Abstract
AIM: To investigate the significance of Twist2 for colorectal cancer (CRC).
METHODS: In this study, 93 CRC patients were included who received curative surgery in Eastern Hepatobiliary Surgery Hospital from January 1999 to December 2010. Records of patients’ clinicopathological characteristics and follow up data were reviewed. Formalin-fixed, paraffin-embedded tissue blocks were used to observe the protein expression of Twist2 and E-cadherin by immunohistochemistry. Two independent pathologists who were blinded to the clinical information performed semiquantitative scoring of immunostaining. A total score of 3-6 (sum of extent + intensity) was considered as Twist2-positive expression. The expression of E-cadherin was divided into two levels (preserved and reduced). An exploratory statistical analysis was conducted to determine the association between Twist2 expression and clinicopathological parameters, as well as E-cadherin expression. Furthermore, the variables associated with prognosis were analyzed by Cox’s proportional hazards model. Kaplan-Meier analysis was used to plot survival curves according to different expression levels of Twist2.
RESULTS: Twist2-positive expression was observed in 66 (71.0%) samples and mainly located in the cytoplasm. Forty-three (46.2%) samples showed reduced expression of E-cadherin. There were no significant correlations between Twist2 expression and any of the clinicopathological parameters. However, Twist2-positive expression was significantly associated with reduced expression of E-cadherin (P = 0.040). Multivariate analysis revealed that bad M-stage [hazard ratio (HR) = 7.694, 95%CI: 2.927-20.224, P < 0.001] and Twist2-positive (HR = 5.744, 95%CI: 1.347-24.298, P = 0.018) were the independent risk factors for poor overall survival (OS), while Twist2-positive (HR = 3.264, 95%CI: 1.455-7.375, P = 0.004), bad N-stage (HR = 2.149, 95%CI: 1.226-3.767, P = 0.008) and bad M-stage (HR = 10.907, 95%CI: 4.937-24.096, P < 0.001) were independently associated with poor disease-free survival (DFS). Survival curves showed a definite trend for Twist2-negative patients to have longer OS and DFS than Twist2-negative patients, not only overall, but also for patients in different stages, especially for DFS of patients in stage III (P = 0.033) and IV (P = 0.026).
CONCLUSION: Our data suggests, for the first time, that Twist2 is a valuable prognostic biomarker for CRC, particularly for patients in stage III and IV.
Keywords: Colorectal cancer, Prognostic biomarker, Twist2, Epithelial-mesenchymal transition, Immunohistochemstry
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignant tumors and continues to be one of the leading causes of cancer-related death worldwide[1]. Traditionally, the prognosis of patients with CRC is mainly evaluated by the tumor (T) node (N) metastasis (M) stage[2]. However, patients in the same stage frequently had different outcomes, despite similar postoperative treatments. There must be some unknown mechanisms affecting patients’ outcome beyond the clinical stage. Although many efforts have been made to find biomarkers to predict CRC, truly effective clinical biomarkers are rare. Therefore, new and more effective biomarkers are still needed.
Recently, Twist2 (Dermo1), a highly homologous protein of Twist1[3,4], has attracted our attention. Koh et al[5] reported that Twist2 could increase resistance to galectin-1-mediated-apoptosis, which facilitated the progression of some T-cells into tumors. Gasparotto et al[6] found overexpression of Twist2 correlated with the poor prognosis of head and neck squamous cell carcinomas. Zhou et al[7] suggested that Twist2 is associated with the invasion and metastasis of salivary adenoid cystic carcinoma. Li et al[8] found that Twist2 is involved in the cervical malignant conversion and tumor metastasis. Twist2 is also considered an inducer of epithelial-mesenchymal transition (EMT)[9-11], a well-known progression involved in embryogenesis[12,13], tumor invasion and metastasis[14-18], and drug resistance[19]. Evidently Twist2 is a significant biomarker for human tumors. However, until now, the relationship between Twist2 and CRC has remained unknown.
Therefore, we undertook the present investigation to determine the significance of Twist2 for CRC and to verify its function as an EMT inducer.
MATERIALS AND METHODS
Patients and tumor samples
Ninety-three CRC patients were included who underwent curative surgery in Eastern Hepatobiliary Surgery Hospital, the Second Military Medical University of China, from January 1999 to December 2010. The patients met the following criteria: no anti-cancer treatments were given before surgery; all the visible tumor nodules were resected (including the distant metastatic nodules); patients who died during surgery or from serious surgical complications were excluded; the resected nodules were identified as primary CRC or metastasis of CRC and the surgical margin was free of tumor cells by pathological examination; patients with lymphatic metastasis or/and distant metastasis had received postoperative adjuvant chemotherapy, patients who died from non-CRC diseases or accidents were excluded; and the clinicopathological and follow-up data were available. All the formalin-fixed and paraffin-embedded primary CRC samples were obtained from the Department of Pathology of Eastern Hepatobiliary Surgery Hospital. All patients in this study gave written informed consent.
Follow-up and postoperative treatment
Patients were followed up until death or until June 15, 2011. All patients were monitored by physical examination, routine blood tests [including serum carcinoembryonic antigen (CEA) concentration], chest X-ray and abdominal ultrasonography every 2 mo in the first year after surgery, and every 3-6 mo thereafter. A computed tomography scan (CT) or magnetic resonance imaging was performed every 6 mo or immediately when a recurrence/metastasis was suspected. If needed, a whole-body fludeoxyglucose positron emission tomography/CT was performed. The follow-up data were recorded during the postoperative examination in our hospital, while patients who were examined in another hospital were followed up by telephone or letter. Recurrence was determined by at least two imaging examination results. Once recurrent tumors were confirmed, further treatment was implemented, such as a second surgery and palliative chemotherapy. Disease-free survival (DFS) was defined as the period from the tumor resection until the tumor recurrence or the last observation. The overall survival (OS) was the interval between the surgery and death or the last follow-up examination.
Immunohistochemistry
Immunohistochemstry was carried out as described previously[20]. Representative 4-μm serial sections were prepared from 10% formalin-fixed, paraffin-embedded tissue blocks. To increase the immunoreactivity, microwave antigen retrieval was performed in citrate buffer (pH 6.0) for 5 min, then cooled the sections at room temperature for at least 30 min. Subsequently 3% hydrogen peroxide was used for 10 min to block endogenous peroxidase activity. After nonspecific binding sites were blocked for 30 min with goat serum, a monoclonal antibody against Twist2 (1:300, H00117581-M01, Abnova) and polyclonal antibodies against E-cadherin (1:100, BS1098, Bioworld Technology) were used to incubate the sections in a humid chamber at 4 °C overnight. Next, an EnVision Detection kit (GK500705, Gene Tech, China) was used to visualize tissue antigens. Sections were counterstained with hematoxylin for 5 min. Negative control sections were incubated with phosphate buffered solution instead of the primary antibody.
Evaluation of immunohistochemistry
Two independent pathologists (Dong H and Cong WM), who were blinded to clinical information, assessed the expression of Twist2 and E-cadherin semiquantitatively. Twist2 staining was observed only in the cytoplasm of CRC tumor cells (described in the results); therefore, the nucleolus staining was not evaluated. Cytoplasmic staining of Twist2 was scored according to its extent and intensity (extent + intensity), similar to the methods described previously[21-24]. The extent of staining was graded as follows: 0 for < 15% positive cells, 1 for 15%-30%, 2 for 30%-60% and 3 for more than 60% positive cells. The intensity of staining was scored on the following scale: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. The total score was 0 to 6 when summed (extent + intensity) together. Subsequently, a total score of 0-2 was considered to be a negative/low expression, while a score of 3-6 was considered as positive/high expression. For E-cadherin, the scoring was determined as previous studies[25,26]. Preserved expression of E-cadherin was defined where tumor cells were stained as strongly and homogeneously as normal epithelial cells. Heterogeneous staining, weaker staining or completely negative staining of E-cadherin was considered as reduced expression.
Statistical analysis
Pearson’s χ2 test and Fisher’s exact test (wherever was applicable) were performed to determine the relationship between Twist2 expression and clinicopathological parameters and E-cadherin expression. The prognostic factors for OS and DFS were examined by both univariate and multivariate analyses (Cox’s proportional hazards model). Survival curves were plotted by Kaplan-Meier analysis and by a log rank test. A P value < 0.05 (two-sided) was considered statistically significant. All statistical analysis were performed using SPSS version 19 (SPSS Inc., Chicago, IL, United States).
RESULTS
Patients’ clinicopathological characteristics are shown in Table 1. The mean age was 58.9 years, ranging from 16 to 81. Forty-one patients had distant metastasis (M1); all the metastatic nodes were in the liver. The median follow-up period was 32 mo (range 6-144 mo). At the last follow-up, 55 patients had tumor recurrence, including one in rectal anastomotic, two with pelvic metastasis, three in the lung, one in both the liver and the lung and the other 48 only in the liver. Thirty patients had died. The OS and DFS rates were 82.2% and 61.0% at 1 year, 71.3% and 42.4% at 3 years, and 66.2% and 30.3% at 5 years, respectively.
Table 1.
Relationship between Twist2 expression and clinicopathological characteristics n (%)
| Characteristics | Total | Twist2 expression | P value | |
| Negative | Positive | |||
| Gender | ||||
| Female | 52 (55.9) | 18 (66.7) | 34 (51.5) | 0.1821 |
| Male | 41 (44.1) | 9 (33.3) | 32 (48.5) | |
| Age | ||||
| < 59 | 41 (44.1) | 13 (48.1) | 28 (42.4) | 0.6141 |
| ≥ 59 | 52 (55.9) | 14 (51.9) | 38 (57.6) | |
| T-stage | ||||
| T1-2 | 17 (18.3) | 5 (18.5) | 12 (18.2) | 1.0002 |
| T3-4 | 76 (81.7) | 22 (81.5) | 54 (81.8) | |
| N-stage | ||||
| N0 | 52 (55.9) | 17 (63.0) | 35 (53.0) | 0.3811 |
| N1-2 | 41 (44.1) | 10 (37.0) | 31 (47.0) | |
| M-stage | ||||
| M0 | 52 (55.9) | 17 (63.0) | 35 (53.0) | 0.3811 |
| M1 | 41 (44.1) | 10 (37.0) | 31 (47.0) | |
| Tumor differentiation | ||||
| Moderate/good | 83 (89.2) | 26 (96.3) | 57 (86.4) | 0.2712 |
| Poor | 10 (10.8) | 1 (3.7) | 9 (13.6) | |
| Vascular invasion | ||||
| No | 56 (60.2) | 17 (63.0) | 39 (59.1) | 0.7291 |
| Yes | 37 (39.8) | 10 (37.0) | 27 (40.9) | |
| Tumor location | ||||
| Rectum | 19 (20.4) | 4 (14.8) | 15 (22.7) | 0.3901 |
| Colon | 74 (79.6) | 23 (85.2) | 51 (77.3) | |
| CEA level (ng/mL) | ||||
| ≤ 5 | 48 (51.6) | 12 (4.4) | 36 (54.5) | 0.3761 |
| > 5 | 45 (48.4) | 15 (55.6) | 30 (45.5) | |
Pearson’s χ2 test;
Fisher’s exact test. T-, N-, M-stage are tumor, node, and metastasis stage (6th edition), performed according to the American Joint Committee on Cancer; CEA: Serum carcinoembryonic antigen.
Twist2 and E-cadherin expression in CRC
Although some previous investigations found Twist2 was expressed in both the cytoplasm and the nucleus in several tumors[7,8,11,27], we found Twist2 was mainly expressed in the cytoplasm in CRC, not in the nucleus (Figure 1). A similar expression pattern of Twist2 was found in hepatocellular carcinoma (HCC)[28]. By semiquantitative analysis, 66 (71.0%) of the 93 primary CRC tissue samples were positive for Twist2 expression, while the other 27 (39.0%) were negative. Twist2 expression was generally low in normal colon mucosa compared with the cancer tissues. For E-cadherin, as described previously[29,30], normal epithelial cells were strongly and homogeneously stained in the membrane, while tumor cells were stained mainly in the membrane and occasionally in the cytoplasm (Figure 2). E-cadherin was considered as reduced in 43 (46.2%) patients. The other 50 (53.8%) patients were preserved.
Figure 1.
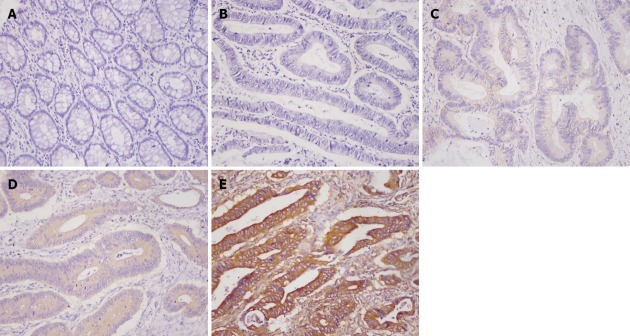
Immunohistochemical images of Twist2. A: Negative staining in the normal mucosa; B: Negative; C: Weak; D: Moderate; E: Strong cytoplasmic staining in colorectal cancer (200× magnification).
Figure 2.
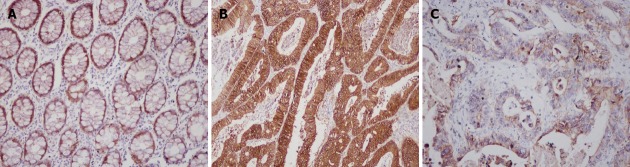
Immunohistochemical images of E-cadherin. A: Strong and homogeneous staining in normal mucosa; B: Preserved expression in colorectal cancer (CRC); C: Reduced expression in CRC (200× magnification).
Relationship between Twist2 expression and clinicopathological parameters and E-cadherin expression
As shown in Table 1, we did not find that Twist2 expression correlated with any of the clinicopathological parameters (gender, age, T-stage, N-stage, M-stage, tumor differentiation, vascular invasion, Tumor location and serum CEA level, all P > 0.05). When the relationship between Twist2 and E-cadherin expression was analyzed, we found a significant correlation: Twist2-positive patients showed a higher percentage of reduced E-cadherin than Twist2-negative ones (53.0% vs 29.6%, P = 0.040, Table 2).
Table 2.
Relationship between Twist2 and E-cadherin expression n (%)
| Twist2 expression | E-cadherin expression | P value | |
| Preserved (n = 50) | Reduced (n = 43) | ||
| Positive (n = 66) | 31 (47.0) | 35 (53.0) | 0.040 |
| Negative (n = 27) | 19 (70.4) | 8 (29.6) | |
Prognostic analysis
For OS on univariate analysis, bad N-stage (lymph node metastasis), bad M-stage (distant metastasis), vascular invasion, serum CEA level (> 5 ng/mL) and Twist2-positive were significantly associated with poor survival (all P < 0.05, Table 3). When adjusted by multivariate analysis by Cox’s proportional hazard model, bad M-stage [hazard ratio (HR) = 7.694, 95%CI: 2.927-20.224, P < 0.001] and Twist2-positive (HR = 5.744, 95%CI: 1.347-24.298, P = 0.018) were considered to independent risk factors for poor OS.
Table 3.
Univariate and multivariate analysis of the prognostic factors for overall survival
| Prognostic factors | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender | ||||||
| Female | 1 | |||||
| Male | 1.011 | 0.490-2.085 | 0.977 | NA | NA | NA |
| Age | ||||||
| < 59 | 1 | |||||
| ≥ 59 | 1.579 | 0.750-3.326 | 0.229 | NA | NA | NA |
| T-stage | ||||||
| T1-2 | 1 | |||||
| T3-4 | 1.118 | 0.493-2.534 | 0.789 | NA | NA | NA |
| N-stage | ||||||
| N0 | 1 | |||||
| N1-2 | 2.172 | 1.056-4.468 | 0.035 | NS | NS | NS |
| M-stage | ||||||
| M0 | 1 | 1 | ||||
| M1 | 6.324 | 2.659-15.041 | < 0.001 | 7.694 | 2.927-20.224 | < 0.001 |
| Tumor differentiation | ||||||
| Moderate/good | 1 | |||||
| Poor | 1.290 | 0.491-3.384 | 0.229 | NA | NA | NA |
| Vascular invasion | ||||||
| No | 1 | |||||
| Yes | 3.398 | 1.601-7.211 | 0.001 | NS | NS | NS |
| Tumor location | ||||||
| Rectum | 1 | |||||
| Colon | 1.188 | 0.517-2.729 | 0.685 | NA | NA | NA |
| CEA level (ng/mL) | ||||||
| ≤ 5 | 1 | |||||
| > 5 | 3.173 | 1.475-6.827 | 0.003 | NS | NS | NS |
| Twist2 expression | ||||||
| Negative | 1 | 1 | ||||
| Positive | 4.964 | 1.181-20.863 | 0.029 | 5.744 | 1.347-24.298 | 0.018 |
Multivariate analysis included adjustment for N-stage, M-stage, vascular invasion, serum carcinoembryonic antigen level and Twist2 expression. T: Tumor; N: Node; M: Metastasis; HR: Hazard ratio; NA: Not available; NS: Not significant.
We also analyzed the risk factors for DFS (Table 4). The result of univariate analysis was similar to OS: bad N-stage, bad M-stage, vascular invasion, serum CEA level (> 5 ng/mL) and Twist2-positive were risk factors for poor DFS (all P < 0.05). After adjustment, multivariate analysis revealed bad N-stage (HR = 2.149, 95%CI: 1.226-3.767, P = 0.008), bad M-stage (HR = 10.907, 95%CI: 4.937-24.096, P < 0.001) and Twist2-positive (HR = 3.264, 95%CI: 1.455-7.375, P = 0.004) were independent risk factors for poor DFS, while vascular invasion and serum CEA level were not.
Table 4.
Univariate and multivariate analysis of the prognostic factors for disease-free survival
| Prognostic factors | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender | ||||||
| Female | 1 | |||||
| Male | 1.001 | 0.587-1.706 | 0.998 | NA | NA | NA |
| Age | ||||||
| < 59 | 1 | |||||
| ≥ 59 | 1.060 | 0.621-1.809 | 0.831 | NA | NA | NA |
| T-stage | ||||||
| T1-2 | 1 | |||||
| T3-4 | 1.258 | 0.833-1.899 | 0.276 | NA | NA | NA |
| N-stage | ||||||
| N0 | 1 | 1 | ||||
| N1-2 | 2.511 | 1.468-4.295 | 0.001 | 2.149 | 1.226-3.767 | 0.008 |
| M-stage | ||||||
| M0 | 1 | 1 | ||||
| M1 | 11.737 | 5.442-25.313 | < 0.001 | 10.907 | 4.937-24.096 | < 0.001 |
| Tumor differentiation | ||||||
| Moderate/good | 1 | |||||
| Poor | 1.140 | 0.537-2.418 | 0.733 | NA | NA | NA |
| Vascular invasion | ||||||
| No | 1 | |||||
| Yes | 3.246 | 1.874-5.625 | < 0.001 | NS | NS | NS |
| Tumor location | ||||||
| Rectum | 1 | |||||
| Colon | 1.557 | 0.810-2.992 | 0.184 | NA | NA | NA |
| CEA level (ng/mL) | ||||||
| ≤ 5 | 1 | |||||
| > 5 | 2.958 | 1.692-5.172 | < 0.001 | NS | NS | NS |
| Twist2 expression | ||||||
| Negative | 1 | 1 | ||||
| Positive | 2.632 | 1.184-5.809 | 0.017 | 3.264 | 1.455-7.375 | 0.004 |
Multivariate analysis included adjustment for N-stage, M-stage, vascular invasion, serum carcinoembryonic antigen level and Twist2 expression. T: Tumor; N: Node; M: Metastasis; HR: Hazard ratio; NA: Not available; NS: Not significant.
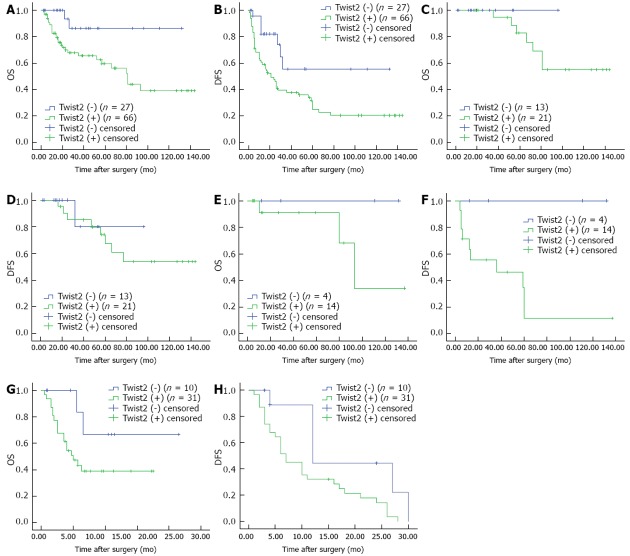
Survival curves plotted according to different expression levels of Twist2 are shown in Figure 3. Significantly, Twist2-negative patients had a higher 5-year OS (86.2% vs 59.6%, P = 0.015, Figure 3A) and 5-year DFS (55.4% vs 24.8%, P = 0.012, Figure 3B) than the Twist2-positive patients. Interestingly, further analysis of the value of Twist2 for CRC patients in different stages showed that for patients in stage I-II (n = 34), there were no differences in OS or DFS (both P > 0.05, Figure 3C and D). For patients in stage III (n = 18) and IV (n = 41), Kaplan-Meier curves showed a clear trend that Twist2-negative patients had a more favorable outcome. Although the differences in OS were not statistically significant (both P > 0.05, Figure 3E and G), we found significant differences in DFS for both stage III and IV (P = 0.033 and P = 0.026 respectively, Figure 3F and H).
Figure 3.
Kaplan-Meier analysis of overall survival and disease-free survival, according to the expression levels of Twist2. A, B: All patients (A, OS, P = 0.015 and B, DFS, P = 0.012); C, D: Patients in stage I-II (C, OS, P = 0.351 and D, DFS, P = 0.652); E, F: Patients in stage III (E, OS, P = 0.178 and F, DFS, P = 0.033); G, H: Patients in stage IV (G, OS, P = 0.101 and H, DFS, P = 0.026). OS: Overall survival; DFS: Disease-free survival.
DISCUSSION
This study, which investigated the significance of Twist2 protein expression in CRC, identified some variables that affected the patients’ prognosis. Bad N-stage, bad M-stage (liver metastasis in our study), vascular invasion, serum CEA level (> 5 ng/mL) and Twist2-positive were valuable predictors for both OS and DFS by univariate analysis. After adjustment by multivariate analysis, bad M-stage and Twist2-positive remained as independent risk factors for poor OS, while bad N-stage, bad M-stage and Twist2-positive were the independent risk factors for poor DFS. Twist2-positive was identified to be an independent risk factor for both poor OS and DFS. Kaplan-Meier analysis showed that patients with Twist2-negative expression had significantly longer OS and DFS than the positive patients. When considering the prognostic value of Twist2 for CRC patients in different stages, we observed a trend for Twist2-negative patients to have a more favorable prognosis compared with Twist2-positive patients, especially for the patients in stage III and IV. Although the P values for OS in stage III and IV didn’t reach significance, the P values for DFS in stage III and IV were statistically significant.
To the best of our knowledge, our study is the first report on the prognostic value of Twist2, based on the protein level, for human CRC. Currently, there is a lack of clinical biomarkers for effectively and routinely predicting CRC, especially for the patients in stage IV. Therefore, the findings of this study are very useful, as we found that Twist2 could be an effective biomarker for predicting the prognosis of CRC, even for patients in stage IV. Furthermore, the expression of Twist2 protein is easily detected by immunohistochemistry. For these reasons, Twist2 is potentially an extremely useful clinical biomarker for predicting the prognosis of CRC patients.
Reduced expression of E-cadherin, which is a hallmark of EMT[14] and plays a significant role in multistage carcinogenesis[31], generally represents a common feature of EMT inducers if these biomarkers were also upregulated[8]. In this study, Twist2-positive expression was significantly associated with reduced expression of E-cadherin, which supports the view that Twist2 is an EMT inducer in CRC. Unfortunately, we did not find a significant correlation between Twist2 expression and the adverse biological behaviors of CRC (bad T, N, M-stage, poor differentiation and vascular invasion). Thus, the mechanism remains unclear. However, other prognostic biomarkers share similar features with Twist2, such as vimentin[32], a-smooth muscle actin[33] and S100A4[29] for CRC, and osteopontin for HCC[34].
Considering the previous reports and the present study, several mechanisms probably contribute to the function of Twist2. Crucially, as an inducer of EMT, Twist2 can activate the EMT program, which is frequently involved in tumor progression and correlates with acquisition of therapeutic resistance[14-19]. In addition, hypoxia may participate in Twist2 function, as Zhou et al[7] found that positive expression of hypoxia-inducible factor-2α was significantly associated with Twist2 overexpression in salivary adenoid cystic carcinoma. Furthermore, Twist2 also correlates with methylation[35,36] and cancer stem cell self-renewal[11], as well as drug resistance[37], which may explain the different outcomes of patients in the same stage. As EMT, cancer stem cells and drug resistance together comprise an axis of evil during tumor progression[19], we speculate that Twist2 is a key component of this axis. In summary, the mechanism of Twist2’s function in CRC is likely to be complex rather than simple.
In conclusion, the results of this study suggest that Twist2 is an independent prognostic factor for CRC. In particular, Twist2 exhibits a prognostic value for CRC in stage III and IV. Future studies with larger samples and functional experiments are needed to confirm the function of Twist2 in CRC.
COMMENTS
Background
Colorectal cancer (CRC) is one of the most common malignant tumors and continues to be one of the most common causes of cancer death worldwide. It is important to identify biomarkers to predict patients’ outcomes. Twist2 is a potential prognostic biomarker, but its value for CRC is unknown.
Research frontiers
Twist2 is a regulatory factor of epithelial-mesenchymal transition, a well-known progression involved in embryogenesis, tumor invasion, metastatic dissemination and acquisition of therapeutic resistance. Hypoxia, methylation, cancer stem cell self-renewal and drug resistance correlate with Twist2 function. Therefore, Twist2 is a potential prognostic biomarker for tumors, and its prognostic value has also been identified for head and neck squamous cell carcinomas.
Innovations and breakthroughs
This study revealed that Twist2 was overexpressed in CRC at the protein level. Twist2-positive expression correlated with the poor prognosis of CRC, particularly for patients in stage III and IV (tumor-node-metastasis stage).
Applications
These results suggest that overexpression of Twist2 can probably serve as a prognostic factor for patients with CRC.
Terminology
EMT is an important change in cell phenotype, which allows the escape of epithelial cells from the structural constraints imposed by tissue architecture, and was first recognized as a central process in early embryonic morphogenesis. Over recent decades, a series of studies have identified the involvement of EMT in solid tissue epithelial cancers’ invasiveness and metastasis.
Peer review
This study investigated Twist2 expression in 93 CRC patients and evaluated its value as a prognostic biomarker based on relapse and survival data of patients. The results indicate that Twist2 could be used as an effective prognostic biomarker for CRC. This paper is generally well designed and the result looks reliable.
Footnotes
Supported by National Natural Science Foundation of China, grant, No. 81201937 and 81070359
P- Reviewer Chung YJ S- Editor Jiang L L- Editor Stewart GJ E- Editor Li JY
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Cserjesi P, Olson EN. Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev Biol. 1995;172:280–292. doi: 10.1006/dbio.1995.0023. [DOI] [PubMed] [Google Scholar]
- 4.Lee MS, Lowe G, Flanagan S, Kuchler K, Glackin CA. Human Dermo-1 has attributes similar to twist in early bone development. Bone. 2000;27:591–602. doi: 10.1016/s8756-3282(00)00380-x. [DOI] [PubMed] [Google Scholar]
- 5.Koh HS, Lee C, Lee KS, Park EJ, Seong RH, Hong S, Jeon SH. Twist2 regulates CD7 expression and galectin-1-induced apoptosis in mature T-cells. Mol Cells. 2009;28:553–558. doi: 10.1007/s10059-009-0150-8. [DOI] [PubMed] [Google Scholar]
- 6.Gasparotto D, Polesel J, Marzotto A, Colladel R, Piccinin S, Modena P, Grizzo A, Sulfaro S, Serraino D, Barzan L, et al. Overexpression of TWIST2 correlates with poor prognosis in head and neck squamous cell carcinomas. Oncotarget. 2011;2:1165–1175. doi: 10.18632/oncotarget.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou C, Liu J, Tang Y, Zhu G, Zheng M, Jiang J, Yang J, Liang X. Coexpression of hypoxia-inducible factor-2α, TWIST2, and SIP1 may correlate with invasion and metastasis of salivary adenoid cystic carcinoma. J Oral Pathol Med. 2012;41:424–431. doi: 10.1111/j.1600-0714.2011.01114.x. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Wang W, Wang W, Yang R, Wang T, Su T, Weng D, Tao T, Li W, Ma D, et al. Correlation of TWIST2 up-regulation and epithelial-mesenchymal transition during tumorigenesis and progression of cervical carcinoma. Gynecol Oncol. 2012;124:112–118. doi: 10.1016/j.ygyno.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, Hu GF. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68:10377–10386. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Fang X, Cai Y, Liu J, Wang Z, Wu Q, Zhang Z, Yang CJ, Yuan L, Ouyang G. Twist2 contributes to breast cancer progression by promoting an epithelial-mesenchymal transition and cancer stem-like cell self-renewal. Oncogene. 2011;30:4707–4720. doi: 10.1038/onc.2011.181. [DOI] [PubMed] [Google Scholar]
- 12.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66:2219–2230. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin GZ, Li Y, Cong WM, Yu H, Dong H, Shu H, Liu XH, Yan GQ, Zhang L, Zhang Y, et al. iTRAQ-2DLC-ESI-MS/MS based identification of a new set of immunohistochemical biomarkers for classification of dysplastic nodules and small hepatocellular carcinoma. J Proteome Res. 2011;10:3418–3428. doi: 10.1021/pr200482t. [DOI] [PubMed] [Google Scholar]
- 21.Koomägi R, Volm M. Expression of Fas (CD95/APO-1) and Fas ligand in lung cancer, its prognostic and predictive relevance. Int J Cancer. 1999;84:239–243. doi: 10.1002/(sici)1097-0215(19990621)84:3<239::aid-ijc7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, Ono T, Yamanoi A, Kohno H, Nagasue N. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325–1332. [PubMed] [Google Scholar]
- 23.Cheng AL, Huang WG, Chen ZC, Peng F, Zhang PF, Li MY, Li F, Li JL, Li C, Yi H, et al. Identification of novel nasopharyngeal carcinoma biomarkers by laser capture microdissection and proteomic analysis. Clin Cancer Res. 2008;14:435–445. doi: 10.1158/1078-0432.CCR-07-1215. [DOI] [PubMed] [Google Scholar]
- 24.Zhao N, Sun BC, Zhao XL, Liu ZY, Sun T, Qiu ZQ, Gu Q, Che N, Dong XY. Coexpression of Bcl-2 with epithelial-mesenchymal transition regulators is a prognostic indicator in hepatocellular carcinoma. Med Oncol. 2012;29:2780–2792. doi: 10.1007/s12032-012-0207-y. [DOI] [PubMed] [Google Scholar]
- 25.Shiozaki H, Tahara H, Oka H, Miyata M, Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol. 1991;139:17–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Shioiri M, Shida T, Koda K, Oda K, Seike K, Nishimura M, Takano S, Miyazaki M. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94:1816–1822. doi: 10.1038/sj.bjc.6603193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang X, Zheng M, Jiang J, Zhu G, Yang J, Tang Y. Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncol. 2011;47:92–97. doi: 10.1016/j.oraloncology.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Zhao XL, Sun T, Che N, Sun D, Zhao N, Dong XY, Gu Q, Yao Z, Sun BC. Promotion of hepatocellular carcinoma metastasis through matrix metalloproteinase activation by epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med. 2011;15:691–700. doi: 10.1111/j.1582-4934.2010.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak JM, Lee HJ, Kim SH, Kim HK, Mok YJ, Park YT, Choi JS, Moon HY. Expression of protein S100A4 is a predictor of recurrence in colorectal cancer. World J Gastroenterol. 2010;16:3897–3904. doi: 10.3748/wjg.v16.i31.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanczak A, Stec R, Bodnar L, Olszewski W, Cichowicz M, Kozlowski W, Szczylik C, Pietrucha T, Wieczorek M, Lamparska-Przybysz M. Prognostic significance of Wnt-1, β-catenin and E-cadherin expression in advanced colorectal carcinoma. Pathol Oncol Res. 2011;17:955–963. doi: 10.1007/s12253-011-9409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, Ikeda JI, Konishi K, Takemasa I, Ikeda M, Sekimoto M, et al. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007;96:986–992. doi: 10.1038/sj.bjc.6603651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–2090. doi: 10.1158/1078-0432.CCR-06-2191. [DOI] [PubMed] [Google Scholar]
- 34.Yang GH, Fan J, Xu Y, Qiu SJ, Yang XR, Shi GM, Wu B, Dai Z, Liu YK, Tang ZY, et al. Osteopontin combined with CD44, a novel prognostic biomarker for patients with hepatocellular carcinoma undergoing curative resection. Oncologist. 2008;13:1155–1165. doi: 10.1634/theoncologist.2008-0081. [DOI] [PubMed] [Google Scholar]
- 35.Raval A, Lucas DM, Matkovic JJ, Bennett KL, Liyanarachchi S, Young DC, Rassenti L, Kipps TJ, Grever MR, Byrd JC, et al. TWIST2 demonstrates differential methylation in immunoglobulin variable heavy chain mutated and unmutated chronic lymphocytic leukemia. J Clin Oncol. 2005;23:3877–3885. doi: 10.1200/JCO.2005.02.196. [DOI] [PubMed] [Google Scholar]
- 36.Mao Y, Toh HB, Ding Z, Sun L, Hong L, Chen CS, Wu X. Differential methylation of CpG islands within the dermo1 gene promoter in several cancer cell lines. Oncol Rep. 2011;25:107–111. [PubMed] [Google Scholar]
- 37.Pham CG, Bubici C, Zazzeroni F, Knabb JR, Papa S, Kuntzen C, Franzoso G. Upregulation of Twist-1 by NF-kappaB blocks cytotoxicity induced by chemotherapeutic drugs. Mol Cell Biol. 2007;27:3920–3935. doi: 10.1128/MCB.01219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]