Abstract
Dipeptidyl peptidase-4 (DPP-4) is a membrane-associated peptidase, also known as CD26. DPP-4 has widespread organ distribution throughout the body and exerts pleiotropic effects via its peptidase activity. A representative target peptide is glucagon-like peptide-1, and inactivation of glucagon-like peptide-1 results in the development of glucose intolerance/diabetes mellitus and hepatic steatosis. In addition to its peptidase activity, DPP-4 is known to be associated with immune stimulation, binding to and degradation of extracellular matrix, resistance to anti-cancer agents, and lipid accumulation. The liver expresses DPP-4 to a high degree, and recent accumulating data suggest that DPP-4 is involved in the development of various chronic liver diseases such as hepatitis C virus infection, non-alcoholic fatty liver disease, and hepatocellular carcinoma. Furthermore, DPP-4 occurs in hepatic stem cells and plays a crucial role in hepatic regeneration. In this review, we described the tissue distribution and various biological effects of DPP-4. Then, we discussed the impact of DPP-4 in chronic liver disease and the possible therapeutic effects of a DPP-4 inhibitor.
Keywords: Incretin, Viral hepatitis, Insulin resistance, Steatohepatitis, Cancer, Sitagliptin, Vildagliptin, Alogliptin, Teneligliptin, Linagliptin
Core tip: Dipeptidyl peptidase-4 (DPP-4) is a membrane-associated peptidase, also known as CD26. DPP-4 has widespread organ distribution throughout the body and exerts pleiotropic effects via its peptidase activity. In this review, we described the tissue distribution and various biological effects of DPP-4. Then, we discussed the impact of DPP-4 in chronic liver disease and the possible therapeutic effects of a DPP-4 inhibitor.
DISTRIBUTION OF DIPEPTIDYL PEPTIDASE-4
Dipeptidyl peptidase-4 (DPP-4, enzyme code number 3.4.14.5) is a 110 kDa membrane-associated peptidase, which was originally identified in 1966 as a dipeptide naphthylamidase that hydrolyzed glycyl-prolyl-beta-naphthylamide[1]. DPP-4, also known as CD26[2-4], is expressed on the apical surfaces of epithelial and acinar cells and in endothelial cells, fibroblasts, and lymphocytes[5-8]. DPP-4 also exists as a soluble circulating form in plasma[9].
DPP-4 occurs in all organs including the small intestine, biliary tract, exocrine pancreas, spleen, and brain in both rodents and humans[10-12]. This widespread organ distribution indicates that DPP-4 has pleiotropic biological activities. The liver is one of the organs that highly expresses DPP-4[8]. In the healthy human liver, intense staining for DPP-4 is seen in hepatic acinar zones 2 and 3, but not in zone 1. Similar lobular heterogeneity is also seen in the expression of cytochrome p450, gamma-glutamyltranspeptidase (GGT), and glutamine synthetase[13-15]. This heterogeneous lobular distribution suggests that DPP-4 may be involved in the regulation of hepatic metabolism.
BIOLOGICAL ACTIVITIES OF DPP-4
Peptidase
DPP-4 is an enzyme that cleaves N-terminal dipeptides of proline or alanine-containing peptides including incretin, appetite-suppressing hormones (neuropeptide), and chemokines as listed in Table 1. Representative targets are glucagon-like peptide (GLP)-1, GLP-2, peptide YY, chemokine ligand 12/stromal-derived factor-1 (CXCL12/SDF-1), and substance P. Thus, DPP-4 exerts pleiotropic effects on glucose metabolism, gut motility, appetite regulation, inflammation, immune system function, and pain regulation though its peptidase activity (Figure 1).
Table 1.
Target peptide of dipeptidyl peptidase-4
| Category | Peptide | Ref. |
| Glucose metabolism | GLP-1 | [94-97] |
| GIP | [97-99] | |
| Glucagon | [97,100,101] | |
| PACAP-38 | [102-104] | |
| Gut motility | GLP-2 | [97,105,106] |
| VIP | [107-109] | |
| NPY | [109,110] | |
| GRP | [103] | |
| Peptide histidine methionine | [94,102] | |
| Appetite regulation | Peptide YY | [107,111] |
| Chemokine | CCL5/RANTES | [112-114] |
| CCL11/eotaxin | [115,116] | |
| CCL22/MDC | [112] | |
| CXCL9/MIg | [117-119] | |
| CXCL10/IP10 | [114,119,120] | |
| CXCL11/I-TAC | [121,122] | |
| CXCL12/SDF-1 | [93,113] | |
| Growth | IGF-1 | [123,124] |
| GHRH | [94,125] | |
| Reproduction | Prolactin | [126-128] |
| hCGα | [129] | |
| LHα | [123] | |
| Vasodilation | CGRP | [107,110] |
| Bradykinin | [107,108] | |
| Pain regulation | Enkephalin | [130] |
| Endomorphins | [109,130-132] | |
| Substance P | [109,110,133,134] | |
| Homeostasis | Thyotropin α | [123,135] |
| Inhibition of endothelial cell growth | Vasostatin-I | [136] |
GLP: Glucagon-like peptide; GIP: Glucose-dependent insulinotoropic peptide; PACAP-38: Pituitary adenylate cyclase-activating polypeptide-38; VIP: Vasoactive intestinal peptide; NPY: Neuro-peptide Y; GRP: Gastrin-releasing peptide; CCL: Chemokine (C-C motif) ligand; RANTES: Regulated upon activation; MDC: Macrophage-derived chemokine; CXCL: Chemokine (C-X-C motif) ligand; MIg: Monokine induced by gamma interferon; IP-10: Protein 10 from interferon (γ)-induced cell line; I-TAC: Interferon-inducible T-cell α chemoattractant; SDF-1: Stromal-derived factor-1; IGF-1: Insulin-like growth factor-1; GHRH: Growth hormone releasing hormone; hCGα: Human chorionic gonadotropin α subunit; LHα: Leutinizing hormone α chain; CGRP: Calcitonin-related peptide.
Figure 1.
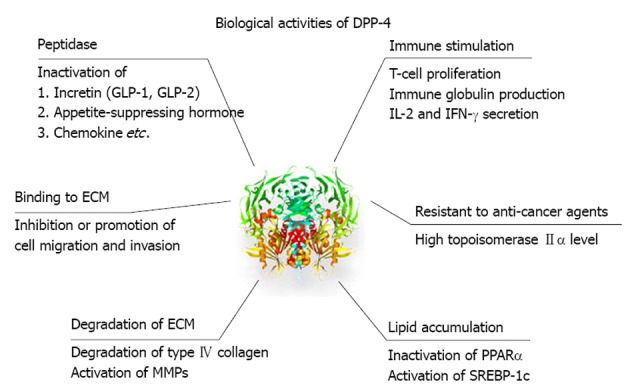
Pleiotropic effects of dipeptidyl peptidase-4. Dipeptidyl peptidase-4 (DPP-4) exerts various effects on metabolism and chemokine through peptidase activity. In addition, DPP-4 is involved in immune stimulation, binding to and degradation of extracellular matrix, and resistant to anti-cancer agents. DPP-4 also directly affects lipid accumulation. GLP: Glucagon-like peptide; ECM: Extracellular matrix; MMPs: Metalloproteinases; IL: Interleukin; IFN: Interferon; PPAR: Peroxisome proliferator-activated receptor; SREBP: Sterol regulatory element binding protein.
Immune stimulation
DPP-4 expression is downregulated in the resting state of T-cells; however, expression is upregulated by antigenic or mitogenic stimulation via an interleukin-12-dependent mechanism[16-18]. DPP-4 activates intracellular molecules including p56lck, phospholipase C-γ, and mitogen-activated protein kinase (MAPK)[11]. This activation enhances T-cell maturation and migration, cytokine secretion, antibody production, immunoglobulin isotype switching of B cells, and activation of cytotoxic T cells[19,20]. In addition, soluble CD26 binds to mannose 6-phosphate receptor and is taken up by CD14 positive monocytes, increasing their antigen presenting activity and T-cell proliferation[11,21,22].
Binding to extracellular matrix
DPP-4 has the ability to bind to the Binding to extracellular matrix (ECM), preferentially to the collagens I and III, and fibronectin[23,24], and is involved in hepatocyte-extracellular matrix interactions[23,25]. The putative collagen binding site of DPP-4 is located at the C-terminal portion of the molecule, separate from the peptidase catalytic site[26].
In a mouse xenograft model, treatment with anti-DPP-4 monoclonal antibody inhibits the growth of renal cell carcinoma via disruption of binding to the extracellular matrix[27]. On the other hand, over expression of DPP-4 induces apoptosis of prostate cancer cells by inhibition of cell migration and invasion through down-regulation of MAPK-extracellular signal-regulated kinase-1/2 activation[28]. Thus, the role of DPP-4 may differ in different types of cancer.
Degradation of ECM
DPP-4 binds to adenosine deaminase and activates plasminogen-2, leading to an increase in plasmin levels[11]. The increased plasmin degrades type IV collagen, fibronectin, laminin, and proteoglycan, and activates matrix metalloproteinases. These changes result in the degradation of the ECM[29,30].
Resistance to anti-cancer agents
DPP-4 is thought to be associated with sensitivity to anti-cancer agents in hematologic malignancies. DPP-4 has been linked to high topoisomerase IIα levels, resistance to anti-cancer agents, and the malignant potential of T-cell lymphoma[11,21,22]. Treatment with anti-DPP-4 monoclonal antibody causes dephosphorylation of both MAPK and integrin β1 in T-cell lymphoma, leading increased sensitivity to anti-cancer agents and greater survival[31]. Similar beneficial effects of anti-DPP-4 monoclonal antibody have been reported in renal cell carcinoma[27] and malignant mesothelioma tumors[32].
Lipid accumulation
DPP-4 affects lipid metabolism by the inactivation of peptides such as GLP-1, neuropeptide Y, and peptide YY. In addition, DPP-4 is known to directly affect lipid metabolism. Knock-out of the gene encoding DPP-4 directly causes activation of the peroxisome proliferator-activated receptor-α pathway and inactivation of the sterol regulatory element binding protein-1 pathway[33], thereby increasing lipid oxidation, reducing lipogenesis, and resulting in the prevention of high-fat diet-induced hepatic steatosis.
CHANGES IN DPP-4 IN PATIENTS WITH LIVER DISEASE
Serum level of DPP-4 is elevated in patients with liver cirrhosis[34,35] and up-regulation of hepatic DPP-4 expression is thought to be responsible for this elevation[36]. Here, we describe the effects of DPP-4 according to each pathophysiology.
Hepatitis C virus infection
Patients with hepatitis C virus (HCV) infection show increased serum DPP-4 expression in hepatocytes[37,38]. Lymphocyte subset analysis has also shown that CD8+ T-cells, which express DPP-4, are present in the portal and periportal areas in patients with HCV infection[39]. Since HCV infects CD8+ T-cells[39-41], HCV-infected T-cells may be responsible for the increased serum DPP-4 activity in patients with HCV infection.
In addition, glucose intolerance with insulin resistance is a feature of HCV infection and is associated with disease progression as well as prognosis[42-52]. Besides hepatic inflammation and steatosis, HCV itself is involved in the development of insulin resistance through the impairment of insulin receptor substrate-1/2[52-54]. Moreover, HCV infection is known to be associated with increased DPP-4 expression in the ileum, liver, and serum[38]. Transfection with cDNA encoding part of the HCV non-structural genome region 4B/5A induces expression of DPP-4 in hepatocyte cell lines[55]. Furthermore, eradication of HCV by interferon therapy results in a decrease in serum DPP-4 levels[56-61] and administration of sitagliptin significantly improves HCV-related glucose intolerance[62]. Since there is no significant association between serum DPP-4 activity and severity of liver disease in patients with HCV infection[38], HCV infection may directly upregulate DPP-4 activity, leading to impairment in glucose metabolism.
Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome and the most common cause of chronic liver disease[63-66]. Although various factors are responsible for the development of NAFLD, a high glucose load is known to induce DPP-4 expression in HepG2 cells[67] and hepatic DPP-4 mRNA expression level in the livers is significantly higher in patients with NAFLD, compared to healthy subjects[67]. In fact, serum DPP-4 activity and hepatic expression of DPP-4 are correlated with hepatic steatosis and NAFLD grading[68]. Moreover, DPP-4 deficient rats show lower levels of hepatic proinflammatory and profibrotic cytokines and reduced hepatic steatosis compared to wild type rats. These favorable changes in lipid metabolism are independent of glucose metabolism[69]. Similar to these results from animal experiments, in patients with NAFLD, DPP-4 activity in serum and liver specimens correlate with markers of liver damage such as serum GGT and alanine aminotransferase levels, but do not correlate with fasting blood glucose levels and glycosylated hemoglobin (HbA1c) values[68,70]. Thus, hepatic DPP-4 expression in NAFLD may be directly associated with hepatic lipogenesis and liver injury.
Recently, DPP-4 inhibitor has been reported to improve hepatic steatosis in mice and humans[71]. We also experienced a case of refractory NAFLD that was successfully treated with sitagliptin, a DDP-4 inhibitor[72]. Moreover, it is reported that sitagliptin ameliorates liver enzymes and hepatocyte ballooning in patients with nonalcoholic steatohepatitis[73]. Taken together, these findings may indicate that DPP-4 inhibitors ameliorate hepatic injury and glucose impairment in patients with NAFLD.
Hepatocellular carcinoma
Increased DPP-4 expression is seen in various malignant tumors, such as breast cancer[74,75], brain glioma[76], malignant mesothelioma[77], and squamous cell laryngeal carcinoma[78]. In hepatocellular carcinoma (HCC), increased DPP-4 expression is also seen in liver specimens and serum from both rats[79] and humans[80].
Inhibition of DPP-4 in human hepatoma cells is reported to suppress tyrosine kinase, leading to anti-apoptotic effects[81]. However, Yamamoto et al[82] recently reported a case in which dramatic regression of HCC was seen after four weeks’ treatment with DPP-4 inhibitor in a patient with HCV-related chronic hepatitis. Although it is unclear whether DPP-4 inhibitor is directly involved in the regression of HCC, marked invasion of CD8+ T-cells was seen around the HCC tissue[82], suggesting that the DPP-4 inhibitor may have improved immune response, which has been impaired by chronic HCV infection[38]. Although exogenous insulin or sulfonylurea treatment increases the risk of HCC[83], treatment with DPP-4 inhibitor does not show any tumor promoting effects in mice[84]. Thus, a DPP-4 inhibitor may safely exert beneficial effects on HCV-related HCC through modulation of immunity.
Stem cell and hepatic regeneration
Increased hepatic DPP-4 expression has been reported to occur in the cirrhotic liver[85,86]. Although the impact of increased DPP-4 expression remains unclear, Lee et al[87] recently reported that human liver stem cells express DPP-4, but not CD34 and CD45, which are hematopoietic stem cell and endothelial progenitor cell markers. Thus, DPP-4 is a specific marker of adult hepatic stem and progenitor cells, indicating that DPP-4 may be involved in the regeneration in chronically inflamed liver.
CXCL12/SDF-1 causes hematopoietic stem cell (HSC) homing and is an important chemokine for hepatic regeneration[88-91]. CXCL12/SDF-1 is a target peptide of DPP-4 and the inhibition of the cell-surface DPP-4 activity of HSC/hematopoietic progenitor cell populations increases their CXCL12/SDF-1 directed chemotaxis, homing, and engraftment[92]. Therefore, inhibition of DPP-4 may be an effective therapy for increasing the efficacy and success of HSC/hematopoietic progenitor cell transplantation[92]. DPP-4 inhibition also increases number of progenitor cells, and stabilization of endogenous CXCL12/SDF-1 by DPP-4 inhibition is achievable and may be a promising strategy to intensify sequestration of regenerative stem cells[93].
CONCLUSION
In this review, we described the tissue distribution and biological effects of DPP-4. Then, we discussed the impact of DPP-4 in chronic liver disease and the possible effects of a DPP-4 inhibitor. DPP-4 plays crucial roles in the development of various chronic liver diseases, and DPP-4 inhibition seems to have beneficial effects in chronic liver diseases. However, DPP-4 inhibitors also modulate the immune system, and further studies will be focused on the effects of long-term administration of a DPP-4 inhibitor on infection and carcinogenesis.
Footnotes
P- Reviewers Tao YX, Kristine F S- Editor Huang XZ L- Editor A E- Editor Li JY
References
- 1.Hopsu-Havu VK, Glenner GG. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-beta-naphthylamide. Histochemie. 1966;7:197–201. doi: 10.1007/BF00577838. [DOI] [PubMed] [Google Scholar]
- 2.Misumi Y, Hayashi Y, Arakawa F, Ikehara Y. Molecular cloning and sequence analysis of human dipeptidyl peptidase IV, a serine proteinase on the cell surface. Biochim Biophys Acta. 1992;1131:333–336. doi: 10.1016/0167-4781(92)90036-y. [DOI] [PubMed] [Google Scholar]
- 3.Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 4.Morrison ME, Vijayasaradhi S, Engelstein D, Albino AP, Houghton AN. A marker for neoplastic progression of human melanocytes is a cell surface ectopeptidase. J Exp Med. 1993;177:1135–1143. doi: 10.1084/jem.177.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 6.Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologia. 2006;49:253–260. doi: 10.1007/s00125-005-0107-1. [DOI] [PubMed] [Google Scholar]
- 7.McCaughan GW, Gorrell MD, Bishop GA, Abbott CA, Shackel NA, McGuinness PH, Levy MT, Sharland AF, Bowen DG, Yu D, et al. Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev. 2000;174:172–191. doi: 10.1034/j.1600-0528.2002.017420.x. [DOI] [PubMed] [Google Scholar]
- 8.Mentzel S, Dijkman HB, Van Son JP, Koene RA, Assmann KJ. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem. 1996;44:445–461. doi: 10.1177/44.5.8627002. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi M, Fukuyama K, Epstein WL. Soluble dipeptidyl peptidase IV from terminal differentiated rat epidermal cells: purification and its activity on synthetic and natural peptides. Arch Biochem Biophys. 1988;266:369–376. doi: 10.1016/0003-9861(88)90268-8. [DOI] [PubMed] [Google Scholar]
- 10.Heike M, Möbius U, Knuth A, Meuer S, Meyer zum Büschenfelde KH. Tissue distribution of the T cell activation antigen Ta1. Serological, immunohistochemical and biochemical investigations. Clin Exp Immunol. 1988;74:431–434. [PMC free article] [PubMed] [Google Scholar]
- 11.Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol. 2001;54:249–264. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 12.Dinjens WN, ten Kate J, Wijnen JT, van der Linden EP, Beek CJ, Lenders MH, Khan PM, Bosman FT. Distribution of adenosine deaminase-complexing protein in murine tissues. J Biol Chem. 1989;264:19215–19220. [PubMed] [Google Scholar]
- 13.Moody DE, Taylor LA, Smuckler EA. Immunofluorescent determination of the lobular distribution of a constitutive form of hepatic microsomal cytochrome P-450. Hepatology. 1985;5:440–451. doi: 10.1002/hep.1840050317. [DOI] [PubMed] [Google Scholar]
- 14.Irie M, Suzuki N, Sohda T, Anan A, Iwata K, Takeyama Y, Watanabe H, Fischer P, Scherberich JE, Sakisaka S. Hepatic expression of gamma-glutamyltranspeptidase in the human liver of patients with alcoholic liver disease. Hepatol Res. 2007;37:966–973. doi: 10.1111/j.1872-034X.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 15.Häussinger D, Gerok W. Hepatocyte heterogeneity in glutamate uptake by isolated perfused rat liver. Eur J Biochem. 1983;136:421–425. doi: 10.1111/j.1432-1033.1983.tb07759.x. [DOI] [PubMed] [Google Scholar]
- 16.Fox DA, Hussey RE, Fitzgerald KA, Acuto O, Poole C, Palley L, Daley JF, Schlossman SF, Reinherz EL. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984;133:1250–1256. [PubMed] [Google Scholar]
- 17.Hegen M, Niedobitek G, Klein CE, Stein H, Fleischer B. The T cell triggering molecule Tp103 is associated with dipeptidyl aminopeptidase IV activity. J Immunol. 1990;144:2908–2914. [PubMed] [Google Scholar]
- 18.Yamabe T, Takakura K, Sugie K, Kitaoka Y, Takeda S, Okubo Y, Teshigawara K, Yodoi J, Hori T. Induction of the 2B9 antigen/dipeptidyl peptidase IV/CD26 on human natural killer cells by IL-2, IL-12 or IL-15. Immunology. 1997;91:151–158. doi: 10.1046/j.1365-2567.1997.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohnuma K, Takahashi N, Yamochi T, Hosono O, Dang NH, Morimoto C. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front Biosci. 2008;13:2299–2310. doi: 10.2741/2844. [DOI] [PubMed] [Google Scholar]
- 20.Matteucci E, Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16:2943–2951. doi: 10.2174/092986709788803114. [DOI] [PubMed] [Google Scholar]
- 21.Ohnuma K, Yamochi T, Uchiyama M, Nishibashi K, Yoshikawa N, Shimizu N, Iwata S, Tanaka H, Dang NH, Morimoto C. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc Natl Acad Sci USA. 2004;101:14186–14191. doi: 10.1073/pnas.0405266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohnuma K, Uchiyama M, Yamochi T, Nishibashi K, Hosono O, Takahashi N, Kina S, Tanaka H, Lin X, Dang NH, et al. Caveolin-1 triggers T-cell activation via CD26 in association with CARMA1. J Biol Chem. 2007;282:10117–10131. doi: 10.1074/jbc.M609157200. [DOI] [PubMed] [Google Scholar]
- 23.Piazza GA, Callanan HM, Mowery J, Hixson DC. Evidence for a role of dipeptidyl peptidase IV in fibronectin-mediated interactions of hepatocytes with extracellular matrix. Biochem J. 1989;262:327–334. doi: 10.1042/bj2620327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng HC, Abdel-Ghany M, Pauli BU. A novel consensus motif in fibronectin mediates dipeptidyl peptidase IV adhesion and metastasis. J Biol Chem. 2003;278:24600–24607. doi: 10.1074/jbc.M303424200. [DOI] [PubMed] [Google Scholar]
- 25.Walborg EF, Tsuchida S, Weeden DS, Thomas MW, Barrick A, McEntire KD, Allison JP, Hixson DC. Identification of dipeptidyl peptidase IV as a protein shared by the plasma membrane of hepatocytes and liver biomatrix. Exp Cell Res. 1985;158:509–518. doi: 10.1016/0014-4827(85)90474-4. [DOI] [PubMed] [Google Scholar]
- 26.Löster K, Zeilinger K, Schuppan D, Reutter W. The cysteine-rich region of dipeptidyl peptidase IV (CD 26) is the collagen-binding site. Biochem Biophys Res Commun. 1995;217:341–348. doi: 10.1006/bbrc.1995.2782. [DOI] [PubMed] [Google Scholar]
- 27.Inamoto T, Yamochi T, Ohnuma K, Iwata S, Kina S, Inamoto S, Tachibana M, Katsuoka Y, Dang NH, Morimoto C. Anti-CD26 monoclonal antibody-mediated G1-S arrest of human renal clear cell carcinoma Caki-2 is associated with retinoblastoma substrate dephosphorylation, cyclin-dependent kinase 2 reduction, p27(kip1) enhancement, and disruption of binding to the extracellular matrix. Clin Cancer Res. 2006;12:3470–3477. doi: 10.1158/1078-0432.CCR-06-0361. [DOI] [PubMed] [Google Scholar]
- 28.Wesley UV, McGroarty M, Homoyouni A. Dipeptidyl peptidase inhibits malignant phenotype of prostate cancer cells by blocking basic fibroblast growth factor signaling pathway. Cancer Res. 2005;65:1325–1334. doi: 10.1158/0008-5472.CAN-04-1852. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Gronow M, Hershfield MS, Arredondo-Vega FX, Pizzo SV. Cell surface adenosine deaminase binds and stimulates plasminogen activation on 1-LN human prostate cancer cells. J Biol Chem. 2004;279:20993–20998. doi: 10.1074/jbc.M401023200. [DOI] [PubMed] [Google Scholar]
- 30.Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci (Lond) 2005;108:277–292. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 31.Sato T, Yamochi T, Yamochi T, Aytac U, Ohnuma K, McKee KS, Morimoto C, Dang NH. CD26 regulates p38 mitogen-activated protein kinase-dependent phosphorylation of integrin beta1, adhesion to extracellular matrix, and tumorigenicity of T-anaplastic large cell lymphoma Karpas 299. Cancer Res. 2005;65:6950–6956. doi: 10.1158/0008-5472.CAN-05-0647. [DOI] [PubMed] [Google Scholar]
- 32.Inamoto T, Yamada T, Ohnuma K, Kina S, Takahashi N, Yamochi T, Inamoto S, Katsuoka Y, Hosono O, Tanaka H, et al. Humanized anti-CD26 monoclonal antibody as a treatment for malignant mesothelioma tumors. Clin Cancer Res. 2007;13:4191–4200. doi: 10.1158/1078-0432.CCR-07-0110. [DOI] [PubMed] [Google Scholar]
- 33.Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, Liu F, Woods J, Zycband E, Moller DE, et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:6825–6830. doi: 10.1073/pnas.0631828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eggstein S, Kreisel W, Gerok W, Eggstein M. [Dipeptidyl aminopeptidase IV in hospitalized patients and in galactosamine hepatitis of the rat: Activity and lectin affinity chromatography in serum and hepatic plasma membranes] J Clin Chem Clin Biochem. 1989;27:547–554. [PubMed] [Google Scholar]
- 35.Nilius R, Stuhec K, Dietrich R. Changes of dipeptidylpeptidase IV as a membrane marker of lymphocytes in acute and chronic liver diseases--biochemical and cytochemical investigations. Physiol Res. 1991;40:95–102. [PubMed] [Google Scholar]
- 36.Matsumoto Y, Bishop GA, McCaughan GW. Altered zonal expression of the CD26 antigen (dipeptidyl peptidase IV) in human cirrhotic liver. Hepatology. 1992;15:1048–1053. doi: 10.1002/hep.1840150613. [DOI] [PubMed] [Google Scholar]
- 37.Grüngreiff K, Hebell T, Gutensohn K, Reinhold A, Reinhold D. Plasma concentrations of zinc, copper, interleukin-6 and interferon-γ, and plasma dipeptidyl peptidase IV activity in chronic hepatitis C. Mol Med Report. 2009;2:63–68. doi: 10.3892/mmr_00000062. [DOI] [PubMed] [Google Scholar]
- 38.Itou M, Kawaguchi T, Taniguchi E, Sumie S, Oriishi T, Mitsuyama K, Tsuruta O, Ueno T, Sata M. Altered expression of glucagon-like peptide-1 and dipeptidyl peptidase IV in patients with HCV-related glucose intolerance. J Gastroenterol Hepatol. 2008;23:244–251. doi: 10.1111/j.1440-1746.2007.05183.x. [DOI] [PubMed] [Google Scholar]
- 39.Bonacini M, Govindarajan S, Kohla M, Lai MM, Lindsay KL. Intrahepatic lymphocyte phenotypes in hepatitis C virus infection: a comparison between cirrhotic and non-cirrhotic livers. Minerva Gastroenterol Dietol. 2007;53:1–7. [PubMed] [Google Scholar]
- 40.Dimitropoulou D, Karakantza M, Tsamandas AC, Mouzaki A, Theodorou G, Gogos CA. T-lymphocyte subsets in peripheral blood and liver tissue of patients with chronic hepatitis B and C. In Vivo. 2011;25:833–840. [PubMed] [Google Scholar]
- 41.Rahman W, Huang P, Belov L, Chrisp JS, Christopherson RI, Stapelberg PM, Warner FJ, George J, Bowen DG, Strasser SI, et al. Analysis of human liver disease using a cluster of differentiation (CD) antibody microarray. Liver Int. 2012;32:1527–1534. doi: 10.1111/j.1478-3231.2012.02854.x. [DOI] [PubMed] [Google Scholar]
- 42.Sakata M, Kawahara A, Kawaguchi T, Akiba J, Taira T, Taniguchi E, Abe M, Koga H, Kage M, Sata M. Decreased expression of insulin and increased expression of pancreatic transcription factor PDX-1 in islets in patients with liver cirrhosis: a comparative investigation using human autopsy specimens. J Gastroenterol. 2013;48:277–285. doi: 10.1007/s00535-012-0633-9. [DOI] [PubMed] [Google Scholar]
- 43.Miyajima I, Kawaguchi T, Fukami A, Nagao Y, Adachi H, Sasaki S, Imaizumi T, Sata M. Chronic HCV infection was associated with severe insulin resistance and mild atherosclerosis: a population-based study in an HCV hyperendemic area. J Gastroenterol. 2013;48:93–100. doi: 10.1007/s00535-012-0610-3. [DOI] [PubMed] [Google Scholar]
- 44.Eslam M, Kawaguchi T, Del Campo JA, Sata M, Khattab MA, Romero-Gomez M. Use of HOMA-IR in hepatitis C. J Viral Hepat. 2011;18:675–684. doi: 10.1111/j.1365-2893.2011.01474.x. [DOI] [PubMed] [Google Scholar]
- 45.Eslam M, Aparcero R, Kawaguchi T, Del Campo JA, Sata M, Khattab MA, Romero-Gomez M. Meta-analysis: insulin resistance and sustained virological response in hepatitis C. Aliment Pharmacol Ther. 2011;34:297–305. doi: 10.1111/j.1365-2036.2011.04716.x. [DOI] [PubMed] [Google Scholar]
- 46.Fukushima N, Kuromatsu R, Arinaga-Hino T, Ando E, Takata A, Sumie S, Nakano M, Kawaguchi T, Ide T, Torimura T, et al. Adipocytokine involvement in hepatocellular carcinoma after sustained response to interferon for chronic hepatitis C. Hepatol Res. 2010;40:911–922. doi: 10.1111/j.1872-034X.2010.00699.x. [DOI] [PubMed] [Google Scholar]
- 47.Kawaguchi T, Taniguchi E, Itou M, Sumie S, Yamagishi S, Sata M. The pathogenesis, complications and therapeutic strategy for hepatitis C virus-associated insulin resistance in the era of anti-viral treatment. Rev Recent Clin Trials. 2010;5:147–157. doi: 10.2174/157488710792007257. [DOI] [PubMed] [Google Scholar]
- 48.Kawaguchi T, Sata M. Importance of hepatitis C virus-associated insulin resistance: therapeutic strategies for insulin sensitization. World J Gastroenterol. 2010;16:1943–1952. doi: 10.3748/wjg.v16.i16.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sumie S, Kawaguchi T, Komuta M, Kuromatsu R, Itano S, Okuda K, Taniguchi E, Ando E, Takata A, Fukushima N, et al. Significance of glucose intolerance and SHIP2 expression in hepatocellular carcinoma patients with HCV infection. Oncol Rep. 2007;18:545–552. [PubMed] [Google Scholar]
- 50.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–576. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 51.Kawaguchi T, Nagao Y, Tanaka K, Ide T, Harada M, Kumashiro R, Sata M. Causal relationship between hepatitis C virus core and the development of type 2 diabetes mellitus in a hepatitis C virus hyperendemic area: a pilot study. Int J Mol Med. 2005;16:109–114. [PubMed] [Google Scholar]
- 52.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 54.Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164–1171. doi: 10.1002/hep.21634. [DOI] [PubMed] [Google Scholar]
- 55.Harada T, Kim DW, Sagawa K, Suzuki T, Takahashi K, Saito I, Matsuura Y, Miyamura T. Characterization of an established human hepatoma cell line constitutively expressing non-structural proteins of hepatitis C virus by transfection of viral cDNA. J Gen Virol. 1995;76(Pt 5):1215–1221. doi: 10.1099/0022-1317-76-5-1215. [DOI] [PubMed] [Google Scholar]
- 56.Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Meltzer H. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6:475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Macquillan GC, Keane NM, Flexman J, Jeffrey GP, French MA, Brochier J, Price P. Immunological markers predicting outcome in patients with hepatitis C treated with interferon-alpha and ribavirin. Immunol Cell Biol. 2002;80:391–397. doi: 10.1046/j.1440-1711.2002.01102.x. [DOI] [PubMed] [Google Scholar]
- 58.Stone SF, Lee S, Keane NM, Price P, French MA. Association of increased hepatitis C virus (HCV)-specific IgG and soluble CD26 dipeptidyl peptidase IV enzyme activity with hepatotoxicity after highly active antiretroviral therapy in human immunodeficiency virus-HCV-coinfected patients. J Infect Dis. 2002;186:1498–1502. doi: 10.1086/344892. [DOI] [PubMed] [Google Scholar]
- 59.Andrieu T, Thibault V, Malet I, Laporte J, Bauvois B, Agut H, Cahour A. Similar increased serum dipeptidyl peptidase IV activity in chronic hepatitis C and other viral infections. J Clin Virol. 2003;27:59–68. doi: 10.1016/s1386-6532(02)00128-2. [DOI] [PubMed] [Google Scholar]
- 60.Maes M, Bonaccorso S. Lower activities of serum peptidases predict higher depressive and anxiety levels following interferon-alpha-based immunotherapy in patients with hepatitis C. Acta Psychiatr Scand. 2004;109:126–131. doi: 10.1046/j.0001-690x.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 61.Yang SS, Fu LS, Chang CS, Yeh HZ, Chen GH, Kao JH. Changes of soluble CD26 and CD30 levels correlate with response to interferon plus ribavirin therapy in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:1789–1793. doi: 10.1111/j.1440-1746.2006.04677.x. [DOI] [PubMed] [Google Scholar]
- 62.Arase Y, Suzuki F, Kobayashi M, Suzuki Y, Kawamura Y, Matsumoto N, Akuta N, Imai N, Kobayashi M, Sezaki H, et al. Efficacy and safety in sitagliptin therapy for diabetes complicated by chronic liver disease caused by hepatitis C virus. Hepatol Res. 2011;41:524–529. doi: 10.1111/j.1872-034X.2011.00798.x. [DOI] [PubMed] [Google Scholar]
- 63.Iwasaki T, Yoneda M, Inamori M, Shirakawa J, Higurashi T, Maeda S, Terauchi Y, Nakajima A. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology. 2011;58:2103–2105. doi: 10.5754/hge11263. [DOI] [PubMed] [Google Scholar]
- 64.Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, Eguchi Y, Suzuki Y, Imai S, Kanemasa K, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257–268. doi: 10.1007/s00535-010-0305-6. [DOI] [PubMed] [Google Scholar]
- 65.Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 66.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyazaki M, Kato M, Tanaka K, Tanaka M, Kohjima M, Nakamura K, Enjoji M, Nakamuta M, Kotoh K, Takayanagi R. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5:729–733. doi: 10.3892/mmr.2011.707. [DOI] [PubMed] [Google Scholar]
- 68.Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, Hascelik G, Asan E, Hamaloglu E, Tatar G. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol. 2007;6:242–250. [PubMed] [Google Scholar]
- 69.Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, Barzilai N, Oren R, Fishman S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214–1223. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 70.Firneisz G, Varga T, Lengyel G, Fehér J, Ghyczy D, Wichmann B, Selmeci L, Tulassay Z, Rácz K, Somogyi A. Serum dipeptidyl peptidase-4 activity in insulin resistant patients with non-alcoholic fatty liver disease: a novel liver disease biomarker. PLoS One. 2010;5:e12226. doi: 10.1371/journal.pone.0012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, et al. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011;60:1246–1257. doi: 10.2337/db10-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Itou M, Kawaguchi T, Taniguchi E, Oriishi T, Sata M. Dipeptidyl Peptidase IV Inhibitor Improves Insulin Resistance and Steatosis in a Refractory Nonalcoholic Fatty Liver Disease Patient: A Case Report. Case Rep Gastroenterol. 2012;6:538–544. doi: 10.1159/000341510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg. 2012;75:240–244. [PubMed] [Google Scholar]
- 74.Leccia F, Nardone A, Corvigno S, Vecchio LD, De Placido S, Salvatore F, Veneziani BM. Cytometric and biochemical characterization of human breast cancer cells reveals heterogeneous myoepithelial phenotypes. Cytometry A. 2012;81:960–972. doi: 10.1002/cyto.a.22095. [DOI] [PubMed] [Google Scholar]
- 75.Wilson CH, Abbott CA. Expression profiling of dipeptidyl peptidase 8 and 9 in breast and ovarian carcinoma cell lines. Int J Oncol. 2012;41:919–932. doi: 10.3892/ijo.2012.1522. [DOI] [PubMed] [Google Scholar]
- 76.Mareš V, Stremeňová J, Lisá V, Kozáková H, Marek J, Syrůček M, Šoula O, Šedo A. Compartment- and malignance-dependent up-regulation of γ-glutamyltranspeptidase and dipetidylpeptidase-IV activity in human brain gliomas. Histol Histopathol. 2012;27:931–940. doi: 10.14670/HH-27.931. [DOI] [PubMed] [Google Scholar]
- 77.Aoe K, Amatya VJ, Fujimoto N, Ohnuma K, Hosono O, Hiraki A, Fujii M, Yamada T, Dang NH, Takeshima Y, et al. CD26 overexpression is associated with prolonged survival and enhanced chemosensitivity in malignant pleural mesothelioma. Clin Cancer Res. 2012;18:1447–1456. doi: 10.1158/1078-0432.CCR-11-1990. [DOI] [PubMed] [Google Scholar]
- 78.Starska K, Głowacka E, Kulig A, Lewy-Trenda I, Bryś M, Lewkowicz P. The role of tumor cells in the modification of T lymphocytes activity--the expression of the early CD69+, CD71+ and the late CD25+, CD26+, HLA/DR+ activation markers on T CD4+ and CD8+ cells in squamous cell laryngeal carcinoma. Part I. Folia Histochem Cytobiol. 2011;49:579–592. [PubMed] [Google Scholar]
- 79.McCaughan GW, Siah CL, Abbott C, Wickson J, Ballesteros M, Bishop GA. Dipeptidyl peptidase IV is down-regulated in rat hepatoma cells at the mRNA level. J Gastroenterol Hepatol. 1993;8:142–145. doi: 10.1111/j.1440-1746.1993.tb01505.x. [DOI] [PubMed] [Google Scholar]
- 80.Stecca BA, Nardo B, Chieco P, Mazziotti A, Bolondi L, Cavallari A. Aberrant dipeptidyl peptidase IV (DPP IV/CD26) expression in human hepatocellular carcinoma. J Hepatol. 1997;27:337–345. doi: 10.1016/s0168-8278(97)80180-8. [DOI] [PubMed] [Google Scholar]
- 81.Gaetaniello L, Fiore M, de Filippo S, Pozzi N, Tamasi S, Pignata C. Occupancy of dipeptidyl peptidase IV activates an associated tyrosine kinase and triggers an apoptotic signal in human hepatocarcinoma cells. Hepatology. 1998;27:934–942. doi: 10.1002/hep.510270407. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto S, Tokuhara T, Nishikawa M, Nishizawa S, Nishioka T, Nozawa A, Takahashi A, Watanabe Y, Wada R, Wakasa K, et al. Spontaneous regression of hepatocellular carcinoma after improving diabetes mellitus: possiblly responsible for immune system. Kanzo. 2012;53:164–167. [Google Scholar]
- 83.Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479–486. doi: 10.1111/j.1478-3231.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 84.Kissow H, Hartmann B, Holst JJ, Viby NE, Hansen LS, Rosenkilde MM, Hare KJ, Poulsen SS. Glucagon-like peptide-1 (GLP-1) receptor agonism or DPP-4 inhibition does not accelerate neoplasia in carcinogen treated mice. Regul Pept. 2012;179:91–100. doi: 10.1016/j.regpep.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Müller E, Rettig WJ, Gorrell MD. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology. 1999;29:1768–1778. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- 86.Reynard MP, Turner D, Navarrete CV. Allele frequencies of polymorphisms of the tumour necrosis factor-alpha, interleukin-10, interferon-gamma and interleukin-2 genes in a North European Caucasoid group from the UK. Eur J Immunogenet. 2000;27:241–249. doi: 10.1046/j.1365-2370.2000.00227.x. [DOI] [PubMed] [Google Scholar]
- 87.Lee JH, Park HJ, Kim YA, Lee DH, Noh JK, Kwon CH, Jung SM, Lee SK. The phenotypic characteristic of liver-derived stem cells from adult human deceased donor liver. Transplant Proc. 2012;44:1110–1112. doi: 10.1016/j.transproceed.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 88.Mavier P, Martin N, Couchie D, Préaux AM, Laperche Y, Zafrani ES. Expression of stromal cell-derived factor-1 and of its receptor CXCR4 in liver regeneration from oval cells in rat. Am J Pathol. 2004;165:1969–1977. doi: 10.1016/S0002-9440(10)63248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng D, Oh SH, Jung Y, Petersen BE. Oval cell response in 2-acetylaminofluorene/partial hepatectomy rat is attenuated by short interfering RNA targeted to stromal cell-derived factor-1. Am J Pathol. 2006;169:2066–2074. doi: 10.2353/ajpath.2006.060211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55:1215–1226. doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsuchiya A, Imai M, Kamimura H, Takamura M, Yamagiwa S, Sugiyama T, Nomoto M, Heike T, Nagasawa T, Nakahata T, et al. Increased susceptibility to severe chronic liver damage in CXCR4 conditional knock-out mice. Dig Dis Sci. 2012;57:2892–2900. doi: 10.1007/s10620-012-2239-8. [DOI] [PubMed] [Google Scholar]
- 92.Campbell TB, Broxmeyer HE. CD26 inhibition and hematopoiesis: a novel approach to enhance transplantation. Front Biosci. 2008;13:1795–1805. doi: 10.2741/2800. [DOI] [PubMed] [Google Scholar]
- 93.Jungraithmayr W, De Meester I, Matheeussen V, Baerts L, Arni S, Weder W. CD26/DPP-4 inhibition recruits regenerative stem cells via stromal cell-derived factor-1 and beneficially influences ischaemia-reperfusion injury in mouse lung transplantation. Eur J Cardiothorac Surg. 2012;41:1166–1173. doi: 10.1093/ejcts/ezr180. [DOI] [PubMed] [Google Scholar]
- 94.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 95.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 96.Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–5363. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 97.Brubaker PL, Drucker DJ. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Receptors Channels. 2002;8:179–188. [PubMed] [Google Scholar]
- 98.Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab. 2000;85:3575–3581. doi: 10.1210/jcem.85.10.6855. [DOI] [PubMed] [Google Scholar]
- 99.Deacon CF, Danielsen P, Klarskov L, Olesen M, Holst JJ. Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs. Diabetes. 2001;50:1588–1597. doi: 10.2337/diabetes.50.7.1588. [DOI] [PubMed] [Google Scholar]
- 100.Ballantyne GH. Peptide YY(1-36) and peptide YY(3-36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 101.Ballantyne GH. Peptide YY(1-36) and peptide YY(3-36): Part II. Changes after gastrointestinal surgery and bariatric surgery. Obes Surg. 2006;16:795–803. doi: 10.1381/096089206777346619. [DOI] [PubMed] [Google Scholar]
- 102.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38) J Biol Chem. 2003;278:22418–22423. doi: 10.1074/jbc.M212355200. [DOI] [PubMed] [Google Scholar]
- 103.Ahrén B, Hughes TE. Inhibition of dipeptidyl peptidase-4 augments insulin secretion in response to exogenously administered glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pituitary adenylate cyclase-activating polypeptide, and gastrin-releasing peptide in mice. Endocrinology. 2005;146:2055–2059. doi: 10.1210/en.2004-1174. [DOI] [PubMed] [Google Scholar]
- 104.Green BD, Irwin N, Flatt PR. Pituitary adenylate cyclase-activating peptide (PACAP): assessment of dipeptidyl peptidase IV degradation, insulin-releasing activity and antidiabetic potential. Peptides. 2006;27:1349–1358. doi: 10.1016/j.peptides.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 105.Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol. 1997;272:E1050–E1058. doi: 10.1152/ajpendo.1997.272.6.E1050. [DOI] [PubMed] [Google Scholar]
- 106.Walsh NA, Yusta B, DaCambra MP, Anini Y, Drucker DJ, Brubaker PL. Glucagon-like peptide-2 receptor activation in the rat intestinal mucosa. Endocrinology. 2003;144:4385–4392. doi: 10.1210/en.2003-0309. [DOI] [PubMed] [Google Scholar]
- 107.Byrd JB, Touzin K, Sile S, Gainer JV, Yu C, Nadeau J, Adam A, Brown NJ. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. 2008;51:141–147. doi: 10.1161/HYPERTENSIONAHA.107.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lambeir AM, Durinx C, Proost P, Van Damme J, Scharpé S, De Meester I. Kinetic study of the processing by dipeptidyl-peptidase IV/CD26 of neuropeptides involved in pancreatic insulin secretion. FEBS Lett. 2001;507:327–330. doi: 10.1016/s0014-5793(01)02982-9. [DOI] [PubMed] [Google Scholar]
- 109.Mentlein R, Roos T. Proteases involved in the metabolism of angiotensin II, bradykinin, calcitonin gene-related peptide (CGRP), and neuropeptide Y by vascular smooth muscle cells. Peptides. 1996;17:709–720. doi: 10.1016/0196-9781(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 110.Hernanz A, Medina S, de Miguel E, Martín-Mola E. Effect of calcitonin gene-related peptide, neuropeptide Y, substance P, and vasoactive intestinal peptide on interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha production by peripheral whole blood cells from rheumatoid arthritis and osteoarthritis patients. Regul Pept. 2003;115:19–24. doi: 10.1016/s0167-0115(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 111.Reinehr T, Roth CL, Enriori PJ, Masur K. Changes of dipeptidyl peptidase IV (DPP-IV) in obese children with weight loss: relationships to peptide YY, pancreatic peptide, and insulin sensitivity. J Pediatr Endocrinol Metab. 2010;23:101–108. doi: 10.1515/jpem.2010.23.1-2.101. [DOI] [PubMed] [Google Scholar]
- 112.Lun SW, Wong CK, Ko FW, Hui DS, Lam CW. Increased expression of plasma and CD4+ T lymphocyte costimulatory molecule CD26 in adult patients with allergic asthma. J Clin Immunol. 2007;27:430–437. doi: 10.1007/s10875-007-9093-z. [DOI] [PubMed] [Google Scholar]
- 113.Liu Z, Christensson M, Forslöw A, De Meester I, Sundqvist KG. A CD26-controlled cell surface cascade for regulation of T cell motility and chemokine signals. J Immunol. 2009;183:3616–3624. doi: 10.4049/jimmunol.0804336. [DOI] [PubMed] [Google Scholar]
- 114.Rai AK, Thakur CP, Kumar P, Mitra DK. Impaired expression of CD26 compromises T-cell recruitment in human visceral leishmaniasis. Eur J Immunol. 2012;42:2782–2791. doi: 10.1002/eji.201141912. [DOI] [PubMed] [Google Scholar]
- 115.Struyf S, Proost P, Schols D, De Clercq E, Opdenakker G, Lenaerts JP, Detheux M, Parmentier M, De Meester I, Scharpé S, et al. CD26/dipeptidyl-peptidase IV down-regulates the eosinophil chemotactic potency, but not the anti-HIV activity of human eotaxin by affecting its interaction with CC chemokine receptor 3. J Immunol. 1999;162:4903–4909. [PubMed] [Google Scholar]
- 116.Forssmann U, Stoetzer C, Stephan M, Kruschinski C, Skripuletz T, Schade J, Schmiedl A, Pabst R, Wagner L, Hoffmann T, et al. Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxin-mediated recruitment of eosinophils in vivo. J Immunol. 2008;181:1120–1127. doi: 10.4049/jimmunol.181.2.1120. [DOI] [PubMed] [Google Scholar]
- 117.Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, Scharpé S, Van Damme J, De Meester I. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276:29839–29845. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- 118.Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med. 2005;11:35–42. doi: 10.1097/01.mcp.0000144502.50149.e0. [DOI] [PubMed] [Google Scholar]
- 119.Wong PT, Wong CK, Tam LS, Li EK, Chen DP, Lam CW. Decreased expression of T lymphocyte co-stimulatory molecule CD26 on invariant natural killer T cells in systemic lupus erythematosus. Immunol Invest. 2009;38:350–364. doi: 10.1080/08820130902770003. [DOI] [PubMed] [Google Scholar]
- 120.Crane M, Oliver B, Matthews G, Avihingsanon A, Ubolyam S, Markovska V, Chang JJ, Dore GJ, Price P, Visvanathan K, et al. Immunopathogenesis of hepatic flare in HIV/hepatitis B virus (HBV)-coinfected individuals after the initiation of HBV-active antiretroviral therapy. J Infect Dis. 2009;199:974–981. doi: 10.1086/597276. [DOI] [PubMed] [Google Scholar]
- 121.Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G, Detheux M, Parmentier M, Durinx C, Lambeir AM, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98:3554–3561. doi: 10.1182/blood.v98.13.3554. [DOI] [PubMed] [Google Scholar]
- 122.Ludwig A, Schiemann F, Mentlein R, Lindner B, Brandt E. Dipeptidyl peptidase IV (CD26) on T cells cleaves the CXC chemokine CXCL11 (I-TAC) and abolishes the stimulating but not the desensitizing potential of the chemokine. J Leukoc Biol. 2002;72:183–191. [PubMed] [Google Scholar]
- 123.Mentlein R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 124.Liu X, Murali SG, Holst JJ, Ney DM. Enteral nutrients potentiate the intestinotrophic action of glucagon-like peptide-2 in association with increased insulin-like growth factor-I responses in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1794–R1802. doi: 10.1152/ajpregu.90616.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Faidley TD, Leiting B, Pryor KD, Lyons K, Hickey GJ, Thompson DR. Inhibition of dipeptidyl-peptidase IV does not increase circulating IGF-1 concentrations in growing pigs. Exp Biol Med (Maywood) 2006;231:1373–1378. doi: 10.1177/153537020623100811. [DOI] [PubMed] [Google Scholar]
- 126.Nausch I, Mentlein R, Heymann E. The degradation of bioactive peptides and proteins by dipeptidyl peptidase IV from human placenta. Biol Chem Hoppe Seyler. 1990;371:1113–1118. doi: 10.1515/bchm3.1990.371.2.1113. [DOI] [PubMed] [Google Scholar]
- 127.Ohta N, Takahashi T, Mori T, Park MK, Kawashima S, Takahashi K, Kobayashi H. Hormonal modulation of prolyl endopeptidase and dipeptidyl peptidase IV activities in the mouse uterus and ovary. Acta Endocrinol (Copenh) 1992;127:262–266. doi: 10.1530/acta.0.1270262. [DOI] [PubMed] [Google Scholar]
- 128.Rovenský J, Buc M, Lojda Z, Ruzicková M, Blazícková S, Rauová L, Mistina T, Vigas M, Lackovic V. Effect of domperidone-induced hyperprolactinemia on selected immune parameters in healthy women. Arch Immunol Ther Exp (Warsz) 1995;43:221–227. [PubMed] [Google Scholar]
- 129.Fujiwara H, Fukuoka M, Yasuda K, Ueda M, Imai K, Goto Y, Suginami H, Kanzaki H, Maeda M, Mori T. Cytokines stimulate dipeptidyl peptidase-IV expression on human luteinizing granulosa cells. J Clin Endocrinol Metab. 1994;79:1007–1011. doi: 10.1210/jcem.79.4.7962267. [DOI] [PubMed] [Google Scholar]
- 130.Sakurada C, Sakurada S, Hayashi T, Katsuyama S, Tan-No K, Sakurada T. Degradation of endomorphin-2 at the supraspinal level in mice is initiated by dipeptidyl peptidase IV: an in vitro and in vivo study. Biochem Pharmacol. 2003;66:653–661. doi: 10.1016/s0006-2952(03)00391-5. [DOI] [PubMed] [Google Scholar]
- 131.Rónai AZ, Király K, Szebeni A, Szemenyei E, Prohászka Z, Darula Z, Tóth G, Till I, Szalay B, Kató E, et al. Immunoreactive endomorphin 2 is generated extracellularly in rat isolated L4,5 dorsal root ganglia by DPP-IV. Regul Pept. 2009;157:1–2. doi: 10.1016/j.regpep.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 132.Király K, Szalay B, Szalai J, Barna I, Gyires K, Verbeken M, Rónai AZ. Intrathecally injected Ile-Pro-Ile, an inhibitor of membrane ectoenzyme dipeptidyl peptidase IV, is antihyperalgesic in rats by switching the enzyme from hydrolase to synthase functional mode to generate endomorphin 2. Eur J Pharmacol. 2009;620:21–26. doi: 10.1016/j.ejphar.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 133.Guieu R, Fenouillet E, Devaux C, Fajloun Z, Carrega L, Sabatier JM, Sauze N, Marguet D. CD26 modulates nociception in mice via its dipeptidyl-peptidase IV activity. Behav Brain Res. 2006;166:230–235. doi: 10.1016/j.bbr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 134.Busek P, Stremenová J, Krepela E, Sedo A. Modulation of substance P signaling by dipeptidyl peptidase-IV enzymatic activity in human glioma cell lines. Physiol Res. 2008;57:443–449. doi: 10.33549/physiolres.931231. [DOI] [PubMed] [Google Scholar]
- 135.Tian L, Gao J, Hao J, Zhang Y, Yi H, O’Brien TD, Sorenson R, Luo J, Guo Z. Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology. 2010;151:3049–3060. doi: 10.1210/en.2010-0068. [DOI] [PubMed] [Google Scholar]
- 136.Zhang XY, De Meester I, Lambeir AM, Dillen L, Van Dongen W, Esmans EL, Haemers A, Scharpé S, Claeys M. Study of the enzymatic degradation of vasostatin I and II and their precursor chromogranin A by dipeptidyl peptidase IV using high-performance liquid chromatography/electrospray mass spectrometry. J Mass Spectrom. 1999;34:255–263. doi: 10.1002/(SICI)1096-9888(199904)34:4<255::AID-JMS752>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]


