Abstract
The thymus is the central site of T-cell development and thus is of fundamental importance to the immune system, but little information exists regarding molecular regulation of thymus development in humans. Here we demonstrate, via spatial and temporal expression analyses, that the genetic mechanisms known to regulate mouse thymus organogenesis are conserved in humans. In addition, we provide molecular evidence that the human thymic epithelium derives solely from the third pharyngeal pouch, as in the mouse, in contrast to previous suggestions. Finally, we define the timing of onset of hematopoietic cell colonization and epithelial cell differentiation in the human thymic primordium, showing, unexpectedly, that the first colonizing hematopoietic cells are CD45+CD34int/-. Collectively, our data provide essential information for translation of principles established in the mouse to the human, and are of particular relevance to development of improved strategies for enhancing immune reconstitution in patients.
Keywords: Thymus development, Human, Hematopoietic, Epithelium, Mesenchyme, Colonization
INTRODUCTION
T-cell development occurs in the thymus and depends on a diverse array of functionally distinct epithelial cell types within the thymic stroma. These thymic epithelial cell (TEC) types originate from the endoderm of the third (and in some species fourth) pharyngeal pouches (PPs), transient bilateral endodermal structures that generate both the thymus and parathyroid glands (Le Douarin and Jotereau, 1975; Gordon et al., 2004). The molecular and cellular processes that govern thymus organogenesis are best understood in the mouse (Blackburn and Manley, 2004; Manley and Condie, 2010). By contrast, knowledge of human thymic organogenesis is based primarily on histological studies, with molecular insight currently limited to analysis of genetic abnormalities affecting thymus development; principally, DiGeorge Syndrome, the human nude syndrome and APECED, which are caused by mutations in TBX1, FOXN1 and AIRE, respectively (Frank et al., 1999; Baldini, 2005; Villaseñor et al., 2005).
The human thymus is thought to develop from either the third PP (Norris, 1938) or from both the third and fourth PP (Van Dyke, 1941). As in the mouse, the bilateral human primordia initially each comprise thymic and parathyroid domains, and are encased in a neural crest-derived mesenchymal capsule from early in development. From week 7 [Carnegie stage (CS) 18-19] to mid-week 8 (CS20-21) of gestation the thymic component of this primordium migrates ventrally, such that the leading tip of the migrating thymic rudiment remains attached to the parathyroid by a thin, elongated, highly lobulated, cord-like structure. This structure then resolves, separating the parathyroid and thymic primordia (Norris, 1938), and the two thymic primordia meet and attach at the pericardium by mid-week 8 (Norris, 1938). It is well documented that an accessory thymus can exist in the cervical region in humans (Norris, 1938; Van Dyke, 1941) and, similarly, the occurrence of cervical thymi in the mouse has recently been reported (Dooley et al., 2006; Terszowski et al., 2006).
The early human thymic primordium is proposed to contain undifferentiated epithelial cells, as reported for the mouse (Lampert and Ritter, 1988; Bennett et al., 2002; Rossi et al., 2006). Medullary development has been observed from week 8 and distinct cortical and medullary compartments by week 16. The presence of other intrathymic cell types, including mesenchymal, vascular and lymphoid cells, has been reported from mid-week 8 (Haynes et al., 1984; Haynes and Heinly, 1995). Between 14 and 16 weeks, mature lymphocytes begin to leave the thymus to seed the peripheral immune system (Van Dyke, 1941; Lobach and Haynes, 1987). However, although the morphological events in human thymus organogenesis are well documented and some information exists regarding TEC differentiation, molecular regulation of these processes remains poorly understood. In particular, only very limited information exists regarding the extent to which mechanisms known to govern thymus development and function in the mouse are conserved in human. Furthermore, neither the time of onset of TEC differentiation nor the identity of markers defining thymic epithelial progenitor cells (TEPCs) has been determined in the human fetal thymus. Similarly, neither the timing of initial hematopoietic colonization nor the identity of the colonizing cells has been reported. Improved understanding is urgently required, owing to the clinical need for improved strategies for enhancing or replacing thymus function in patients (Wils and Cornelissen, 2005; Lynch et al., 2009; Awong et al., 2010).
Here, we demonstrate that the major mechanisms currently known to underpin thymus organogenesis are conserved between mouse and human. We further define the age at which overt TEC differentiation is initiated in human fetal thymus, and establish the timing of expression of known mediators of TEC function, including the autoimmune regulator AIRE and the chemokines CCL21 and CCL25. These observations delineate the stage in thymus development at which the thymus is composed principally of undifferentiated thymic epithelial progenitor cells. Finally, we determine the stage of thymus organogenesis at which colonization by hematopoietic, endothelial and mesenchymal cells first occurs, identifying the first colonizing hematopoietic cells as CD45+CD7-CD34lo/- and the first thymus-seeding T-lineage progenitors as CD45+CD7+CD34int cells, in contrast to expectations from previous studies (Haddad et al., 2006). These data, to our knowledge, constitute the first detailed analysis of human thymus organogenesis and TEC development that is not based solely on morphological and histological data, and thus provide essential information for the translation of principles established in the mouse to the human.
MATERIALS AND METHODS
Human tissue
First and second trimester human fetuses were obtained following elective medical termination of pregnancy, and all were morphologically normal. Ethical approval for use of human fetal tissue in these studies was granted by the Lothian Research Ethics Committee or the Ethics Committee of the Erasmus University Medical Center. Consent was obtained from all women in writing, and the tissue was anonymized before being made available for research. All experiments using human tissue were performed at the University of Edinburgh or the Erasmus University Medical Center. Embryos were aged according to the standard head/rump measurement and Carnegie stage was determined (Gasser, 1975; O’Rahilly and Muller, 1987). Embryos used in this study were from mid- to late week 6 (CS17), week 7 (CS18-19), early to mid-week 8 (CS20-21), and weeks 9, 10, 15-16 and 17, with days/weeks indicating days/weeks post-fertilization. Note that Carnegie staging does not apply to second trimester fetuses.
In situ hybridization
Whole-mount in situ hybridizations were performed as previously described (Gordon et al., 2001). Probes were made by PCR amplification from human fetal tissue using primers as listed in supplementary material Table S1.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Gordon et al., 2004). Primary antibodies used were: anti-cytokeratin (rabbit polyclonal anti-keratin, Dako); anti-CLDN4 (3E2C1, Zymed); anti-human CD45 (clone HI30, BD Pharmingen); anti-keratin 5 (AF138 rabbit IgG, Covance); anti-keratin 8 (CAM 5.2, Becton Dickinson); anti-keratin 14 (AF64, Covance); TE7 (Developmental Studies Hybridoma Bank); UEA1 (biotinylated, Vector Labs); anti-AIRE (D-17, Santa Cruz); anti-CD205 (MG38, Serotec); CDR2 (provided by B. Kyewski, German Cancer Research Center, Heidelberg, Germany); anti-HLA-DR/DP/DQ (clone TU39, BD Pharmingen); anti-EPCAM (Ber-EP4, DakoCytomation); anti-CCL21 (anti-6Ckine, R&D Systems); anti-CCL25/TECK (R&D Systems); mouse anti-CD11b/Mac1 (ICRF44, BD Pharmingen); mouse anti-CD31 (WM59, BD Pharmingen); mouse anti-CD144 PE (16B1, eBiosciences); mouse anti-CD34 FITC (581, BD Pharmingen); mouse anti-CD7 (M-T701, BD Pharmingen). Unconjugated antibodies were detected using goat anti-mouse Alexa488, goat anti-rabbit Alexa647, goat anti-rat Alexa488, chicken anti-goat Alexa488, chicken anti-rabbit Alexa594, donkey anti-goat IgG Alexa488, streptavidin-Alexa647 (all Invitrogen). Appropriate isotype-control antibodies (BD Pharmingen) provided negative controls in all experiments. DAPI was used as a nuclear counterstain.
Microscopy
Thymic tissue was microdissected from embryos using an Olympus SZH dissecting microscope. Images were captured using a ZEISS Stemi SVII stereomicroscope, an Olympus BX61 stereomicroscope, a Leica AOBS confocal microscope or a Leica DMRXA microscope under the appropriate excitation conditions, and were processed using Adobe Photoshop CS4.
Flow cytometry
Thymic single cell suspensions were generated by using enzymatic dissociation as described for mouse fetal thymus (Nowell et al., 2011) or by mincing thymic fragments through a 100-μm filter (BD). Flow cytometric analysis was performed on a LSRII Flow Cytometer (BD) and analyzed using FlowJo software (Tree Star). Antibodies used were: mouse anti-CD19 DY590 (Exbio Praha), mouse anti-BDCA2 APC (Miltenyi Biotech), rat anti-CD7 (MCA344, Serotec), mouse anti-human CD45 (clone HI30), mouse anti-human CD34 FITC (clone 581), mouse anti-CD3 Alexa700, mouse anti-CD45 PerCP-Cy5, mouse anti-HLA-DR APC-Cy7, mouse anti-CD123 PE (all BD Pharmingen). Unconjugated antibodies were detected with goat anti-mouse Alexa647 and goat anti-mouse Alexa 568 (Invitrogen).
Grafted re-aggregate fetal thymic organ culture
Two human thymic lobes (early week 8) were lightly dissociated as described, mixed with 150,000 mouse primary embryonic fibroblasts and re-aggregated as described previously (Sheridan et al., 2009). The re-aggregates were cultured on a filter for 24 hours and then grafted under the kidney capsule of NOD/SCID recipient mice the next morning. Grafts were recovered after 2 weeks for analysis.
Affymetrix microarray analysis
RNA was prepared from three independent mid-week 8 thymic lobes microdissected free of any other cells or tissues (CB094, CS20; CB388, CS21/22; CB106, CS22). Note that the name of each sample includes both an identifier (e.g. CB094) and the Carnegie stage of that sample (e.g. CS20). Samples were amplified, labeled and hybridized to Affymetrix Human Genome U133Plus2 GeneChip arrays according to standard Affymetrix protocols. The microarray data were normalized independently using RMA (Irizarry et al., 2003) and Micro Analysis Suite 5.0 (MAS5) (Liu et al., 2002), and the probe sets were then ranked based on the resulting expression values, with the probe set having the highest expression given rank-1. The data are deposited in the Stem Cell Database under accession number StemDB-2316 (http://www.stemdb.org/stemdb/utils/show?id=StemDB-2316). The data presented show ranks for selected probe sets along with the corresponding MAS5 PMA flag [present (P), marginal (M) and absent (A)]. For a given probe set, the lower the rank the higher the probability of the gene being present in the sample, whereas MAS5 predicts the presence or absence of a gene using different statistics. Data were analyzed using Limma and Affymetrix Bioconductor packages and visualized using Genespring GX (Agilent).
RESULTS
Spatial and temporal expression of essential regulators of mouse thymus organogenesis is conserved in human thymus development
To determine whether molecular mechanisms that regulate thymus development are conserved between mouse and human, we investigated the detailed spatial and temporal expression profiles of known regulators of mouse thymus organogenesis in human embryos. We first confirmed the relative staging of human and mouse thymus development. The earliest human tissue available for this study was early week 6 (CS16). At this stage, the third PP had formed and morphological criteria indicated that third PP/thymic primordium development was at an equivalent stage to that at embryonic day (E)10.5 in CBAxC57BL/6 F1 mouse embryos. By early week 7 (CS18), outgrowth of the third PP to form the common thymus/parathyroid primordium (Gordon et al., 2001) had occurred, equivalent to ∼E11.5 in the mouse (Fig. 1A). Migration of the thymus component of the primordium along the carotid artery was observed by mid-week 8 (CS21), as described, and at this and subsequent stages the thymic primordium was surrounded by a mesenchymal capsule (Fig. 1B-B″). By week 10, the thymic primordium comprised numerous lobules (Fig. 1C,C′; note that the mid-week 8 thymus is not lobulated), and lobules could also be seen within the elongated cord connecting the thymus and parathyroid primordia (Fig. 1C,C′). Of note is that from week 8, the mesenchyme surrounding the human thymic primordium was less closely associated with the primordium than is the mesenchymal capsule in the mouse (Fig. 1C, arrow).
Fig. 1.
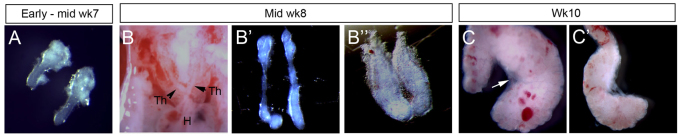
Morphological features of early human thymus development. (A-C′) Images show microdissected human fetal thymic primordia at the ages shown, except for B, which shows anatomical details of the exposed chest cavity to indicate relative locations of thymic primordia, carotid artery and heart. B″ shows detail from lower part of thymic primordium (i.e. the leading edge during migration). Arrowheads indicate thymic primordial; white arrow in C indicates mesenchymal capsule. Images are representative of at least two independent analyses. H, heart; Th, thymus; Wk, week.
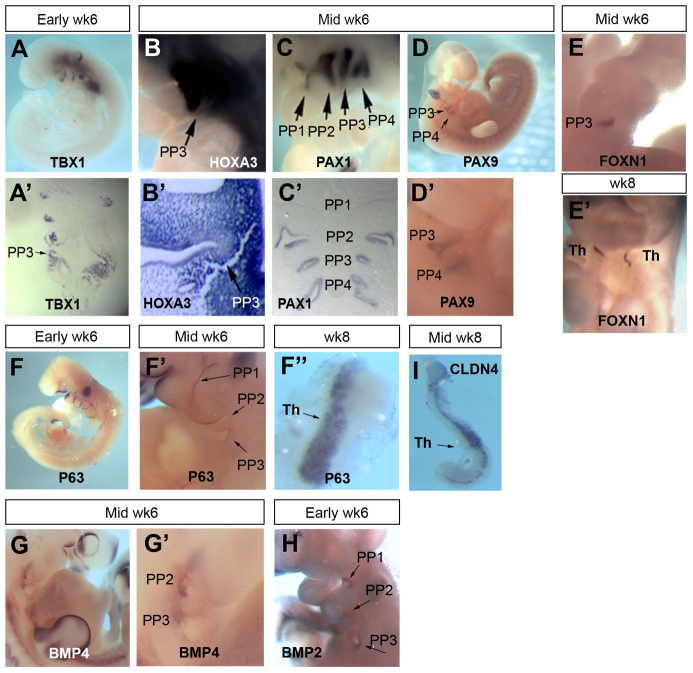
We then investigated the expression pattern in week 6 human embryos of TBX1, HOXA3, PAX1 and PAX9 genes, which encode proteins with genetically defined roles in patterning of the pharyngeal endoderm (PE) and/or formation of the third PP. Tbx1 is required for development of the mouse foregut endoderm, where it regulates segmentation of the PE and PP patterning (Xu et al., 2005). Conserved stage-specificity and localization of TBX1 expression between mouse and human embryos was demonstrated (ISH; Fig. 2A,A′). In the early week 6 (CS16) human embryo, TBX1 expression was detected in the core mesenchyme of the first, second and third pharyngeal arches (PAs) and in the third PP endoderm (Fig. 2A,A′). Expression was also detected in the epithelium of the caudal half of the otic vesicle (Fig. 2A).
Fig. 2.
Expression profile of known regulators of mouse thymus organogenesis in human thymus development. Images show whole or sectioned human embryos or microdissected thymic primordia after in situ hybridization with the probes indicated. Ages of embryos or dissected thymus lobes are as shown. (A-D′) TBX1, HOXA3 and PAX1 images show side view of whole embryos (top) and coronal sections (bottom). PAX9 images show side view of whole embryo; lower panel shows detail of upper panel. (E,E′) FOXN1 images showing ventral view of whole embryos. (F-F″) P63 images showing side view of whole embryos (left and middle) and dissected thymic lobe. (G-H) BMP4 and BMP2 images showing side view of whole embryos. (I) CLDN4 image showing dissected thymic lobe. Images are representative of at least two independent analyses. PP1, first PP; PP2, second PP; PP3, third PP; PP4, fourth PP; Th, thymus; Wk, week.
Hoxa3 is expressed in the mouse from E9.5 in the third pharyngeal cleft ectoderm, in the third and fourth PA neural crest cells, and in the third PP endoderm, and is required for thymus and parathyroid formation (Chisaka and Capecchi, 1991; Manley and Capecchi, 1995). HOXA3 expression was also conserved between mouse and human; the anterior boundary of HOXA3 expression in the week 6 (CS16-17) human embryo was the third PA and, as in the E10.5 mouse embryo, expression was observed throughout the third PP and surrounding mesenchyme (Fig. 2B,B′).
In the mouse, both Pax1 and Pax9 are expressed in the foregut endoderm from E8.5 (Neubüser et al., 1995; Wallin et al., 1996) and are then expressed in all four PPs and in the thymus throughout organogenesis, becoming restricted to subpopulations of TECs postnatally (Neubüser et al., 1995; Wallin et al., 1996). Here, PAX1 expression was investigated in a week 6 human embryo; expression was observed in all four PPs (Fig. 2C,C′). PAX9 expression was also examined in human embryos at week 6; expression was evident in the third and fourth PPs but was not detected in the first and second pouches (Fig. 2D,D′). Thus, whereas the expression pattern of PAX1 is conserved between mouse and human, PAX9 expression in the human PE is more restricted than in the mouse at the equivalent developmental stage.
Foxn1 is required cell-autonomously for development of mouse TECs but is not required for initiation of thymus organogenesis (Blackburn et al., 1996; Nehls et al., 1996; Nowell et al., 2011). Null mutations in Foxn1 result in athymia and hairlessness in mice, rats and humans (Flanagan, 1966; Festing et al., 1978; Nehls et al., 1994; Frank et al., 1999). In the mouse, Foxn1 expression initiates at the ventral tip of the third PP at E11.25 (in CBAxC57BL/6 embryos) and spreads to the entire thymus domain by E11.5 (Gordon et al., 2001). We could not detect FOXN1 expression in the third PP of early week 6 (CS16) human embryos (not shown) but observed strong expression throughout the thymus domain of the third PP by mid-week 6 (CS16-17; Fig. 2E), suggesting that timing of initiation of high-level FOXN1 expression occurs at equivalent developmental stages in human and mouse. FOXN1 expression was also seen throughout the thymic lobes at week 8 (CS20-21; Fig. 2E′). The human thymus has been suggested to develop from both the third and fourth PPs (Norris, 1938; Van Dyke, 1941). However, we did not detect FOXN1 expression in the fourth PP at any stage of development (Fig. 2E,E′). This establishes that in the human, as in the mouse, the thymus arises solely from the third PP, indicating that the mechanisms of early thymus organogenesis are closely conserved between these species. Of note is that GCM2, which delineates the parathyroid domain within the pouch, is expressed in both the third and fourth PPs in human (Liu et al., 2010), in keeping with the proposition that human parathyroids derive from both the third and fourth PPs.
We also determined the expression pattern of P63 (TP63 - Human Gene Nomenclature Database), because in the mouse p63 (Trp63 - Mouse Genome Informatics) has a pivotal role in many stratified epithelia, including the thymus (Candi et al., 2007; Senoo et al., 2007). In the early week 6 (CS16-17) human embryo, P63 was expressed by the ectoderm surrounding the pharyngeal arches (Fig. 2F), consistent with its expression in the pharyngeal ectoderm in the mouse from E9.5 (Mills et al., 1999). By mid-week 6, P63 expression was observed in the endoderm of the third PP (Fig. 2F′) and by late week 8, it appeared to be expressed by all TECs (Fig. 2F″). Thus, P63 expression in the pharyngeal region is also conserved between mouse and human.
Finally, we analyzed the expression of BMP2 and BMP4, because BMP signaling has been implicated both in thymus development (Gordon et al., 2010) and as a possible regulator of Foxn1 (Tsai et al., 2003; Senoo et al., 2007). BMP4 expression was observed in the second and third PP endoderm and adjacent mesenchyme in the mid-week 6 (CS16-17) human embryo (Fig. 2G,G′), consistent with its expression pattern at E10.5 in the mouse (Patel et al., 2006), whereas BMP2 was expressed in the third and fourth PP endoderm of the early week 6 (CS16) embryo (Fig. 2H), as observed in the mouse from E9.5 (Lyons et al., 1990).
Collectively, these data demonstrate that the spatial and temporal expression of transcription factors and signaling molecules involved in mouse thymus organogenesis is highly conserved in human thymus development, albeit with some minor regional differences. This strongly suggests that the genetic mechanisms controlling thymus development are conserved between mouse and human. Our previous studies have shown that Rhox4 and Plet1 (1600029D21Rik - Mouse Genome Informatics), specific markers of the mouse third PP/early thymus primordium, are not expressed in this region during human thymus organogenesis (Morris et al., 2006; Depreter et al., 2008). Thus, our data indicate that although the expression patterns of all genes implicated in thymus organogenesis by functional studies are conserved between mouse and human, not all gene expression patterns in this region are conserved between species.
Onset and progression of epithelial cell differentiation in the human thymic primordium
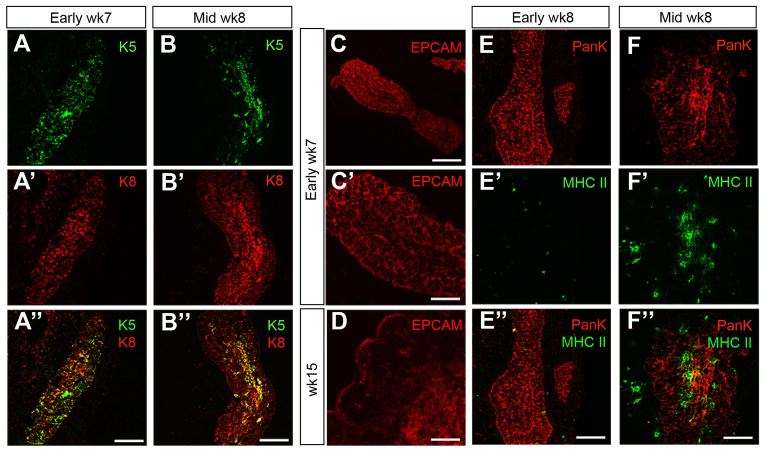
We next investigated whether patterning of the human thymic primordium into cortical and medullary compartments occurred in a similar manner to in the mouse, and at what stage the onset of TEC differentiation occurred. In the adult mouse thymus, the structural proteins cytokeratin 8 (K8; Krt8 - Mouse Genome Informatics) and keratin 5 (K5; Krt5 - Mouse Genome Informatics) are broadly restricted to cortical TECs (cTECs) and medullary TECs (mTECs), respectively, and additionally are co-expressed by a minor TEC population found at the cortico-medullary junction and scattered throughout the cortex (Klug et al., 1998). These markers are also co-expressed by most TECs in the E11.5 mouse thymic primordium (Bennett et al., 2002); by E12.5, high level K5 expression marks a medullary thymic epithelial progenitor cell population that also expresses high levels of claudin 4 (Cldn4) and, from ∼E13.5, co-stains with the mTEC marker lectin UEA1 (Hamazaki et al., 2007).
At early week 7 (CS18), K5 and K8 were co-expressed by most, if not all, TECs in the human thymic primordium, consistent with the expression pattern seen at the equivalent stage (E11.5) in mouse (Fig. 3A-A″). By mid-week 8 (CS21), these markers had begun to segregate such that, although most TEC expressed K8, K5 was more highly expressed by cells along the midline of the thymic lobe and in some scattered cells (Fig. 3B-B″). Expression of EPCAM, a pan-epithelial marker in the mouse thymus that is expressed at higher levels in mouse mTECs than cTECs (Farr and Anderson, 1985), was also similar in the mouse and human thymus. EPCAM was expressed by all TECs in the week 7 human thymic primordium (Fig. 3C-C′) and by week 15 was expressed strongly by mTECs and weakly by cTECs (Fig. 3D). These data suggested that the onset of overt TEC differentiation in human thymus development occurs around mid-week 8 (CS21), demonstrating that, as in mouse, human TEC development is initiated after the initiation of FOXN1 expression (Nowell et al., 2011). Consistent with this conclusion, expression of a functional marker of TEC maturation, major histocompatibility complex (MHC) Class II (HLA-DR/DP/DQ), expression of which is Foxn1-dependent in the early mouse thymic priordium (Nowell et al., 2011), was not detected in human TECs until mid-week 8 (Fig. 3E-F″).
Fig. 3.
Onset of TEC differentiation in the human thymic primordium. (A-F″) Immunohistochemical staining of thymic primordia at the developmental stages shown with anti-Keratin 5 (K5) and anti-Keratin 8 (K8) (A-B″), anti-EPCAM (C-D), or anti-HLA-DR/DP/DQ (MHC II) and anti-pan-Cytokeratin (PANK) (E-F″). Images are representative of at least two independent analyses. Wk, week. Scale bars: 150 μm in A-C,D-E″; 75 μm in C′,F-F″.
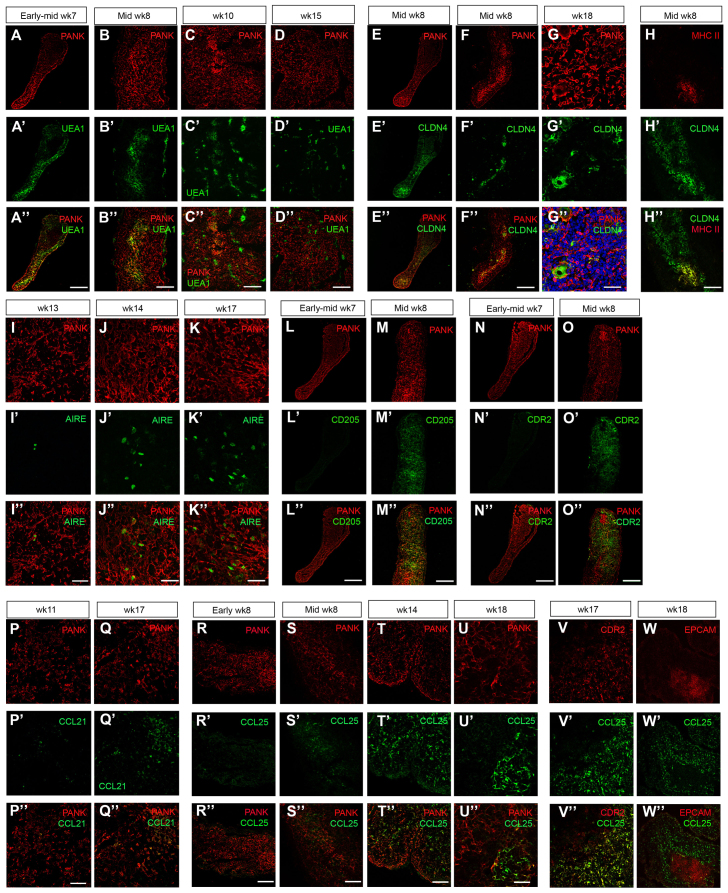
We next determined the pattern of UEA1 staining in human thymus development, because in the mouse UEA1 binding identifies mTECs in the thymic primordium from E13.5 (Hamazaki et al., 2007) and, subsequently, most postnatal mTECs (Farr and Anderson, 1985). Surprisingly, as no UEA1 staining is observed at the equivalent stage in the mouse, UEA1 bound most, if not all, TECs within the week 7 human thymus (Fig. 4A-A″). By mid-week 8, UEA1 staining on TEC was restricted to the central area of the thymic lobes (Fig. 4B-B″), suggesting that medullary epithelial progenitor cells had arisen by mid-week 8, and a subpopulation of mTECs binding UEA1 at high intensity were present by week 15 of human fetal development (Fig. 4D-D″). Of note is that some non-epithelial UEA1+ cells were also observed in the human fetal thymus, unlike in the mouse. These cells were initially seen lining week 8 thymic lobes (Fig. 4B-B″) and were found throughout the thymus at week 10 and week 15, possibly indicating UEA1 staining of vasculature-associated cells (Fig. 4C-D″).
Fig. 4.
Expression of markers of cortical and medullary TECs in human thymus development. (A-K″) Medullary TEC differentiation. Images show staining with UEA1 (A-D″), anti-CLDN4 (E-G″), anti-CLDN4 and anti-HLA-DR/DP/DQ (MHC II) (H-H″) and anti-AIRE (I-K″). (L-O″) Cortical TEC differentiation. Images show staining with CD205 (L-M″) and CDR2 (N-O″). (P-W″) Chemokine expression. Images show staining with anti-CCL21 (P-Q″) and anti-CCL25 (R-W″). Developmental stages are as shown. Anti-pan-Cytokeratin (PANK) co-stain reveals the entire thymus primordium. Images are representative of at least two independent analyses. Wk, week. Scale bars: 150 μm in A-B″,D-F″,H-H″,N-W″; 75 μm in C-C″; 47.6 μm in G-G″; 50 μm in I-I″,L-M″; 55 μm in J-K″.
We also investigated the expression of CLDN4, as the Cldn4+UEA1+ population in the fetal mouse thymus contains progenitors that give rise to Aire+ mTECs (Hamazaki et al., 2007). By week 8, CLDN4 was expressed by TECs in the central area of the thymic lobes (Fig. 2I; Fig. 4E-G″). These CLDN4+ cells also expressed MHC class II (HLA-DR/DP/DQ; Fig. 4H-H″), suggesting that, as in the mouse (Hamazaki et al., 2007), these are the first cells to differentiate into mature medullary TECs. CLDN4 expression remained restricted to a subpopulation of mTECs and, by week 18, identified both small clusters of nucleated mTECs and swirls of cells surrounding an enucleated region that were strongly suggestive of Hassall’s corpuscles (Fig. 4G-G″).
The autoimmune regulator Aire is essential for induction of central tolerance in mice and humans (Mathis and Benoist, 2009) and therefore we analyzed AIRE expression during human thymus development. AIRE+ TECs were not present in the week 10 human thymic primordium (not shown), but were observed in medullary areas by week 13 (Fig. 4I-I″) and had increased in number by week 17 (Fig. 4J-K″).
To investigate differentiation towards the cTEC fate, we determined the expression pattern of CD205 (LY75 - Human Gene Nomenclature Database) (Shakib et al., 2009) and CDR2 (Rouse et al., 1988). CD205 has recently been shown to mark the onset of cTEC differentiation in the mouse and is expressed as early as E12.5 in mouse thymus development (Shakib et al., 2009); later in fetal development, it may mark a common TEPC (Baik et al., 2013). CDR2 is restricted to cTECs in the human adult thymus (Rouse et al., 1988). We were unable to detect CD205 or CDR2 expression in the early or mid-week 7 thymic primordium by immunohistochemistry (Fig. 4L-L″,N-N″). However by mid-week 8, extensive expression of both markers was observed throughout the thymic lobes (Fig. 4M-M″,O-O″). CDR2+ TECs within the mid-week 8 thymus did not express K5, suggesting that they may represent cortical sub-lineage-restricted epithelial progenitor cells. Again, these data are consistent with the onset of TEC differentiation occurring around mid-week 8 in the human thymic primordium.
The chemokine CCL21, which plays a role in hematopoietic colonization of the thymus in mice (Liu et al., 2006), was detected in scattered cells in the human thymic primordium at week 11 (Fig. 4P-P″). The number of CCL21+ TECs was substantially increased by week 17 and appeared to be largely restricted to medullary regions (Fig. 4Q-Q″), consistent with its localization in the mouse (Kwan and Killeen, 2004). CCL25, a chemokine expressed by mouse TECs in a Foxn1-dependent manner that also plays a role in hematopoietic colonization of the thymus (Liu et al., 2006; Nowell et al., 2011), was first detected at mid-week 8 (Fig. 4R-S″). CCL25 expression was initially detected in scattered cells throughout the human thymic primordium. By week 17, CCL25 expression was detected only in cortical TECs (Fig. 4U-W″), as shown by co-staining with CDR2 and EPCAM (Fig. 4V-W″), which mark cortical and medullary regions, respectively, by immunohistochemistry (Farr and Anderson, 1985; Rouse et al., 1988).
Immigration of mesenchymal cells and endothelial progenitors into the human thymic primordium
Neural crest-derived mesenchymal cells form the capsule and trabeculae of the fetal thymus and also generate the intrathymic pericytes (Foster et al., 2008). It is well established that thymic mesenchyme is at least partly responsible for regulating expansion (Auerbach, 1960; Jenkinson et al., 2003; Anderson et al., 2006) and migration (Griffith et al., 2009; Foster et al., 2010) of the early thymic primordium. However, the timing of the initial immigration of mesenchymal cells into the human thymic primordium has not been determined. TE7 is a marker of human thymic mesenchymal cells that has been reported to stain the mesenchymal cells surrounding the thymic lobes at weeks 7 and 10, and to mark interlobular fibrous septae, vessels and thymic fibrous capsule at week 15 (Haynes et al., 1984). At early week 7, we observed cells stained with TE7 surrounding the thymic primordium, but no stained cells were seen within the thymic epithelium (Fig. 5A,A′). By mid-week 7, formation of trabeculae was apparent and TE7 expression was also seen in the anterior end of the thymic/parathyroid primordium, where it appeared to be in the luminal spaces (Fig. 5B,B′). However, mesenchymal cells were not evident within the thymic epithelium region itself until mid-week 8 (Fig. 5C,C′). At subsequent stages, TE7+ thymic mesenchyme divided the thymus into discrete lobules, and TE7+ cells were also present throughout the epithelial regions (Fig. 5D-E′) (Haynes et al., 1984). Thus, the timing of onset of migration of mesenchymal cells into the human thymic primordium closely follows the onset of FOXN1 expression (Fig. 2E), consistent with the timing of this event in the mouse (Foster et al., 2008).
Fig. 5.
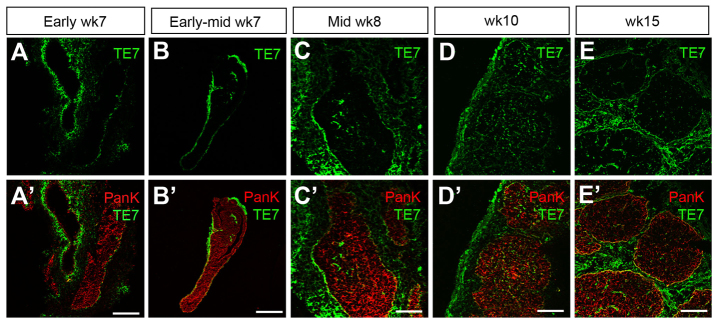
Colonization of the human thymic primordium by mesenchymal cells. (A-E′) Images show staining with TE7 at the developmental stages shown. Anti-pan-Cytokeratin (PANK) reveals the entire thymus primordium. Images are representative of at least two independent analyses. Wk, week. Scale bars: 150 μm in A-B′,D-E′; 75 μm in C,C′.
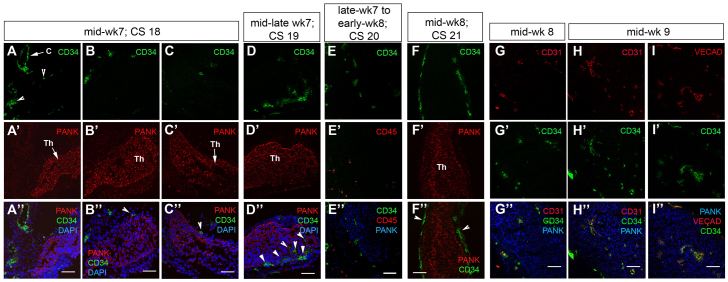
Immigration of vascular progenitors into the mouse thymus is closely linked to that of neural crest-derived cells, and both of these processes are Foxn1 dependent (Mori et al., 2010; Nowell et al., 2011). However, again, the timing of vascular endothelial progenitor entry into the human thymus has not been determined. Our analysis identified the presence of CD34hiCD31+ cells in the connective tissue between the carotid artery and the thymus primordium at mid-week 7 (Fig. 6A-C″); occasional cells at this stage were found within the epithelial component of the primordium (Fig. 6D-D″). By late week 7 to mid-week 8, CD34hi cells were found within the thymic lobes (Fig. 6E-F″) and all co-expressed both CD31 (PECAM1 - Human Gene Nomenclature Database) and VE cadherin (CDH5 - Human Gene Nomenclature Database), identifying them as endothelial progenitors (Fig. 6G-G″). Of note is that the CD34hi cells present within the thymic primordium at late week 7 to early week 8 did not express CD45 (PTPRC - Human Gene Nomenclature Database) (Fig. 6E-E″). Blood vessels per se were present by mid-week 9 (note long blood vessel-like structures at mid-week 9) (Fig. 6H-I″).
Fig. 6.
Colonization of the human thymic primordium by vascular endothelial cells. (A-I″) Images show staining with anti-CD34 at the developmental stages shown. Anti-pan-Cytokeratin (PANK) reveals the entire thymus primordium. Anti-CD45 reveals hematopoietic cells. CD31 (G-I″) and VE cadherin (VEcad; I-I″) staining reveal vascular endothelial cells. DAPI reveals nuclei. White arrowheads in A-F″ indicate vascular endothelial cells. Images are representative of at least two independent analyses. C, carotid artery; Th, thymus; CS, Carnegie stage. Scale bars: 75 μm.
Some variation in timing of colonization with endothelial progenitors was observed: in the thymus primordium from a CS19 fetus (mid to late week 7; 48-51 days old) and a CS20 fetus (late week 7 to early week 8), several CD34hi cells were present within the epithelial compartment, and were localized to one discrete region (Fig. 6D-E″). However, in a CS21 fetus (mid to late week 8; 53-54 days old) CD34hi cells were present in the mesenchymal capsule but were not detected within the epithelial component of the thymus (Fig. 6F-F″). These differences in kinetics of colonization between different fetal thymi are likely to reflect the effects of genetic variation within the human population on fetal thymus development; in this context, it is well established that thymus organogenesis proceeds at different rates in different inbred mouse strains.
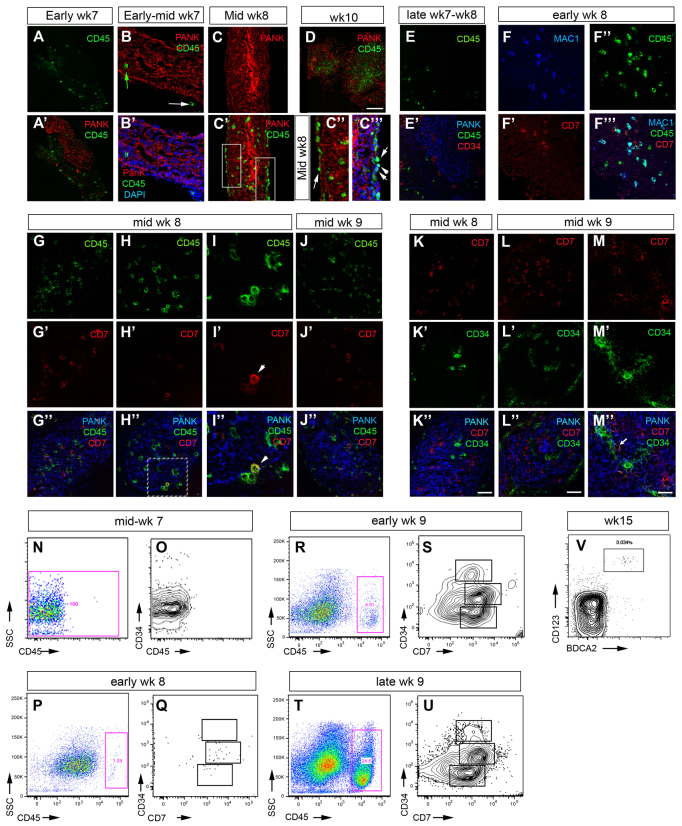
CD45+CD7+ T-lineage progenitors are first present at week 8 and lack high level CD34 expression
Hematopoietic colonization of the human fetal thymus has previously been proposed to occur during early week 8, and the week 7 human primordium was reported as being devoid of hematopoietic cells (Haynes and Heinly, 1995). However, as acknowledged by the authors, this study was compromised by the limited material available (Haynes and Heinly, 1995) and, thus, although the timing at which subsequent stages in T-cell development are first observed has been determined (Res and Spits, 1999), the stage at which the earliest hematopoietic cells colonize the human thymus remains unknown. We therefore sought to determine the kinetics of hematopoietic colonization of the human thymus using markers associated with human hematopoietic progenitor cells, including early T-lineage precursors. Of note is that the earliest T-lineage restricted precursors in human are thought to be bone marrow-derived CD45+CD34hiCD7+ cells based on functional analysis of hematopoietic cells at week 8-9 of fetal development, the earliest time-point examined (Haddad et al., 2006).
We observed CD45+ hematopoietic cells in the perithymic space at early week 7 (Fig. 7A,A′), and by early to mid-week 7 we detected occasional CD45+ cells within the thymic primordium of some embryos (Fig. 7B,B′, green arrow). By early to mid-week 8, CD45+ cells lined the perithymic space and were also distributed throughout the thymic lobes, and by week 10 the numbers of CD45+ cells within the thymus had increased dramatically (Fig. 7C,D). However, no CD34hiCD45+ cells could be detected prior to week 9 (Fig. 7E-M″). Co-staining with additional markers indicated that at mid-week 8 a proportion of the CD45+ cells were Mac1 (IGTAM)+ macrophages (Fig. 7F-F″′). Of note is that comparison of immunohistochemistry and flow cytometric staining data indicated that only cells expressing high levels of CD34 were detected by immunohistochemistry.
Fig. 7.
Colonization of the human thymic primordium by hematopoietic cells. (A-M″) Images show staining of microdissected human thymic primordia with anti-CD45, anti-CD34, anti-CD7 and Mac1 as indicated, at the developmental stages shown. Anti-pan-Cytokeratin (PANK) reveals the entire thymus primordium. Green arrow in B indicates CD45+ cell within the thymic epithelial compartment; white arrows indicate CD45+ cells present in the perithymic mesenchyme. C′,C″ show detail from boxed regions on mid-week 8 image (C′). I-I″ show boxed area from H-H″. Images are representative of at least two independent analyses. Scale bars: 75 μm in K-M″; 150 μm in D. (N-V) Flow cytometric analysis of human thymic primordia at the developmental stages shown. (N-U) Plots show staining with anti-CD45 and anti-CD34 and anti-CD7 after gating on live cells (i.e. against DAPI). Q,S,U show cells after gating on CD45+ cells (P,R,T). Boxes on O,Q,S,U at each age indicate CD34hi, CD34int and CD34lo/neg cells. (N-U) n=1 for each age shown. (V) Plot shows staining with markers for plasmacytoid (CD123+BDCA2+) dendritic cells after gating on CD45+CD19-CD3- cells and is representative of at least two independent analyses. SSC, side scatter; Wk, week. See also supplementary material Table S2.
Surprisingly, given the absence of CD34hiCD45+ cells at this stage, CD45+CD7+ cells were observed within the thymic lobes from early week 8, when they comprised a minor subpopulation of intrathymic CD45+ cells (Fig. 7G-I″; CD45+CD7+Mac1-; CD45+CD7-Mac1+ and CD45+CD7-Mac1- cells were all present at this stage). Consistent with previous reports that CD7 identifies extra-thymic hematopoietic precursor cells with T-lineage potential (Haynes and Heinly, 1995; Haynes et al., 2000), we observed some CD7int cells located in the perithymic space. These cells were sometimes juxtaposed to CD7hi cells that appeared to be actively entering the thymus, suggesting that CD7 upregulation might occur upon migration into the thymic epithelium (Fig. 7H-I″). However, intrathymic CD34hiCD7hi cells were not detected until mid-week 9, when they were observed closely associated with the blood vessels (Fig. 7K-U). Most intra-thymic CD7+ cells at early week 8 were CD34int, consistent with the presence of CD34int cells in the fetal liver (FL) (Fig. 7P,Q). A minor population of CD34lo/negCD7+ cells were also present at week 8, which might represent the CD34negCD7+CD56+ natural killer (NK) cell population previously described in the FL (Phillips et al., 1992; Bárcena et al., 1993). However, the week 8 hematopoietic population contained T-cell precursors, as culture of week 8 thymic lobes microdissected free of any other tissues resulted in a robust expansion of CD45+ thymocytes (supplementary material Fig. S1). Furthermore, expression of c-Kit (KIT - Human Gene Nomenclature Database), IL7R, pre Tα (PTCRA - Human Gene Nomenclature Database), RAG1, CD3δ (CD3D - Human Gene Nomenclature Database), and TCR β and γ (TRB and TRG - Human Gene Nomenclature Database) constant region mRNA was detected in microarray analysis of whole mid-week 8 fetal human thymic lobes (supplementary material Table S2). Collectively, these data pinpoint the stage of human thymus development at which colonization by hematopoietic progenitors occurs, and identify a novel subpopulation of CD34intCD7+ cells that might represent intrathymic T lineage-restricted progenitors.
Subsequent analyses confirmed previous studies indicating the appearance of CD4 and CD8 single positive and γδ TCR+ thymocytes, and CD4+CD25+ T regulatory cells in the human fetal thymus by week 15 (Lobach and Haynes, 1987; Galy et al., 1993; Haynes and Heinly, 1995; Cupedo et al., 2005) (data not shown). Low numbers of conventional dendritic cells (cDCs) were detected at week 15, consistent with a previous report indicating their presence at week 12 (Janossy et al., 1986). CD11c+CD11b+ cDCs increased proportionally by week 16 (not shown), together with a numerical increase in the entire cDC population. Of note is that plasmacytoid dendritic cells were already evident at week 15 (Fig. 7V).
DISCUSSION
The data presented above address the current profound gap in understanding of human thymus development. Collectively, they demonstrate that the principal genetic mechanisms currently known to regulate thymus organogenesis in the mouse are conserved in humans, and that key cellular processes, including the developmental origin of the thymus in the third pharyngeal pouches, are also conserved. They establish that overt differentiation of cortical and medullary TECs occurs from mid-week 8 in the human thymic primordium and closely mirrors the progression seen in mouse in terms of phenotypic and functional marker expression, and that colonization of the human thymic primordium by endothelial and mesenchymal cells occurs from early and mid-week 8, respectively. Finally, they define the timing of onset of hematopoietic cell colonization of the human thymic primordium and reveal, in contrast to expectations from previous studies, that the first colonizing hematopoietic cells that express T lineage affiliated markers are CD45+CD7+CD34int cells, which are present from early week 8. These findings are summarized in supplementary material Table S3.
Conservation of mechanisms of thymus organogenesis between mouse and human
The mouse is the principal model organism used to determine regulatory mechanisms operating to control thymus development and function, and therefore interrogation of its utility for predicting mechanisms that control these processes in the human is essential. The data presented above establish that the spatial and temporal expression pattern of all of the genes tested, which have been demonstrated by genetic analyses to have functional roles in thymus development, are conserved between mouse and human. They therefore validate the mouse as a model for understanding human thymus organogenesis. Of note is that although some variation in expression between mouse and human was observed in the genes analyzed herein, this did not affect expression in the thymus domain; for instance, PAX9 is expressed throughout the pharyngeal pouches in mouse but was detected only in pouches 3 and 4 in human. However, it is of interest that two genes, RHOX4 and PLET1, expression of which in mouse is restricted to the pharyngeal endoderm and the thymus domain of the third pharyngeal pouch, respectively, at this developmental stage, are not expressed in the human pharyngeal pouches or thymic rudiment, indicating that not all gene expression patterns are conserved. In this regard, we note that neither of these genes has been shown to be important for thymus organogenesis by functional studies and, indeed, both are currently of unknown function.
Differentiation of the earliest progenitor cells in the human thymic primordium
The data presented above demonstrate that overt differentiation of cortical and medullary TEC occurs at around mid-week 8. However, the thymic rudiment itself is present from early week 6 and therefore, our data further indicate that the week 6 and week 7 thymic primordia consist mainly of undifferentiated TECs. Thus, cell surface markers expressed by most TECs at these developmental stages will be useful for isolating and characterizing the differentiative potential of early progenitor TECs. Similarly, the expression patterns demonstrated here for CD205 and CLDN4 are consistent with these markers identifying early medullary and cortical sub-lineage-restricted progenitors in the human, as well as mouse, thymus. Collectively, these data pave the way for prospective isolation and functional testing of defined human fetal TEC subpopulations, which will be essential if progress is to be made in defining conditions for in vitro propagation of human TEPCs or for generating these cells from embryonic stem cells for use in cell replacement therapies.
We note that FOXN1, a master regulator of TEC differentiation, is expressed in the human thymic primordia from mid-week 6. In the mouse, high-level Foxn1 expression is initiated at E11.5 and the onset of overt TEC differentiation closely follows this upregulation. The interval between upregulation of FOXN1 and the appearance of differentiating cTECs and mTECs is therefore much longer in human than in mouse. This probably reflects differences in cell cycle; however, it is also possible that other, as yet unknown mechanisms, limiting the time of onset of TEC differentiation are initiated or repressed with different kinetics to FOXN1 in the human.
Kinetics of initial mesenchymal and vascular endothelial cell entry into the human thymus
Our data indicate that entry of vascular endothelial progenitor cells into the human thymus occurs as early to mid-week 8, with migrating cells observed in the connective tissue between the carotid artery and the thymus from early to mid-week 7. They further show that, although formation of trabeculae is observed from mid-week 7, the time of entry of neural crest-derived mesenchymal cells into the epithelial regions of the thymus is mid-week 8. These processes are thus closely linked, as in the mouse. Notably, both events occur after initiation of high-level FOXN1 expression in the human thymus, consistent with the demonstration in mouse that thymic colonization with both mesenchymal and vascular endothelial cells is Foxn1 dependent. However, our data further suggest that entry of vascular endothelial progenitors into the epithelial region of the thymic primordium precedes its colonization with mesenchymal cells, in contrast to the widespread assumption that immigration of vascular endothelial cells follows that of neural crest-derived mesenchyme.
Identity of the first thymus-seeding hematopoietic cells
The data presented in Fig. 7 establish that hematopoietic cell colonization of the human thymus primordium occurs from early to mid-week 7. As in mouse, hematopoietic colonization of the thymus therefore closely follows upregulation of high level FOXN1 expression, consistent with FOXN1-regulation of CCL25 and DLL4, which are required for migration and T lineage commitment of thymus seeding progenitors, respectively (Liu et al., 2006; Hozumi et al., 2008; Koch et al., 2008).
The earliest human thymus seeding T lineage-restricted progenitors have previously been proposed to be CD45+CD34hiCD7+ cells, based on functional analysis of hematopoietic cells in the week 8-9 fetal thymus (Galy et al., 1993; Haynes and Heinly, 1995; Haddad et al., 2006). Our data indicate that the hematopoietic population of the early human fetal thymus is heterogeneous, and that all intrathymic hematopoietic cells are CD34int/- until early to mid-week 9. Indeed, the first colonizing hematopoietic cells appearing at early week 7 are CD45+CD34-CD7-, and some CD45+ at least as early as early week 8 are Mac1+ macrophages. Intrathymic CD45+CD34intCD7+ and CD45+CD34lo/-CD7+ cells are present from early week 8; this was surprising, because the earliest intrathymic T-lineage progenitors were expected to express CD34 (Galy et al., 1993; Haynes and Heinly, 1995; Haddad et al., 2006). Although we cannot exclude the possibility that these cells mature within the thymic primordium to generate CD45+CD34hiCD7+ cells, we note that CD45+CD34hiCD7+ cells first appear within the thymus at mid-week 9 coincident with establishment of blood vessels, and furthermore that cells of this phenotype are initially found closely associated with blood vessels. However, the thymus contains T-lineage progenitors at least as early as week 8, as evidenced by the robust development of thymocytes in fetal thymic organ cultures established at this developmental stage and the intrathymic expression of genes associated with early human thymocyte development. Thus, we favor the idea that the thymus-seeding population changes once the vasculature has been established as the primary route of entry, and comprises CD45+CD34hiCD7+ cells only after this point.
CD45+CD34lo/-CD7+ cells have been described previously in the FL; these are NK cells, and also express CD56 (NCAM1 - Human Gene Nomenclature Database) (Phillips et al., 1992; Bárcena et al., 1993). Thus, the minor CD45+CD34lo/-CD7+ population present at week 8 might also be NK cells. The CD45+CD34intCD7+ population present in the pre-vascular thymus is, however, a novel intrathymic hematopoietic population that is probably T lineage committed based on its expression of CD7. In this regard, it is of interest that CD7hi cells were never detected in the connective tissue associated with the pre-vascular thymus or in the perithymic space, but were sometimes observed interspersed with thymic epithelial cells adjacent to the mesenchymal capsule, suggesting that in the pre-vascular thymus, upregulation of CD7 might occur upon contact with thymic epithelial cells. Of note is that the FL contains a population of CD45+CD34int CD7- cells (Haddad et al., 2006) that might constitute precursors for this population. Based on these data, we therefore suggest that the pre-vascular thymus is initially seeded by CD45+CD34intCD7- cells and that upregulation of CD7 in these cells occurs dynamically as part of the thymus-seeding process. We further suggest that this population is superseded by the previously described T lineage-restricted CD45+CD34hiCD7+ population (Haddad et al., 2006) only following establishment of the thymic vasculature.
Supplementary Material
Acknowledgments
We thank Anne Sanderson, Isobel Morton, Joan Creiger and the staff of the Bruntsfield Suite of the Royal Infirmary of Edinburgh and the staff of the CASA clinic in Leiden for patient recruitment for provision of samples for these studies; Simon Monard for flow cytometry; and K. O’Neill (University of Edinburgh) and Sten Eirik Jacobsen (University of Oxford) for critical reading of the manuscript.
Footnotes
Funding
This work was funded by Leukaemia and Lymphoma Research [C.C.B., A.M.F.]; the Medical Research Council, UK [C.C.B., R.A.A.]; the Wellcome Trust [C.C.B., L.X.M., A.M.F.]; the EU FP7 integrated project EuroSystem [C.C.B., S.R.T.]; and the Netherlands Organisation for Scientific Research [Zon-MW Vidi grant #91710377 to T.C.]. Deposited in PMC for immediate release.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.087320/-/DC1
References
- Anderson G., Jenkinson W. E., Jones T., Parnell S. M., Kinsella F. A., White A. J., Pongrac’z J. E., Rossi S. W., Jenkinson E. J. (2006). Establishment and functioning of intrathymic microenvironments. Immunol. Rev. 209, 10–27 [DOI] [PubMed] [Google Scholar]
- Auerbach R. (1960). Morphogenetic interactions in the development of the mouse thymus gland. Dev. Biol. 2, 271–284 [DOI] [PubMed] [Google Scholar]
- Awong G., LaMotte-Mohs R., Zúñiga-Pflücker J. C. (2010). Key players for T-cell regeneration. Curr. Opin. Hematol. 17, 327–332 [DOI] [PubMed] [Google Scholar]
- Baik S., Jenkinson E. J., Lane P. J., Anderson G., Jenkinson W. E. (2013). Generation of both cortical and Aire(+) medullary thymic epithelial compartments from CD205(+) progenitors. Eur. J. Immunol. 43, 589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini A. (2005). Dissecting contiguous gene defects: TBX1. Curr. Opin. Genet.Dev. 15, 279–284 [DOI] [PubMed] [Google Scholar]
- Bárcena A., Muench M. O., Galy A. H., Cupp J., Roncarolo M. G., Phillips J. H., Spits H. (1993). Phenotypic and functional analysis of T-cell precursors in the human fetal liver and thymus: CD7 expression in the early stages of T- and myeloid-cell development. Blood 82, 3401–3414 [PubMed] [Google Scholar]
- Bennett A. R., Farley A., Blair N. F., Gordon J., Sharp L., Blackburn C. C. (2002). Identification and characterization of thymic epithelial progenitor cells. Immunity 16, 803–814 [DOI] [PubMed] [Google Scholar]
- Blackburn C. C., Manley N. R. (2004). Developing a new paradigm for thymus organogenesis. Nat. Rev. Immunol. 4, 278–289 [DOI] [PubMed] [Google Scholar]
- Blackburn C. C., Augustine C. L., Li R., Harvey R. P., Malin M. A., Boyd R. L., Miller J. F. A. P., Morahan G. (1996). The nu gene acts cell-autonomously and is required for differentiation of thymic epithelial progenitors. Proc. Natl. Acad. Sci. USA 93, 5742–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E., Rufini A., Terrinoni A., Giamboi-Miraglia A., Lena A. M., Mantovani R., Knight R., Melino G. (2007). DeltaNp63 regulates thymic development through enhanced expression of FgfR2 and Jag2. Proc. Natl. Acad. Sci. USA 104, 11999–12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. (1991). Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature 350, 473–479 [DOI] [PubMed] [Google Scholar]
- Cupedo T., Nagasawa M., Weijer K., Blom B., Spits H. (2005). Development and activation of regulatory T cells in the human fetus. Eur. J. Immunol. 35, 383–390 [DOI] [PubMed] [Google Scholar]
- Depreter M. G., Blair N. F., Gaskell T. L., Nowell C. S., Davern K., Pagliocca A., Stenhouse F. H., Farley A. M., Fraser A., Vrana J., et al. (2008). Identification of Plet-1 as a specific marker of early thymic epithelial progenitor cells. Proc. Natl. Acad. Sci. USA 105, 961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley J., Erickson M., Gillard G. O., Farr A. G. (2006). Cervical thymus in the mouse. J. Immunol. 176, 6484–6490 [DOI] [PubMed] [Google Scholar]
- Farr A. G., Anderson S. K. (1985). Epithelial heterogeneity in the murine thymus: fucose-specific lectins bind medullary epithelial cells. J. Immunol. 134, 2971–2977 [PubMed] [Google Scholar]
- Festing M. F., May D., Connors T. A., Lovell D., Sparrow S. (1978). An athymic nude mutation in the rat. Nature 274, 365–366 [DOI] [PubMed] [Google Scholar]
- Flanagan S. P. (1966). ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 8, 295–309 [DOI] [PubMed] [Google Scholar]
- Foster K., Sheridan J., Veiga-Fernandes H., Roderick K., Pachnis V., Adams R., Blackburn C., Kioussis D., Coles M. (2008). Contribution of neural crest-derived cells in the embryonic and adult thymus. J. Immunol. 180, 3183–3189 [DOI] [PubMed] [Google Scholar]
- Foster K. E., Gordon J., Cardenas K., Veiga-Fernandes H., Makinen T., Grigorieva E., Wilkinson D. G., Blackburn C. C., Richie E., Manley N. R., et al. (2010). EphB-ephrin-B2 interactions are required for thymus migration during organogenesis. Proc. Natl. Acad. Sci. USA 107, 13414–13419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Pignata C., Panteleyev A. A., Prowse D. M., Baden H., Weiner L., Gaetaniello L., Ahmad W., Pozzi N., Cserhalmi-Friedman P. B., et al. (1999). Exposing the human nude phenotype. Nature 398, 473–474 [DOI] [PubMed] [Google Scholar]
- Galy A., Verma S., Bárcena A., Spits H. (1993). Precursors of CD3+CD4+CD8+ cells in the human thymus are defined by expression of CD34. Delineation of early events in human thymic development. J. Exp. Med. 178, 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser R. F. (1975). Atlas of Human Embryos. Hagerstown, MD: Harper & Row; [Google Scholar]
- Gordon J., Bennett A. R., Blackburn C. C., Manley N. R. (2001). Gcm2 and Foxn1 mark early parathyroid- and thymus-specific domains in the developing third pharyngeal pouch. Mech. Dev. 103, 141–143 [DOI] [PubMed] [Google Scholar]
- Gordon J., Wilson V. A., Blair N. F., Sheridan J., Farley A., Wilson L., Manley N. R., Blackburn C. C. (2004). Functional evidence for a single endodermal origin for the thymic epithelium. Nat. Immunol. 5, 546–553 [DOI] [PubMed] [Google Scholar]
- Gordon J., Patel S. R., Mishina Y., Manley N. R. (2010). Evidence for an early role for BMP4 signaling in thymus and parathyroid morphogenesis. Dev. Biol. 339, 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith A. V., Cardenas K., Carter C., Gordon J., Iberg A., Engleka K., Epstein J. A., Manley N. R., Richie E. R. (2009). Increased thymus- and decreased parathyroid-fated organ domains in Splotch mutant embryos. Dev. Biol. 327, 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R., Guimiot F., Six E., Jourquin F., Setterblad N., Kahn E., Yagello M., Schiffer C., Andre-Schmutz I., Cavazzana-Calvo M., et al. (2006). Dynamics of thymus-colonizing cells during human development. Immunity 24, 217–230 [DOI] [PubMed] [Google Scholar]
- Hamazaki Y., Fujita H., Kobayashi T., Choi Y., Scott H. S., Matsumoto M., Minato N. (2007). Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat. Immunol. 8, 304–311 [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Heinly C. S. (1995). Early human T cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J. Exp. Med. 181, 1445–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Scearce R. M., Lobach D. F., Hensley L. L. (1984). Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J. Exp. Med. 159, 1149–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Markert M. L., Sempowski G. D., Patel D. D., Hale L. P. (2000). The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 18, 529–560 [DOI] [PubMed] [Google Scholar]
- Hozumi K., Mailhos C., Negishi N., Hirano K., Yahata T., Ando K., Zuklys S., Holländer G. A., Shima D. T., Habu S. (2008). Delta-like 4 is indispensable in thymic environment specific for T cell development. J. Exp. Med. 205, 2507–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry R. A., Bolstad B. M., Collin F., Cope L. M., Hobbs B., Speed T. P. (2003). Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Bofill M., Poulter L. W., Rawlings E., Burford G. D., Navarrete C., Ziegler A., Kelemen E. (1986). Separate ontogeny of two macrophage-like accessory cell populations in the human fetus. J. Immunol. 136, 4354–4361 [PubMed] [Google Scholar]
- Jenkinson W. E., Jenkinson E. J., Anderson G. (2003). Differential requirement for mesenchyme in the proliferation and maturation of thymic epithelial progenitors. J. Exp. Med. 198, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug D. B., Carter C., Crouch E., Roop D., Conti C. J., Richie E. R. (1998). Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc. Natl. Acad. Sci. USA 95, 11822–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U., Fiorini E., Benedito R., Besseyrias V., Schuster-Gossler K., Pierres M., Manley N. R., Duarte A., Macdonald H. R., Radtke F. (2008). Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J. Exp. Med. 205, 2515–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan J., Killeen N. (2004). CCR7 directs the migration of thymocytes into the thymic medulla. J. Immunol. 172, 3999–4007 [DOI] [PubMed] [Google Scholar]
- Lampert I. A., Ritter M. A. (1988). The origin of the diverse epithelial cells of the thymus: is there a common stem cell? In The Microenvironment of the Human Thymus (Thymus Update) (ed. Kendall D., Ritter M. A.), pp. 5–25 New York, NY: Harwood Academic Press; [Google Scholar]
- Le Douarin N. M., Jotereau F. V. (1975). Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J. Exp. Med. 142, 17–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. M., Mei R., Di X., Ryder T. B., Hubbell E., Dee S., Webster T. A., Harrington C. A., Ho M. H., Baid J., et al. (2002). Analysis of high density expression microarrays with signed-rank call algorithms. Bioinformatics 18, 1593–1599 [DOI] [PubMed] [Google Scholar]
- Liu C., Saito F., Liu Z., Lei Y., Uehara S., Love P., Lipp M., Kondo S., Manley N., Takahama Y. (2006). Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood 108, 2531–2539 [DOI] [PubMed] [Google Scholar]
- Liu Z., Farley A., Chen L., Kirby B. J., Kovacs C. S., Blackburn C. C., Manley N. R. (2010). Thymus-associated parathyroid hormone has two cellular origins with distinct endocrine and immunological functions. PLoS Genet. 6, e1001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobach D. F., Haynes B. F. (1987). Ontogeny of the human thumus during fetal development. J. Clin. Immunol. 7, 81–97 [DOI] [PubMed] [Google Scholar]
- Lynch H. E., Goldberg G. L., Chidgey A., Van den Brink M. R., Boyd R., Sempowski G. D. (2009). Thymic involution and immune reconstitution. Trends Immunol. 30, 366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K. M., Pelton R. W., Hogan B. L. (1990). Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development 109, 833–844 [DOI] [PubMed] [Google Scholar]
- Manley N. R., Capecchi M. R. (1995). The role of Hoxa-3 in mouse thymus and thyroid development. Development 121, 1989–2003 [DOI] [PubMed] [Google Scholar]
- Manley N. R., Condie B. G. (2010). Transcriptional regulation of thymus organogenesis and thymic epithelial cell differentiation. Prog. Mol. Biol. Transl. Sci. 92, 103–120 [DOI] [PubMed] [Google Scholar]
- Mathis D., Benoist C. (2009). Aire. Annu. Rev. Immunol. 27, 287–312 [DOI] [PubMed] [Google Scholar]
- Mills A. A., Zheng B., Wang X. J., Vogel H., Roop D. R., Bradley A. (1999). p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 [DOI] [PubMed] [Google Scholar]
- Mori K., Itoi M., Tsukamoto N., Amagai T. (2010). Foxn1 is essential for vascularization of the murine thymus anlage. Cell. Immunol. 260, 66–69 [DOI] [PubMed] [Google Scholar]
- Morris L., Gordon J., Blackburn C. C. (2006). Identification of a tandem duplicated array in the Rhox alpha locus on mouse chromosome X. Mamm. Genome 17, 178–187 [DOI] [PubMed] [Google Scholar]
- Nehls M., Pfeifer D., Schorpp M., Hedrich H., Boehm T. (1994). New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 372, 103–107 [DOI] [PubMed] [Google Scholar]
- Nehls M., Kyewski B., Messerle M., Waldschütz R., Schüddekopf K., Smith A. J. H., Boehm T. (1996). Two genetically separable steps in the differentiation of thymic epithelium. Science 272, 886–889 [DOI] [PubMed] [Google Scholar]
- Neubüser A., Koseki H., Balling R. (1995). Characterization and developmental expression of Pax9, a paired-box-containing gene related to Pax1. Dev. Biol. 170, 701–716 [DOI] [PubMed] [Google Scholar]
- Norris E. H. (1938). The morphogenesis and histogenesis of the thymus gland in man: in which the origin of the Hassall’s corpuscle of the human thymus is discovered. Contrib. Embryol. 27, 193–207 [Google Scholar]
- Nowell C. S., Bredenkamp N., Tetelin S., Jin X., Tischner C., Vaidya H., Sheridan J. M., Stenhouse F. H., Heussen R., Smith A. J. H., et al. (2011). Foxn1 regulates lineage progression in cortical and medullary tyhymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genetics 7, e1002348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly R., Muller F., Streeter G. L. (1987). Developmental Stages inHuman Embryos: Including a Revision of Streeter’s Horizons and a Survey of the Carnegie Collection. Washington, DC: Carnegie Institution of Washington; [Google Scholar]
- Patel S. R., Gordon J., Mahbub F., Blackburn C. C., Manley N. R. (2006). Bmp4 and Noggin expression during early thymus and parathyroid organogenesis. Gene Expr. Patterns 6, 794–799 [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Hori T., Nagler A., Bhat N., Spits H., Lanier L. L. (1992). Ontogeny of human natural killer (NK) cells: fetal NK cells mediate cytolytic function and express cytoplasmic CD3 epsilon,delta proteins. J. Exp. Med. 175, 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Res P., Spits H. (1999). Developmental stages in the human thymus. Semin. Immunol. 11, 39–46 [DOI] [PubMed] [Google Scholar]
- Rossi S. W., Jenkinson W. E., Anderson G., Jenkinson E. J. (2006). Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature 441, 988–991 [DOI] [PubMed] [Google Scholar]
- Rouse R. V., Bolin L. M., Bender J. R., Kyewski B. A. (1988). Monoclonal antibodies reactive with subsets of mouse and human thymic epithelial cells. J. Histochem. Cytochem. 36, 1511–1517 [DOI] [PubMed] [Google Scholar]
- Senoo M., Pinto F., Crum C. P., McKeon F. (2007). p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129, 523–536 [DOI] [PubMed] [Google Scholar]
- Shakib S., Desanti G. E., Jenkinson W. E., Parnell S. M., Jenkinson E. J., Anderson G. (2009). Checkpoints in the development of thymic cortical epithelial cells. J. Immunol. 182, 130–137 [DOI] [PubMed] [Google Scholar]
- Sheridan J. M., Taoudi S., Medvinsky A., Blackburn C. C. (2009). A novel method for the generation of reaggregated organotypic cultures that permits juxtaposition of defined cell populations. Genesis 47, 346–351 [DOI] [PubMed] [Google Scholar]
- Terszowski G., Müller S. M., Bleul C. C., Blum C., Schirmbeck R., Reimann J., Pasquier L. D., Amagai T., Boehm T., Rodewald H. R. (2006). Evidence for a functional second thymus in mice. Science 312, 284–287 [DOI] [PubMed] [Google Scholar]
- Tsai P. T., Lee R. A., Wu H. (2003). BMP4 acts upstream of FGF in modulating thymic stroma and regulating thymopoiesis. Blood 102, 3947–3953 [DOI] [PubMed] [Google Scholar]
- Van Dyke J. H. (1941). On the origin of accessory thymus tissue, thymus IV: the occurrence in man. Anat. Rec. 79, 179–209 [Google Scholar]
- Villaseñor J., Benoist C., Mathis D. (2005). AIRE and APECED: molecular insights into an autoimmune disease. Immunol. Rev. 204, 156–164 [DOI] [PubMed] [Google Scholar]
- Wallin J., Eibel H., Neubüser A., Wilting J., Koseki H., Balling R. (1996). Pax1 is expressed during development of the thymus epithelium and is required for normal T-cell maturation. Development 122, 23–30 [DOI] [PubMed] [Google Scholar]
- Wils E. J., Cornelissen J. J. (2005). Thymopoiesis following allogeneic stem cell transplantation: new possibilities for improvement. Blood Rev. 19, 89–98 [DOI] [PubMed] [Google Scholar]
- Xu H., Cerrato F., Baldini A. (2005). Timed mutation and cell-fate mapping reveal reiterated roles of Tbx1 during embryogenesis, and a crucial function during segmentation of the pharyngeal system via regulation of endoderm expansion. Development 132, 4387–4395 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.