Abstract
Diabetic retinopathy (DRP) is a common complication caused by multiple biochemical abnormalities of the underlying metabolic disease. While the incidence of DRP appears to decline due to evidence-based changes in diabetes management, the predicted increase in patients affected in particular by type 2 diabetes may outweigh the positive trend. The diagnosis is based on the alterations of the vessels, usually indicating abnormalities of the blood–retinal barrier and increased vasoregression, but the neuroglial elements appear equally vulnerable to the diabetic condition. Control of blood glucose, blood pressure and timely identification of coincident nephropathy are important to prevent progression to vision-threatening stages. Guidelines give specific indications for laser photocoagulation, in particular when euglycemia is no longer effective in preventing progression to advanced stages. Intravitreal administration of antibodies directed against the single best characterized propagator of clinically significant macular edema, vascular endothelial growth factor (VEGF), has become popular despite uncertainty about the patient subgroups which benefit best and the optimum administration schedule. Multifactorial intervention beyond glycemic control includes antihypertensive, lipid-lowering and antiaggregatory and is effective in type 2 diabetic patients with high-risk profiles, in particular coincident nephropathy.
Keywords: biomarkers, cardiovascular risk, diabetic retinopathy, treatment
Introduction
Diabetic retinopathy is a microvascular complication of diabetes mellitus and affects a substantial proportion of patients suffering from the underlying disease. As the diabetes epidemic has not yet peaked out, the expected numbers of patients affected by vision-threatening stages will also increase, in particular in patient subgroups and in areas in which optimum care is missing.
Diabetic retinopathy used to affect almost every patient with long diabetes duration and poor metabolic control. There is cumulating evidence that some degree of improvement has been achieved in retinopathy treatment recently, based on the implementations of study results obtained during the last 20 years. However, there is not yet a reason for optimism as the treatment of the advanced, sight-threatening end-stage is destructive and burdened by multiple side effects. Innovative intravitreal ophthalmic medication (IVOM) has been introduced and impressed clinically by short-term success regarding improvement of vision and degree of macular edema. However, the question is open about long-term safety and effect of IVOM.
Epidemiology of diabetic retinopathy
For decades, diabetic retinopathy has remained the primary cause of blindness in adults of working age. It is the most frequent microvascular complication in a person with diabetes. The relative risk of developing retinopathy is higher in a person with type 1 diabetes than with type 2 diabetes. However, recent data from a blind person registry of a regional council in Germany indicated that 70% of all cases of blindness were due to diseases of the elderly, that diabetic eye disease accounted for less than 10% of all cases of legal blindness, and that the overall standardized prevalence rate of blindness has declined over the past 15 years [Finger et al. 2012].
The global prevalence of any retinopathy and vision-threatening diabetic retinopathy and the major risk factors of both have been recently examined in a metaanalysis using studies of sufficient quality including retinopathy assessment by fundus photography [Yau et al. 2012]. The overall prevalence of retinopathy was determined as being 35%, the prevalence of proliferative end-stage and of diabetic macular edema was 7%, and that of vision-threatening forms of retinopathy was 10%. Like many other studies before, this metaanalysis confirmed that persons with type 1 diabetes were more prone to retinopathy development and that disease duration, metabolic control and blood pressure were the major risk factors. Smoking and male sex have been identified as additional risk factors for any retinopathy in a large European study of type 1 diabetic patients [Hammes et al. 2011]. Table 1 summarizes these and additional risk factors for retinopathy development or progression.
Table 1.
Risk factors for diabetic retinopathy (onset/progression):
| • Hyperglycemia |
| • Hypertension |
| • Dyslipidemia |
| • Puberty |
| • Gender (male) |
| • Smoking (type 1 diabetes) |
| • Euglycemic reentry |
| • Diabetic nephropathy |
Figure 1.
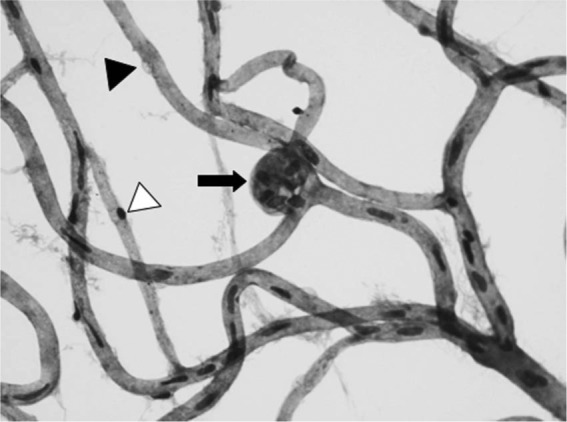
Retinal digest preparation of a human diabetic retina showing acellular capillaries with pericyte ghosts (black arrowhead), acellular capillaries only with pericyte remainder (white arrowhead) and hypercellular microaneurysms (black arrow). Original magnification ×400.
Evidence is emerging from selected cohorts with long-term follow up, such as the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), that the risk of developing any retinopathy and the incidence is declining in groups with more recent disease onset [Klein and Klein, 2010]. Even more so, the annual incidence of sight-threatening proliferative diabetic retinopathy and diabetic macular edema was substantially reduced in groups with more recent onset but comparative disease durations [Klein et al. 2010; Hovind et al. 2003]. The risk reduction is brought about by the implementation of timely diagnosis (in the case of type 2 diabetes), intensified medical therapy of hyperglycemia, hypertension and dyslipidemia, and by increasing the numbers of patients treated for advanced disease using photocoagulation [Klein and Klein 2010]. Public awareness and available sophisticated technologies for blood glucose monitoring cannot belie the fact that a substantial number of persons with diabetes still do not meet recommended treatment targets of metabolic and vascular risk surrogate markers [Anderson et al. 2012; Wong et al. 2012b].
Retinopathy as risk indicator of cardiovascular disease
With the advent of modern fundus imaging and morphometry, the inspection of the retinal vasculature as a ‘window’ to the body’s vasculature has found its palingenesis. From older studies, it was known that advanced retinopathy is associated with premature morbidity and mortality from cardiovascular disease (CVD) [Klein et al. 1999]. However, it was less clear whether milder levels of retinopathy were also predictive for CVD mortality. Recently, Kramer and colleagues determined the predictive value of any degree of retinopathy for all-cause mortality and CVD events [Kramer et al. 2011]. Of 20 studies representing almost 20,000 patients, the presence of any retinopathy more than doubled the risk for these events [odds ratio (OR) 2.34] in type 2 diabetic patients and quadruplicated the risk in type 1 diabetes (OR 4.1), independent of traditional risk factors. Interestingly, diabetic retinopathy was significantly associated with all-cause mortality alone, suggesting that there might factors independent of those influencing CVD, as CVD is the cause of death only in 35% of the studied individuals. Autonomic neuropathy is a suspected link, but other candidate mechanisms may also exist. Clinically, a simple method to identify patients at risk for a progressive disease course is fundus inspection.
Biomarkers for retinopathy
Early detection of retinopathy affects cardiovascular outcome of patients with diabetes. Prediction of an accelerated course of retinopathy would identify patients in which multifactorial intervention reduces morbidity and mortality. One biomarker is the timely detection of microaneurysms as they are reliable predictors of progression of retinopathy to more advanced stages.
To date, diabetic retinopathy is clinically identified by changes produced through either progressive vasoregression or abnormalities in the blood–retinal barrier, limiting the diagnostic and therapeutic focus to the vascular system (Table 2). However, it has been established that diabetic retinopathy involves the neuroglial as well as the vascular compartments. Attempts have been made to identify functional changes of the retina which precede microaneurysms, such as blood flow and defects vascular contractility upon stimulation, but these novel insights have not yet been translated into clinical practice. In fact, high-resolution imaging techniques such as spectral domain optical coherence tomography (SD-OCT) and magnetic resonance imaging (MRI) are able to predict the incidence of clinical retinopathy [Tam et al. 2011; Parravano et al. 2008; Trick et al. 2008]. Optical coherence tomography (OCT) is able to identify the loss of ganglion cells in the retina, which precedes vascular changes.
Table 2.
Retinopathy staging according to [Wilkinson et al. 2003].
| Stage | Leading leasion |
|---|---|
| Nonproliferative | |
| Mild | Microaneuryms (Figure 1) Dot/blot hemorrhage |
| Moderate | Microaneurysms + other retinal lesions, but not severe NPDRP |
| Severe | (More than 20) microaneurysms in four quadrants or venous beadings in two quadrants or intraretinal microvascular anomalies in one quadrant |
| Proliferative | Neovascularization, vitreous/preretinal hemorrhage |
| Macular edema | |
| Absent | No apparent retinal thickening or hard exudates in posterior pole |
| Present | Some apparent retinal thickening or hard exudates in posterior pole |
| Clinically significant | |
| Macular edema | Retinal thickening within 500 μm from centre of macula or hard exudates within 500 μm from centre of macula with adjacent retinal thickening or retinal thickening of more than one optic disc area within one optic disc diameter from centre of macula |
NPDRP: Non-proliferative diabetic retinopathy
Novel psychophysical testing is currently being evaluated as future means to predict retinopathy development. For example, multifocal electroretinogram (mfERG) and frequency doubling technology (FDT) perimetry are being tested for diagnosis and treatment monitoring [Bronson-Castain et al. 2012; Harrison et al. 2011]. As retinal areas that develop neuronal dysfunction found by these tests are more prone to subsequent vascular abnormalities, multiplex testing assessing both the vascular and the neuronal compartments may be more precise in predicting the course and the visual consequence. MRI is another technique that provides a high-resolution image of the retina and visual pathways to the brain [Bissig and Berkowitz, 2011; Trick et al. 2008].
Based on clinical studies showing that retinopathy clusters in families with type 1 diabetes, genetic studies have analysed risk genes for retinopathy [The Diabetes Control and Complications Trial Research Group, 1997]. To date, none of the studies have provided clear evidence for risk genes that determine aggressive retinopathy development [Williams et al. 2012]. A large European consortium is currently investigating this further (Summit, http://www.imi.europa.eu/content/summit).
A somewhat clearer picture results from the analysis of the association between genes and advanced (proliferative) retinopathy. Two associations were found to be consistently involved: one for vascular endothelial growth factor (VEGF) and the other for erythropoietin [Al-Kateb et al. 2007; Tong et al. 2008]. Both VEGF and erythropoetin are elevated in the vitreous of patients with proliferative diabetic retinopathy and the difference in the transcriptional activity may account for the different propensity to growth new vessels under hypoxic conditions.
The link between diabetic retinopathy and diabetic nephropathy
When Friedman and L’Esperance coined the term ‘the renal–retinal syndrome’, they aimed to define a high risk group of patients in which ‘the coincident kidney and eye diseases resulting from diabetic microvasculopathy in retinal and glomerular arterioles and capillaries’ indicated a shared pathophysiology [Friedman and L’Esperance, 1986]. Epidemiologic and clinical studies have demonstrated that patients with diabetic kidney disease take a much more aggressive course in the eye as well [Jensen and Deckert, 1992, Gilbert et al. 1998]. Moreover, the existence of albuminuria in type 1 diabetes increases the risk for severe retinopathy by more than fourfold and the risk further increases with macroalbuminuria [Hammes et al. 2011]. These data are in accordance with the WESDR study and with Scandinavian studies [Kofoed-Enevoldsen et al. 1987; Klein, 2006].
Whether the relation holds also true for type 2 diabetic patients is less clear. In the WESDR, the risk for retinopathy in albuminuric type 2 diabetic patients was not significant in multivariate analysis. Similar calculations revealed that albuminuria increased the risk for any retinopathy by 54% and by 2.3 fold for proliferative retinopathy in patients with macroalbuminuria/impaired renal function in a large dataset of the German/Austrian ‘Diabetes-Patienten-Verlaufsdokumentation’ (DPV) Hammes, Lessmeister, Fach, Wagner, Welp, Best, et al. manuscript in preparation]. An association of equivalent importance is albuminuria and diabetic macular edema [Knudsen et al. 2002]. Both entities may share a common pathogenesis resulting in a generalized vascular hyperpermeability. Clinically significant macular edema (CSME) correlates with gross proteinuria, but not with albuminuria in two independent studies, indicating that factors different from those leading to microalbuminuria are involved [Klein et al. 1998; Knudsen et al. 2007].
Optimum treatment of diabetic retinopathy
Blood glucose
The cornerstone of treating diabetes is to bring blood glucose levels to the lowest range possible without severe side effects. The procedures to achieve this have been recently revised to emphasize the priority of individualized medicine [Inzucchi et al. 2012]. For type 1 diabetes, the lesson that tight glycemic control achieved by multiple daily injections or an insulin pump can reduce retinopathy in a primary prevention setting by 76% and in a secondary intervention setting by 54% had to be modified. Despite the fact that glycemia is the major risk factor for developing retinopathy, its overall contribution is only 11%, i.e. 89% of the risk is explained by (still) unknown factors [Lachin et al. 2008]. However, the Diabetes Control and Complication Trial (DCCT) and the follow-up study, Epidemiology of Diabetes Interventions and Complications (EDIC), showed that the effect of good glycemic control persists for at least 10 years, albeit with a tendency of waning [White et al. 2010]. As the persistent difference of retinopathy risk was maintained despite equal glycemic levels for at least 4 years, the term ‘metabolic memory’ was coined which is the persistence of a phenotypic imprinting despite a change in glycemia as underlying (causal) factor.
Implementation of good glycemic control can cause transient (early) worsening, mostly due to the development of small arteriolar infarcts which result in the well-known cotton wool spots [The Diabetes Control and Complications Trial Research Group, 1998]. In particular, patients with poor control and long-standing disease are at risk, and deserve careful monitoring before and at 3-monthly intervals after implementation of intensified control for at least 1 year. The reason for early worsening is unclear, but despite earlier suspicions, insulin and its analogues have been found not to be responsible. The benefit–risk ratio of intensified glycemic control is positive as the long-term benefits of glucose control far outweigh the risk of early worsening.
It has also been analysed as to which level of retinopathy intensified glucose control is beneficial, at least in type 1 diabetes patients. Level 43 on the Early Treatment of Diabetic Retinopathy Study (ETDRS) severity scale, i.e. moderately severe nonproliferative diabetic retinopathy has been defined as the level beyond which no further effect of intensified insulin therapy achieves any benefit over a 6-year period in type 1 diabetes [The Diabetes Control and Complications Trial Research Group, 1998].
Intensified glucose control has also been studied either alone or as part of a multifactorial intervention in type 2 diabetic patients. The first study to demonstrate an effect of glycemia on retinopathy progression was the UK Prospective Diabetes Study (UKPDS) [Stratton et al. 2001]. This study showed that the overall effect of intensified treatment was modest, and it took 6 years to see a difference between the conventional (mean HbA1c 7.9%) and the intensive group (mean HbA1c 7.0%). Only a proportion of patients (36%) had any retinopathy lesion at onset, suggesting that the modest effect was partly due to the secondary intervention character of the study. In a follow-up study, the authors noted what was called a legacy effect of glycemic control, i.e. that similar to type 1 diabetic patients, the effect of a former intensified glucose treatment translated into a persistent benefit [Holman et al. 2008]. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, type 2 diabetic patients with a high risk of CVD received either intensified or standard treatment for glycemia, aiming at HbA1c of 6% or 7.0–7.9%. A subgroup of 2856 patients was analysed for retinopathy incidence and progression, and it was found that glycemic control reduced the risk for progression by 33% [Chew et al. 2011].
Despite the results of these trials, all pointing into the direction of glycemia being important in the pathogenesis of retinopathy and its lowering being beneficial, a recent metaanalysis came to a different conclusion. Hemmingsen and colleagues did not find a sufficiently strong overall effect of the clinical trials to date to prove or refute a relative risk reduction for retinopathy [Hemmingson et al. 2011]. However, a 30% increase of severe hypoglycemia was noted. Thus it appears that the effect of blood glucose control has been overestimated and needs complementation by other therapies.
Renin–angiotensin system blockade
When discussing the role of the renin–angiotensin system (RAS) in the pathogenesis of diabetic retinopathy and the possible effect of RAS inhibitors, blood pressure dependent and independent mechanisms have to be considered. The EURODIAB Controlled Trial of Lisinopril in IDDM (EUCLID) study, one of the first to demonstrate a beneficial effect of RAS blockade on retinopathy in type 1 diabetes, did not achieve different blood pressure levels in the treated group, while in the UKPDS, as the landmark study in type 2 diabetes, blood pressure treatment showed improvement of retinopathy progression and need for laser therapy by lowering systolic blood pressure by 10 mmHg and diastolic blood pressure by 5 mmHg [UK Prospective Diabetes Study Group 1998; Chaturvedi et al. 1998]. In that study, it was noted that the same effect on retinopathy outcome parameters was achieved with other antihypertensive medications, suggesting that the effect was independent of RAS blockade.
A recent study compared the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril with the angiotensin receptor blocker losartan on two-step progression in retinopathy and nephropathy-naïve type 1 diabetes patients. There was no difference between the two compounds on the odds of retinopathy progression by two steps, which was 65–70% [Mauer et al. 2009]. This suggests that both approaches to RAS blockade are equally effective.
Another study using candesartan cilexetil, DIabetic REtinopathy Candesartan Trials (DIRECT-Prevent and DIRECT-Protect), assessed the impact on retinopathy development and progression in retinopathy-naïve (Prevent) type 1 diabetes patients and in patients with mild retinopathy (Protect) [Chaturvedi et al. 2008; Sjolie et al. 2008]. Neither of the primary aims were met, i.e. incidence and two-step progression. Only in a posthoc analysis was there a 35% inhibition of three-step progression. As the latter is a sign of above-average retinopathy progression, the conclusion is that RAS blockade is beneficial in patients with type 1 diabetes and nephropathy.
An interesting observation was made in the DIRECT trial which describes regression of retinal lesions in type 2 diabetes [Sjolie et al. 2012]. Although not explained mechanistically, the chance of retinopathy regression upon RAS blockade was most associated with the numbers of microaneurysms at study baseline.
In the ACCORD trial, in which a blood pressure of 120 mmHg was aimed for in the intensive group, no effect was found in type 2 diabetes patients with a high risk vascular profile [Chew et al. 2011]. Similar negative results were reported from the Action in Diabetes and VAscular Disease: Preterax and Diamicron MR controlled Evaluation (ADVANCE) study in which retinopathy was advanced beyond the point of no return defined in type 1 diabetes patients [Beulens et al. 2009]. In a follow up of the UKPDS it was found that, in contrast to glycemia, antihypertensive treatment did not achieve any prolonged effect [Holman et al. 2008].
Together, these data suggest that RAS blockade is most effective in patients at above average risk of retinopathy progression, but less effective when the overall risk of retinopathy development is low.
Lipids
Two recent studies have found that addition of fenofibrate either to treatment naïve or to statin-treated type 2 diabetes patients reduces the risk of diabetic macular edema, for laser treatment, or for progression of retinopathy [Chew et al. 2011; Keech et al. 2007]. Evidence has been obtained that lipid deposition in diabetic macular edema (DME) is more likely to occur in type 2 diabetes patients with hyperlipidemia and lipid variations within the normal range are associated with retinopathy development in type 1 diabetes [Chew et al. 1996; Lyons et al. 2004]. However, in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, the protective effect was independent from lipid lowering, and in the ACCORD trial, the absence of an effect on high-density lipoprotein (HDL) and the modest effect on mean triglycerides [-27 mg/dl (-1.7 mmol/l)] in the fibrate treated subgroup may not explain the variation in retinopathy outcome.
Certain limitations were also reported in both studies. In FIELD, the indication for laser treatment was at the discretion of the ophthalmologist at the reporting centre and the numbers of events was small. In ACCORD, there were a considerable number of patients without fundus photographs at year 4. Overall, the data appear promising for those who experience a rapid retinopathy progression, in particular in DME. Mechanisms explaining retinopathy outcome may involve peroxisome proliferator-activated receptor alpha (PPARa) activation, which inhibits inflammatory signalling and hypoxia-induced gene regulation [Chen et al. 2013; Wong et al. 2012b]. Furthermore, peroxisome proliferator-activated receptor α (PPARα) activation is inversely associated with receptor for advanced glycation end products (RAGE) expression, with protection from apoptosis, oxidative stress and inflammation, and may be neuroprotective [Wong et al. 2012b].
Multifactorial treatment
Gaede and colleagues studied microalbuminuric type 2 diabetes patients using an intensified multifactorial approach using agents that target blood glucose, lipids, blood pressure and platelets on top of lifestyle intervention [Gaede et al. 2008]. After 8 years of intensified treatment, patients were followed observationally for another 5.5 years. Intensified treatment had a memory effect on the need for retinal photocoagulation (risk reduction 55%; p < 0.02). It needs to be shown that multimodal intensified therapy in diabetes is beneficial for those patients who develop retinopathy early in the course of diabetes [Gaede et al. 2008].
IVOM
Local restriction of medication to the eye combines multiple pharmacological advantages: prevention of systemic side effects, high local concentration of drug, and specific inhibition of an identified target molecule. Antiangiogenic and antiedemateous properties are a domain of anticancer drugs, limiting tumour growth, metastasis and radiosensitivity. Similar properties are mandatory for activity against proliferative diabetic retinopathy and diabetic macular edema. Although not all patients with DME and proliferative diabetic retinopathy have high levels of angiogenic growth factors in the vitreous, VEGF is currently the most widely addressed target in these two ocular complications.
Anti-VEGF treatment was first performed with bevazicumab as off-label use. In a metaanalysis of four small randomized controlled trials (RCTs), no sufficient high-quality evidence was found to support anti-VEGF IVOM [Parravano et al. 2009]. Bevacizumab is a humanized antibody against all VEGF isoforms. Although cost effective, it was not approved for use in ophthalmic diseases.
The antibody fragment ranibizumab was developed for IVOM and tested in several trials in combination and in comparison with laser therapy. In Ranibizumab in Diabetic Macular Edema (RESOLVE), 0.3 and 0.5 mg ranibizumab were compared with sham injection for the effect on best corrected visual acuity (BCVA) and central retinal thickness (CRT) in patients with type 1 and type 2 diabetes. At 12 months of treatment, patients who had baseline vision of approximately 60 ± 10 lines, gained 7.8 letters while sham-treated patients remained stable over the study period. Mean CRT dropped almost 200 µm in the treatment group and approximately 50 µm in the sham-treated group. Patients were 3.3-fold more likely to gain more than 10 letters with an average of 10 injections of ranibizumab [Massin et al. 2010].
In the Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy for Diabetic Macular Edema (RESTORE) trial, Mitchell and colleagues compared ranibizumab as 0.5 mg monotherapy or combined with laser to laser alone in a 12-month randomized, double masked phase III trial in 345 patients with type 1 and type 2 diabetic patients [Mitchell et al. 2011]. Patients receiving a mean of seven injections gained more vision (6.1 and 5.9 letters, with ranibizumab or ranibizumab plus laser) compared with 0.8 letters with laser alone. CRT in the treated groups dropped 103 and 116.5 µm, respectively, compared with 60 µm in the laser group [Mitchell et al. 2011].
In the Ranibizumab for Edema of the mAcula in Diabetes study (READ-2) study, three groups were compared: a group treated with laser alone, a group that received ranibizumab 0.5 mg over 6 months, and a group with combined therapy. At month 6, the ranibizumab-treated group was significantly superior to the laser-only group (7.2 versus -0.4 letters), while the combined group was intermediate (3.8 letters improvement, p = 0.08).
These patients were included into a follow-up study, in which from month 24 to 36, treatment frequency was increased from injections every second month to monthly. At 24 months, mean improvements in BCVA had been similar in all three groups (n = 22–28 points per group), suggesting that patients were undertreated. More frequent ranibizumab injections resulted in a significant gain of BCVA of 3.1 letters and a reduction in FTH of 70 µm, which was superior to the laser alone and the laser + ranibizumab group. The authors concluded that more aggressive therapy (monthly injections) may be necessary in many patients to optimally control edema and maximize vision [Do et al. 2012].
DRCRnet recently compared deferred (>24 weeks) versus prompt laser in type 1 and type 2 patients with centre-involving DME and impaired BCVA. They noted that prompt laser is not superior and possibly worse to deferred laser for vision outcome [Elman et al. 2012].
Although side effects including local complications such as endophthalmitis or retinal detachment appear to be infrequent, there is considerable concern about VEGF as it is a neurotrophic factor. Ablation of VEGF from retinal pigment epithelial cells induced rapid vision loss and regression of the choriocapillaris, leading to dysfunction of the cone photoreceptors [Kurihara et al. 2012]. Thus, besides systemic adverse effects such as thromboembolism or proteinuria, chronic depletion of VEGF, in particular, if more aggressively needed, must be carefully monitored for retinal degeneration such as those reported in case presentations [Rosenfeld et al. 2011].
An alternative IVOM for diabetic macular edema is the administration of intravitreal steroids. The rationale in DME is evident because of the antiinflammatory and antiedematous potential of steroids. Triamcinolone acetonide is used because of its crystalline form, allowing for a single injection and slow release over a period of several months. The complications of intravitreal triamcinolone therapy include secondary ocular hypertension in 40% of the injected eyes, elevated intraocular pressure requiring antiglaucomatous surgery in about 1–2% of the eyes, cataract surgery in a fifth of eyes in older patients within 1 year after injection, and endophthalmitis at a rate of 1:1000, among others [Tao and Jonas, 2011]. In a metaanalysis, Rudnisky and colleagues analysed the effect of steroids on DME resistant to laser treatment and found that intravitreal triamcinolone (4–8 mg) resulted in a three line improvement of BCVA 1 month after injection with a gradually decreasing benefit that reaches baseline by 6 months [Rudnisky et al. 2009].
DRCRnet recently compared laser treatment and intravitreal triamcinolone for diabetic macular edema in a 3-year follow-up study of 306 eyes. They noted no benefit of long-term steroid injections compared with laser and found a 83% cumulative probability for cataract surgery, which led them to conclude that there is no long-term benefit of intravitreal triamcinolone relative to focal/grid laser therapy [Beck et al. 2009].
On the basis of cost effectiveness analysis, treatment of phakic patients with centre-involving DME with ranibizumab and deferred laser achieved a better vision (six letters) compared with triamcinolone at the cost of a little over US$19,000, which is considered acceptable provided that the gain at 2 years is maintained in subsequent years. In pseudophakic eyes, first line treatment with triamcinolone seems to be the most cost-effective option [Dewan et al. 2012].
As more and more effective anti-VEGF treatments are being introduced into the treatment of patients with DME, particular attention will to be given to the patient characteristics which fit best with the treatment, in particular because no long-term (i.e. > 5 years) visual outcomes are yet available.
Final considerations
It appears that retinopathy is a medical problem in type 1 and type 2 diabetes, for which treatment options have been developed that reduce the overall prevalence and incidence. Implementation of multifactorial care, also for the benefit of other target organs of diabetic damage, is as efficient as continuous screening. The invasiveness of treating advanced stages and the surprisingly modest effect of IVOM, however, translates into a demand for further research. As an immediate question, the role of the kidney in determining results of the multiple IVOM studies using ranibizumab and other compounds needs to be assessed. To reduce costs, it appears mandatory to define appropriate patient cohorts which benefit best from IVOM. When proposing multifactorial approaches for the optimal treatment of diabetic retinopathy, it must be kept in mind that the adherence to medication declines with the numbers of pills that a patient has to take daily [Chapman et al. 2005]. The solution comes with novel multitarget drugs and reasonable application schedules.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
References
- Al-Kateb H., Mirea L., Xie X., Sun L., Liu M., Chen H., et al. (2007) Multiple variants in vascular endothelial growth factor (VEGFA) are risk factors for time to severe retinopathy in type 1 diabetes: the DCCT/EDIC genetics study. Diabetes 56: 2161–2168 [DOI] [PubMed] [Google Scholar]
- Anderson S., Narayanan R., Amlesh J., Qureshi M., Heald A. (2012) Type 1 diabetes in Cheshire: cardiometabolic risk factor trends (2004–2009). Prim Care Diabetes 6: 123–126 [DOI] [PubMed] [Google Scholar]
- Beck R., Edwards A., Aiello L., Bressler N., Ferris F., Glassman A., et al. (2009) Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 127: 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beulens J., Patel A., Vingerling J., Cruickshank J., Hughes A., Stanton A., et al. (2009) Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia 52: 2027–2036 [DOI] [PubMed] [Google Scholar]
- Bissig D., Berkowitz B. (2011) Same-session functional assessment of rat retina and brain with manganese-enhanced MRI. Neuroimage 58: 749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson-Castain K., Bearse M., Jr., Neuville J., Jonasdottir S., King-Hooper B., Barez S., et al. (2012) Early neural and vascular changes in the adolescent type 1 and type 2 diabetic retina. Retina 32: 92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R., Benner J., Petrilla A., Tierce J., Collins S., Battleman D., et al. (2005) Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch Intern Med 165: 1147–1152 [DOI] [PubMed] [Google Scholar]
- Chaturvedi N., Porta M., Klein R., Orchard T., Fuller J., Parving H., et al. (2008) Effect of candesartan on prevention (direct-prevent 1) and progression (direct-protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet 372: 1394–1402 [DOI] [PubMed] [Google Scholar]
- Chaturvedi N., Sjolie A., Stephenson J., Abrahamian H., Keipes M., Castellarin A., et al. (1998) Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The Euclid Study Group. Eurodiab Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet 351: 28–31 [DOI] [PubMed] [Google Scholar]
- Chen Y., Hu Y., Lin M., Jenkins A., Keech A., Mott R., et al. (2013) Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetic models. Diabetes 62: 261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E., Ambrosius W., Davis M., Danis R., Gangaputra S., Greven C., et al. (2011) Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 363: 233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E., Klein M., Ferris F., Remaley N., Murphy R., Chantry K., et al. (1996) Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. early treatment diabetic retinopathy study (ETDRS) report 22. Arch Ophthalmol 114: 1079–1084 [DOI] [PubMed] [Google Scholar]
- Dewan V., Lambert D., Edler J., Kymes S., Apte R. (2012) Cost-effectiveness analysis of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 119: 1679–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do D., Nguyen Q., Khwaja A., Channa R., Sepah Y., Sophie R., et al. (2012) Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. Arch Ophthalmol: 1–7 [DOI] [PubMed] [Google Scholar]
- Elman M., Qin H., Aiello L., Beck R., Bressler N., Ferris F., et al. (2012) Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology 119: 2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger R., Bertram B., Wolfram C., Holz F. (2012) Blindness and visual impairment in Germany: a slight fall in prevalence. Dtsch Arztebl Int 109: 484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L., Jr., L’Esperance F. (1986) Diabetic Renal-Retinal Syndrome: Therapy. New York: Grune & Stratton [Google Scholar]
- Gaede P., Lund-Andersen H., Parving H., Pedersen O. (2008) Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591 [DOI] [PubMed] [Google Scholar]
- Gilbert R., Tsalamandris C., Allen T., Colville D., Jerums G. (1998) Early nephropathy predicts vision-threatening retinal disease in patients with type I diabetes Mellitus. J Am Soc Nephrol 9: 85–89 [DOI] [PubMed] [Google Scholar]
- Hammes H., Kerner W., Hofer S., Kordonouri O., Raile K., Holl R. (2011) Diabetic retinopathy in type 1 diabetes – a contemporary analysis of 8,784 patients. Diabetologia 54: 1977–1984 [DOI] [PubMed] [Google Scholar]
- Harrison W., Bearse M., Jr., Ng J., Jewell N., Barez S., Burger D., et al. (2011) Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Via Sci 52: 772–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen B., Lund S., Gluud C., Vaag A., Almdal T., Hemmingsen C., et al. (2011) Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ 343: d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman R., Paul S., Bethel M., Matthews D., Neil H. (2008) 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577–1589 [DOI] [PubMed] [Google Scholar]
- Holman R., Paul S., Bethel M., Neil H., Matthews D. (2008) Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 359: 1565–1576 [DOI] [PubMed] [Google Scholar]
- Hovind P., Tarnow L., Rossing K., Rossing P., Eising S., Larsen N., et al. (2003) Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care 26: 1258–1264 [DOI] [PubMed] [Google Scholar]
- Inzucchi S., Bergenstal R., Buse J., Diamant M., Ferrannini E., Nauck M., et al. (2012) Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 55: 1577–1596 [DOI] [PubMed] [Google Scholar]
- Jensen T., Deckert T. (1992) Diabetic retinopathy, nephropathy and neuropathy. generalized vascular damage in insulin-dependent diabetic patients. Horm Metab Res Suppl 26: 68–70 [PubMed] [Google Scholar]
- Keech A., Mitchell P., Summanen P., O’Day J., Davis T., Moffitt M., et al. (2007) Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370: 1687–1697 [DOI] [PubMed] [Google Scholar]
- Klein R. (2006) Diabetic Retinopathy and nephropathy. In: Cortes P., Mogensen C. (eds), The Diabetic Kidney. Totowa, NJ: Humana Press [Google Scholar]
- Klein R., Klein B. (2010) Are individuals with diabetes seeing better? A long-term epidemiological perspective. Diabetes 59: 1853–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Klein B., Moss S., Cruickshanks K. (1998) The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 105: 1801–1815 [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B., Moss S., Cruickshanks K. (1999) Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol 117: 1487–1495 [DOI] [PubMed] [Google Scholar]
- Klein R., Lee K., Gangnon R., Klein B. (2010) The 25-year incidence of visual impairment in type 1 diabetes mellitus. The Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology 117: 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen L., Lervang H., Lundbye-Christensen S., Gorst-Rasmussen A. (2007) The North Jutland County Diabetic Retinopathy Study (NCDRS) 2. Non-ophthalmic parameters and clinically significant macular oedema. Br J Ophthalmol 91: 1593–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen S., Bek T., Poulsen P., Hove M., Rehling M., Mogensen C. (2002) Macular edema reflects generalized vascular hyperpermeability in type 2 diabetic patients with retinopathy. Diabetes Care 25: 2328–2334 [DOI] [PubMed] [Google Scholar]
- Kofoed-Enevoldsen A., Jensen T., Borch-Johnsen K., Deckert T. (1987) Incidence of retinopathy in type i (insulin-dependent) diabetes: association with clinical nephropathy. J Diabet Complications 1: 96–99 [DOI] [PubMed] [Google Scholar]
- Kramer C., Rodrigues T., Canani L., Gross J., Azevedo M. (2011) Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta-analysis of observational studies. Diabetes Care 34: 1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T., Westenskow P., Bravo S., Aguilar E., Friedlander M. (2012) Targeted deletion of VEGFA in adult mice induces vision loss. J Clin Invest 122: 4213–4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachin J., Genuth S., Nathan D., Zinman B., Rutledge B. (2008) Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial –revisited. Diabetes 57: 995–1001 [DOI] [PubMed] [Google Scholar]
- Lyons T., Jenkins A., Zheng D., Lackland D., Mcgee D., Garvey W., et al. (2004) Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Via Sci 45: 910–918 [DOI] [PubMed] [Google Scholar]
- Massin P., Bandello F., Garweg J., Hansen L., Harding S., Larsen M., et al. (2010) Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 33: 2399–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer M., Zinman B., Gardiner R., Suissa S., Sinaiko A., Strand T., et al. (2009) Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 361: 40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Bandello F., Schmidt-Erfurth U., Lang G., Massin P., Schlingemann R., et al. (2011) The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118: 615–625 [DOI] [PubMed] [Google Scholar]
- Parravano M., Menchini F., Virgili G. (2009) Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev: CD007419. [DOI] [PubMed] [Google Scholar]
- Parravano M., Oddone F., Mineo D., Centofanti M., Borboni P., Lauro R., et al. (2008) The role of humphrey matrix testing in the early diagnosis of retinopathy in type 1 diabetes. Br J Ophthalmol 92: 1656–1660 [DOI] [PubMed] [Google Scholar]
- Rosenfeld P., Shapiro H., Tuomi L., Webster M., Elledge J., Blodi B. (2011) Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 118: 523–530 [DOI] [PubMed] [Google Scholar]
- Rudnisky C., Lavergne V., Katz D. (2009) Visual acuity after intravitreal triamcinolone for diabetic macular edema refractory to laser treatment: a meta-analysis. Can J Ophthalmol 44: 587–593 [DOI] [PubMed] [Google Scholar]
- Sjolie A., Klein R., Porta M., Orchard T., Fuller J., Parving H., et al. (2008) Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-PROTECT 2): a randomised placebo-controlled trial. Lancet 372: 1385–1393 [DOI] [PubMed] [Google Scholar]
- Sjolie A., Klein R., Porta M., Orchard T., Fuller J., Parving H., et al. (2012) Retinal microaneurysm count predicts progression and regression of diabetic retinopathy. post-hoc results from the DIRECT programme. Diabet Med 28: 345–351 [DOI] [PubMed] [Google Scholar]
- Stratton I., Kohner E., Aldington S., Turner R., Holman R., Manley S., et al. (2001) UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 44: 156–163 [DOI] [PubMed] [Google Scholar]
- Tam J., Dhamdhere K., Tiruveedhula P., Manzanera S., Barez S., Bearse M., Jr., et al. (2011) Disruption of the retinal parafoveal capillary network in type 2 diabetes before the onset of diabetic retinopathy. Invest Ophthalmol Via Sci 52: 9257–9266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Jonas J. (2011) Intravitreal triamcinolone. Ophthalmologica 225: 1–20 [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group (1997) Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes 46: 1829–1839 [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group (1998) Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol 116: 874–886 [DOI] [PubMed] [Google Scholar]
- Tong Z., Yang Z., Patel S., Chen H., Gibbs D., Yang X., et al. (2008) Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci U S A 105: 6998–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick G., Edwards P., Desai U., Morton P., Latif Z., Berkowitz B. (2008) MRI retinovascular studies in humans: research in patients with diabetes. NMR Biomed 21: 1003–1012 [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Br Med J 317: 703–713 [PMC free article] [PubMed] [Google Scholar]
- White N., Sun W., Cleary P., Tamborlane W., Danis R., Hainsworth D., et al. (2010) Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes 59: 1244–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson C., Ferris F., Klein R., Lee P., Agardh C., Davis M., et al. (2003) Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110: 1677–1682 [DOI] [PubMed] [Google Scholar]
- Williams W., Salem R., Mcknight A., Sandholm N., Forsblom C., Taylor A., et al. (2012) Association testing of previously reported variants in a large case-control meta-analysis of diabetic nephropathy. Diabetes 61: 2187–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T., Simo R., Mitchell P. (2012b) Fenofibrate - a potential systemic treatment for diabetic retinopathy? Am J Ophthalmol 154: 6–12 [DOI] [PubMed] [Google Scholar]
- Yau J., Rogers S., Kawasaki R., Lamoureux E., Kowalski J., Bek T., et al. (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35: 556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]


