Abstract
Objectives:
To compare the response between Chinese children with growth hormone deficiency (GHD) born either small for gestational age (SGA) or appropriate for gestational age (AGA) after 4 weeks of recombinant human growth hormone (r-hGH) therapy.
Methods:
This was a phase IV, open-label, multicenter, interventional study (NCT01187550). Prepubertal children with GHD received open-label treatment with daily r-hGH (0.033 mg/kg) for 4 weeks. Serum levels of insulin-like growth factor I (IGF-I) and insulin-like growth factor-binding protein 3 (IGFBP3), and metabolic markers (including fasting glucose, insulin, total cholesterol, and homeostasis model assessment of insulin resistance) were assessed at baseline and after 4 weeks of treatment, and were analyzed according to patient subgroup (SGA or AGA).
Results:
A total of 205 children with GHD (mean age 10.4 years; 175 AGA, 30 SGA) were included in the analysis. Mean baseline serum IGF-I and IGFBP3 standard deviation scores (SDS) across the whole patient population were lower than the population norms (mean values: -2.1 SDS for IGF-I and -1.2 SDS for IGFBP3), with no significant differences between the two patient subgroups. After 4 weeks, IGF-I and IGFBP3 levels increased by 1.0 SDS (p < 0.001) and 0.34 SDS (p < 0.001), respectively, but no significant differences were found between the two patient subgroups for growth-related or metabolic markers.
Conclusions:
For children with GHD born SGA, IGF-I and IGFBP3 are short-term biomarkers of responsiveness to treatment with growth hormone, as for children with GHD born AGA.
Keywords: biomarker response, insulin-like growth factor I, insulin-like growth factor-binding protein 3, recombinant human growth hormone
Introduction
Newborn infants are classified as small for gestational age (SGA) if their weight or length is below −2 standard deviation scores (SDS) for their gestational age and sex [Lee et al. 2003; Saenger et al. 2007]. In most babies born SGA, normal height is achieved following a period of catch-up growth before the age of 2 years [Hokken-Koelega et al. 1995; Karlberg and Albertsson-Wikland, 1995; Clayton et al. 2007]. However, in 10–15% of babies born SGA, catch-up growth is absent or limited, and they remain below -2 SDS for height throughout childhood, adolescence, and into adulthood [Leger et al. 1998]. Children who are born SGA and who do not experience postnatal catch-up growth before 2 years of age are at significantly higher risk of short adult stature than children who are born appropriate for gestational age (AGA) [Albertsson-Wikland and Karlberg, 1994; Hokken-Koelega et al. 1995; Karlberg and Albertsson-Wikland, 1995; Labarta et al. 2009]. Lam and colleagues reported that 33–35% of Chinese children born SGA were short at 6–12 months compared with 7–12% born AGA [Lam et al. 1995]. In a longitudinal study, 13.6% of children born SGA had short stature at final height compared with only 1.8% of those born AGA [Leger et al. 1998]. The reasons for poor postnatal growth in a proportion of children born SGA are poorly understood. Functional defects in the growth hormone–insulin-like growth factor (GH–IGF) axis may account for some of the growth retardation: up to 60% of children with short stature who were born SGA have GH-secretion abnormalities and/or reduced levels of IGF-I [Albertsson-Wikland, 1989; Rochiccioli et al. 1989; Stanhope et al. 1989; de Waal et al. 1994; Boguszewski et al. 1995, 1996; Völkl et al. 2004].
Children with short stature who were born SGA often have GH levels lower than normal, but do not meet the standard criteria for growth hormone deficiency (GHD). However, children born SGA who do not show adequate catch-up growth may be eligible for treatment with recombinant human growth hormone (r-hGH), which can help achieve rapid catch-up growth and maintain a normal growth pattern during childhood, leading to the ultimate goal of normal adult height [de Zegher et al. 1996; Ranke and Lindberg, 1996; de Zegher et al. 1997; Boguszewski et al. 1998; Sas et al. 1999; Saenger et al. 2007; Labarta et al. 2009; Maiorana and Cianfarani, 2009; Bannink et al. 2010; Prasad et al. 2012].
There are few publications on the response of children with GHD born SGA to r-hGH treatment [Cacciari et al. 1999; Di Cesare et al. 2005; Ranke et al. 2011]. Here we report the findings from an open-label, multicenter, phase IV study in which patients previously diagnosed with GHD were treated for 4 weeks with r-hGH. The overall aim of this study was to compare the short-term response to treatment with r-hGH in Chinese children with GHD born AGA or SGA, as measured by changes in surrogate markers of GH action. Here, we present data on changes in serum growth-related biomarkers and metabolic markers after 4 weeks of treatment in a population of children with a diagnosis of GHD, and compare the response in those born SGA with those born AGA.
Materials and methods
Study design
This was a phase IV, open-label, multicenter, interventional study of prepubertal children with GHD treated with r-hGH (NCT01187550). The study was conducted at eight centers in China (Ruijin Hospital [Shanghai], Xinhua Hospital [Shanghai], Children’s Hospital of Fudan University [Shanghai], Beijing Children’s Hospital [Beijing], Tongji Hospital [Wuhan], First Affiliated Hospital of Sun Yat-sen University [Guangzhou], Children’s Hospital of Zhejiang University School of Medicine [Hangzhou], and Union Hospital [Wuhan]) between May 2007 and April 2009. The study was approved by the medical ethics committees of the individual centers and conducted in accordance with Good Clinical Practice guidelines.
Participants
Eligible patients were prepubertal (Tanner stage 1), with a documented diagnosis of GHD (peak GH response of < 10 μg/ml with two stimulation tests without priming with estradiol), and normal thyroid function (or adequate substitution therapy for at least 3 months). Patients who had acquired GHD attributable to central nervous system tumor, trauma, infection, or infiltration, or a history of irradiation or cranial surgery were excluded. Other exclusion criteria included previous treatment with GH, GH-releasing hormone, anabolic steroids, or any treatment affecting growth, or with corticosteroids (except topical or inhaled corticosteroids for atopic disease), and severe associated pathology affecting growth such as malnutrition, malabsorption, or bone dysplasia. Written informed consent was obtained from the parents of the children treated. At enrolment, demographic data and medical history were obtained along with physical measurements (including weight, height, and body mass index). The total population of patients with GHD (GHD-all) was divided into two subgroups: those who were born SGA (birth weight and/or length below -2 SDS for their gestational age; GHD-SGA), and those who were born AGA (GHD-AGA) (all children who were not classified as being born SGA) [Qin et al. 1988; Li, 2009]. SDS was defined as (actual value - mean)/standard deviation (SD), where the mean and SD are from reference standards.
Study treatment
All patients were treated according to the standard care and medical practice of the study site. Patients were screened prior to the start of treatment to establish GHD and normal thyroid function. From week 0, patients received open-label treatment with r-hGH (Saizen®, Merck Serono SA, Geneva, Switzerland, a branch of Merck Serono S.A., Coinsins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany) administered subcutaneously daily at bedtime using an autoinjector device (one.click®, Merck Serono SA, Geneva, Switzerland, a branch of Merck Serono S.A., Coinsins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany) for 4 weeks, at 0.033 mg/kg/day (the approved dosage in China for GHD).
Laboratory analyses
Physical examinations and blood sampling for central laboratory analysis (MDS Pharma Services [China] Inc., Beijing, China) were performed before the start and after the end of treatment.
Efficacy analyses
The primary endpoint was the change in serum IGF-I SDS at 4 weeks. Secondary endpoints included changes at 4 weeks in insulin-like growth factor-binding protein 3 (IGFBP3) SDS, levels of IGF-I, IGFBP3, fasting glucose, and fasting insulin, and in insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR] test), and lipid profiles (total, low-density lipoprotein and high-density lipoprotein cholesterol, and triglycerides). IGF-I SDS and IGFBP3 SDS scores were calculated based on (log10 actual value - log10 mean reference value)/log10 reference SD from Elmlinger and colleagues [Elmlinger et al. 2004].
The intent-to-treat (ITT) population (all children who received at least one dose of study medication) was used for all efficacy analyses.
Statistical analysis
The study was designed to test the null hypothesis that the median change from baseline for IGF-I SDS was the same for children with GHD born AGA and SGA after 4 weeks of treatment with r-hGH. Subjects were enrolled in a 3:1 ratio for AGA:SGA children. A total of 168 evaluable subjects were required to provide an 80% power to test the hypothesis at the 0.05 two-sided level of significance for a point difference of 0.9 in the change in IGF-I SDS after 4 weeks of treatment between children with GHD born AGA and SGA, based on a point difference of 2.3 at 28 days after treatment with an SD of 1.78 [Ranke et al. 2005]. Height standards were obtained from Chinese growth reference data [Li, 2009]. Differences between the baseline characteristics of the GHD-AGA group and GHD-SGA group were assessed using the Wilcoxon rank sum test for continuous variables and Fisher’s exact test for gender. For each efficacy endpoint, the signed rank sum test was used to assess whether the within-group change from baseline was different from zero, and the Wilcoxon rank sum test was used to assess whether the between-group difference in change from baseline was different from zero. A test result was considered to be significant if p < 0.05.
Results
Patient baseline clinical characteristics
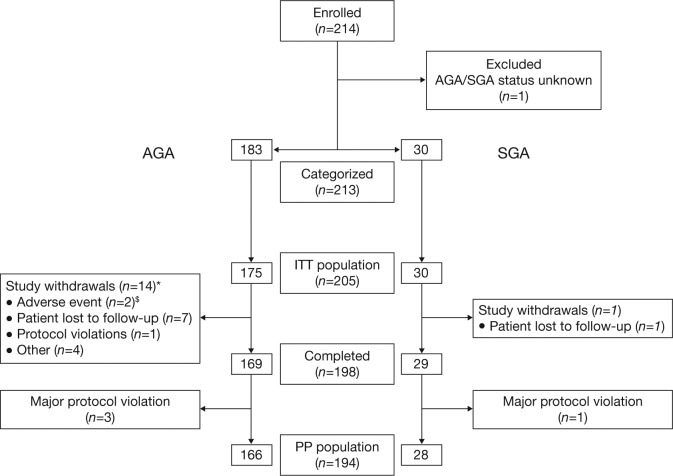
In total, 214 children with GHD were enrolled into the study. Of these, 205 were included in the ITT population: 175 children born AGA (129 males [73.7%]; mean [SD] age 10.5 [3.84] years), and 30 born SGA (24 males [80.0%]; mean [SD] age 9.4 [4.35] years) (Figure 1). The mean (SD) peak GH level in response to provocative testing was 4.4 (3.1) μg/ml, confirming a diagnosis of GHD. Children were categorized as having severe GHD (GH peak values < 5 μg/ml; n = 114; 55.6%), or less severe GHD (GH peak values ≥ 5 μg/ml and < 10 μg/ml; n = 91; 44.4%).
Figure 1.
Patient flow diagram.
*Of these 14 study withdrawals, 8 did not receive at least one dose of recombinant human growth hormone; $bronchitis and headache.
AGA, appropriate for gestational age; ITT, intent-to-treat; PP, per protocol; SGA: small for gestational age.
In the GHD-all group, the height SDS, and serum IGF-I SDS and IGFBP3 SDS values were below those of the reference population (Table 1). The mean height SDS was significantly lower in the GHD-SGA than in the GHD-AGA subgroup at baseline. There were no significant differences between the GHD-AGA and GHD-SGA subgroups for IGF-I SDS and IGFBP3 SDS at baseline.
Table 1.
Clinical characteristics of children at birth and before treatment.
| Parameter | GHD–all (N = 205) | GHD–AGA (n = 175) | GHD–SGA (n = 30) | p value* |
|---|---|---|---|---|
| At birth | ||||
| Male (%) | 153 (74.6) | 129 (73.7) | 24 (80.0) | 0.6499 |
| Gestational age (weeks) | 38.86 (2.19)a | 38.8 (2.32)b | 39.4 (1.02)c | 0.2363 |
| Birth length SDS | −0.64 (1.48)d | −0.24 (0.69)e | −3.0 (2.48)f | <0.0001 |
| Birth weight SDS | −0.42 (1.25)g | −0.13 (1.03)h | −2.2 (1.02)i | <0.0001 |
| At start of treatment with r–hGH | ||||
| Chronological age (years) | 10.4 (3.93) | 10.5 (3.84) | 9.4 (4.35) | 0.1012 |
| Height SDS | −3.5 (1.70) | −3.3 (1.57) | −4.52 (2.07) | 0.0006 |
| BMI (kg/m2) | 16.2 (2.75) | 16.2 (2.75) | 16.0 (2.77) | 0.7153 |
| IGF–I (ng/ml) | 115.1 (75.44) | 117.1 (74.02) | 103.8 (83.73) | 0.2024 |
| IGF–I SDS | −2.1 (2.34) | −2.0 (2.34) | −2.2 (2.39) | 0.7681 |
| IGFBP3 (μg/ml) | 3.1 (1.42) | 3.2 (1.39) | 2.6 (1.53) | 0.0487 |
| IGFBP3 SDS | −1.2 (2.40) | −1.2 (2.37) | −1.7 (2.59) | 0.3458 |
Data are mean (standard deviation) values unless noted otherwise.
p values calculated using the Wilcoxon rank sum test, with the exception of that for gender (Fisher’s exact test). a n = 202; b n = 173; c n = 29; d n = 116; e n = 99; f n = 17; g n = 197; h n = 169; i n = 28.
AGA, appropriate for gestational age; BMI, body mass index; GHD, growth hormone deficiency; IGF–I, insulin–like growth factor I; IGFBP3, insulin–like growth factor–binding protein 3; r–hGH, recombinant human growth hormone; SDS, standard deviation score; SGA, small for gestational age.
Changes in serum IGF-1 and IGFBP3 levels after r-hGH treatment
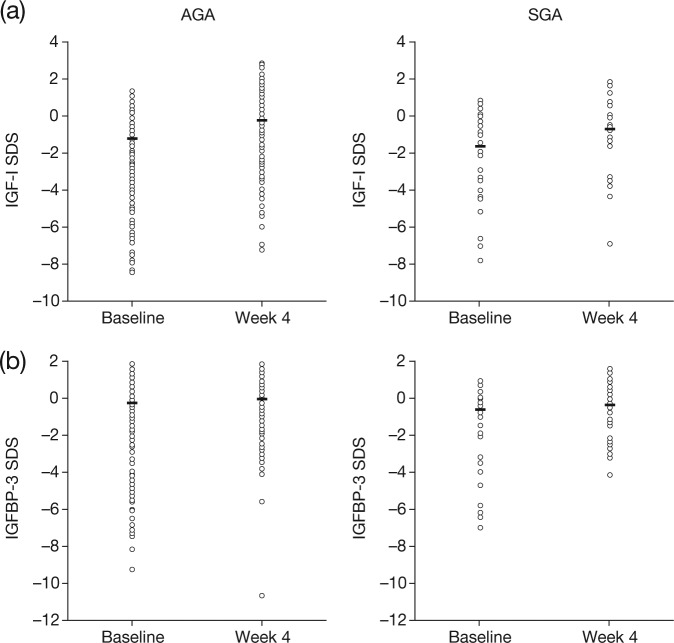
Individual patient data for IGF-I SDS and IGFBP3 at baseline and week 4 are shown in Figure 2(a) and (b), respectively. The median (interquartile range [IQR]) serum IGF-I SDS in the GHD-all group at baseline and after 4 weeks of treatment were −1.27 (−3.5; −0.3) and −0.36 (−1.6; 0.7) SDS, respectively, a median (IQR) increase of 1.00 (0.4; 1.9) SDS (p < 0.001) (Table 2). Although the median change from baseline in IGF-I SDS in the GHD-AGA subgroup was numerically greater than in the GHD-SGA subgroup, the difference between the subgroups did not reach significance (Table 2).
Figure 2.
Growth-related biomarkers in children with growth hormone deficiency born appropriate for gestational age and small for gestational age at baseline and after 4 weeks of treatment with recombinant human growth hormone: (a) insulin-like growth factor I standard deviation score; (b) insulin-like growth factor-binding protein 3 standard deviation score.
Individual patient values for growth-related biomarkers are shown for each patient subgroup/time point. Median values are indicated by the line within each dataset.
IGF-I, insulin-like growth factor I; IGFBP3, insulin-like growth factor-binding protein 3; SDS, standard deviation score.
Table 2.
Comparative changes in serum growth–related biomarkers and metabolic markers after 4 weeks of treatment with recombinant human growth hormone within* and between** patient groups.
| Parameter | GHD–all (N = 205) | GHD–AGA (n = 175) | GHD–SGA (n = 30) | p value** (GHD–AGA versus GHD–SGA) |
|---|---|---|---|---|
| IGF–I SDS | 1.00 (0.4; 1.9)a | 1.01 (0.5; 2.0)b | 0.92 (0.2; 1.6)c | 0.194 |
| p value* | < 0.001 | < 0.001 | < 0.001 | |
| IGFBP3 SDS | 0.34 (0.0; 1.3)a | 0.29 (0.0; 1.0)b | 0.59 (0.1; 1.6)c | 0.232 |
| p value* | < 0.001 | < 0.001 | < 0.001 | |
| Fasting glucose (mmol/L) | 0.20 (−0.2; 0.5)d | 0.20 (−0.2; 0.5)e | 0.15 (−0.3; 0.5)c | 0.710 |
| p value* | < 0.001 | < 0.001 | 0.405 | |
| Fasting insulin (pmol/L) | 3.50 (0.0; 23.5)f | 5.00 (0.0; 24.0)g | 0.00 (−5.0; 19.0)h | 0.265 |
| p value* | < 0.001 | < 0.001 | 0.097 | |
| Insulin resistance (HOMA–IR) | 1.00 (−0.3; 5.7)i | 1.14 (−0.2; 5.9)j | 0.25 (−0.6; 4.8)h | 0.308 |
| p value* | < 0.001 | < 0.001 | 0.168 | |
| Fasting total cholesterol (mmol/L) | −0.18 (−0.6; 0.1)a | −0.21 (−0.6; 0.1)b | −0.04 (−0.4; 0.2)c | 0.078 |
| p value* | < 0.001 | < 0.001 | 0.574 | |
| Triglycerides (mmol/L) | 0.12 (−0.2; 0.4)k | 0.14 (−0.1; 0.4)b | 0.02 (−0.3; 0.2)l | 0.091 |
| p value* | < 0.001 | < 0.001 | 0.873 |
Data are median (interquartile range) values.
Signed rank sum test used to assess that the *within–group change from baseline was different from zero, and the Wilcoxon rank sum test used to assess that the **between–group difference of change from baseline was different from zero. a n = 196; b n = 168; c n = 28; d n = 195; e n = 167; f n = 188; g n = 165; h n = 23; i n = 187; j n = 164; k n = 194; l n = 26.
AGA, appropriate for gestational age; GHD, growth hormone deficiency; HOMA–IR, homeostatic model assessment of insulin resistance; IGF–I, insulin–like growth factor I; IGFBP3, IGF–binding protein 3; SDS, standard deviation score; SGA, small for gestational age.
The median (IQR) serum IGFBP3 SDS values in the GHD-all group at baseline and 4 weeks were −0.23 (−2.2; 0.5) and −0.06 (−0.9; 0.7) SDS, respectively, with a median (IQR) increase of 0.34 (0.0; 1.3) SDS (p < 0.001) (Table 2). Although the change in the GHD-AGA subgroup was numerically less than that of the GHD-SGA subgroup, the difference between the subgroups did not reach significance (Table 2).
Changes in fasting glucose, fasting insulin, and insulin resistance after r-hGH treatment
Fasting glucose, insulin levels, and insulin resistance (HOMA-IR test) all increased after 4 weeks of GH treatment in the GHD-all group (p < 0.001) (Table 2). The magnitudes of the median changes in these parameters were significantly different from zero in the GHD-AGA subgroup (all p < 0.001), but not for the GHD-SGA subgroup. The changes in these parameters were not significantly different between the GHD-AGA and GHD-SGA subgroups.
Changes in lipid profile after r-hGH treatment
In the GHD-all group, fasting total cholesterol levels decreased from baseline to week 4 (Table 2). Changes in total cholesterol levels were significantly different from zero in the GHD-all group and the GHD-AGA subgroup (p < 0.001), but were not significantly different from zero for the GHD-SGA subgroup. The between-subgroup (GHD-AGA versus GHD-SGA) difference in change in total cholesterol from baseline was not significantly different from zero.
The median level of triglycerides in the GHD-all group and GHD-AGA subgroup significantly increased from baseline to week 4 (p < 0.001), but not in the GHD-SGA subgroup. The between-subgroup (GHD-AGA versus GHD-SGA) difference in change in triglycerides from baseline between the GHD-AGA subgroup and the GHD-SGA subgroup was not significantly different from zero.
Discussion
This study provides an insight into the changes observed in serum markers after short-term treatment with r-hGH in Chinese children with GHD categorized as AGA or SGA on the basis of their birth weight or length relative to gestational age. We found that in the study population with GHD (GHD-all), and also the subgroup of patients with GHD who were born AGA, significant changes from baseline were observed for all growth-related biomarkers and metabolic markers studied after 4 weeks of r-hGH therapy. In those children born SGA, r-hGH therapy significantly increased serum levels of growth-related biomarkers (IGF-I and IGFBP3), but not metabolic markers. However, there were no significant differential effects between the GHD-SGA and GHD-AGA subgroups for any marker. An increase in IGF-I and IGFBP3 after short-term GH treatment in children with GHD is well established; our data confirm that these are markers of sensitivity to GH treatment in children with GHD who were born SGA, as well as those born AGA.
As there is an increased prevalence of metabolic disorders in later life among subjects who were born SGA, monitoring of glucose homeostasis is recommended in children born SGA who are treated with r-hGH [Chatelain et al. 2007]. Although alterations in insulin sensitivity have been observed after long-term GH therapy in short children born SGA, no adverse effects on glucose levels are thought to occur [Sas, 2001; Hokken-Koelega et al. 2004; Cutfield et al. 2006; Chatelain et al. 2007]. In our study, insulin resistance was not significantly changed from baseline in children born SGA, or after 4 weeks of r-hGH treatment in those born SGA relative to those born AGA, suggesting that short-term GH therapy did not lead to changes in glucose metabolism.
The majority of children born SGA who have persistent short stature do not meet the criteria for GHD, and there are few publications on the response to r-hGH treatment in children born SGA who also have GHD. In a retrospective study of 108 Italian children, birth weight was reported to influence final height in children receiving GH therapy for GHD [Cacciari et al. 1999]. However, in a smaller study of 35 children, no difference in adult height was reported between AGA and SGA groups after treatment with GH, although the median GH dosage was higher, and treatment was for longer in the SGA group [Di Cesare et al. 2005]. In common with our study, a recent retrospective study of short Italian children with GHD born SGA or AGA reported no significant difference between these subgroups in IGF-I and growth responses after 1 year of r-hGH therapy; in this study, all children had a low birth weight [Ranke et al. 2011].
Our study population provides a rare opportunity to study a population of short children born SGA with an established diagnosis of GHD and, to our knowledge, is the first study to compare the response to 4 weeks of r-hGH treatment in children with GHD born SGA or AGA. Furthermore, this is the largest cohort study to date to evaluate changes in serum biomarkers after short-term r-hGH treatment in Chinese children with GHD, and the largest study to date to compare children with GHD born SGA and AGA. In addition, the fact that the same dose of r-hGH was used in the AGA and SGA populations meant that any differences in sensitivity to treatment could be explored.
However, the conclusions that can be drawn from the data presented here are limited. Several factors may have contributed to the observation that there were no differences between the two subgroups in response to treatment in terms of changes in serum markers. The number of patients in the GHD-SGA was lower than planned due to recruitment challenges, and a high level of variability was seen between individuals in both subgroups. In addition, to permit comparisons, the same dose of r-hGH was used in the SGA and AGA subgroups; thus, the dose used in the SGA subgroup was suboptimal. Furthermore, this was a short-term study; longer follow-up to obtain relevant longitudinal data for biomarkers, metabolic markers, and growth would be of interest. We also acknowledge that the reference ranges used to calculate SDS values for IGF-I and IGFBP3 were based on a European, not Chinese, population [Elmlinger et al. 2004].
In summary, serum IGF-I and IGFBP3 levels increased after 4 weeks of GH therapy in children with GHD born SGA or AGA. This confirms the role of these markers of responsiveness to treatment with r-hGH in this patient population and supports monitoring serum IGF levels as a practical means of managing r-hGH therapy, even in patients less sensitive to treatment, such as those born SGA.
Acknowledgments
Merck Serono China (an affiliate of Merck KGaA, Darmstadt, Germany) supported the design and conduct of the study, and data collection, management, and analysis. The authors thank for their contribution to the conduct of the study: F. Luo, R. Ye, R. Cheng and Z. Zhao (Children’s Hospital of Fudan University); M. Liu and Y. Li (Beijing Children’s Hospital); H. Ma and H. Chen (First Affiliated Hospital of Sun Yat-sen University); Y. Hao (Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology); L. Liang, X.Q. Chen, J.T. Lou and X.P. Zheng (Children’s Hospital of Zhejiang University School of Medicine); L. Han (Xinhua Hospital, Shanghai Jiaotong University School of Medicine); D. Zhou (Union Hospital, Tongji Medical College of Huazhong University of Science and Technology). The authors thank M. Wong, Caudex Medical (supported by funding from Merck Serono SA, Geneva, Switzerland, a branch of Merck Serono SA, Coinsins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany), for assistance in the preparation of this manuscript.
Footnotes
Funding: This work was supported by Merck Serono China (an affiliate of Merck KGaA, Darmstadt, Germany).
Conflict of interest statement: M. D. has received honoraria and research funding from the study sponsor for clinical studies. Q.Z. is an employee of Merck Serono China. W.L., S.S., X.L., C.G., X.G., Y.L., R.J. and W.W. have nothing to disclose.
Contributor Information
Wenli Lu, Ruijin Hospital, Jiaotong University School of Medicine, Shanghai, China.
Shuixian Shen, Children’s Hospital of Fudan University, Shanghai, China.
Xiaoping Luo, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China.
Chunxiu Gong, Beijing Children’s Hospital, Capital Medical University, Beijing, China.
Xuefan Gu, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Yun Li, Children’s Hospital of Zhejiang University School of Medicine, Hangzhou, China.
Minlian Du, First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Beijing, China.
Runming Jin, Union Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China.
Queena Zhou, Merck Serono China, Beijing, China.
Wei Wang, Ruijin Hospital, Jiaotong University School of Medicine, No. 197, 2nd Ruijin Road, Shanghai, Shanghai City 200025, China.
References
- Albertsson-Wikland K. (1989) Growth hormone secretion and growth hormone treatment in children with intrauterine growth retardation. Swedish Paediatric Study Group for Growth Hormone Treatment. Acta Paediatr Scand Suppl 349: 35–41 [DOI] [PubMed] [Google Scholar]
- Albertsson-Wikland K., Karlberg J. (1994) Natural growth in children born small for gestational age with and without catch-up growth. Acta Paediatr Suppl 399: 64–70 [DOI] [PubMed] [Google Scholar]
- Bannink E., Djurhuus C., Christensen T., Jøns K., Hokken-Koelega A. (2010) Adult height and health-related quality of life after growth hormone therapy in small for gestational age subjects. J Med Econ 13: 221–227 [DOI] [PubMed] [Google Scholar]
- Boguszewski M., Albertsson-Wikland K., Aronsson S., Gustafsson J., Hagenäs L., Westgren U., et al. (1998) Growth hormone treatment of short children born small-for-gestational-age: the Nordic Multicentre Trial. Acta Paediatr 87: 257–263 [DOI] [PubMed] [Google Scholar]
- Boguszewski M., Jansson C., Rosberg S., Albertsson-Wikland K. (1996) Changes in serum insulin-like growth factor I (IGF-I) and IGF-binding protein-3 levels during growth hormone treatment in prepubertal short children born small for gestational age. J Clin Endocrinol Metab 81: 3902–3908 [DOI] [PubMed] [Google Scholar]
- Boguszewski M., Rosberg S., Albertsson-Wikland K. (1995) Spontaneous 24-hour growth hormone profiles in prepubertal small for gestational age children. J Clin Endocrinol Metab 80: 2599–2606 [DOI] [PubMed] [Google Scholar]
- Cacciari E., Zucchini S., Cicognani A., Pirazzoli P., Balsamo A., Salardi S., et al. (1999) Birth weight affects final height in patients treated for growth hormone deficiency. Clin Endocrinol (Oxf) 51: 733–739 [DOI] [PubMed] [Google Scholar]
- Chatelain P., Carrascosa A., Bona G., Ferrandez-Longas A., Sippell W. (2007) Growth hormone therapy for short children born small for gestational age. Horm Res 68: 300–309 [DOI] [PubMed] [Google Scholar]
- Clayton P., Cianfarani S., Czernichow P., Johannsson G., Rapaport R., Rogol A. (2007) Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab 92: 804–810 [DOI] [PubMed] [Google Scholar]
- Cutfield W., Lindberg A., Rapaport R., Wajnrajch M., Saenger P. (2006) Safety of growth hormone treatment in children born small for gestational age: the US trial and KIGS analysis. Horm Res 65(Suppl 3): 153–159 [DOI] [PubMed] [Google Scholar]
- de Waal W., Hokken-Koelega A., Stijnen T., de Muinck Keizer-Schrama S., Drop S. (1994) Endogenous and stimulated GH secretion, urinary GH excretion, and plasma IGF-I and IGF-II levels in prepubertal children with short stature after intrauterine growth retardation. The Dutch Working Group on Growth Hormone. Clin Endocrinol (Oxf) 41: 621–630 [DOI] [PubMed] [Google Scholar]
- de Zegher F., Butenandt O., Chatelain P., Albertsson-Wikland K., Jonsson B., Löfström A., et al. (1997) Growth hormone treatment of short children born small for gestational age: reappraisal of the rate of bone maturation over 2 years and metanalysis of height gain over 4 years. Acta Paediatr Suppl 423: 207–212 [DOI] [PubMed] [Google Scholar]
- de Zegher F., Maes M., Gargosky S., Heinrichs C., Du Caju M., Thiry G., et al. (1996) High-dose growth hormone treatment of short children born small for gestational age. J Clin Endocrinol Metab 81: 1887–1892 [DOI] [PubMed] [Google Scholar]
- Di Cesare M., Bozzola E., Castelnovi C., Chiabotto L., Costante C., De Sanctis L., et al. (2005) Adult height in patients treated for isolated growth hormone deficiency: role of birth weight. Horm Res 63: 102–106 [DOI] [PubMed] [Google Scholar]
- Elmlinger M., Kühnel W., Weber M., Ranke M.(2004) Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-1) and IGF binding protein 3 (IGFBP-3). Clin Chem Lab Med 42: 654–664 [DOI] [PubMed] [Google Scholar]
- Hokken-Koelega A., De Ridder M., Lemmen R., Den Hartog H., de Muinck Keizer-Schrama S., Drop S. (1995) Children born small for gestational age: do they catch up? Pediatr Res 38: 267–271 [DOI] [PubMed] [Google Scholar]
- Hokken-Koelega A., de Waal W., Sas T., Van Pareren Y., Arends N. (2004) Small for gestational age (SGA): endocrine and metabolic consequences and effects of growth hormone treatment. J Pediatr Endocrinol Metab 17(Suppl 3): 463–469 [PubMed] [Google Scholar]
- Karlberg J., Albertsson-Wikland K. (1995) Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res 38: 733–739 [DOI] [PubMed] [Google Scholar]
- Labarta J., Ruiz J., Molina I., De Arriba A., Mayayo E., Longás A. (2009). Growth and growth hormone treatment in short stature children born small for gestational age. Pediatr Endocrinol Rev 6(Suppl 3): 350–357 [PubMed] [Google Scholar]
- Lam B., Karlberg J., Low L., Yeung C. (1995) Incomplete catch-up growth in length in low birthweight Chinese infants in Hong Kong. J Paediatr Child Health 31: 428–434 [DOI] [PubMed] [Google Scholar]
- Lee P., Chernausek S., Hokken-Koelega A., Czernichow P. (2003) International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24–October 1, 2001. Pediatrics 111: 1253–1261 [DOI] [PubMed] [Google Scholar]
- Leger J., Limoni C., Collin D., Czernichow P. (1998) Prediction factors in the determination of final height in subjects born small for gestational age. Pediatr Res 43: 808–812 [DOI] [PubMed] [Google Scholar]
- Li H. (2009) Growth Chart of 0–18-Year-old Chinese Children and Adolescents. Shanghai: Second Military Medical University Press [Google Scholar]
- Maiorana A., Cianfarani S. (2009) Impact of growth hormone therapy on adult height of children born small for gestational age. Pediatrics 124: e519–e531 [DOI] [PubMed] [Google Scholar]
- Prasad H., Khadilkar V., Chiplonkar S., Khadilkar A. (2012) Growth of short children born small for gestational age and their response to growth hormone therapy. Indian Pediatrpii S097475591100905. [DOI] [PubMed] [Google Scholar]
- Qin Z., Jin H., Huang D. (1988) Newborn growth and development study group in 15 China urban cities. Chin J Pediatr 26: 206–208 [Google Scholar]
- Ranke M., Lindberg A. (1996). Growth hormone treatment of short children born small for gestational age or with Silver–Russell syndrome: results from KIGS (Kabi International Growth Study), including the first report on final height. Acta Paediatr Suppl 417: 18–26 [DOI] [PubMed] [Google Scholar]
- Ranke M., Martin D., Ehehalt S., Schwarze C., Serra F., Wollmann H., et al. (2011) Short children with low birth weight born either small for gestational age or average for gestational age show similar growth response and changes in insulin-like growth factor-1 to growth hormone treatment during the first prepubertal year. Horm Res Paediatr 76: 104–112 [DOI] [PubMed] [Google Scholar]
- Ranke M., Traunecker R., Martin D., Schweizer R., Schwarze C., Wollmann H., et al. (2005) IGF-I and IGF binding protein-3 levels during initial GH dosage step-up are indicators of GH sensitivity in GH-deficient children and short children born small for gestational age. Horm Res 64: 68–76 [DOI] [PubMed] [Google Scholar]
- Rochiccioli P., Tauber M., Moisan V., Pienkowski C. (1989) Investigation of growth hormone secretion in patients with intrauterine growth retardation. Acta Paediatr Scand Suppl 349: 42–46 [DOI] [PubMed] [Google Scholar]
- Saenger P., Czernichow P., Hughes I., Reiter E. (2007) Small for gestational age: short stature and beyond. Endocr Rev 28: 219–251 [DOI] [PubMed] [Google Scholar]
- Sas T. (2001) Carbohydrate metabolism during long-term growth hormone treatment in children with short stature born small for gestational age. Clin Endocrinol (Oxf) 54: 243–251 [DOI] [PubMed] [Google Scholar]
- Sas T., de Waal W., Mulder P., Houdijk M., Jansen M., Reeser M., et al. (1999) Growth hormone treatment in children with short stature born small for gestational age: 5-year results of a randomized, double-blind, dose-response trial. J Clin Endocrinol Metab 84: 3064–3070 [DOI] [PubMed] [Google Scholar]
- Stanhope R., Ackland F., Hamill G., Clayton J., Jones J., Preece M. (1989) Physiological growth hormone secretion and response to growth hormone treatment in children with short stature and intrauterine growth retardation. Acta Paediatr Scand Suppl 349: 47–52 [DOI] [PubMed] [Google Scholar]
- Völkl T., Schwöbel K., Simm D., Beier C., Rohrer T., Dörr H. (2004) Spontaneous growth hormone secretion and IGF1:IGFBP3 molar ratios in children born small for gestational age (SGA). Growth Horm IGF Res 14: 455–461 [DOI] [PubMed] [Google Scholar]