth salicylate in bone cement with a 2/1 solid/liquid ratio [MMA, 1% (v/v) dimethyl-4-toluidine, 1.25% (w/w) benzoyl peroxide, Merck] had higher radiopacity than standard admixtures containing barium sulphate (Merck)[30]. Furthermore, 10% bismuth salicylate preparations had a higher percentage of injectability than their 10% barium sulphate counterpart (85.89% vs 81.90%; no reported significance)[30]. The addition of contrast agents, such as gadolinium and manganese, to produce a signal-inducing bone cement formulation has also been useful for magnetic resonance imaging. Gadolinium in gadoterate meglumine-water cement (Dotarem 0.5 mmol/mL; Laboratory Guerbet, Paris, France, 12 g PMMA and 5 mL MMA) had a higher contrast-to-noise ratio (CNR) in air than the manganese-containing cement (5 mL MnCl2 solution, 100 mg/L deionised water) with a maximum CNR of 157.5 in a fast T1W turbo-spin echo sequence[31].
Antibiotic additives
There is a high incidence of post-operative infections (0.25%-2.0%) in individuals receiving total joint replacements[32]. In cases where PMMA is used this rate increases to 13%[33]. Use of antibiotic-loaded bone cement for prophylaxis and prosthesis related infections has been documented since the 1970s, with erythromycin one of the earliest additives used[34,35]. Despite achieving clinical efficacy, erythromycin was found to diffuse poorly from the cement matrix into surrounding bone[34,35]. Aminoglycosides, such as gentamicin and tobramycin have since become popular additives for bone cements, due to their broad spectrum activity and low allergy profiles[36,37].
One study found that addition of gentamicin (2/60 g cement) did not significantly alter compressive or diametral tensile strength compared to control PMMA (Simplex-P; Figure 1). However, higher gentamicin levels of 5/60 g or 10/60 g, significantly reduced compressive strength (P < 0.05), although results for tensile strength could not be interpreted[38]. Although higher doses of gentamicin mean greater antibiotic availability, the mechanical properties of the additive are adversely affected.
Figure 1.
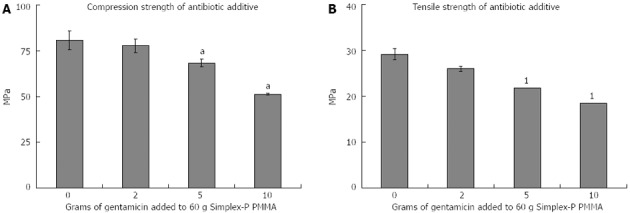
Mechanical strength of antibiotic (gentamicin) additives[38]. A: Compression strength, aP < 0.05 vs 0 g addition of Gentamicin; B: Tensile Strength, 1invalid result as cements failed to fracture in a non-brittle manner.
Another study compared four antibiotics (sodium oxacillin, sodium cefazolin powder, gentamicin powder and gentamicin sulphate aqueous solution; 40 mg/mL of PMMA mixture), evaluating them for compressive (80, 70 and 65 MPa; 2g gentamicin powder, 250 mg aqueous gentamicin and 800 mg aqueous gentamicin solution respectively) and diametral tensile strength (27, 23 and 15 MPa; 2 g gentamicin powder, 250 mg aqueous gentamicin and 800mg aqueous gentamicin solution respectively) in comparison to control PMMA (Simplex-P)[39]. Powered gentamicin (2/40 g) made no statistically significant difference to compressive or diametral tensile strengths whereas aqueous forms produced weakened bone cements, as result attributed to the water in the mixture[39]. We recommend use of 2/60 g, or less, of antibiotic in powdered form. This lowers post-operative infection rates while only causing modest reductions in compressive (< 5% reduction) and tensile (< 5% reduction) strength.
Vancomycin has also been used as a bone cement additive, with concentrations less than 5% having no effect on the mechanical properties of the bone cement[40,41]. However, this has been found to be less efficacious than similar concentrations of tobramycin and gentamicin[37,42]. Interestingly, when used in combination with tobramycin, a synergistic effect appeared[43,44], with a 68% greater elution of tobramycin (P = 0.024), and 103% greater elution of vancomycin from the bone cement (P = 0.007), compared to controls containing only one antibiotic[43].
Vitamin E additives
The polymerisation process utilises a redox system, comprising benzoyl peroxide (BPO) as an initiator and N,N-dimethyl-4-toluidine (DMT) as an activator. This produces benzoate and amine free radicals which are thought to induce local inflammation and alter macrophage activity[45]. Vitamin E is a free radical “scavenger” in the oxidative process[46]. Mixed Vitamin E (MVE) additive (1 part liquid MVE: 1.8 part solid cement) shows increased cytocompatibility (as measured by total cellular DNA, cellular proliferation and differentiation vs control PMMA group) and decreased exothermic activity (peak temperature: 15% wt MVE-MMA 53 °C vs PMMA 76 °C), reducing the likelihood of bone necrosis. However, setting time is increased (20.7 min 15% wt MVE-MMA mixture vs 12.2 min PMMA control), which exposes the operative site to the environment for longer[46]. Compositions of > 25% wt MVE-MMA have no effect on compressive strength, but significantly reduce tensile strength (Figure 2), although this still remains within the range for clinical usage[46]. The use of 15% vitamin E yields a lower compressive strength compared to additive concentrations of 10% and 20% (Figure 2), though this could be attributed to experimental error. Greatest clinical scope exists for 10% vitamin E additives as they have a positive effect on free radical oxidation and exothermic activity, with only modest reduction (< 5%) in tensile strength.
Figure 2.
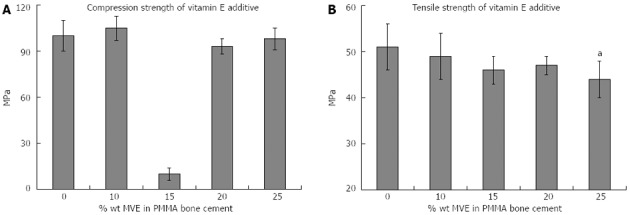
Mechanical strength of vitamin E additives[46]. A: Compression strength, great reduction at 15% appears to be an anomaly, but requires further review; B: Tensile strength, aP < 0.05 vs 0%.
Monomer and nanoparticle additives
The co-polymer [poly (methylmethacrylate-acrylic acid-allylmethacrylate) or poly (MMA-AA-AMA); MMA, Kanton Chemical Co. Japan; AA, Alfa Aesar, Ward Hill, MA, United States; AMA, Acros Organics, Morris Planes, NJ, United States] reduces bone cement shrinkage (a problem in traditional compositions) as it absorbs body fluids and swells to compensate for shrinkage. An MMA:AA:AMA ratio of 80:20:10 resulted in improved mechanical strength (Table 2). In contrast, 70:30:10 did not yield any significant improvements, possibly due to increased acrylic acid concentration[47]. Co-polymerisation with MMA:AA:AMA also resulted in improved fracture toughness, due to a roughened surface, as identified with scanning electron microscopy. Further, cross-linked poly (MMA-AA-AMA) copolymer is able to induce bone ingrowths at the interface of bone and copolymer[48].
Table 2.
Diametral tensile strength of polymethylmethacrylate and MMA:AA: MA co-polymer mixtures[47]
| PMMA | MMA:AA:AMA | MMA:AA:AMA | Tensile strength |
| quantity (g) | quantity (g) | ratio | (Mpa) |
| 20 | 0 | - | 31.3 ± 9.0 |
| 19 | 1 | 80:20:10 | 39.3 ± 3.0 |
| 17 | 3 | 80:20:10 | 36.2 ± 4.7 |
| 19 | 1 | 70:30:10 | 33.1 ± 4.2 |
| 17 | 3 | 70:30:10 | 26.6 ± 6.1 |
PMMA: Polymethylmethacrylate.
Bone cement composites have been trialed with nanoparticle additives, such as multi-walled carbon nanotubes and nano-sized titanium fibers. While there were measurable improvements in the flexural strength and bending capacity by 12.8% and 3.7% respectively, adverse effects on surrounding cell in vitro biocompatibility were observed[9] At the optimal concentration of 1% by wt, nano-titania fibers-give a significant increase in fracture toughness (67%), flexural strength (20%) and flexural modulus (22%), compared with control PMMA cement, while retaining handling properties and in vitro biocompatibility[9].
Recently, nanoparticles have been trialed in vitro as bactericidal agents. PMMA (DePuy International Ltd., UK and Biomet, Merck, Germany) with and without gentamicin was loaded with chitosan (CSNP, CarboMec Inc) and quaternary ammonium CS derived nanoparticles (QCSNP) at weight ratios of 15% and 30%, and then examined for their antibacterial (Staphylococcus aureus and Staphylococcus epidermidis, analysed by sphectrophotometry), mechanical (tensile and three point bending test, Young’s and bending modulus) and cytotoxic properties (3T3 mouse fibroblast assay)[49]. Bone cement mixed with CSNP and QCSNP significantly (P < 0.05) decreased cell count for both strains (500 to 200 CFU/cm2 for CSNP; 500 to 40 CFU/cm2 for QCSNP)[49]. Cytoxicity assay and mechanical testing showed no significant difference between CSNP, QCSNP and control PMMA[49]. Further in vivo assessment of CSNP and QCSNP as potential bone cement additives is suggested for future studies.
Silver ions (AgNP) inactivate enzymes vital to bacteria and disable the mechanism for bacterial DNA replication[50]. Clinical application is limited by the difficulty of incorporating and dispersing AgNP into acrylics. In situ generation of AgNP (University of Texas Health Science Center, Texas) has been trialed[51]. Silver benzoate (AgBz; 1.0% w/w of total monomer; Sigma Aldrich) was blended with PMMA and extra benzoyl peroxide (B; 0.5%, 1.0%, 1.5% and 2.0% w/w; Sigma Aldrich) and diamethyl-p-toludine (D; 0.5, 1.0, 1.5 and 2.0% w/w; Sigma Aldrich) added. AgNP released silver ions in vitro for over 28 d (analysed by Atomic Absorption Spectrometry), inhibited 99.9% of bacterial growth at 48 h (Acinetobacter baumannii, Pseudomonas aeruginosa, Proteus mirabilis and Staphylococcus aureus; in vitro antimicrobial assay) and showed a continued antibacterial effect against P. aeruginosa for over 28 d (1.5B: 0.5D 1% AgBz, 1B: 1D 1% AgBz and 0% AgBz; 4.8, 6.3 and 0 mm inhibition; long term antimicrobial assay)[51]. However, AgNP (1%) mixtures have reduced mechanical strength (three point bending flexural test) compared to controls. Further work is needed to assess optimum loading, other mechanical properties and long term antimicrobial activity against other bacterial strains.
Nanosilver (5-50 nm; 0.1%, 0.5% and 1.0% w/w monomer) mixed with PMMA (Coripharm, Dieberg, Germany), PMMA mixed with 2% w/w gentamicin sulphate (Schering-Plough, Brussels, Belgium) and PMMA control were compared for antimicrobial activity (on microplate proliferation assays) against S. epidermidis, methicillin-resistant S. epidermidis (MRSE) and methicillin-resistant S. aureus (MRSA)[52]. PMMA control had no antimicrobial effect, whereas 1% Nanosilver and 2% gentamicin loaded cements completely inhibited S. epidermidis. Furthermore, 1% Nanosilver completely inhibited MRSA and MRSE growth whereas gentamicin had no effect. This may be due to gentamicin resistance in tested strains[52]. The antimicrobial effect of Nanosilver was dose dependent, with higher concentrations of Nanosilver having higher antimicrobial effect. In vitro cytotoxicity was not significantly different (human osteoblast quantitative elusion testing and qualitative growth) between Nanosilver and PMMA controls[35]. Further, biocompatibility (measured by human osteoblast on growth) was similar between Nanosilver and the control group.
FUTURE APPROACHES
The focus of bone cement research is better mechanical quality, curing time and biocompatibility. Biomaterials, such as calcium phosphates and hydroxyapatite, more efficiently induce bone growth. Advances in the biocompatibility of PMMA bone cements might be achieved by introducing osteogenic agents, such as bone morphogenic proteins or transforming growth factors, to cement surfaces that contact the surrounding bone[53].
PMMA for vertebroplasty has greater stiffness than vertebral cancellous bone, causing higher incidences of fracture of neighboring vertebral bodies[54]. More porous bone cement has been developed by introducing an aqueous phase in PMMA cements, which is released in vivo with powder particles and thus increases risk of embolism. Beck and Boger (2009) showed that delaying the addition of the aqueous phase to acrylate mixture minimizes the amount of particles released[54].
CONCLUSION
As demonstrated in this review, there are many bone cement additives, none of which is perfect as strength often being adversely affected with minor additions of an additive (Table 3). There is scant data focusing on the effect of combining various additives. We suggest that this approach may yield bone cements that display the beneficial properties of each additive, while still maintaining structural integrity. Low index (< 15%) vitamin E and low index (< 5 g) antibiotic impregnated additives should be investigated further. These target inflammatory and infective pathologies, respectively, related to long term failure in bone cements, with only modest reductions in mechanical strength of the cement matrix. Mechanical strength and interface integrity should be improved through the use of rubber-toughened cements, amphiphilic bonders and/or increasing trabecular bone concentration in the cement matrix. Chitosan (15% w/w PMMA) and silver (1% w/w PMMA) nanoparticles have strong antibacterial activity with no significant reduction in mechanical strength. The field of nanoparticle technology holds promise.
Table 3.
Summary of polymethylmethacrylate bone cement additives
| Additive | Summary |
| Gentamicin | Reduces post-operative infection rates. Powdered format (2/60 g or 2/40 g) shows no significant impact on mechanical strength, however increased gentamicin concentration decreases mechanical strength |
| Vitamin E | Improves cement cytocompatibility and reduces peak temperature. 10% vitamin E concentration does not significantly affect mechanical strength. Increasing concentrations associated with increased setting time and decreased mechanical strength |
| Polymer MMA:AA:AMA | Reduces bone cement shrinkage and improves fracture toughness. 80:20:10 significantly improves mechanical strength vs control |
| NanoMgO and NanoBaSO4 | Improves osteoblast adhesion, nanoMgO (12.8 nm) minimizes tissue necrosis and nanoBaSO4 (100 nm) improves mechanical strength |
| Barium sulfate | Allows radiological identification of cement. 10% concentration is not associated with significant decrease in mechanical strength vs control. As concentration increases, mechanical strength decreases |
| Chitosan nanoparticles | In vitro studies show significant antibacterial activity against S. aureus and S. epidermidis with no significant difference in cytoxicity and mechanical strength vs control PMMA |
| Silver nanoparticles | AgNP (1%) has strong and continued antibacterial activity (against A. baumannii, P. aeruginosa, P. mirabilis and S. aureus) but with reduction in mechanical strength. Nanosilver (5-50 nm) has antibacterial activity against S. epidermidis, MRSE and MRSA with no significant difference in cytotoxicity vs control |
PMMA: Polymethylmethacrylate; MRSE: Methicillin-resistant S. epidermidis; MRSA: Methicillin-resistant S. aureus.
Footnotes
P- Reviewer Cheung WH S- Editor Huang XZ L- Editor Hughes D E- Editor Zhang DN
References
- 1.Nottrott M. Acrylic bone cements: influence of time and environment on physical properties. Acta Orthop Suppl. 2010;81:1–27. doi: 10.3109/17453674.2010.487929. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn KD. Chapter 3. 1: Properties of Bone Cement-What is Bone Cement? The well-cemented total hip arthroplasty: theory and practice, Germany: Springer; 2005. pp. 51–59. [Google Scholar]
- 3.Charnley J. Anchorage of the femoral head prosthesis to the shaft of the femur. J Bone Joint Surg Br. 1960;42-B:28–30. doi: 10.1302/0301-620X.42B1.28. [DOI] [PubMed] [Google Scholar]
- 4.Charnley J. Acrylic cement in orthopaedic surgery. Brit J Surg. 1970;57:874. [Google Scholar]
- 5.Waugh W. John Charnley-The Man and the Hip. 1st ed. Germany: Springer; 1990. pp. 1–268. [Google Scholar]
- 6.Lewis G. Alternative acrylic bone cement formulations for cemented arthroplasties: present status, key issues, and future prospects. J Biomed Mater Res B Appl Biomater. 2008;84:301–319. doi: 10.1002/jbm.b.30873. [DOI] [PubMed] [Google Scholar]
- 7.Sylvain GM, Kassab S, Coutts R, Santore R. Early failure of a roughened surface, precoated femoral component in total hip arthroplasty. J Arthroplasty. 2001;16:141–148. doi: 10.1054/arth.2001.20541. [DOI] [PubMed] [Google Scholar]
- 8.Bowman AJ, Manley TR. The elimination of breakages in upper dentures by reinforcement with carbon fibre. Br Dent J. 1984;156:87–89. doi: 10.1038/sj.bdj.4805275. [DOI] [PubMed] [Google Scholar]
- 9.Khaled SM, Charpentier PA, Rizkalla AS. Physical and mechanical properties of PMMA bone cement reinforced with nano-sized titania fibers. J Biomater Appl. 2011;25:515–537. doi: 10.1177/0885328209356944. [DOI] [PubMed] [Google Scholar]
- 10.Stipho HD. Effect of glass fiber reinforcement on some mechanical properties of autopolymerizing polymethyl methacrylate. J Prosthet Dent. 1998;79:580–584. doi: 10.1016/s0022-3913(98)70180-5. [DOI] [PubMed] [Google Scholar]
- 11.Puckett AD, Roberts B, Bu L, Mays JW. Improved orthopaedic bone cement formulations based on rubber toughening. Crit Rev Biomed Eng. 2000;28:457–461. doi: 10.1615/critrevbiomedeng.v28.i34.180. [DOI] [PubMed] [Google Scholar]
- 12.Frigstad JR, Park JB. Reinforcement of PMMA bone cement with a continuous wire coil--a 3D finite element study. Biomed Mater Eng. 1996;6:429–439. [PubMed] [Google Scholar]
- 13.Marx R, Faramarzi R, Jungwirth F, Kleffner BV, Mumme T, Weber M, Wirtz DC. [Silicate coating of cemented titanium-based shafts in hip prosthetics reduces high aseptic loosening] Z Orthop Unfall. 2009;147:175–182. doi: 10.1055/s-0029-1185456. [DOI] [PubMed] [Google Scholar]
- 14.Stone JJ, Rand JA, Chiu EK, Grabowski JJ, An KN. Cement viscosity affects the bone-cement interface in total hip arthroplasty. J Orthop Res. 1996;14:834–837. doi: 10.1002/jor.1100140523. [DOI] [PubMed] [Google Scholar]
- 15.Müller-Rath R, Andereya S, Gravius S, Wirtz DC, Marx R, Mumme T. [ Improvement of femoral bone-cement adhesion in cemented revision hip arthroplasty by application of an amphiphilic bonder in a dynamic femur expulsion testing in vitro ] Biomed Tech (Berl) 2007;52:391–397. doi: 10.1515/BMT.2007.064. [DOI] [PubMed] [Google Scholar]
- 16.Mann KA, Ayers DC, Werner FW, Nicoletta RJ, Fortino MD. Tensile strength of the cement-bone interface depends on the amount of bone interdigitated with PMMA cement. J Biomech. 1997;30:339–346. doi: 10.1016/s0021-9290(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 17.Frost HM. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Relat Res. 1989;(248):283–293. [PubMed] [Google Scholar]
- 18.Frost HM. The biology of fracture healing. An overview for clinicians. Part II. Clin Orthop Relat Res. 1989;(248):294–309. [PubMed] [Google Scholar]
- 19.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10 Suppl 2:S96–101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukeoka T, Suzuki M, Ohtsuki C, Sugino A, Tsuneizumi Y, Miyagi J, Kuramoto K, Moriya H. Mechanical and histological evaluation of a PMMA-based bone cement modified with gamma-methacryloxypropyltrimethoxysilane and calcium acetate. Biomaterials. 2006;27:3897–3903. doi: 10.1016/j.biomaterials.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Endogan T, Kiziltay A, Hasirci V, Hasirci N. Modification of acrylic bone cements with oxygen plasma and additives. JBT. 2012;2:236–243. [Google Scholar]
- 22.Cheng Y, Yang HC, Cho JH, Lee SH, Lim BS. The effect of N-acetylcysteine addition on the polymerization behavior of PMMA bone cement. Macromol Res. 2012;20:928–938. [Google Scholar]
- 23.Gillani R, Ercan B, Qiao A, Webster TJ. Nanofunctionalized zirconia and barium sulfate particles as bone cement additives. Int J Nanomedicine. 2010;5:1–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Cisneros-Pineda OG, Cauich-Rodríguez JV, Cervantes-Uc JM, Vázquez B, Román JS. Combined influence of barium sulfate content and co-monomer concentration on properties of PMMA bone cements for vertebroplasty. J Biomater Sci Polym Ed. 2011;22:1563–1580. doi: 10.1163/092050610X516780. [DOI] [PubMed] [Google Scholar]
- 25.Ricker A, Liu-Snyder P, Webster TJ. The influence of nano MgO and BaSO4 particle size additives on properties of PMMA bone cement. Int J Nanomedicine. 2008;3:125–132. [PMC free article] [PubMed] [Google Scholar]
- 26.Makita M, Yamakado K, Nakatsuka A, Takaki H, Inaba T, Oshima F, Katayama H, Takeda K. Effects of barium concentration on the radiopacity and biomechanics of bone cement: experimental study. Radiat Med. 2008;26:533–538. doi: 10.1007/s11604-008-0269-0. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi N, Togawa D, Fujishiro T, Powell KA, Turner AS, Seim HB, Bauer TW. Histological and radiographic evaluation of polymethylmethacrylate with two different concentrations of barium sulfate in a sheep vertebroplasty model. J Biomed Mater Res A. 2005;75:123–127. doi: 10.1002/jbm.a.30388. [DOI] [PubMed] [Google Scholar]
- 28.van Hooy-Corstjens CS, Bulstra SK, Knetsch ML, Geusens P, Kuijer R, Koole LH. Biocompatibility of a new radiopaque iodine-containing acrylic bone cement. J Biomed Mater Res B Appl Biomater. 2007;80:339–344. doi: 10.1002/jbm.b.30602. [DOI] [PubMed] [Google Scholar]
- 29.Gomoll AH, Fitz W, Scott RD, Thornhill TS, Bellare A. Nanoparticulate fillers improve the mechanical strength of bone cement. Acta Orthop. 2008;79:421–427. doi: 10.1080/17453670710015349. [DOI] [PubMed] [Google Scholar]
- 30.Hernández L, Fernández M, Collía F, Gurruchaga M, Goñi I. Preparation of acrylic bone cements for vertebroplasty with bismuth salicylate as radiopaque agent. Biomaterials. 2006;27:100–107. doi: 10.1016/j.biomaterials.2005.05.074. [DOI] [PubMed] [Google Scholar]
- 31.Wichlas F, Bail HJ, Seebauer CJ, Schilling R, Pflugmacher R, Pinkernelle J, Rump J, Streitparth F, Teichgräber UK. Development of a signal-inducing bone cement for magnetic resonance imaging. J Magn Reson Imaging. 2010;31:636–644. doi: 10.1002/jmri.22074. [DOI] [PubMed] [Google Scholar]
- 32.Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92 Suppl 2:36–46. doi: 10.2106/JBJS.J.01046. [DOI] [PubMed] [Google Scholar]
- 33.Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg. 2003;14:144–153. doi: 10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63-B:342–353. doi: 10.1302/0301-620X.63B3.7021561. [DOI] [PubMed] [Google Scholar]
- 35.Buchholz HW, Elson RA, Heinert K. Antibiotic-loaded acrylic cement: current concepts. Clin Orthop Relat Res. 1984;(190):96–108. [PubMed] [Google Scholar]
- 36.Hanssen AD. Prophylactic use of antibiotic bone cement: an emerging standard--in opposition. J Arthroplasty. 2004;19:73–77. doi: 10.1016/j.arth.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am. 2006;88:2487–2500. doi: 10.2106/JBJS.E.01126. [DOI] [PubMed] [Google Scholar]
- 38.Lautenschlager EP, Jacobs JJ, Marshall GW, Meyer PR. Mechanical properties of bone cements containing large doses of antibiotic powders. J Biomed Mater Res. 1976;10:929–938. doi: 10.1002/jbm.820100610. [DOI] [PubMed] [Google Scholar]
- 39.Marks KE, Nelson CL, Lautenschlager EP. Antibiotic-impregnated acrylic bone cement. J Bone Joint Surg Am. 1976;58:358–364. [PubMed] [Google Scholar]
- 40.Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop. 1998;22:171–177. doi: 10.1007/s002640050235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang YH, Wang JC, Pei YL. Adding vancomycin to bone cement: research on its influence on mechanical and fixation strength using rabbit femoral prostheses. Orthop Surg. 2011;3:265–267. doi: 10.1111/j.1757-7861.2011.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertazzoni Minelli E, Caveiari C, Benini A. Release of antibiotics from polymethylmethacrylate cement. J Chemother. 2002;14:492–500. doi: 10.1179/joc.2002.14.5.492. [DOI] [PubMed] [Google Scholar]
- 43.Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939–944. doi: 10.1016/s0883-5403(96)80135-5. [DOI] [PubMed] [Google Scholar]
- 44.González Della Valle A, Bostrom M, Brause B, Harney C, Salvati EA. Effective bactericidal activity of tobramycin and vancomycin eluted from acrylic bone cement. Acta Orthop Scand. 2001;72:237–240. doi: 10.1080/00016470152846547. [DOI] [PubMed] [Google Scholar]
- 45.Lewis G. Properties of acrylic bone cement: state of the art review. J Biomed Mater Res. 1997;38:155–182. doi: 10.1002/(sici)1097-4636(199722)38:2<155::aid-jbm10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 46.Méndez JA, Aguilar MR, Abraham GA, Vázquez B, Dalby M, Di Silvio L, San Román J. New acrylic bone cements conjugated to vitamin E: curing parameters, properties, and biocompatibility. J Biomed Mater Res. 2002;62:299–307. doi: 10.1002/jbm.10296. [DOI] [PubMed] [Google Scholar]
- 47.Nien YH, Chen J. Studies of the mechanical and thermal properties of crosslinked poly (methylmethacrylate acrylic acid allylmethacrylate) modified bone cement. J Appl Polym Sci. 2006;100:3727–3732. [Google Scholar]
- 48.Gualtieri GM, Siegler S, Hume EL, Kalidindi SR. Biological and mechanical characteristics of the interface between a new swelling anchor and bone. J Orthop Res. 2000;18:494–499. doi: 10.1002/jor.1100180324. [DOI] [PubMed] [Google Scholar]
- 49.Shi Z, Neoh KG, Kang ET, Wang W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials. 2006;27:2440–2449. doi: 10.1016/j.biomaterials.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 50.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 51.Oei JD, Zhao WW, Chu L, Desilva MN, Ghimire A, Rawls HR, Whang K. Antimicrobial acrylic materials with in situ generated silver nanoparticles. J Biomed Mater Res B Appl Biomater. 2011:Epub ahead of print. doi: 10.1002/jbm.b.31963. [DOI] [PubMed] [Google Scholar]
- 52.Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25:4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 53.Hallman M, Thor A. Bone substitutes and growth factors as an alternative/complement to autogenous bone for grafting in implant dentistry. Periodontol 2000. 2008;47:172–192. doi: 10.1111/j.1600-0757.2008.00251.x. [DOI] [PubMed] [Google Scholar]
- 54.Beck S, Boger A. Evaluation of the particle release of porous PMMA cements during curing. Acta Biomater. 2009;5:2503–2507. doi: 10.1016/j.actbio.2009.04.002. [DOI] [PubMed] [Google Scholar]


