Abstract
Dysphagia lusoria is a term used to describe dysphagia secondary to vascular compression of the oesophagus. The various embryologic anomalies of the arterial brachial arch system often remain unrecognised and asymptomatic, but in 30%-40% of cases can result in tracheo-oesophageal symptoms, which in the majority of cases manifest as dysphagia. Diagnosis of dysphagia lusoria is via barium swallow and chest Computed tomography scan. Manometric abnormalities are variable, but age-related manometric changes may contribute to clinically relevant dysphagia lusoria in patients who present later in life. Our report describes a case of late-onset dysphagia secondary to a right aortic arch with an aberrant left subclavian artery, which represents a rare variant of dysphagia lusoria. The patient had proven additional oesophageal dysmotility with solid bolus only and a clinical response to dietary modification.
Keywords: Dysphagia, Dysphagia Lusoria, Oesophagus, Dysmotility, Endoscopy
Core tip: Dysphagia lusoria is a term used to describe dysphagia as a consequence of vascular compression of the oesophagus. Our case describes a rare anatomical variant of a right-sided aortic arch with aberrant left subclavian artery with late onset dysphagia. Manometric studies were abnormal with solid bolus, likely contributing to the worsening of the patient’s symptoms over time. The patient is managing to maintain weight and nutrition through dietary modification and no operative intervention is currently planned.
INTRODUCTION
Dysphagia lusoria is a term used to describe dysphagia as a consequence of vascular compression of the oesophagus. Bayford coined the term itself meaning “freak or jest of nature” in 1761 in describing a case of longstanding dysphagia leading to emaciation and eventual death of a 62-year old female patient. On autopsy the patient was found to have an aberrant right subclavian artery (ARSA) running anterior to and causing compression of her oesophagus[1]. The majority of cases of dysphagia lusoria are due to ARSA causing posterior oesophageal compression; yet only 20%-40% of aberrant arteries are thought to cause tracheo-oesophageal symptoms including dysphagia[2,3]. In patients who present at an advanced age, decreased vascular compliance is thought to be the most predominant factor; however the additional contribution of age-related oesophageal dysmotility to their symptoms needs to be considered. Our report describes a case of late-onset dysphagia secondary to a right aortic arch with an aberrant left subclavian artery, which represents a rare variant of dysphagia lusoria.
CASE REPORT
A 68-year-old male presented to the Gastroenterology Outpatient Department with a five-year history of dysphagia. The initial presenting symptom was that of intermittent dysphagia to solids, which had recently worsened and become more progressive in nature. The patient also reported a sensation of food sticking in the left side of his chest. There was no weight loss reported. Past medical history was noteworthy for well-controlled gastroesophageal reflux and cardiovascular disease including both ischaemic heart disease and peripheral vascular disease. Physical examination was unremarkable.
Initial investigations some 5 years ago included routine laboratory blood tests and chest X-ray, which were within normal limits. A barium swallow and modified barium swallow (MBS) were obtained suggestive of oesophageal dysmotility, which was treated with several empirical oesophageal dilatations over the subsequent years with transient improvement. Throughout the MBS assessment the patient repeatedly cleared his throat and complained of perceived left-sided food residue in the absence of the actual presence of pharyngeal or oesophageal residue. Oesophageal manometry was normal for liquid swallows by Chicago criteria. Solid bolus swallows demonstrated shortened distal latency and a rapid contractile front velocity. The lower oesophageal sphincter relaxed appropriately with all swallows during manometry (Figure 1). A non-contrast computed tomography (CT) scan of the abdomen and chest was not reported as showing any abnormal pathology. A therapeutic trial of a proton pump inhibitor and domperidone was commenced, but failed to improve his symptoms.
Figure 1.
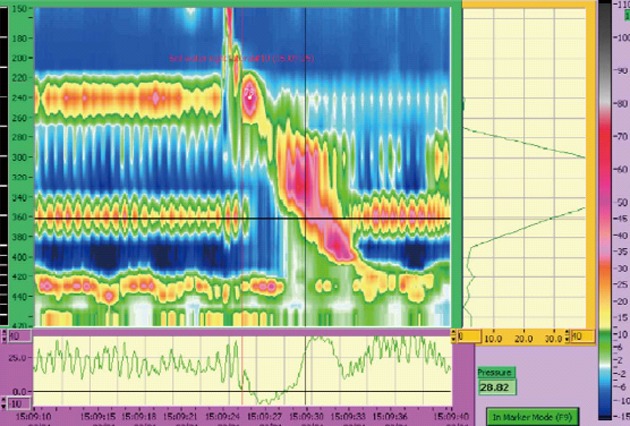
Manometry study demonstrating a high pressure band in the lower oesophagus (at the 360 mm mark/36 cm) consistent with arterial pulsations. This pressure band does not manometrically cause obstruction with a clear relaxation across the region of interest during the swallow.
Due to the progression of his symptoms, a repeat video-fluoroscopic barium swallow was performed. This demonstrated passage of barium freely through the pharynx and upper oesophagus (Figure 2). An extrinsic impression running obliquely across the upper oesophagus just below the level of the aortic arch and a right-sided aortic arch was noted. A contrast CT scan of the chest demonstrated an aberrant subclavian artery running posteriorly to the oesophagus, as well as a Kommerell’s diverticulum at the origin of the attenuated vessel (Figure 3).
Figure 2.
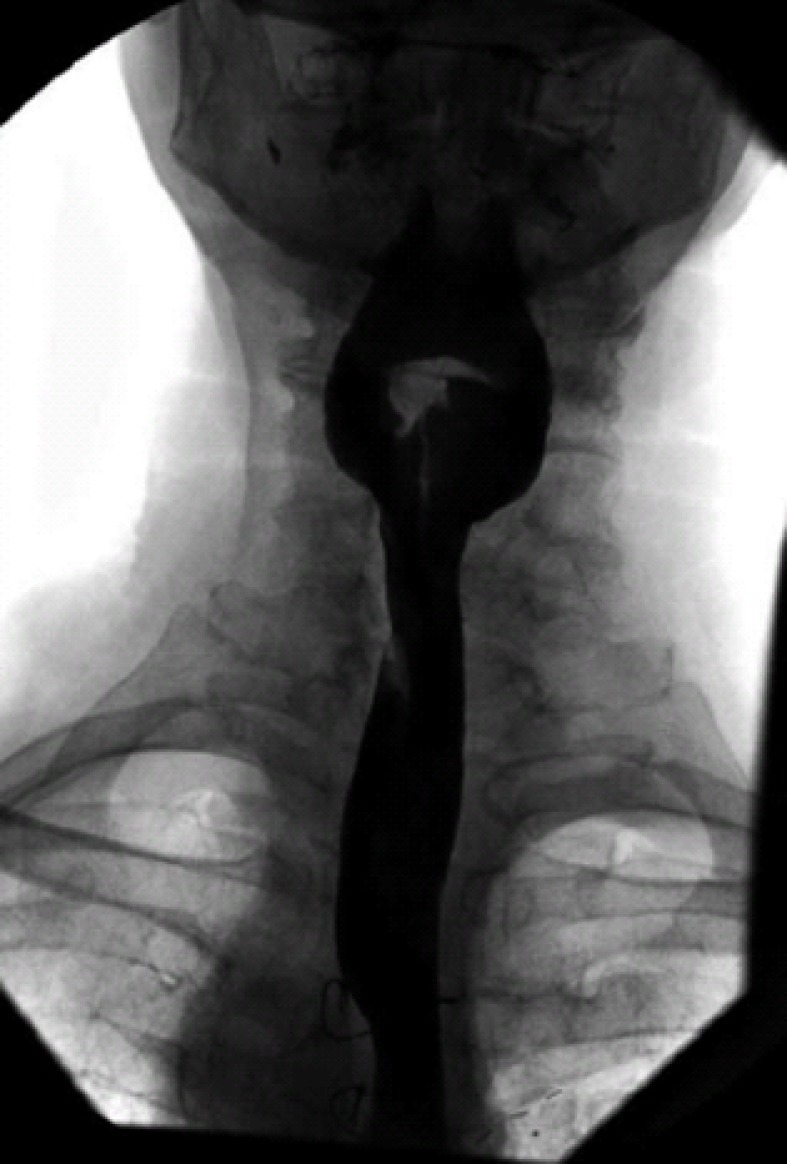
Barium oesophogram demonstrating the extrinsic impression on the oesophagus secondary to the aberrant left subclavian artery superiorly and right aortic arch distally.
Figure 3.
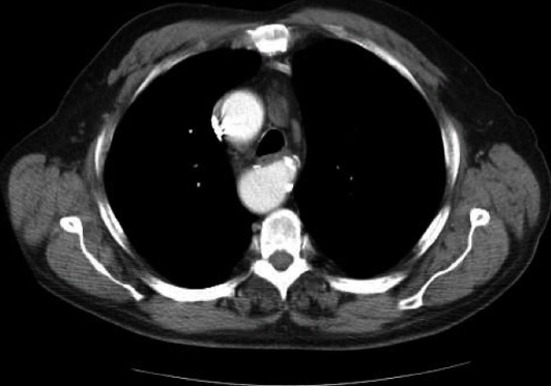
Computed tomography chest demonstrating the right aortic arch and dilatation at the origin of the aberrant left subclavian artery (Komerrell’s diverticulum).
Given the patient’s co-morbidities, age and considering the ability to maintain weight and nutrition, it was decided that the patient would not be a suitable candidate for vascular revision of his aberrant vessel and he was managed conservatively. Lifestyle and diet issues were addressed in conjunction with modification of atherosclerotic risk factors.. It was thought that the progressive symptoms were likely due to a combination of the oesophageal dysmotility demonstrated with solids and artherosclerotic progression with vascular non-compliance.
DISCUSSION
ARSA is a common variant of embryonic aortic arch involution within the general population[3]. This occurs as a consequence of a persistent 7th intersegmental artery with involution of the 4th vascular arch with the right dorsal aorta[4,5]. In the majority of cases the aberrant artery has a course posterior to the oesophagus, but in a small percentage may run anterior to the oesophagus, as in the original description of the syndrome by Bayford[1]. At times a broad base, known as Kommerell’s diverticulum, accompanies ARSA[3]. In rare cases Kommerell’s diverticulum may become aneurysmal with subsequent oesophageal compression and dysphagia[6,7].
The prevalence in the general population of an aberrant subclavian artery is estimated at 0.4% to 0.7% in the majority of the published literature. A study by Haesemeyer et al[8] found 29 cases in 7174 chest CT scans performed in trauma patients. Abhaichand et al[9] found 14 in 3730 patients undergoing transradial coronary angiography. Fockens et al[10] found 6 cases out of 1629 examined via endoscopic ultrasound. Kelly[11] found a single case in 223 patients undergoing upper gastrointestinal endoscopy for dysphagia. Molz et al[12] described a prevalence of 0.7% during autopsies. Aberrant subclavian arteries are present on routine endoscopy as a pulsatile posterior indentation in the upper oesophagus. However, endoscopic diagnosis is rare.
The majority of lesions are a right subclavian artery originating from the left-sided aortic arch. A similar abnormality can occur as a consequence of a left-sided aberrant subclavian artery with a right-sided aortic arch, although this arterial abnormality is much rarer[13,14]. This anomaly develops when the right dorsal artery remains patent and either the left 4th arch or left dorsal aorta regress abnormally. A complete vascular ring is formed when the anomaly is associated with a left sided ductus arteriousus passing from the left subclavian artery to the proximal left pulmonary artery. 30%-40% of patients with vascular anomalies have dysphagia as a consequence, with the majority presenting with solid bolus dysphagia[3]. Patients may at times report the dysphagia to be one-sided, such as in our case. However, these anomalies largely remain asymptomatic and are often an incidental finding on imaging.
Dynamic barium swallow studies, including assessment of solid bolus swallow, serve as diagnostic screening for dysphagia lusoria. CT chest or magnetic resonance imaging with vascular reconstruction are used to define the vascular lesion and plan surgical interventions. Manometry may show variable abnormalities and is not helpful in diagnosis. Manometric studies on six patients with dysphagia lusoria by Janssen et al[2]. showed abnormalities in five out of six patients with two studies showing diminished amplitude contractions, two showing a high pressure zone (increase in intrabolus pressure) above the aberrant artery and one a hypocontractile zone proximal to the aberrant artery. When dysphagia occurs with ageing it seems probable that non-specific age-related manometric abnormalities (such as an increased prevalence of peristaltic failure)[2,15,16] may be contributory to dysphagia in these patients.
Several explanations exist for patients who present with late onset of symptoms. Motility abnormalities and oesophageal stiffening, which occur as a consequence of ageing represent one possibility[2]. Several additional mechanisms have been proposed, including atherosclerosis induced vascular changes leading to stiffening of the obstructing artery, aortic elongation with increased traction on the obstructing artery or aneurysmal dilatation in the presence of Kommerell’s diverticulum[2].
The management of patients with dysphagia lusoria is dependent on the degree of symptoms and impact on the ability of the patients to maintain their weight and nutrition. It would appear approximately half of patients can be managed through dietary modification and through eating slower and chewing well. Severe symptoms, not amenable to interventional dietary and swallowing strategies may warrant surgical treatment.
Gross[17] first reported surgical management of this condition, describing the division and ligation of an aberrant right subclavian artery in a 4-mo old infant via left thoracotomy. Lichter[18] described surgery on an adult patient. The most common approach to repair of a right sided aortic arch and aberrant left subclavian artery is a left postero-lateral thoracotomy followed by division of the liagamentum with dissection. This allows the mediastinal structures to be freed in order to assume a less constricting position[19]. The decision to ligate or re-implant the aberrant vessel to avoid steal syndrome remains an intra-operative one.
Our case describes a rare anatomical variant of a right-sided aortic arch with aberrant left subclavian artery with late onset dysphagia. Manometric studies were abnormal with solid bolus, likely contributing to the worsening of the patient’s symptoms over time. The patient is managing to maintain weight and nutrition through dietary modification and no operative intervention is currently planned.
Footnotes
P- Reviewer Clave P S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Asherson N. David Bayford. His syndrome and sign of dysphagia lusoria. Ann R Coll Surg Engl. 1979;61:63–67. [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen M, Baggen MG, Veen HF, Smout AJ, Bekkers JA, Jonkman JG, Ouwendijk RJ. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95:1411–1416. doi: 10.1111/j.1572-0241.2000.02071.x. [DOI] [PubMed] [Google Scholar]
- 3.Levitt B, Richter JE. Dysphagia lusoria: a comprehensive review. Dis Esophagus. 2007;20:455–460. doi: 10.1111/j.1442-2050.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 4.Taylor M, Harris KA, Casson AG, DeRose G, Jamieson WG. Dysphagia lusoria: extrathoracic surgical management. Can J Surg. 1996;39:48–52. [PMC free article] [PubMed] [Google Scholar]
- 5.Dandelooy J, Coveliers JP, Van Schil PE, Anguille S. Dysphagia lusoria. CMAJ. 2009;181:498. doi: 10.1503/cmaj.081651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triantopoulou C, Ioannidis I, Komitopoulos N, Papailiou J. Aneurysm of aberrant right subclavian artery causing Dysphagia lusoria in an elderly patient. AJR Am J Roentgenol. 2005;184:1030–1032. doi: 10.2214/ajr.184.3.01841030. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Grewal PD, Symons J, Ahmed A, Khosla S, Arora R. Adult-onset dysphagia lusoria secondary to a dissecting aberrant right subclavian artery associated with type B acute aortic dissection. Can J Cardiol. 2008;24:63–65. doi: 10.1016/s0828-282x(08)70552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haesemeyer SW, Gavant ML. Imaging of acute traumatic aortic tear in patients with an aberrant right subclavian artery. AJR Am J Roentgenol. 1999;172:117–120. doi: 10.2214/ajr.172.1.9888750. [DOI] [PubMed] [Google Scholar]
- 9.Abhaichand RK, Louvard Y, Gobeil JF, Loubeyre C, Lefèvre T, Morice MC. The problem of arteria lusoria in right transradial coronary angiography and angioplasty. Catheter Cardiovasc Interv. 2001;54:196–201. doi: 10.1002/ccd.1266. [DOI] [PubMed] [Google Scholar]
- 10.Fockens P, Kisman K, Tytgat GNJ. Endosonographic imaging of an aberrant right subclavian (lusorian) artery. Gastrointestinal Endosc. 1996;43:419. [Google Scholar]
- 11.Kelly MD. Endoscopy and the aberrant right subclavian artery. Am Surg. 2007;73:1259–1261. [PubMed] [Google Scholar]
- 12.Molz G, Burri B. Aberrant subclavian artery (arteria lusoria): sex differences in the prevalence of various forms of the malformation. Evaluation of 1378 observations. Virchows Arch A Pathol Anat Histol. 1978;380:303–315. doi: 10.1007/BF00431315. [DOI] [PubMed] [Google Scholar]
- 13.McNally PR, Rak KM. Dysphagia lusoria caused by persistent right aortic arch with aberrant left subclavian artery and diverticulum of Kommerell. Dig Dis Sci. 1992;37:144–149. doi: 10.1007/BF01308358. [DOI] [PubMed] [Google Scholar]
- 14.Panduranga P, Al-Delamie T, Ratnam L, Al-Mukhaini M, Zachariah S. Repair of Kommerell’s diverticulum with aberrant left subclavian artery in an elderly patient with right aortic arch and dysphagia lusoria. J Card Surg. 2011;26:637–640. doi: 10.1111/j.1540-8191.2011.01344.x. [DOI] [PubMed] [Google Scholar]
- 15.Grishaw EK, Ott DJ, Frederick MG, Gelfand DW, Chen MY. Functional abnormalities of the esophagus: a prospective analysis of radiographic findings relative to age and symptoms. AJR Am J Roentgenol. 1996;167:719–723. doi: 10.2214/ajr.167.3.8751689. [DOI] [PubMed] [Google Scholar]
- 16.Tack J, Vantrappen G. The aging oesophagus. Gut. 1997;41:422–424. doi: 10.1136/gut.41.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross RE. Surgical treatment for dysphagia lusoria. Ann Surg. 1946;124:532–534. [PubMed] [Google Scholar]
- 18.Lichter I. The treatment of dysphagia lusoria in the adult. Br J Surg. 1963;50:793–796. doi: 10.1002/bjs.18005022605. [DOI] [PubMed] [Google Scholar]
- 19.Morris CD, Kanter KR, Miller JI. Late-onset dysphagia lusoria. Ann Thorac Surg. 2001;71:710–712. doi: 10.1016/s0003-4975(00)02241-4. [DOI] [PubMed] [Google Scholar]


