Abstract
AIM: To explore the relationship of patient comfort and experience to commonly used performance indicators for colonoscopy.
METHODS: All colonoscopies performed in our four endoscopy centres are recorded in two reporting systems that log key performance indicators. From 2008 to 2011, all procedures performed by qualified endoscopists were evaluated; procedures performed by trainees were excluded. The following variables were measured: Caecal intubation rate (CIR), nurse-reported comfort levels (NRCL) on a scale from 1 to 5, polyp detection rate (PDR), patient experience of the procedure (worse than expected, as expected, better than expected), and use of sedation and analgesia. Pearson’s correlation coefficient was used to identify relationships between performance indicators.
RESULTS: A total of 17027 colonoscopies were performed by 23 independent endoscopists between 2008 and 2011. Caecal intubation rate varied from 79.0% to 97.8%, with 18 out of 23 endoscopists achieving a CIR of > 90%. The percentage of patients experiencing significant discomfort during their procedure (defined as NRCL of 4 or 5) ranged from 3.9% to 19.2% with an average of 7.7%. CIR was negatively correlated with NRCL-45 (r = -0.61, P < 0.005), and with poor patient experience (r = -0.54, P < 0.01). The average dose of midazolam (mean 1.9 mg, with a range of 1.1 to 3.5 mg) given by the endoscopist was negatively correlated with CIR (r = -0.59, P < 0.01). CIR was positively correlated with PDR (r = 0.44, P < 0.05), and with the numbers of procedures performed by the endoscopists (r = 0.64, P < 0.01).
CONCLUSION: The best colonoscopists have a higher CIR, use less sedation, cause less discomfort and find more polyps. Measuring patient comfort is valuable in monitoring performance.
Keywords: Endoscopy, Colonoscopy, Quality, Comfort, Performance
INTRODUCTION
Colonoscopy is a very common procedure performed to investigate colonic symptoms and screen for cancer and polyps[1]. It has always been known that colonoscopy can cause harm and even death, but poor quality colonoscopy has only been linked to other important outcomes in the last decade. Back-to-back colonoscopies identified important missed lesions[2], fast withdrawal times were associated with lower adenoma detection rates[3,4], and low adenoma detection rates are associated with higher rates of missed cancer[5]. Several studies have shown that colonoscopy misses, and fails to “protect” individuals from, cancer[6-10]. Thus there has been increasing attention on the quality of colonoscopy[11,12], especially in the context of colorectal cancer screening where there is potential for causing harm to otherwise healthy people.
In order to assess quality, the British Society of Gastroenterology (BSG) has defined a set of indicators and auditable outcomes for colonoscopy[13]. Important key performance indicators are an unadjusted caecal intubation rate (CIR) of > 90% and an adenoma detection rate of > 10%. CIR is globally recognised as the main measure of competence in colonoscopy in a non-screening setting and is one of the key measures used in a colorectal cancer screening. It is an absolute requirement for total colonoscopy, and poor completion rates may be one reason why colonoscopy does not prevent cancer in the right colon[14-16]. However, there are several factors that can influence the CIR and thus the performance of an endoscopist[17].
A possible consequence of having CIR as a prime indicator of quality is that individuals with poor technique may push harder and persist for longer to achieve the standard. This could lead to more pain and the administration of more sedation. Clearly this could cause unnecessary harm to patients, including more perforations and sedation related complications[18].
To prevent this eventuality the BSG proposed that other key performance indicators should be sedation and comfort[13]. Standards were set for sedation, particularly for older patients, but there is no standard for comfort so it was designated an essential “auditable outcome”: a standard that should be measured, reviewed and acted upon, but not one for which an absolute performance level could be defined.
Various studies have addressed patient pain or discomfort during colonoscopy, and identified predictive factors of pain[19-22]. However, none have explored the use of sedation and patient comfort as measures of performance.
This study aims to analyse the different factors affecting an individual’s performance in diagnostic colonoscopy and to explore the use of patient comfort scores as performance indicators for colonoscopy.
MATERIALS AND METHODS
All colonoscopies performed in the four endoscopy units in one healthcare organisation are recorded on two electronic endoscopy reporting systems (SQL scope and Unisoft), which log the key performance indicators defined by the BSG: CIR; polyp detection rate (PDR) (adenomatous and hyperplastic); and sedation (invariably opiates and midazolam). Colonoscopies performed by all independently practicing endoscopists during the four year period of 2008 to 2011 were included in the analysis. Throughout the United Kingdom (and in this study) an unadjusted CIR is used: the rate is not adjusted at all, even for obstructions and poor bowel preparation.
Comfort is assessed using nurse-reported comfort levels (NRCL) on a 5-point scale, which is shown in Table 1. The attending endoscopy nurses assess the comfort of the patient during the procedure without discussing it with the endoscopist, and record it immediately. For this study, significant discomfort was defined as a NRCL of either level 4 or 5 (NRCL-45).
Table 1.
Five-point scale of nurse-reported comfort levels
| Nurse-reported comfort levels | Descriptors |
| No discomfort | Talking/comfortable throughout |
| Minimal discomfort | 1 or 2 episodes of mild discomfort with no distress |
| Mild discomfort | More than 2 episodes of discomfort without distress |
| Moderate discomfort | Significant discomfort experienced several times with some distress |
| Severe discomfort | Frequent discomfort with significant distress |
The patient experience (PE) is captured by the recovery nurse before the patient leaves the unit. Patients are asked whether their experience was: better than expected, as expected, or worse than expected. Both the comfort scores and the PE are recorded on the hospital administration system. The colonoscopists are identified in the reporting system so that all data can be linked to individuals.
The influence of midazolam and opiate analgesia on NRCL and worse patient experience (PE-W) was also explored. A further variable used in this analysis was PDR. The dataset for PDR was less complete as our endoscopic reporting systems did not mandate the input of PDR until September 2010.
A complete dataset was not available for all variables. Table 2 lists the numbers of colonoscopies where data was not documented.
Table 2.
Data completeness on colonoscopies performed from 2008-2011
| Variable | Total number of colonoscopies with missing data | Percent of colonoscopies with complete data |
| CIR | 0 | 100% |
| NRCL | 520 | 95% |
| PE | 1647 | 84% |
| Midazolam | 62 | 99% |
| Opiates | 65 | 99% |
| Polyp detection | 3863 | 71% |
CIR: Caecal intubation rate; NRCL: Nurse-reported comfort levels; PE: Patient experience.
Statistical analysis
Relationships of CIR to comfort (NRCL-45), sedation and PE-W were explored using Pearson’s correlation coefficient. The relationship between the number of procedures performed per year and CIR was also studied using Pearson’s correlation coefficient. Only endoscopists performing colonoscopies for the full four year period were included in this analysis. A Mann-Whitney U test was used to assess whether there was a difference in the number of colonoscopies performed by those with a higher CIR.
RESULTS
During the four year period from 1 January 2008 to 28 December 2011, 17027 colonoscopies were performed by 23 colonoscopists; 88.8% of procedures were performed on service lists; 11.2% of procedures were performed on bowel cancer screening lists. Data is reported as performance data for these colonoscopists.
Colonoscopy completion
CIR varied from 79.0% to 97.8%, with 18 out of 23 endoscopists achieving > 90%. Four endoscopists completed colonoscopy in 85%-89% of the procedures and 1 locum endoscopist in 79%. The effect of the number of colonoscopies performed on CIR was studied. Only endoscopists performing colonoscopy during the whole period were included in this analysis alone (n = 16). CIR was positively correlated with the average number of procedures performed per annum (r = 0.64, P < 0.01) (Figure 1A). The average CIR for these 16 endoscopists was 94.3%. Endoscopists with a CIR of less than 94.3% performed an average of 139.9 colonoscopies per year whereas those with a CIR of greater than 94.3% performed an average of 245.9 procedures (P < 0.05).
Figure 1.
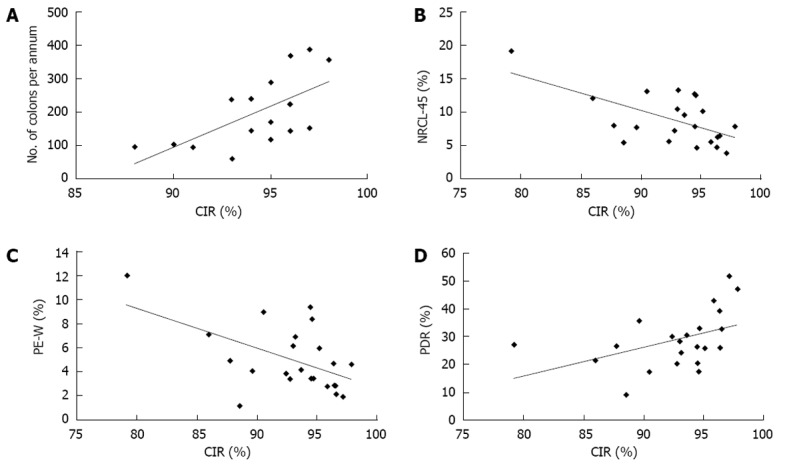
The figure shows correlations of caecal intubation rate with number of annual colonoscopies (A), nurse-reported comfort level of 4-5 (B), patient experience worse than expected (C) and polyp detection rate (D). CIR: Caecal intubation rate; NRCL: Nurse-reported comfort levels; PE-W: Worse patient experience; PDR: Polyp detection rate.
Patient comfort
The percentage of patients experiencing significant discomfort during their procedure (defined as NRCL of 4 or 5) ranged from 3.9% to 19.2% with an average of 7.7%. There was significant negative correlation between NRCL-45 and CIR (r = -0.61, P < 0.005) (Figure 1B).
Patient experience
A worse than expected patient experience (PE-W) was recorded in 4.3% of procedures (1.2%-12.0%). PE-W correlated negatively with CIR (r = -0.54, P < 0.01) (Figure 1C). There was strong correlation between NRCL-45 and PE-W (r = 0.92, P < 0.0001). Only 2% of patients with a NRCL of 1, 2 or 3 rated the procedure as worse than expected compared to 28% of patients with a NRCL of 4 or 5.
Sedation
The sedation used in our endoscopy units for colonoscopy is usually a combination of an opiate (either pethidine or fentanyl) and midazolam. An increasing proportion of procedures are done without sedation.
The average amount of midazolam used per procedure was 1.9 mg, varying from 1.1 mg to 3.5 mg. Average dose of midazolam was negatively correlated with CIR (r = -0.59, P < 0.01). To assess whether this was due to higher doses of midazolam being used by colonoscopists with worse CIRs or to a higher rate of no sedation being used by those with better CIRs, the analysis was repeated for the sedated colonoscopies only. In this sedated group (n = 14870) there was a significant correlation between average midazolam usage and CIR (r = -0.60, P < 0.005). The percentage of colonoscopies performed without sedation was not significantly correlated with CIR (r = 0.30, P = 0.13). There was also a correlation between midazolam dose and NRCL-45 (r = 0.54, P < 0.01) but not for midazolam and PE-W (r = 0.37, P = 0.08). In unsedated patients, there was no correlation between CIR with either NRCL-45 (r = -0.09, P > 0.05) or PE-W (r = -0.01, P > 0.05). However, the numbers were smaller in this group, especially for colonoscopists who rarely performed colonoscopy without sedation. Furthermore, the more uncomfortable procedures would have led to patients being given sedation thereby introducing bias.
There were 4 endoscopists who used fentanyl and 19 who used pethidine as their opiate of preference. To ensure uniformity, the endoscopists using fentanyl were excluded from the analysis on analgesia. There was no significant correlation between average pethidine dose, and CIR (r = -0.39, P > 0.05), NRCL-45 (r = 0.17, P > 0.05) or PE-W (r = 0.06, P > 0.05).
Polyp detection
In this study, the average PDR (including both hyperplastic and adenomatous polyps) was 31.8% (range 9.2%-51.9%). There was a positive correlation between PDR and CIR (r = 0.44; P < 0.05) (Figure 1D).
Performance indicators over time
Table 3 shows data on the CIR, NRCL-45, PE-W, midazolam usage and PDR for each year. A consistent improvement is seen in all variables between 2008 and 2011.
Table 3.
Improvements in key performance indicators between 2008-2011
| Year | CIR | NRCL-45 | PE-W | Midazolam, mg (mean dose) | PDR |
| 2008 | 93.3% | 10.0% | 5.6% | 2.3 | 29.6% |
| 2009 | 93.4% | 7.8% | 4.2% | 2.0 | 27.4% |
| 2010 | 94.6% | 7.6% | 4.1% | 1.8 | 31.9% |
| 2011 | 95.9% | 5.8% | 3.7% | 1.7 | 37.7% |
CIR: Caecal intubation rate; NRCL: Nurse-reported comfort levels; PE-W: Worse patient experience; PDR: Polyp detection rate.
DISCUSSION
In this study, we explored factors that predict high performance in colonoscopy. Ideally a colonoscopy should be safe, complete and comfortable. It should also detect and remove safely and completely all important lesions. The CIR has become the most universally recognised performance indicator. While striving to achieve and exceed target CIRs there is a potential danger that a colonoscopist will cause more discomfort, or put the patient at risk of perforation and excessive sedation. The results of this study indicate the reverse: those colonoscopists with the highest CIR use less sedation, cause less discomfort and achieve a better patient experience. Furthermore, it appears they are more vigilant, identifying more polyps than those with lower intubation rates. The results also show that better colonoscopists perform more colonoscopies. In this study, colonoscopists with a CIR of greater than 94.3% performed an average of 245.9 procedures per annum compared with 139.9 for the endoscopists with a CIR lower than 94.3%. This is consistent with previously published data[23]. This study adds further weight to the argument that there should be a minimum number of procedures performed by an endoscopist per annum to maintain their skills.
There are very large variations in the use of sedation across the world ranging from virtually none in Scandinavian countries to increasing use of deep sedation with propofol in Australia, France, Germany and the United States. The use of sedation is still not as safe as we would like[24]. In the United States, it is now common to perform a colonoscopy with propofol and it has been shown that patient satisfaction is higher than with other types of sedation[25,26]. Conversely, a Scandinavian study showed that high sedation rates were not associated with less painful colonoscopies[21]. Another Scandinavian group showed that sedation is not necessary for screening individuals, and an American group clearly believes unsedated colonoscopy has a place and has coined the phrase “sedation-risk-free colonoscopy”[27].
In our study, the average midazolam dose used was negatively correlated with CIR: the more often the caecum was reached, the less midazolam was used and, furthermore, patients did not experience more discomfort. These findings demonstrate that colonoscopy can be performed without deep sedation and without significant discomfort in the majority of patients.
Sedation alters the perception and recollection of discomfort experienced during colonoscopy. Thus the patient cannot necessarily provide an accurate guide of pain during the procedure. An alternative to the patient assessing discomfort is for the endoscopist or endoscopy nurse to make the assessment. We ask the nurse to make this assessment because they are more likely to be objective and have the benefit of observing all colonoscopists perform colonoscopy. Our comfort scale has not been formally validated but it assesses three components of discomfort: severity, frequency and the extent to which it is distressing the patient. Interestingly there was strong correlation of this nurse-assessed scale with patient reports (r = 0.92, P < 0.0001). Only 2% of patients with a NRCL of 1, 2 or 3 rated the experience as worse than expected. It is likely that different nurses rate discomfort differently but that discrepancy would be applied to all colonoscopists. There are always two nurses in the procedure room during a colonoscopy and the nurses are encouraged to discuss the comfort score with each other before making a final decision.
The assessment of patient experience is different from that of discomfort by a health professional. Because of the effect of sedation on experience and recall, we chose not to ask patients to rate comfort but to rate their experience of the procedure compared to what they expected. This measure was chosen on the assumption that a worse experience than expected was unacceptable and a better or as expected experience was acceptable. Clearly a patient’s rating will be affected by the way they are prepared for the procedure and hearsay. It is possible that the patients of a colonoscopist who routinely tells them that they will experience terrible pain will rarely report the experience worse than expected. We cannot control or assess this possibility. It seems very unlikely that the colonoscopists with high CIR tell their patients that they will have a bad experience when the nurses rate them as causing less pain than their colleagues.
Sedation practice varies but the majority use a combination of opiates and sedatives, and an increasing number use no sedation. It is therefore difficult to make meaningful comparisons. However, whichever way the data was examined the same conclusion was drawn: colonoscopists with high CIR use less sedation (midazolam). One argument against using CIR (especially an unadjusted rate) as a performance indicator is that endoscopists may use excessive force to ensure that the caecum is intubated. However, data from this study shows that comfort scores were better in colonoscopists with a higher CIR and there was no evidence that they were using more opiate analgesia.
A possible bias in this study is case mix. It is possible that the colonoscopists with the highest CIR were colonoscoping the easiest patients. Previous studies have identified factors that predict lower CIR: female sex, older patient and the presence of diverticular disease[19,28]. Until recently our reporting system was not capturing diagnoses according to a recognised coding system so it is not possible to determine the proportion of patients with diverticular disease in each of the colonoscopist cohorts. About 30% of patients listed for colonoscopy are pooled and listed with the endoscopist that is first available. This sharing of patients reduces the likelihood that an individual will be scoping a particularly difficult group of patients. Furthermore, colonoscopists with a higher CIR are often asked to scope “difficult” patients meaning case mix is more likely to affect them adversely. Another possible source of case mix bias is bowel cancer screening (FOBT positive) patients because only accredited colonoscopists are allowed to colonoscope them. These patients are usually asymptomatic and may therefore be easier to colonoscope; there is however no data available on this topic. They certainly have more polyps than other patients, which may bias polyp detection data. Whilst only 10% of all colonoscopies are performed on screen positive patients, up to 50% of the procedures performed by the bowel cancer screening colonoscopists are on screened patients. However, only 2 of the 23 colonoscopists for the majority of the study period were screening accredited and several of the high performing (high CIR, low sedation, low discomfort) colonoscopists were not screening colonoscopists. Another possible confounder is the use of unadjusted CIR instead of the CIR being adjusted for poor bowel preparation or obstruction. CIR would invariably have been higher if adjusted. We chose to use unadjusted CIR as this is standard practice in the United Kingdom for quality assessment. The number of cases with poor bowel preparation or obstruction was probably low and there is no reason to believe that one endoscopist was exposed to all those cases especially as the bowel preparation was standardised across all four units. Therefore, we feel that it is unlikely that the use of adjusted CIR would influence the main findings in this study.
Adenoma detection rate is a key performance indicator and has been shown to be related to the chance of post colonoscopy colorectal cancer[5]. Ideally, adenoma detection rate should be recorded but linking endoscopic with pathology databases is difficult, and late entry of pathology data into an endoscopic database is fraught with problems. In view of this difficulty, we have used polyp rather than adenoma detection in this study whilst recognising the limitations of this approach. However, recent studies have shown that PDR can be used as a marker for ADR because they are highly correlated[29,30]. A recent study of colonoscopies performed on the United Kingdom Bowel Cancer Screening programme also found a positive correlation between adenoma detection rate and caecal intubation rate[31].
In each of the endoscopy units included in this study there is a robust quality assurance process for colonoscopy. All colonoscopists are fed back their performance indicators on a quarterly basis. If any colonoscopist underperforms, the endoscopy lead will discuss this with them and, if appropriate, offer further support and training. Furthermore most of the colonoscopists in this study have completed a training the trainer course during which there is detailed discussion of colonoscopy technique and ways to improve it. These approaches are likely to have contributed to the consistent improvements in CIR, patient comfort/experience and PDR. One aspect of quality assurance we did not address in this study is occurrence of complications in colonoscopy. Our study explores the intubation performance, not performance of therapy. There were no diagnostic perforations during the period of this study and no procedure related deaths. Literature tells us that less than 1:1000 patients will suffer from a complication of colonoscopy without biopsies or polypectomy[32]. A much larger sample size would be required to test the relationship of key performance indicators and complication rates.
In conclusion, this study demonstrates that the best colonoscopists are doing more colonoscopies per year, get to the caecum more often, use less sedation, cause less discomfort, achieve a better patient experience and find more polyps. We believe that measurement of patient comfort and experience, use of sedation, together with CIR, could provide a richer picture of a colonoscopist’s performance, at least of intubation skills.
This study shows that the best colonoscopists, i.e., the ones that have the highest CIR and PDR, also have the best comfort scores, despite using less sedation. Measurement of patient comfort during sedated or non sedated colonoscopy may provide useful information on endoscopist performance.
COMMENTS
Background
Caecal intubation rate (CIR), use of sedation and adenoma detection rate are key performance indicators for colonoscopy. CIR is the most widely recognised measure of performance. Patient comfort is not routinely assessed; it is unknown whether higher intubation rates are achieved at the expense of greater patient discomfort, deeper sedation and possibly higher risk.
Research frontiers
Quality in colonoscopy is an important topic, especially with the introduction of bowel cancer screening programs in different countries. Caecal intubation rate is a key performance indicator of quality. Patient comfort is an auditable outcome, but there are little data on the topic.
Innovations and breakthroughs
Measuring patient comfort through nurse assessment provides valuable information about performance of endoscopists. Performing colonoscopies under deep sedation is not necessary to achieve good patient comfort. The colonoscopists that get to the caecum most often and see and remove the most polyps, have the best patient comfort scores.
Applications
Measuring patient comfort through nurse assessment is a valuable addition in measuring performance. Nurse assessment correlates well with patient experience. People believe that, in the future, assessment of patient comfort, next to CIR and ADR, could be a good performance indicator.
Terminology
Nurse-reported comfort level: assessment of patient comfort during the procedure by endoscopy nurses. Patient experience: the patient’s experience of the procedure, assessed by the patient himself directly after the colonoscopy.
Peer review
The paper about patient comfort and quality in colonoscopy is very interesting. Questions were raised on influence of using adjusted CIR on the results, and on the relationship of age, gender and previous surgical procedures with the nurse-reported comfort levels and patient experience.
Footnotes
P- Reviewers Figueiredo P, Chamberlain SM S- Editor Song XX L- Editor A E- Editor Li JY
References
- 1.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 3.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091–1098. doi: 10.1016/j.cgh.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 6.Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Ferrández A, Navarro M, Díez M, Sopena F, Roncalés P, Polo-Tomas M, Sáinz R, Lanas A. Risk factors for advanced lesions undetected at prior colonoscopy: not always poor preparation. Endoscopy. 2010;42:1071–1076. doi: 10.1055/s-0030-1255868. [DOI] [PubMed] [Google Scholar]
- 8.Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588–2596. doi: 10.1038/ajg.2010.390. [DOI] [PubMed] [Google Scholar]
- 9.Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858–864. doi: 10.1016/j.cgh.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Leung K, Pinsky P, Laiyemo AO, Lanza E, Schatzkin A, Schoen RE. Ongoing colorectal cancer risk despite surveillance colonoscopy: the Polyp Prevention Trial Continued Follow-up Study. Gastrointest Endosc. 2010;71:111–117. doi: 10.1016/j.gie.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faigel DO, Pike IM, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT, et al. Quality indicators for gastrointestinal endoscopic procedures: an introduction. Am J Gastroenterol. 2006;101:866–872. doi: 10.1111/j.1572-0241.2006.00677.x. [DOI] [PubMed] [Google Scholar]
- 12.de Jonge V, Sint Nicolaas J, Cahen DL, Moolenaar W, Ouwendijk RJ, Tang TJ, van Tilburg AJ, Kuipers EJ, van Leerdam ME. Quality evaluation of colonoscopy reporting and colonoscopy performance in daily clinical practice. Gastrointest Endosc. 2012;75:98–106. doi: 10.1016/j.gie.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Valori R, Barton R. BSG Quality and safety indicators for endoscopy. Available from: http://www.thejag.org.uk/downloads%5CUnit%20Resources%5CBSG%20Quality%20and%20Safety%20Indicators.pdf.
- 14.Leaper M, Johnston MJ, Barclay M, Dobbs BR, Frizelle FA. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy. 2004;36:499–503. doi: 10.1055/s-2004-814399. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman DA, Weiss DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555–560. doi: 10.1056/NEJMoa010328. [DOI] [PubMed] [Google Scholar]
- 16.Neerincx M, Terhaar sive Droste JS, Mulder CJ, Räkers M, Bartelsman JF, Loffeld RJ, Tuynman HA, Brohet RM, van der Hulst RW. Colonic work-up after incomplete colonoscopy: significant new findings during follow-up. Endoscopy. 2010;42:730–735. doi: 10.1055/s-0030-1255523. [DOI] [PubMed] [Google Scholar]
- 17.Radaelli F, Meucci G, Sgroi G, Minoli G. Technical performance of colonoscopy: the key role of sedation/analgesia and other quality indicators. Am J Gastroenterol. 2008;103:1122–1130. doi: 10.1111/j.1572-0241.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 18.Arrowsmith JB, Gerstman BB, Fleischer DE, Benjamin SB. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37:421–427. doi: 10.1016/s0016-5107(91)70773-6. [DOI] [PubMed] [Google Scholar]
- 19.Chung YW, Han DS, Yoo KS, Park CK. Patient factors predictive of pain and difficulty during sedation-free colonoscopy: a prospective study in Korea. Dig Liver Dis. 2007;39:872–876. doi: 10.1016/j.dld.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Kim WH, Cho YJ, Park JY, Min PK, Kang JK, Park IS. Factors affecting insertion time and patient discomfort during colonoscopy. Gastrointest Endosc. 2000;52:600–605. doi: 10.1067/mge.2000.109802. [DOI] [PubMed] [Google Scholar]
- 21.Seip B, Bretthauer M, Dahler S, Friestad J, Huppertz-Hauss G, Høie O, Kittang E, Nyhus S, Pallenschat J, Sandvei P, et al. Patient satisfaction with on-demand sedation for outpatient colonoscopy. Endoscopy. 2010;42:639–646. doi: 10.1055/s-0030-1255612. [DOI] [PubMed] [Google Scholar]
- 22.Eckardt AJ, Swales C, Bhattacharya K, Wassef WY, Phelan NP, Zubair S, Martins N, Patel S, Moquin B, Anwar N, et al. Open access colonoscopy in the training setting: which factors affect patient satisfaction and pain? Endoscopy. 2008;40:98–105. doi: 10.1055/s-2007-995469. [DOI] [PubMed] [Google Scholar]
- 23.Harewood GC. Relationship of colonoscopy completion rates and endoscopist features. Dig Dis Sci. 2005;50:47–51. doi: 10.1007/s10620-005-1276-y. [DOI] [PubMed] [Google Scholar]
- 24.Bell GD, Quine A. Preparation, premedication, and surveillance. Endoscopy. 2006;38:105–109. doi: 10.1055/s-2005-921205. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, Dominitz JA, Fanelli RD, Gan SI, Harrison ME, Ikenberry SO, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:815–826. doi: 10.1016/j.gie.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675–701. doi: 10.1053/j.gastro.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Thiis-Evensen E, Hoff GS, Sauar J, Vatn MH. Patient tolerance of colonoscopy without sedation during screening examination for colorectal polyps. Gastrointest Endosc. 2000;52:606–610. doi: 10.1067/mge.2000.109804. [DOI] [PubMed] [Google Scholar]
- 28.Aslinia F, Uradomo L, Steele A, Greenwald BD, Raufman JP. Quality assessment of colonoscopic cecal intubation: an analysis of 6 years of continuous practice at a university hospital. Am J Gastroenterol. 2006;101:721–731. doi: 10.1111/j.1572-0241.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- 29.Francis DL, Rodriguez-Correa DT, Buchner A, Harewood GC, Wallace M. Application of a conversion factor to estimate the adenoma detection rate from the polyp detection rate. Gastrointest Endosc. 2011;73:493–497. doi: 10.1016/j.gie.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Williams JE, Le TD, Faigel DO. Polypectomy rate as a quality measure for colonoscopy. Gastrointest Endosc. 2011;73:498–506. doi: 10.1016/j.gie.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Lee TJ, Rutter MD, Blanks RG, Moss SM, Goddard AF, Chilton A, Nickerson C, McNally RJ, Patnick J, Rees CJ. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050–1057. doi: 10.1136/gutjnl-2011-300651. [DOI] [PubMed] [Google Scholar]
- 32.Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, Schulman J. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]


