Abstract
All components of the Drosophila intestinal tract, including the endodermal midgut and ectodermal hindgut/Malpighian tubules, maintain populations of dividing stem cells. In the midgut and hindgut, these stem cells originate from within larger populations of intestinal progenitors that proliferate during the larval stage and form the adult intestine during metamorphosis. The origin of stem cells found in the excretory Malpighian tubules (‘renal stem cells’) has not been established. In this paper, we investigate the migration patterns of intestinal progenitors that take place during metamorphosis. Our data demonstrate that a subset of adult midgut progenitors (AMPs) move posteriorly to form the adult ureters and, consecutively, the renal stem cells. Inhibiting cell migration by AMP-directed expression of a dominant-negative form of Rac1 protein results in the absence of stem cells in the Malpighian tubules. As the majority of the hindgut progenitor cells migrate posteriorly and differentiate into hindgut enterocytes, a group of the progenitor cells, unexpectedly, invades anteriorly into the midgut territory. Consequently, these progenitor cells differentiate into midgut enterocytes. The midgut determinant GATAe is required for the differentiation of midgut enterocytes derived from hindgut progenitors. Wingless signaling acts to balance the proportion of hindgut progenitors that differentiate as midgut versus hindgut enterocytes. Our findings indicate that a stable boundary between midgut and hindgut/Malpighian tubules is not established during early embryonic development; instead, pluripotent progenitor populations cross in between these organs in both directions, and are able to adopt the fate of the organ in which they come to reside.
Keywords: Stem cell, Migration, Intestine, Malpighian tubule, Metamorphosis, Drosophila
INTRODUCTION
To compensate for cell loss, most animal tissues maintain slowly proliferating populations of stem cells that are able to differentiate on demand. Given the great potential of stem cells for the management of many human diseases, it is important to understand in detail the molecular pathways that control the biology of stem cells, including their origin, pattern of proliferation and migration during development. In this paper, we focus on the development of intestinal stem cells (ISCs) that form part of the gut and excretory system of the fruit fly Drosophila melanogaster. Stem cells have been described for the Drosophila endodermal midgut, as well as for the ectodermal Malpighian tubules and hindgut. In the midgut and Malpighian tubules, stem cells are scattered more or less evenly over the outer (basal) surface of the epithelium (Ohlstein and Spradling, 2006; Micchelli and Perrimon, 2006; Singh et al., 2007). In the hindgut, proliferating cells are confined to a narrow segment that forms the hindgut-midgut boundary (hindgut proliferation zone, HPZ) (Takashima et al., 2008). A similar ring of proliferating cells also exists in the adult foregut (Singh et al., 2011). Stem cells develop as part of the adult gut progenitors that can be already distinguished in the embryonic and larval gut (Jiang and Edgar, 2009; Mathur et al., 2010; Takashima et al., 2011a; Takashima et al., 2011b). Small clusters (‘nests’) of dividing adult midgut progenitors (AMPs) are distributed over the larval midgut. Two ring-shaped domains of proliferating cells flanking the midgut anteriorly and posteriorly, form the primordia of the adult foregut and hindgut, respectively. During pupal development, most of the larval gut undergoes programmed cell death, similar to what has been described for some vertebrate systems undergoing metamorphosis (Ishizuya-Oka and Shi, 2007; Hasebe et al., 2011). The adult gut primordia spread, fuse together and differentiate as the adult foregut, midgut and hindgut. Only the larval Malpighian tubules, according to previous reports, survive metamorphosis and become the adult tubules.
Recent genetic studies have elucidated several of the signaling pathways that control the proliferation and differentiation of gut progenitors in the larva, and ISCs in the adult. Among these are: the Notch and Wnt/Wingless pathways, which keep gut progenitors and ISCs in a dividing non-differentiated state (Ohlstein and Spradling, 2006; Ohlstein and Spradling, 2007; Micchelli and Perrimon, 2006; Lin et al., 2008; Lee et al., 2009; Xu et al., 2011); EGFR and JAK/STAT, which act upstream of Notch to trigger proliferation and promote enterocyte survival in the midgut (Jiang et al., 2009; Jiang et al., 2011; Liu et al., 2010; Xu et al., 2011); and Hedgehog, which promotes enterocyte differentiation in the hindgut (Takashima et al., 2008). However, many of the mechanisms that specify ISCs, in particular the signaling events that, during metamorphosis, select these cells from among the adult gut progenitors and keep them undifferentiated, are still unknown. It is also not clear how the ISCs, once determined, migrate to their final position. Notably, the site of origin of ISCs populating the adult Malpighian tubules has remained unknown so far.
In this paper, we have investigated the origin of stem cells that form near the boundary between midgut, hindgut and Malpighian tubules. Our findings show that, during early stages of metamorphosis, two unsuspected, major movements of gut progenitors take place. First, adult midgut progenitors (AMPs) give rise not only to the adult midgut epithelium, but also move posteriorly to form the adult ureters. During later pupal stages, subsets of AMPs migrate from the ureters onto the Malpighian tubules to establish the population of renal stem cells associated with these structures in the adult. Blocking cell migration by directed expression of a Rac dominant-negative form results in the lack of the stem cells in the Malpighian tubules. A second major movement of presumptive stem cells takes place during early pupal development when cells of the hindgut proliferation zone, instead of extending posteriorly to generate the adult hindgut, move anteriorly to form the posterior segment of the adult midgut. Our findings indicate that the boundary between the endodermal midgut and ectodermal hindgut/Malpighian tubules that appears in the embryo is not maintained during metamorphosis: pluripotent progenitor populations cross between these domains in both directions and are able to adopt the fate of the domain they come to reside in.
MATERIALS AND METHODS
Fly stocks
Flies used in this study were (sources in parentheses): byn-Gal4 (Dr J. Lengyel, University of California, Los Angeles, USA); esg-Gal4, pros-Gal4 (National Institute of Genetics, Japan); OregonRw1118, tub-Gal80ts7, tub-Gal80ts20, UAS-mCD8GFP, UAS-flp, UAS-GFP, UAS-myr-mRFP, UAS-mitoGFP, UAS-Rac1N17 (a dominant-negative form of Rac1), and Act5C >Stop >lacZ (Act5C promoter-FRT-phl[+]-FRT-lacZ.nls) (Bloomington Stock Center); 10XStat92E-GFP (Dr E. Bach, New York University School of Medicine, NY, USA); UAS-dGATAeRNAi (VDRC, Austria; #10418, #10420); and UAS-wg (Dr H. Krause, University of Toronto, Ontario, Canada). All flies were reared with normal fly food at room temperature or in incubators at 18°C, 25°C or 29°C.
Lineage trace experiments
Flies carrying genotypes of tub-Gal80ts/+; esg-Gal4 UAS-myr-mRFP/UAS-flp; Act5C >Stop >lacZ/+ or tub-Gal80ts/UAS-flp, byn-Gal4 UAS-GFP/Act5C >Stop >lacZ were used for tracing the lineage of adult midgut progenitors (esg+) or HPZ cells (byn+), respectively. Animals raised at 18°C were transferred to at 29°C to inactivate Gal80ts repressor enabling flip-out ‘Stop cassette’ of Act5C >Stop >lacZ transgene to label permanently the cells of given lineages. To trace the lineages during metamorphosis, third instar larvae were transferred to 29°C and then dissected at the desired pupal stages. In some experiments, the temperature shift was applied as a 6-hour pulse to restrict the labeled lineage, resulting in labeling of subsets of progeny (e.g. Fig. 5C). Without a temperature shift, we observed no or a very limited number of labeled cells (supplementary material Fig. S2). When overexpressing GATAe combined with the lineage tracing, animals with genotype of tub-Gal80ts/UAS-GATAe; byn-Gal4 UAS-GFP/UAS-flp Act5C >Stop >lacZ were used.
Fig. 5.
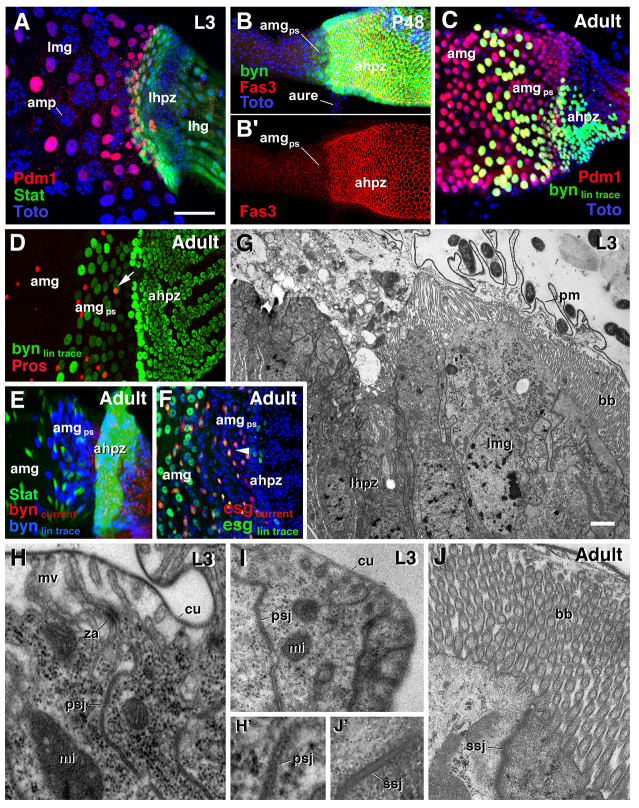
Differentiation of HPZ-derived ectodermal cells into midgut. (A) In late third instar larva (L3), Pdm1 (red) marks differentiated midgut enterocytes (large polyploid cells), whereas undifferentiated adult midgut progenitors (amp; recognizable by their small cell size) are not Pdm1 positive. (B,B′) At 48 hours APF (P48), posterior terminal midgut (amgps) derived from HPZ (marked by faint green; byn-Gal4 >UAS-GFP) loses high Fas3 expression. (C) A 6-hour heat pulse with byn-GAL4 at third instar labels a subset of HPZ-derived cells in the posterior terminal midgut, which are positive for Pdm1. (D) The posterior terminal midgut is not pros positive, indicating that endocrine cells (e.g. white arrow) in this region are derived from midgut pISCs (the lineage was traced from third instar to adult with byn-GAL4). (E) 10XStat92E-GFP-positive ISCs (green) are also negative for the byn lineage. (F) esg lineage-traced stem cells (green; e.g. arrowhead) populate the posterior terminal midgut. (G-J) Transmission electron microscopy of the hindgut-midgut boundary region of the larva (G-I) and adult (J). Hindgut cells have an apical cuticle (cu) topping irregular microvilli (mv) and a junctional complex consisting of a sub-apical zonula adherens (za) and pleated septate junctions (psj) (H). Hindgut cells of the HPZ directly adjacent to the hindgut-midgut boundary (G,I) exhibit the same characteristics, with the exception of a much thinner cuticle (compare cu in I,H). Enterocytes of the posterior terminal midgut of the larva (lmg in G) and adult (J) form an apical brush border (bb), consisting of long, densely packed microvilli oriented in parallel. They lack the zonula adherens and have smooth septate junctions (ssj; compare appearance of ssj in J′ with that of pleated septate junctions in H′). ahpz, adult hindgut proliferation zone; amg, adult midgut; amgps, posterior terminal midgut; amp, adult midgut progenitor; aure, adult ureter; bb, brush border; cu, cuticle; lhg, larval hindgut; lhpz, larval hindgut proliferation zone; lmg, larval midgut; mi, mitochondrion; mv, microvilli; pm, peritrophic membrane; psj, pleated septate junction; ssj, smooth septate junction; za, zonula adherens. Scale bars: 50 μm.
In situ hybridization
Gut samples were fixed with 4% formaldehyde for 45 minutes at room temperature and stored in 100% methanol at -20°C until use. Hybridization was performed with a digoxigenin-labeled RNA probe prepared against GATAe cDNA following standard protocols (Takashima et al., 2011a).
Immunohistochemistry
Antibodies used in this study were: mouse anti-Arm (1:10), mouse anti-Cut (1:10) and mouse anti-Prospero (1:50) (all purchased from Developmental Studies Hybridoma Bank, University of Iowa); mouse anti-β-galactosidase (1:100, Promega); rabbit anti-phoshorylated-histone H3 (1:1000, Cell Signaling Technology, Danvers, MA); rabbit anti-Pdm1/nubbin (1:1000, Yeo et al., 1995); goat anti-mouse IgG Alexa488 (1:100); goat anti-mouse IgG Alexa546 (1:300); goat anti-rabbit IgG Alexa488 (1:100) (Invitrogen, Carlsbad, CA); and goat anti-rabbit IgG Cy3 (1:200) (Jackson ImmunoResearch, West Grove, PA). Antibody staining was performed as described previously (Takashima et al., 2011a). TOTO-3 or TOPRO-3 nuclear dye (Invitrogen) was added to the mounting medium when necessary.
Transmission electron microscopy
Guts were dissected in PBS and fixed with 2.5% glutaraldehyde and then with 1% osmium tetroxide. Samples were dehydrated with ascending ethanol series and acetone, embedded in Epon resin, and sectioned with a Leica ultramicrotome at a thickness of ∼50-70 nm. The specimens were stained with uranyl acetate and lead citrate. Images were observed and photographed with a JEOL 100CX transmission electron microscope (JEOL, Peabody, MA).
RESULTS
Adult gut progenitors migrate across organ boundaries during metamorphosis
To trace the origin of adult gut tissues, we used a lineage-tracing construct (Act5C >Stop >lacZ; see Materials and methods), driven by either esg-Gal4 [expressed in adult midgut progenitors (AMPs; supplementary material Fig. S1A)] or byn-Gal4 (expressed in the hindgut; supplementary material Fig. S1B). Flies with the genotype of esg-Gal4/UAS-flp; tub-Gal80ts/Act5C >Stop >lacZ or tub-Gal80ts/UAS-flp; byn-Gal4, UAS-GFP/Act5C >Stop >lacZ were heat treated from the first instar larva resulting in the stable expression of lacZ reporter in cells expressing esg-Gal4 or byn-Gal4, respectively. In preparations fixed at the adult stage, we observed that, after activating the construct in AMPs by esg-Gal4, the entire midgut was labeled, with the exception of a short midgut segment (termed ‘posterior terminal midgut’ in the following) located right in front of the hindgut. To our surprise, labeling also extended to the ureters and the stem cells of the Malpighian tubules (renal stem cells) (Fig. 1A), neither of which expresses esg in the larva (supplementary material Fig. S1A) or embryo (data not shown). Expression of lacZ by byn-Gal4 in the larval HPZ was not only confined to the adult hindgut, but also extended anteriorly to the adjacent posterior terminal midgut (Fig. 1B). These findings suggest that during metamorphosis, adult gut progenitors migrate and cross the boundaries between midgut, hindgut and Malpighian tubules. AMPs, which are located in the larval midgut, form the ureters and the renal stem cell population of the Malpighian tubules; the HPZ, part of the larval hindgut, crosses anteriorly and gives rise to the posterior terminal midgut (Fig. 1C).
Fig. 1.
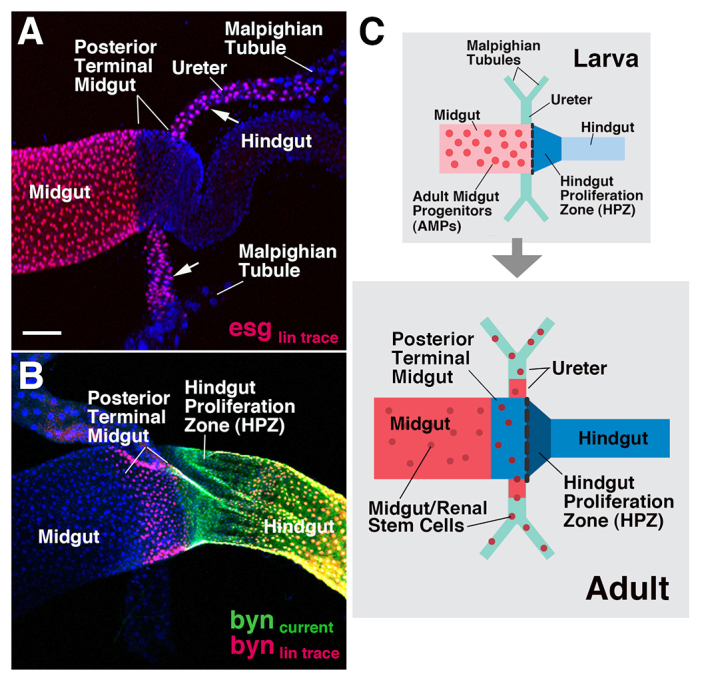
Lineage tracing of adult midgut and hindgut progenitors. (A,B) Gut progenitor lineages labeled by lacZ. Progenitors were labeled from early larval stage by a lineage-reporter gene (lacZ, red) activated by esg-Gal4 (A; esg lin trace) or byn-Gal4 (B; byn lin trace), respectively. Labeled progeny of progenitors in adult gut are depicted. Arrows in A indicate labeled renal stem cells on Malpighian tubules. Current expression of byn-Gal4 >UAS-GFP in adult hindgut is shown in green (byn current). Nuclei are stained with TOTO-3 or TOPRO-3 dye (shown in blue) in all confocal pictures of all figures hereafter. (C) Schematic depiction of larval and adult hindgut-midgut boundary region, illustrating the movement of gut progenitors that is inferred from lineage-tracing experiments. Scale bar: 50 μm.
Origin of renal stem cells from migrating midgut progenitor cells
To reconstruct in detail when and how the migration of gut progenitors takes place, we used the lineage-tracing approach introduced above, but in this case lineage labeling was performed within restricted time window such as from first to third instar, or from third instar to different stages of metamorphosis up to 72 hours after puparium formation (APF; Fig. 2). In the late third instar larva right before metamorphosis, esg-positive AMPs formed clusters of 8-12 cells each scattered throughout the midgut. No AMPs were seen in the larval ureters or Malpighian tubules (Fig. 2A). In the 6-hour pupa, the esg-positive AMPs formed a complete outer epithelial layer: the presumptive adult midgut (Fig. 2B). This layer enclosed the esg-negative larval midgut layer. A third tissue layer, the transient pupal midgut (tPMG), which derives from the peripheral cells of the AMP clusters, was sandwiched in between the adult and larval midgut (Takashima et al., 2011a; Takashima et al., 2011b; Fig. 2B′). We found that the tPMG layer projects into the base of the larval ureters, engulfing cells of the proximal segment of the larval ureter (Fig. 2E). The esg-positive outer epithelium also reached posteriorly, forming a new proximal segment of the ureter (Fig. 2B-D,F-H). This esg-positive, AMP-derived ureter segment, which (aside from esg expression) can be distinguished from the distal (larval) ureter by its small cell size, lengthened until it acquired its full extension, covering approximately half of the length of the ureter (Fig. 2H), by 72 hours APF.
Fig. 2.
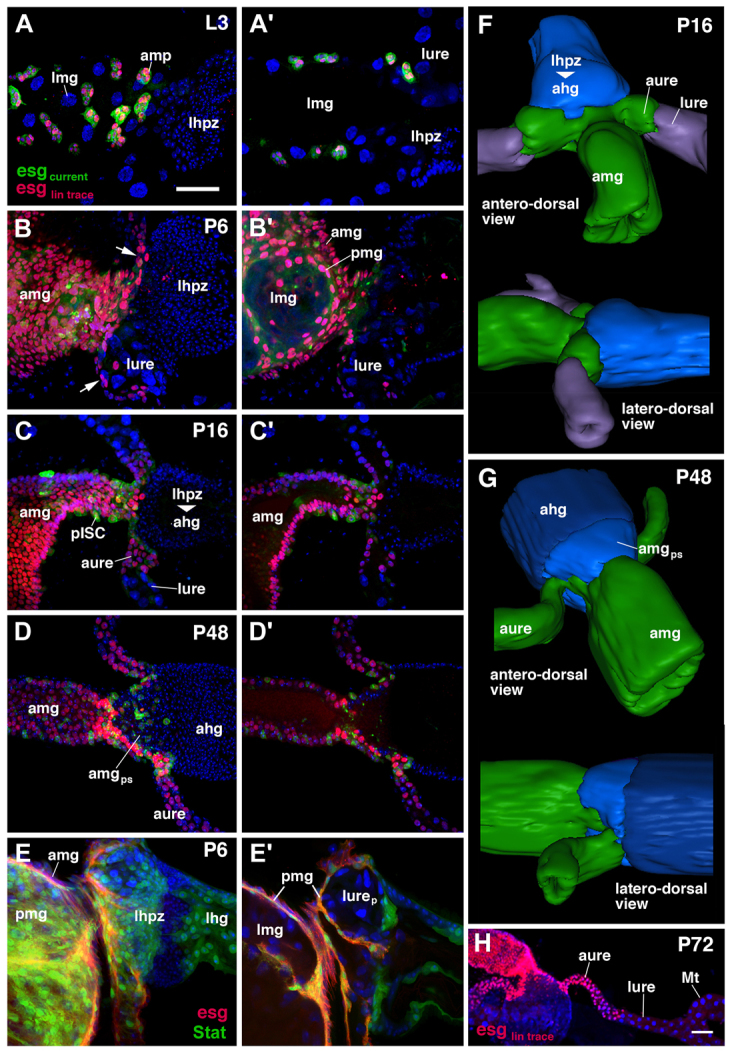
Movement of midgut progenitors during metamorphosis. (A-D′,H) Z-projections of confocal sections of the hindgut-midgut boundary at different developmental stages. esg-Gal4 driven lineage tracing at the stages of (A,A′) wandering third instar larva (L3), (B,B′) prepupa at 6 hours APF (P6), (C,C′) early pupa at 16 hours APF (P16), (D,D′) middle pupa at 48 hours APF (P48) and (H) late pupa at 72 hours APF. (E) Expression of esg (red) and 10XStat92E-GFP (green) at 6 hours APF prepupa (P6). (A-E,H) Sections tangentially through gut wall (‘surface view’); (A′-E′) sections through gut midline (‘sagittal view’). AMPs and their progeny were labeled by lacZ reporter (red) using esg-Gal4 driver from first instar stage onwards (A) or from third instar stage (B-D,H), respectively. Current expression of esg-Gal4 is visualized by UAS-myr-mRFP (shown in green). (E,E′) 10XStat92E-GFP (green) highlights the transient pupal midgut (pmg) (Takashima et al., 2011b). At the larval stage (A,A′), midgut progenitors (amp) form clusters throughout the larval midgut (lmg). Shortly after the onset of metamorphosis (B,B′,E,E′), AMP-derived cells constitute the adult midgut epithelium (amg), the transient pupal midgut (pmg), which engulfs the larval midgut, and the base of the ureter (lmg and lurep in E′). From 16 hours APF onwards, AMP-derived cells also populate the proximal ureter (aure) and form intestinal stem cells (pISCs) upregulating the expression of esg (C,D,H). (F,G) Three-dimensional digital models of the hindgut-midgut boundary region in laterodorsal view and anterodorsal view at 16 hours APF (P16) (F) and at 48 hours APF (P48) (G), respectively. Tissues descended from midgut progenitors are rendered green; the adult hindgut (ahg) and posterior terminal midgut (amgps), produced by the hindgut proliferation zone (hpz), are rendered in blue. ahg, adult hindgut; amg, adult midgut; amgps, posterior terminal midgut; amp, adult midgut progenitor; aure, adult ureter; lhg, larval hindgut; lhpz, larval hindgut proliferation zone; lmg, larval midgut; lure, larval ureter; lurep, proximal larval ureter; Mt, Malpighian tubule; pISC, presumptive intestinal stem cell; pmg, transient pupal midgut. Scale bars: 50 μm.
During the time between 6 and 24 hours APF, the majority of the AMP-derived cells of the midgut and ureters expressed specific differentiation markers, such as Pdm1 and Cut (Fig. 3A-C), respectively, and lost the expression of esg (Fig. 3D). However, a subset of cells maintained high esg levels. Most of them were found on the midgut epithelium and are presumptive adult intestinal stem cells (pISCs) of the midgut (Jiang et al., 2009). Interestingly, we also found some of the esg-positive cells on ureters and Malpighian tubules. Initially at 16 hours APF they were found at the bases of the ureters (supplementary material Fig. S1A); at 24 hours APF, they distributed among the cells of ureters (Fig. 3E). At 48 and 72 hours APF, increasing numbers of esg-positive cells were found on the proximal tubules (Fig. 3F-H; supplementary material Fig. S1A). Based on their small size and pattern of distribution on the Malpighian tubules and ureters, we find that they are renal stem cells (Singh et al., 2007) developing from pISCs.
Fig. 3.
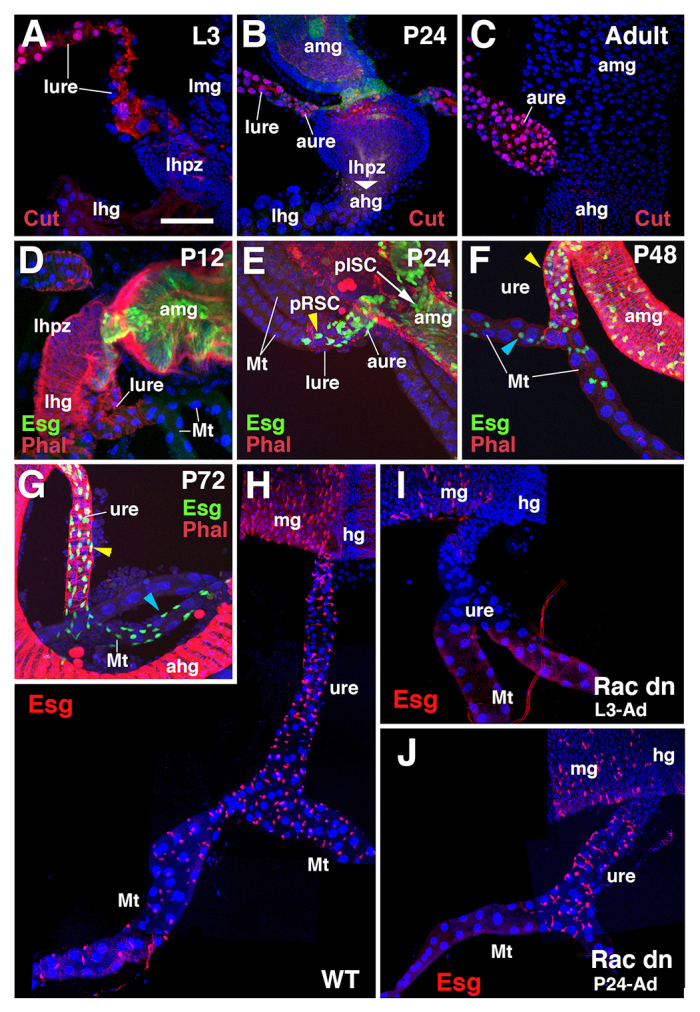
Origin and migration of renal stem cells. (A-C) Expression of Cut protein (red) at the hindgut-midgut boundary in wandering larvae (A), 24 hour pupa (B) and adult (C). In the larva, Cut is confined to the Malpighian tubules and ureter (A). In the early pupa, AMP-derived adult ureter cells (aure; distinguishable from larval ureter cells by their much smaller size) turn on Cut expression (B) and maintain it in the adult (C). (D-G) Z-projections of confocal sections of the ureter and Malpighian tubules at different pupal stages. Prepupa at 12 hours APF (D; P12), pupa at 24 hours (E; P24), 48 hours (F; P48) and 72 hours APF (G; P72). Visceral muscle is stained with Rhodamine-Phalloidin (red). esg-positive presumptive intestinal stem cells (pISCs) and renal stem cells (pRSCs) are shown in green (esg-Gal4 >UAS-mCD8GFP). pRSCs populate the proximal ureter by P24 (E) and spread on to the Malpighian tubules (F,G; yellow arrowheads indicate pRSCs on ureters; cyan arrowheads indicate pRSCs on Malpighian tubules). (H-J) Z-projections of the ureter and Malpighian tubules in which midgut pISCs and pRSCs are labeled with esg-Gal4 >UAS-mRFP (red). (H) Wild-type control. (I,J) Rac1N17 is overexpressed by esg-Gal4 from third instar larva (I) or from 24 hours APF (J) to adult. ahg, adult hindgut; amg, adult midgut; aure, adult ureter; hg, hindgut; lhg, larval hindgut; lhpz, larval hindgut proliferation zone; lmg, larval midgut; lure, larval ureter; mg, midgut; Mt, Malpighian tubule; pISC, presumptive intestinal stem cell; pRSC, presumptive renal stem cell; ure, ureter. Scale bar: 50 μm.
Rac1 is a small GTPase that mediates actin filament reorganization and its disruption has been shown to interfere with cell motility in Drosophila (Paladi and Tepass, 2004). We used esg-Gal4 driver line to express a dominant-negative construct of Drosophila Rac1 (Rac1N17) during the pupal period, and assayed for the distribution of pISCs in the adult gut and pISC-derived presumptive renal stem cells (pRSCs) on the Malpighian tubules (Fig. 3H-J). Flies with the genotype of esg-Gal4, UAS-mRFP/+; tub-Gal80ts/UAS-Rac1N17 were heat treated from late third instar and their phenotype was observed after adult eclosion. The overexpression of Rac1N17 caused loss of renal stem cells on the adult ureter and Malpighian tubules (Fig. 3I). By contrast, when Rac1N17 was overexpressed starting at 24 hours APF, by which time the adult ureter is partially formed and some pRSCs already reside in it (see Fig. 3E), we found esg-positive renal stem cells on the ureter but not in the Malpighian tubules (Fig. 3J). The results show that early Rac1 inhibition interferes with the extension of the adult ureter, as well as subsequent distal migration of pRSCs on to the tubules, whereas later treatment does not interfere with the formation of the proximal ureter, but with only the further migration of pRSC.
The hindgut proliferation zone forms the posterior terminal midgut of the adult
Lineage-tracing experiments using byn-Gal4 driver temperature-activated from third instar larvae allowed us to follow the fate of the hindgut proliferation zone (HPZ) (Fig. 4A,D-F) during metamorphosis. Throughout the embryonic and larval stages, byn expression was confined to the hindgut, including the HPZ, which is set apart from the differentiated larval hindgut as a ring-shaped domain of small, columnar cells (Fig. 4A,B; supplementary material Fig. S1B). During the pupal stage, around 24-30 hours APF, the HPZ telescoped posteriorly and replaced the degenerating larval hindgut (data not shown) (Takashima et al., 2008). A subset of cells of the HPZ delaminated around 6 hours APF and formed an interior ‘plug’ that occluded the lumen at the hindgut-midgut boundary (Fig. 4C,D′-E′). At 48 hours APF, the plug had expanded anteriorly, reaching a level anterior to the ureter (Fig. 4F). Up until 16 hours APF, these cells maintained expression of byn (green and red cells in Fig. 4E). Subsequently, by 48 hours APF, they lost byn expression (red cells in Fig. 4F; supplementary material Fig. S1B). At this stage, cells of the plug (trans)differentiated into midgut enterocytes, expressing molecular markers (e.g. Pdm1; Fig. 5A,C) and structural markers of midgut enterocytes. Before delamination, all cells of the HPZ showed hallmarks of ectoderm/hindgut: they had an apical junctional complex consisting of a zonula adherens and pleated septate junctions, and secreted a cuticle layer (Fig. 5G-I). After transitioning into posterior midgut enterocytes (48 hours APF), plug-derived cells exhibited an apical brush border (long microvilli without cuticle; Fig. 5J) and smooth septate junctions, characteristic of midgut/endoderm (Fig. 5J′). The change in junctional complex was accompanied by the relocalization of structural proteins, such as Fasciclin 3 (Fas3) (Fig. 5B). Cells of the hindgut, including the HPZ, express high levels of Fas3 around their basolateral membrane, as described for ectodermal epithelia in general. By contrast, enterocytes of the midgut, including the plug-derived posterior terminal midgut, showed strongly reduced levels of Fas3, localized around their apical membrane (Fig. 5B,B′).
Fig. 4.
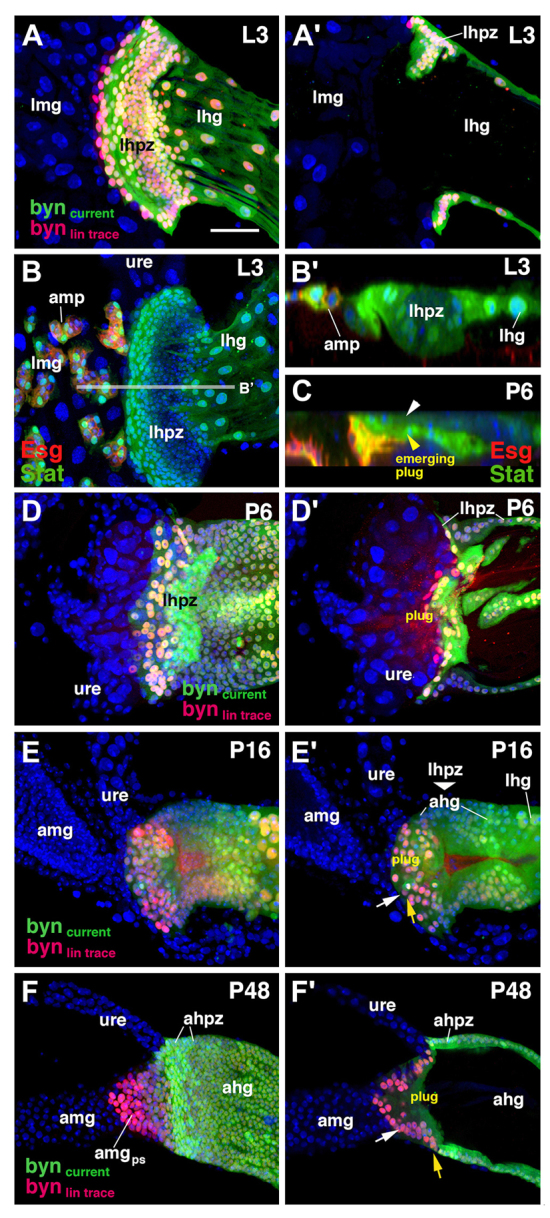
Movement of hindgut progenitors during metamorphosis. (A-F′) Z-projections of confocal sections of the hindgut-midgut boundary at different developmental stages. (A-B′) Wandering larva (L3); (C-D′) prepupa at 6 hours APF (P6); (E,E′) early pupa at 16 hours APF (P16); (F,F′) middle pupa at 48 hours APF (P48). Surface views (left column) and sagittal views (right column) are shown. The lineage of the adult hindgut progenitors detected by a lineage-tracing lacZ reporter (red), activated by byn-Gal4 from early third instar (A) or from late third instar (D-F) to the time of the observation. Current expression of byn-Gal4 is visualized by UAS-GFP (green). (B,C) Green label visualizes the expression of 10XStat92E-GFP. At the larval stage (A-B′), adult hindgut progenitors are confined to the larval hindgut proliferation zone (lhpz) at the boundary between larval midgut (lmg) and hindgut (lhg). Hindgut progenitors are cylindrical epithelial cells integrated in the gut epithelium (B′). In the prepupa (C,D), a subset of cells of the HPZ delaminate, resulting in an outer layer (white arrowhead in C) and an inner layer (yellow arrowhead), which goes on to form a solid ‘plug’ that occludes the gut lumen at the hindgut-midgut boundary (D′,E′). Beyond 24 hours APF, cells of the former plug have moved anteriorly, re-epithelialized and become the posterior terminal midgut (amgps in F; outlined by white and yellow arrows in F′). ahg, adult hindgut; ahpz, adult hindgut proliferation zone; amg, adult midgut; amgps, posterior terminal midgut; amp, adult midgut progenitor; lhpz, larval hindgut proliferation zone; lhg, larval hindgut; lmg, larval midgut; ure, ureter. Scale bar: 50 μm.
Interestingly, endocrine cells, which can be labeled by prospero (pros), settling in the posterior terminal midgut were negative for the byn-lineage marker (Fig. 5D). Likewise, ISCs (labeled by 10XStat92E-GFP) also did not co-label with the byn-lineage marker in the posterior terminal midgut (Fig. 5E); instead, they were descended from the AMPs (Fig. 5F). These findings indicate that pISCs migrate into the posterior terminal midgut from anteriorly adjacent midgut regions.
The HPZ-midgut transformation requires the function of GATAe
As byn expression is gradually lost from the plug when the plug transforms into midgut enterocytes, we hypothesized that its downregulation is accompanied by upregulation of a gene that instructs midgut cell differentiation. GATAe is a C2H2 type zinc-finger protein that is required for cell differentiation of embryonic endoderm in the late embryo and its action antagonizes byn (Okumura et al. 2005). In the embryo, the expression of GATAe was detected in the midgut and Malpighian tubules (Fig. 6A,B; Okumura et al., 2005). In the larva, we found that GATAe appears not only in the midgut cells, including AMPs, but also in the anterior part of the HPZ, where it overlaps with the expression of byn (Fig. 6C,D). We speculated that GATAe expression may enable cells of the anterior HPZ to be transformed into midgut cells. We tested this idea by downregulating GATAe using RNAi-mediated gene knockdown directed by the byn-Gal4 driver. Animals with the genotype of tub-Gal80ts/UAS-GATAeRNAi; byn-Gal4, UAS-GFP/+ were reared at 18°C and transferred to 29°C from late third instar in order to ablate GATAe specifically in hindgut cells, including the anterior part of the HPZ, and the phenotype was examined after adult eclosion. In these animals, the anterior HPZ was able to form a plug, but subsequent anterior extension and transition into the posterior terminal midgut were disrupted, remaining within the lumen at the anterior hindgut (Fig. 6E-H). These findings demonstrate that GATAe is indeed required for both the forward expansion and epithelialization of the HPZ-derived plug, as well as their subsequent differentiation into endodermal midgut enterocytes.
Fig. 6.
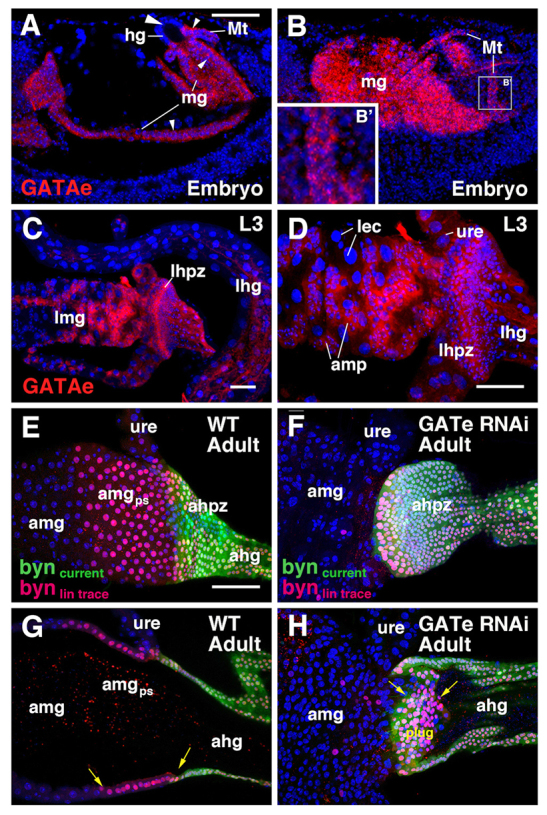
Role of GATAe in the formation of the posterior terminal midgut. (A-D) Confocal sections of gut of late embryo (A,B) and third instar larva (C,D), showing expression of GATAe mRNA (red). Embryonic expression is found in the midgut (mg; small arrowheads) and Malpighian tubules (Mt) (B′), but not in the hindgut (hg; big arrowhead). In the larva, GATAe is expressed strongly in adult midgut progenitors (amp) and the hindgut proliferation zone (hpz); faint expression is seen in the Malpighian tubules and ureter (ure), and differentiated midgut enterocytes (lec). (E-H) Confocal z-projection images of the adult hindgut-midgut boundary of wild-type (E,G) and GATAe knock-down animals (F,H). Surface views (E,F) and sagittal views (G,H) are shown. Cells of the hindgut proliferation zone are lineage traced from late third instar using byn-Gal4 driver. Current expression of byn is shown in green, the lineage is shown in red. In wild type, HPZ-derived cells come to lie within the posterior terminal midgut (amgps; arrows in G). Upon GATAe knock-down, HPZ-derived cells do not epithelialize and form a solid cluster of cells (plug; arrows) in the lumen of the hindgut (F,H). ahg, adult hindgut; ahpz, adult hindgut proliferation zone; amg, adult midgut; amgps, posterior terminal midgut; amp, adult midgut progenitor; hg, hindgut; lec, larval enterocyte; lhg, larval hindgut; lhpz, larval hindgut proliferation zone; lmg, larval midgut; mg, midgut; Mt, Malpighian tubule; ure, ureter. Scale bars: 50 μm.
Balanced Wingless activity is required to adjust the appropriate forward-versus-backward expansion of the HPZ
In a previous study (Takashima et al., 2008) we had shown that the activity of Wingless (Wg) signal promotes the continued proliferation of the HPZ, and inhibits the differentiation of hindgut enterocytes. wg is normally expressed in the anterior HPZ during the larval and early pupal stages, and is required for maintaining the stem/progenitor state of hindgut cells (Takashima et al., 2008). Overexpressing wg in the HPZ during metamorphosis results in an expansion of stem/progenitor cells and a lack of cell differentiation (Takashima et al., 2008). When we overexpressed wg in earlier time points from the early larval period onwards (tub-Gal80ts/UAS-wg; byn-GAL4 UAS-GFP/+) and animals were fixed as late larvae, pupae or adults, an additional phenotype became apparent. In late larvae, the posterior part of the HPZ, which in wild-type larvae becomes sculpted into several longitudinal folds or columns (Fig. 7B), remained flat and undifferentiated (Fig. 7A). At pupal stages, this was followed by a dramatic change in HPZ morphogenesis. First, the size of the plug that forms from the HPZ was strongly increased. Second, at around 52 hours APF, most of the cells of the HPZ formed a plug that extended abnormally far anteriorly (Fig. 7C). At the same time, the larval hindgut epithelium (which in wild-type control pupae 52 hours APF or younger had been completely replaced by the HPZ; Fig. 7D), persists in the wg-overexpressed animals (Fig. 7C). In consequence, a massively increased posterior terminal midgut was observed in the adult (Fig. 7E). At that stage, the enlarged plug had adopted an epithelial phenotype, but the cells remained immature in terms of reduced size and absence of Pdm1 expression (Fig. 7F). However, in other respects, the cells showed clear attributes of midgut rather than of hindgut; for example, expression of Fas3 was low and apically polarized, as in midgut cells (Fig. 7G-I). As in wild-type posterior terminal midgut, esg-positive pISCs and pros-positive entero-endocrine cells were present in the enlarged posterior terminal midgut of wg-activated flies (Fig. 7J-L). We conclude that the level of Wg expression, aside from its basic role in maintaining proliferating cells, forms part of the mechanism that determines what part of the HPZ gives rise to midgut versus hindgut.
Fig. 7.
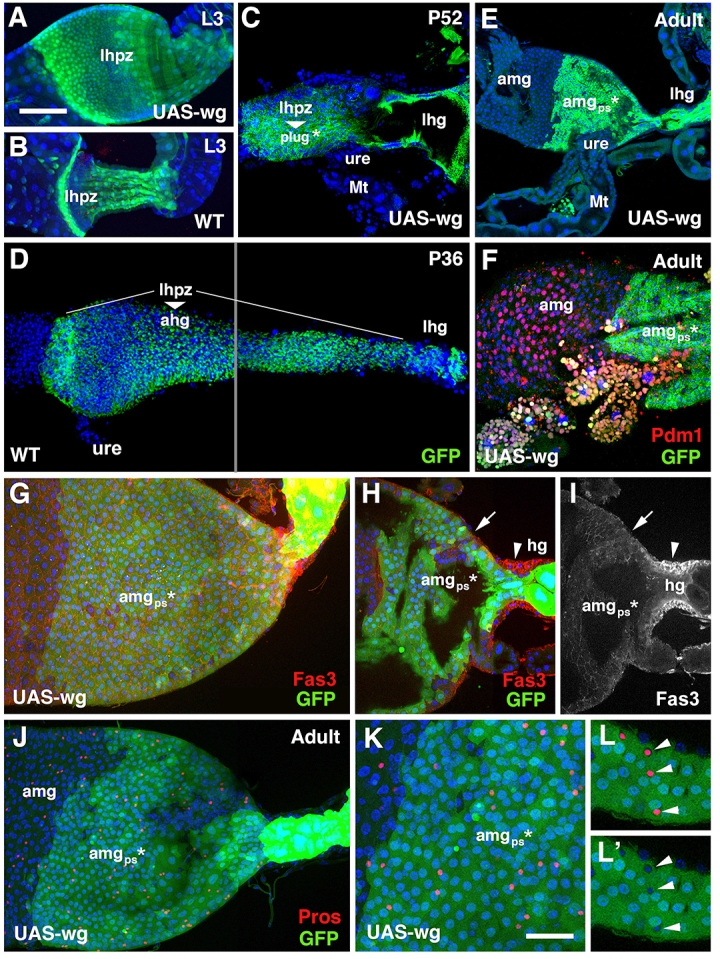
Role of wingless in the formation of the posterior terminal midgut. (A-L) Z-projections of the hindgut-midgut boundary region following byn-Gal4-driven wg overexpression (A,C,E-L) and wild-type controls (B,D). HPZ-derived cells (lineage) are detected by the expression of mito-GFP (green), which has a long half life (C-F), or by use of anti-GFP antibody against conventional GFP to enhance the signal (G,H,J-L). (A,B) Wg overexpression from first to third instar causes expansion of the HPZ (anterior HPZ is detected by 10XStat92E-GFP). (C) During mid-pupal stage (52 hours APF), the HPZ transforms into an enlarged plug (*) that has expanded anteriorly relative to the ureter (ure). (D) The HPZ of a wild-type pupa (36 hours APF) has expanded posteriorly to form the adult hindgut (ahg), which replaces the larval hindgut (lhg). (E-I) In adults of animals in which Wg was overexpressed from L1 onwards (E,F), cells of the plug become epithelial and form an abnormal, enlarged posterior terminal midgut (amgps*) lacking Pdm1 (F), but showing other midgut specific attributes, including low Fas3 (G-I). Thus, Fas3 is expressed at low levels at apical side of the midgut epithelium (arrows in H,I) and at high levels in the hindgut (arrowheads in H,I) (z-projection shown in G represents a surface view of the hindgut-midgut boundary, H and I represent sagittal sections). (J-L′) Pros-positive endocrine cells (arrowheads) form in the posterior terminal midgut as in wild type, but lack GFP expression, confirming that they are not of HPZ origin. ahg, adult hindgut; amg, adult midgut; amgps, posterior terminal midgut; hg, hindgut; lhg, larval hindgut; lhpz, larval hindgut proliferation zone; Mt, Malpighian tubule; ure, ureter. Scale bars: 50 μm in A-J; 25 μm in K-L′.
DISCUSSION
Drosophila midgut progenitors give rise to renal stem cells
Our current study revealed that the intestinal progenitor cells of the Drosophila midgut (AMPs) show a dynamic behavior that is unparalleled among adult progenitor cell populations described thus far. Even though they appear to originate from a single, endodermal cell population in the embryo and remain confined to the midgut during the larval period, AMPs spread out in the pupa and intermingle with cells of different germ layer origin. They reconstitute part of the ureter of the adult, and from there migrate onto the Malpighian tubules and then become the renal stem cells (Singh et al., 2007). Throughout the adult phase, these renal stem cells maintain molecular and cellular characteristics of midgut stem cells; they remain as small diploid mesenchymal cells attached to the basal membrane of the Malpighian tubule epithelium and are positive for esg expression. Renal stem cells do not express markers specific for Malpighian tubules, such as Cut (Singh et al., 2007) (S.T. and V.H., unpublished). Only post-mitotic progeny of renal stem cells, once integrated in the tubule epithelium, turn on these markers. Whereas they share common origin and similar molecular characteristics with midgut ISCs, the repertoire of cells that they produce is entirely distinct from that of midgut ISCs, indicating that there must be an extrinsic signal(s) that specify the fate of their progeny according to where they reside.
The origin of Drosophila adult renal stem cells from midgut progenitors differs strongly from what has been reported for vertebrate kidney stem cells; this may in part reflect the fact that the developmental pathways by which the excretory systems of vertebrates and insects are formed show very little resemblance. Vertebrate kidney tubules arise from the metanephrogenic mesenchyme, an embryonic cell population that forms part of the intermediate mesoderm. In response to complex signaling mechanisms from the ureteric bud, as well as the ingrowing vascular system, these cells undergo a mesenchymal-epithelial transition to become polarized, epithelial cells (Schmidt-Ott et al., 2006). Vertebrate kidney tubules show a high degree of regenerative capacity. Following damage, tubule cells partially dedifferentiate and become proliferative (Humphreys and Bonventre, 2007; Maeshima, 2007). Recent fate-mapping experiments strongly support the notion that tubule epithelial cells are responsible for the generation of new kidney cells (Duffield and Humphreys, 2011). This then differs markedly from the situation in Drosophila, where mesenchymal cells originating from outside the excretory organs represent stem cells that replace renal cells under normal conditions and following injury (Singh and Hou, 2009).
A transition of ectoderm to endoderm: formation of the posterior terminal midgut
Cells of the posterior terminal midgut arise by delamination from the HPZ during the first few hours after puparium formation. Until about 24 hours into the pupal phase they form a plug of mesenchymal cells that fills the lumen of the HPZ. After another 24 hours, plug cells regain their polarity, epithelialize and form the posterior terminal midgut, whereas the lumen of this region is closed so that the midgut and the hindgut tubes are still disconnected, which opens right before eclosion. The sequential loss-and re-acquisition of epithelial properties of cells forming the posterior terminal midgut is reminiscent of midgut formation in the embryo. During gastrulation, the posterior endoderm and hindgut primordium form a common epithelial invagination (Skaer, 1993). Around stage 10, the posterior endoderm undergoes an epithelial-mesenchymal transition, which then reverses during stage 12/13 with the formation of the definitive midgut epithelium (Tepass and Hartenstein, 1994b). It is tempting to speculate that the peculiar development of the epithelial HPZ giving rise to a small part of the midgut via a mesenchymal plug recapitulates a morphogenetic program that is active during early embryogenesis.
Interestingly, only enterocytes of the posterior terminal midgut are formed from the anterior HPZ. Midgut ISCs and endocrine cells found in this area emigrate from an anteriorly adjacent midgut region that is derived from endodermal AMPs. The boundary between hindgut and posterior midgut forms a conspicuous border at which the spread of pISCs stops. It is known that migrating cells interact with their environment via cell-cell adhesion molecules (e.g. cadherins, fasciclins) or cell-substrate adhesion molecules (e.g. integrins), and differential expression of these factors is likely to be involved in controlling pISC movement, causing them to stop at the hindgut-midgut boundary. In line with this hypothesis, several adhesion molecules, including DE-cadherin and Fas3, are expressed very highly in the HPZ, and abruptly decline in the midgut (Fig. 5B and data not shown), which might prevent pISCs from invading into the hindgut territory.
Wg apparently has a role in controlling the balance between midgut and hindgut derivatives of the HPZ. It is well established that Wg promotes the maintenance of intestinal stem cells and inhibits differentiation in both vertebrates and Drosophila (Lin et al., 2008; Takashima et al., 2008; Faro et al., 2009; Haegebarth and Clevers, 2009; Silva et al., 2011). Overexpression of Wg in the Drosophila late larval HPZ causes these cells to continue to proliferate and prevents hindgut enterocytes from differentiating (Takashima et al., 2008). If Wg activity is enhanced in the HPZ long enough prior to metamorphosis, an additional phenotype is observed, consisting of an increased fraction of HPZ-derived cells developing as midgut. We speculate that the early overexpression of Wg prevents a ‘determinative event’ in the HPZ that predisposes all cells in the (larval) HPZ to initiate hindgut differentiation. Thus, the characteristic columnarization that one normally observes in the posterior HPZ of late larvae can be interpreted as the first step of hindgut differentiation. Cells undergoing this determinative step (in wild-type) are thereby unable to delaminate and contribute to the plug, which will become midgut. Overexpression of Wg, in line with its basic pro-proliferative and anti-differentiative function, prevents the hindgut determination and columnarization. As a result, most cells form a plug, and subsequently differentiate as midgut enterocytes. Why are cells allowed to differentiate (into midgut), rather than stay undifferentiated? The most likely answer is that expression of byn-Gal4 driving Wg recedes from the plug, which then removes the block on differentiation.
Germ layers and the origin of the Malpighian tubules
In most animals, the specification of cell fates appears to be a long-lasting stepwise process. From an initial state of totipotency or pluripotency, cells become progressively more restricted in their fate. Gastrulation, the process by which cells are separated into three germ layers (ectoderm, endoderm, mesoderm) is widely considered as one of the most decisive, early occurring steps by which cell lineages with different fates become restricted. Subsequently, germ layers split into smaller units with even more restricted fates. Gastrulation in insects is a process by which the ventral domain and polar domain of the blastoderm become internalized to form the ‘gastral groove’ or ‘ventral furrow’ (Anderson, 1973; Alwes and Scholtz, 2006; Biffis et al., 2009; Wolff and Hilbrant, 2011; Brenneis et al., 2011). Cells of the gastral groove form the mesoderm (in the center) and the endoderm (anterior and posterior tip). After gastrulation, the outer surface epithelium adjacent to the anterior and posterior endoderm invaginates and forms the stomodeum (future foregut) and proctodeum (future hindgut). Their later time point of origin, as well as the fact that foregut and hindgut remain epithelial throughout development and later form cuticle, like the epidermis, were sufficient to consider these organs ectodermal, rather than endodermal, in much of the classical literature (e.g. Hertwig and Hertwig, 1881; Korschelt, 1936). The Malpighian tubules, including ureters, arise from the proctodeum, sometimes even before this tissue invaginates, and are therefore also usually counted as ectodermal structures. In Dipterans, matters are made difficult because invagination of the proctodeum occurs at the time of gastrulation, and the exact boundary between ectoderm and posterior endoderm is blurred. This has led some authors in the past to propose that the Malpighian tubules arise from the endoderm (e.g. Poulson, 1950). It is interesting to note that spiders, which became terrestrial independently of insects, have Malpighian tubules that are similar in ultrastructure and function to those of insects, but that are endodermal in origin, evaginating from the gastral groove well before the appearance of the proctodeum (Korschelt, 1936; Anderson, 1973).
The data presented in this paper as well as in previous genetic and molecular studies, suggest that a stable boundary between ectoderm and endoderm does not form in Drosophila, and that Malpighian tubules/ureters are supplied from all three germ layers. In the embryo, principal Malpighian tubule and ureter cells originate from the (structurally defined) ectoderm and mesodermal stellate cells, which later intercalate into tubule epithelium via mesenchymal-epithelial transition (Denholm et al., 2003). During metamorphosis, part of the ureter, as well as renal stem cells populating the (proximal) Malpighian tubules, are derived from endodermal midgut progenitors. Genetically, Malpighian tubules/ureters are also closely related to endoderm: transcriptional regulators associated with midgut (srp, GATAe) or Malpighian tubules (Kr) are transiently or permanently expressed in both tissues (Liu and Jack, 1992; Reuter, 1994; Okumura et al., 2005; Okumura et al., 2007) (see Fig. 6). Finally, ultrastructural features (lack of cuticle, presence of the brush border and smooth septate junctions) support the endodermal nature of the Malpighian tubules. Interestingly, these structural features are acquired secondarily during embryogenesis: Malpighian tubule progenitors start out like the ectodermal hindgut, as epithelial cells with pleated septate junctions and signs of apical cuticle secretion (Tepass and Hartenstein, 1994a), and, at a later time point, switch to an ‘endodermal phenotype’, replacing pleated septate junctions with smooth septate junctions and an apical brush border.
We speculate around the boundary between endoderm and ectoderm, a domain of germ layer of undefined identity exists that gives rise to the Malpighian tubules and posterior terminal midgut. It is puzzling what the developmental significance of this domain may be. In other words, why do Malpighian tubules, which at the anlagen stage as well as in their differentiated state express (at least some) attributes of endoderm, undergo a transient phase where they appear ectodermal? Comparative developmental-genetic studies, looking in other arthropods at the processes that shape the Malpighian tubules and adjacent intestine, may provide the answer to this puzzle. It will be particularly enlightening to gain more insight into the molecular mechanism that specifies Malpighian tubules in chelicerates, where these structures appear to be endodermal from start to finish, but where little is known about aspects of morphogenesis and gene expression patterns.
Supplementary Material
Acknowledgments
We thank Bloomington Stock Center, Vienna Drosophila Resource Center (VDRC) and the National Institute of Genetics (Japan) for providing flies. We are grateful to all our lab members for critical and helpful discussions.
Footnotes
Funding
This work is supported by the National Institutes of Health [1 R01 GM087373 to V.H.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.082933/-/DC1
References
- Alwes F., Scholtz G. (2006). Stages and other aspects of the embryology of the parthenogenetic Marmorkrebs (Decapoda, Reptantia, Astacida). Dev. Genes Evol. 216, 169–184 [DOI] [PubMed] [Google Scholar]
- Anderson D. T. (1973). Embryology and Phylogeny in Annelids and Arthropods. New York, NY: Pergamon Press; [Google Scholar]
- Biffis C., Alwes F., Scholtz G. (2009). Cleavage and gastrulation of the dendrobranchiate shrimp Penaeus monodon (Crustacea, Malacostraca, Decapoda). Arthropod Struct. Dev. 38, 527–540 [DOI] [PubMed] [Google Scholar]
- Brenneis G., Arango C. P., Scholtz G. (2011). Morphogenesis of Pseudopallene sp. (Pycnogonida, Callipallenidae) II: postembryonic development. Dev. Genes Evol. 221, 329–350 [DOI] [PubMed] [Google Scholar]
- Denholm B., Sudarsan V., Pasalodos-Sanchez S., Artero R., Lawrence P., Maddrell S., Baylies M., Skaer H. (2003). Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr. Biol. 13, 1052–1057 [DOI] [PubMed] [Google Scholar]
- Duffield J. S., Humphreys B. D. (2011). Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney Int. 79, 494–501 [DOI] [PubMed] [Google Scholar]
- Faro A., Boj S. F., Clevers H. (2009). Fishing for intestinal cancer models: unraveling gastrointestinal homeostasis and tumorigenesis in zebrafish. Zebrafish 6, 361–376 [DOI] [PubMed] [Google Scholar]
- Haegebarth A., Clevers H. (2009). Wnt signaling, lgr5, and stem cells in the intestine and skin. Am. J. Pathol. 174, 715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T., Kajita M., Iwabuchi M., Ohsumi K., Ishizuya-Oka A. (2011). Thyroid hormone-regulated expression of nuclear lamins correlates with dedifferentiation of intestinal epithelial cells during Xenopus laevis metamorphosis. Dev. Genes Evol. 221, 199–208 [DOI] [PubMed] [Google Scholar]
- Hertwig O., Hertwig R. (1881). Die Coelomtheorie: Versuch Einer Erkla”Rung Des Mittleren Keimblattes, pp. 68–76 Jena: Verlag von Gustav Fischer; [Google Scholar]
- Humphreys B. D., Bonventre J. V. (2007). The contribution of adult stem cells to renal repair. Nephrol. Ther. 3, 3–10 [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A., Shi Y. B. (2007). Regulation of adult intestinal epithelial stem cell development by thyroid hormone during Xenopus laevis metamorphosis. Dev. Dyn. 236, 3358–3368 [DOI] [PubMed] [Google Scholar]
- Jiang H., Edgar B. A. (2009). EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136, 483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., McEwen D. G., Edgar B. A. (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Grenley M. O., Bravo M. J., Blumhagen R. Z., Edgar B. A. (2011). EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8, 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korschelt E. (1936). Vergleichende Entwicklungsgeschichte der Tiere. Jena, Germany: G. Fischer; [Google Scholar]
- Lee W. C., Beebe K., Sudmeier L., Micchelli C. A. (2009). Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development 136, 2255–2264 [DOI] [PubMed] [Google Scholar]
- Lin G., Xu N., Xi R. (2008). Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455, 1119–1123 [DOI] [PubMed] [Google Scholar]
- Liu S., Jack J. (1992). Regulatory interactions and role in cell type specification of the Malpighian tubules by the cut, Krüppel, and caudal genes of Drosophila. Dev. Biol. 150, 133–143 [DOI] [PubMed] [Google Scholar]
- Liu W., Singh S. R., Hou S. X. (2010). JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J. Cell. Biochem. 109, 992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima A. (2007). Label-retaining cells in the kidney: origin of regenerating cells after renal ischemia. Clin. Exp. Nephrol. 11, 269–274 [DOI] [PubMed] [Google Scholar]
- Mathur D., Bost A., Driver I., Ohlstein B. (2010). A transient niche regulates the specification of Drosophila intestinal stem cells. Science 327, 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli C. A., Perrimon N. (2006). Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. (2006). The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. (2007). Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315, 988–992 [DOI] [PubMed] [Google Scholar]
- Okumura T., Matsumoto A., Tanimura T., Murakami R. (2005). An endoderm-specific GATA factor gene, dGATAe, is required for the terminal differentiation of the Drosophila endoderm. Dev. Biol. 278, 576–586 [DOI] [PubMed] [Google Scholar]
- Okumura T., Tajiri R., Kojima T., Saigo K., Murakami R. (2007). GATAe-dependent and -independent expressions of genes in the differentiated endodermal midgut of Drosophila. Gene Expr. Patterns 7, 178–186 [DOI] [PubMed] [Google Scholar]
- Paladi M., Tepass U. (2004). Function of Rho GTPases in embryonic blood cell migration in Drosophila. J. Cell Sci. 117, 6313–6326 [DOI] [PubMed] [Google Scholar]
- Poulson D. F. (1950). Histogenesis, organogenesis and differentiation in the embryo of Drosophila melanogaster Meigen. In Biology of Drosophila (ed. Demerec M.). New York, NY: John Wiley & Sons, Inc. [Google Scholar]
- Reuter R. (1994). The gene serpent has homeotic properties and specifies endoderm versus ectoderm within the Drosophila gut. Development 120, 1123–1135 [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott K. M., Lan D., Hirsh B. J., Barasch J. (2006). Dissecting stages of mesenchymal-to-epithelial conversion during kidney development. Nephron. Physiol. 104, 56–60 [DOI] [PubMed] [Google Scholar]
- Silva A. C., Filipe M., Steinbeisser H., Belo J. A. (2011). Characterization of Cer-1 cis-regulatory region during early Xenopus development. Dev. Genes Evol. 221, 29–41 [DOI] [PubMed] [Google Scholar]
- Singh S. R., Hou S. X. (2009). Multipotent stem cells in the Malpighian tubules of adult Drosophila melanogaster. J. Exp. Biol. 212, 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. R., Liu W., Hou S. X. (2007). The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell 1, 191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. R., Zeng X., Zheng Z., Hou S. X. (2011). The adult Drosophila gastric and stomach organs are maintained by a multipotent stem cell pool at the foregut/midgut junction in the cardia (proventriculus). Cell Cycle 10, 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaer H. (1993). The alimentary canal. In The development of Drosophila melanogaster (ed. Bate M., Martinez-Arias A.), pp. 941–1012 Plainview, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Takashima S., Mkrtchyan M., Younossi-Hartenstein A., Merriam J. R., Hartenstein V. (2008). The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454, 651–655 [DOI] [PubMed] [Google Scholar]
- Takashima S., Adams K. L., Ortiz P. A., Ying C. T., Moridzadeh R., Younossi-Hartenstein A., Hartenstein V. (2011a). Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev. Biol. 353, 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S., Younossi-Hartenstein A., Ortiz P. A., Hartenstein V. (2011b). A novel tissue in an established model system: the Drosophila pupal midgut. Dev. Genes Evol. 221, 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Hartenstein V. (1994a). The development of cellular junctions in the Drosophila embryo. Dev. Biol. 161, 563–596 [DOI] [PubMed] [Google Scholar]
- Tepass U., Hartenstein V. (1994b). Epithelium formation in the Drosophila midgut depends on the interaction of endoderm and mesoderm. Development 120, 579–590 [DOI] [PubMed] [Google Scholar]
- Wolff C., Hilbrant M. (2011). The embryonic development of the central American wandering spider Cupiennius salei. Front. Zool. 8, 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Wang S. Q., Tan D., Gao Y., Lin G., Xi R. (2011). EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev. Biol. 354, 31–43 [DOI] [PubMed] [Google Scholar]
- Yeo S. L., Lloyd A., Kozak K., Dinh A., Dick T., Yang X., Sakonju S., Chia W. (1995). On the functional overlap between two Drosophila POU homeo domain genes and the cell fate specification of a CNS neural precursor. Genes Dev. 9, 1223–1236 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.