Abstract
Recruiting research participants based on genetic information generated about them in a prior study is a potentially powerful way to study the functional significance of human genetic variation, but also presents ethical challenges. To inform policy development on this issue, we conducted a survey of U.S. institutional review board chairs concerning the acceptability of recontacting genetic research participants about additional research and their views on the disclosure of individual genetic results as part of recruitment. Our findings suggest there is unlikely to be a “one-size-fits-all” solution, but rather several ethically acceptable approaches to genotype-driven recruitment depending on context. Disclosures made during the consent process for the original study and the clinical validity of the results are key considerations. Researchers must be prepared to communicate and answer questions in clear, lay language about what is known and not known about the role of genetics in their proposed area of research.
Keywords: Research recruitment, informed consent, disclosure of research results, genetic research, Institutional Review Boards
INTRODUCTION
Genotype-driven research recruitment is a potentially powerful tool for studying the functional significance of human genetic variation.1 With this approach, investigators use an existing study population for which genetic analyses have been conducted to identify individuals who possess a gene variant of interest. Those individuals are then recontacted to invite their participation in further research involving in-depth phenotyping to better understand the relationship between observable traits and that gene variant.2 This kind of “recruitment by genotype” eliminates the time-consuming and expensive step of screening new populations to find subjects who have the variant of interest.3 Such recruitment could be undertaken when investigators want to recontact selected participants in their own studies for further research4 or in the context of biobanks that maintain a link between stored biospecimens and data and identifying information.5 Conceivably, individuals who have particular gene variants could also be identified by searching across multiple datasets stored in centralized databases, such as dbGaP—an approach that could maximize the utility of the massive amounts of data generated in genome-wide association studies, only a tiny fraction of which is related to the disease or condition originally under study.6
Genotype-driven recruitment, however, presents ethical challenges. Concerns about the use and disclosure of genetic information—more commonly associated with participation in genetic research—are shifted to the recruitment phase when genetic information that is generated in one study is used as the basis for identifying and recontacting participants about further research.7 A central issue is the disclosure of individual genetic research results from the first study as part of the recruitment process for the second. There is a fundamental tension between disclosing genetic research results that may be unwanted and/or preliminary and easily misinterpreted, and leaving prospective participants uninformed about the purposes of the second study and why they are eligible to participate.8
Because of the vital role institutional review boards (IRBs) play in reviewing and approving approaches to research recruitment, IRB chairs are one of the stakeholder groups whose input is essential to the development of guidelines on ethical approaches to genotype-driven recruitment. We conducted an online survey to gather data on the opinions, experiences, and concerns of IRB chairs at U.S. institutions that received federal funding for genetics-related research between 2000 and 2010. Our survey focused on whether and under what conditions: (a) recontact for the purpose of genetic research recruitment should be allowed, and (b) individual genetic research results from the first study should be disclosed as part of the recruitment process for a second study. In general, our survey included broad questions to establish baseline opinions on these topics, followed by more nuanced questions concerning contextual factors that could potentially modify such opinions.
METHODS
Sample Assembly
We searched NIH RePORTER (http://projectreporter.nih.gov/reporter.cfm) for new research projects awarded in 2000–2010 using the phrase ‘gene OR genetic OR genome OR genomic.” This search resulted in a list of 599 uniquely-named institutions in the U.S. that had received such funding. We removed institutions from the list (n=65) that were unlikely to have conducted human subjects research involving genetic analyses (e.g., institutions dedicated to wildlife or agriculture, professional societies).
We attempted to match each remaining institution (n=534) to an IRB Organization (IORG) registered in the U.S. using a comprehensive roster of obtained from the Office for Human Research Protections (http://www.hhs.gov/ohrp/assurances/index.html). For 13 of the institutions, the matching IORG’s registration had expired or been deactivated. For 89 institutions, we were unable to identify a matching IORG. To account for the fact that many of these might use a commercial IRB, we added 30 such IRBs to our sample from a publicly available list (http://www.circare.org/info/commercialirb.htm).
The remaining 432 institutions mapped to 376 IORGs. Because an IORG can operate multiple IRBs, our final task was to identify one chair for each of the 376 IORGs and 30 commercial IRBs to whom we could direct our survey. We emailed the Human Protections Administrator at those with multiple chairs and asked for assistance identifying the chair with most experience reviewing human genetics research. In addition, the survey communications that went to all chairs included the statement, “If you are an IRB chair but would prefer to recommend another chair at your institution who has more experience reviewing human genetic research, please let us know and we will direct our invitation to that person.”
Instrument Development
We drafted our survey instrument based on our knowledge of the issues and literature concerning research recruitment, informed consent, disclosure of individual genetic research results, human research protections, and survey methodology. We revised the instrument based on iterative rounds of comments from all co-authors, as well as on feedback from cognitive pre-testing conducted among 9 local IRB chairs and senior members.
The final instrument (available upon request) consisted of 40 questions, primarily multiple choice and 5-point scale items, and took approximately 20 minutes to complete. The survey included a narrative description and diagram explaining the concept of genotype-driven recruitment. Many of the sections also included a lead-in statement, such as:
Imagine that you have a protocol to review where researchers want to undertake genotype-driven recontact for research recruitment (i.e., they would like to contact the subset of participants in one study who were found to have a particular gene variant in order to invite their participation in a second study to learn more about that gene variant). Understanding that your thinking may change based on the details of a particular protocol, what is your general inclination with regard to each of the following statements:
The Duke University Health System IRB determined that this study was exempt under 45 CFR 46.101(b)(2) and served as the IRB of record for the University of North Carolina at Chapel Hill. We did not offer a monetary incentive for participation.
Survey Implementation and Analysis
We implemented the survey on the Web using Checkbox Survey Software. The survey was fielded in October–November, 2010. Responses were downloaded from Checkbox for descriptive analysis using Stata 11.0. We assessed differences in responses to general vs. scenario-specific questions using Fisher’s exact χ2 tests.
RESULTS
Participation Rate
Of the 406 IRB chairs invited, 201 (50%) completed the survey. Most were white, non-Hispanic males, age 50 or older; most reported more than 4 years of service as an IRB chair and had a professional background in medicine or social science (Table 1). Over 80% chose “academic institution” as the best descriptor of their current institution. Over 75% said they were familiar with the review of human genetic research and 17% said they had been personally involved in reviewing a protocol involving genotype-driven recruitment.
Table 1.
Participant characteristics
| n | (%) * | |
|---|---|---|
| Years as IRB chair: Mean = 6.7; range = 1–40 | ||
| Age | ||
| <50 years | 37 | (18) |
| ≥50 years | 149 | (74) |
| Sex | ||
| Male | 129 | (64) |
| Female | 58 | (29) |
| Race | ||
| White | 173 | (86) |
| Asian | 4 | (2) |
| Black | 3 | (2) |
| American Indian/Alaska Native | 1 | (1) |
| Other | 2 | (1) |
| Hispanic | ||
| No | 180 | (90) |
| Yes | 7 | (4) |
| Professional background § | ||
| Medicine | 82 | (41) |
| Social sciences | 51 | (25) |
| Biological sciences | 26 | (13) |
| Epidemiology/public health | 18 | (9) |
| Bioethics | 13 | (7) |
| Humanities | 7 | (4) |
| Law | 3 | (2) |
| Other | 16 | (8) |
| Current institution | ||
| Academic institution | 164 | (82) |
| Independent IRB | 14 | (7) |
| Non-academic hospital/healthcare setting | 11 | (6) |
| Non-academic research institute | 10 | (5) |
| Other | 2 | (1) |
| Familiarity with review of human genetic research | ||
| More familiar † | 156 | (78) |
| Less familiar ‡ | 45 | (22) |
| Ever reviewed protocol involving genotype-driven recruitment | ||
| No | 145 | (72) |
| Yes | 34 | (17) |
| Unsure | 12 | (6) |
May not sum to 100% due to missing data
Respondents were allowed to choose more than one
Includes responses ‘somewhat familiar’, ‘familiar’ and ‘very familiar’
Includes responses ‘not at all familiar’ and ‘not too familiar’
Views about the Acceptability of Recontact for Genetic Research Recruitment
We asked a series of questions to establish baseline opinions about recontact—not necessarily genotype-driven—for the purposes of genetic research recruitment. There was considerable variation in responses to the general statement “Researchers should be allowed to contact participants in one genetic research study in order to invite their participation in another genetic research study.” Although 37% of chairs agreed with this statement, most either disagreed (27%) or selected “neither agree nor disagree” (36%). These findings suggest that other factors might have an important influence when IRBs are reviewing protocols involving recontact. Indeed, in more detailed questions examining specific aspects of planned recontact:
52% said it would be important that the second study focus on the same medical condition as the first
52% said it would be important that the second study involve the same researchers as the first; among those who indicated that it was not necessarily important for the same researchers be involved (n=90); 12% said it would be important that the second study at least be conducted at the same institution as the first
91% said it would be important that the possibility of such contact was disclosed during the consent process for the first study
Among those who said that disclosures about recontact during the original consent process were important (n=183), 91% agreed with the statement “Participants in genetic research should have a choice at the time they consent to one study about whether they are willing to be contacted about other studies in the future.”
In the specific context of genotype-driven research recruitment, a substantial majority of all chairs (90%) again indicated that statements in the original consent form regarding contact about future research would be an important consideration when determining whether such contact should be allowed. However, when we presented respondents with a hypothetical scenario in which a researcher submits a protocol involving genotype-driven recontact (Box 1), a somewhat different view emerged. In response to this scenario, in which the original consent form did not include any statements either allowing or prohibiting the possibility of contact about future research, half of chairs (51%) said they definitely or probably would allow the researcher to contact eligible participants. This represents a statistically significant departure from responses to our question about the general statement “Researchers should be allowed to contact participants in one genetic research study in order to invite their participation in another genetic research study” (p=.004) (Figure 1). Compared to their responses to this general statement, answers to our scenario specific question moved in a positive direction (more accepting of recontact) for 34% of chairs.
Figure 1. General vs. scenario-specific views of the acceptability of recontact for genetic research recruitment.
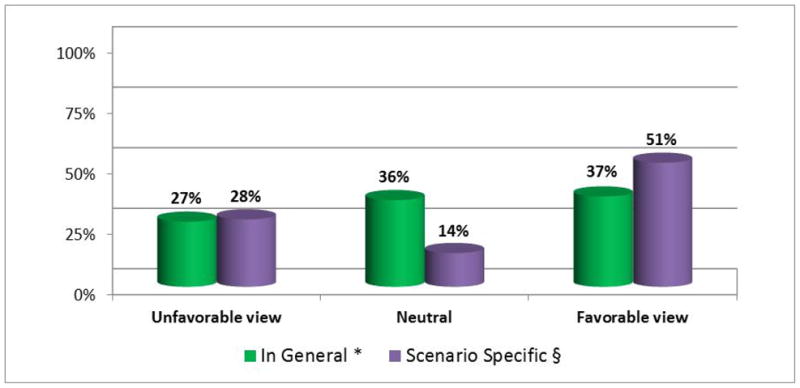
* Based on responses to the general statement, “Researchers should be allowed to contact participants in one genetic research study in order to invite their participation in another genetic research study.” Unfavorable views include those who disagreed or strongly disagreed with this statement; neutral views are those who selected ‘neither agree nor disagree’; favorable views include those who agreed or strongly agreed.
§ Based on responses to the scenario-specific question, “Would you allow Dr. Jones to contact eligible participants to invite their participation in a second study?” Unfavorable views include those who said they definitely or probably would not; neutral views are those who were undecided; favorable views include those who said they definitely or probably would.
We asked how alternate statements that could have been included in the hypothetical original consent form might modify chairs’ opinions about the acceptability of recontact. Excluding respondents who already indicated they “definitely would” allow contact (n=10), 84% said they would be more likely to allow recontact if the original consent form included the explicit statement “We may contact you about participating in other research studies.”
Taken together, these results suggest a high degree of consensus that consent disclosures about the possibility of future contact for the purpose of research recruitment are important and highly preferable, but that not all chairs necessarily view them as imperative. This opinion is captured by a comment offered by one chair in an open-ended text box at the end of our survey where we invited chairs to share any addition thoughts:
Autonomy is the most important principle in my opinion. We should give participants the right to be contacted, to know their results, and to participate in future research at the earliest opportunity (i.e., [during consent for] the original study). If that is not possible, then the decision must be based on other considerations such as the person making the contact, the medium of contact, etc. The latter case is necessarily a more difficult decision, but a blanket disapproval is not warranted, as it not only prevents the advancement of science, but also prevents giving subjects the opportunity to participate in science.
Comments from other chairs, however, demonstrate that some do consider such disclosures essential and may have little tolerance for their absence:
For me the answer depends on whether [participants] were told in the consent for study #1 that they might be contacted again at some future time. If that is not part of the original consent, the researchers would have a very steep slope to climb to convince me they should be allowed to re-contact these people.
If it is so important for future studies, investigators who did not have the simple common sense to ask permission for future contacts can just go out and replicate/extend their critically important research finding that spurs the “need” to contact people based on their private research records.
Disclosure of Research Results in the Context of Genotype-Driven Recontact
Similar to the general statement about the acceptability of recontact, there was considerable variation in responses to the general statement “Each participant should be offered his/her individual genetic results from the first study when contacted about taking part in the second study”. Although 42% agreed with this statement, most either disagreed (21%) or selected “neither agree nor disagree” (32%). This distribution again suggests that other factors may have an important influence on IRBs’ opinions about this issue. When asked:
87% of respondents said that statements in the consent form for the first study concerning disclosure of individual genetic research results would be important
86% said that the level of clinical validity of the first study’s findings would be important (clinical validity was defined as “the accuracy with which the presence of a gene variant predicts the presence of a clinical condition or predisposition”)
76% said that the level of clinical utility of the first study’s findings would be important (clinical utility was defined as “the availability and effectiveness of interventions aimed at avoiding the adverse clinical consequences of a gene variant”)
We also asked respondents to consider disclosure of research results in the context of the hypothetical scenario (Box 1). In response to this scenario—in which the consent form for the first study did not include any statements either allowing or prohibiting such disclosure, and in which the first study’s findings were described as having uncertain validity and utility—42% said the researcher definitely or probably should offer to disclose eligible participants’ individual genetic results from the first study as part of her explanation of the purpose of the second study. More, however, either said she definitely or probably should not (28%) or were undecided (22%). This represents a statistically significant departure from responses to our question about the general statement “Each participant should be offered his/her individual genetic results from the first study when contacted about taking part in the second study” (p<.001) (Figure 2). Compared to their responses to this general statement, answers to our scenario-specific question moved in a negative direction (less favorable toward offering results) for 30% of chairs.
Figure 2. General vs. scenario-specific views about the disclosure of individual genetic research results during the recruitment process.
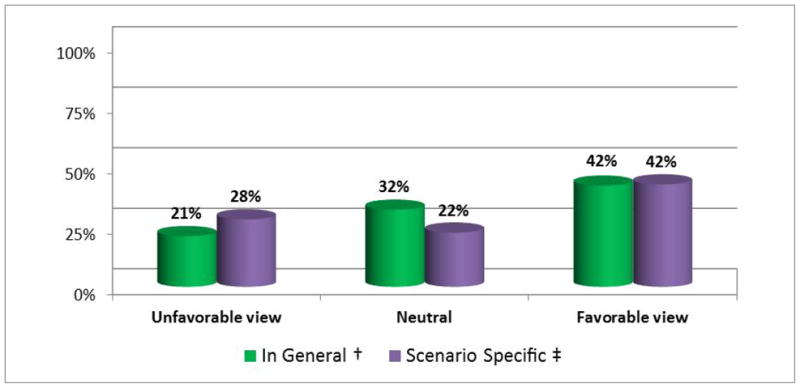
† Based on responses to the general statement, “Each participant should be offered his/her individual genetic results from the first study when contacted about taking part in the second study.” Unfavorable views include those who disagreed or strongly disagreed with this statement; neutral views are those who selected ‘neither agree nor disagree’; favorable views include those who agreed or strongly agreed.
‡ Based on responses to the scenario-specific question, “Should Dr. Jones offer to disclose individual genetic results from the first study as part of her explanation of the purpose of the second study?” Unfavorable views include those who said she definitely or probably should not; neutral views are those who were undecided; favorable views include those who said she definitely or probably should.
We probed about alternate statements concerning the disclosure of results that could have been included in the hypothetical first study’s consent form. Excluding respondents who already indicated that the researcher “definitely should” offer individual results (n=24), 77% were more likely to favor disclosure if the original consent form had stated “We will offer to disclose your individual genetic research results if they have potential clinical consequences for you and/or your family members.”
We also probed about alternate descriptions of the nature of the hypothetical first study’s results. One alternative provided an example of findings with limited clinical utility:
Although additional research is needed, it appears that this gene variant conveys a small increase in risk for heart disease compared to that in the general population. At this time, recommendations for people who have this variant would be the same as for the general population: stop smoking, follow a heart healthy eating plan, be physically active each day, and maintain a healthy weight.
This kind of finding did not have a definitive effect: Excluding chairs who already indicated that the researcher “definitely should” offer results (n=24), only 30% said they would be more likely to favor disclosure. A second alternative provided an example of findings with potentially more utility:
Although additional research is needed, this particular gene variant is in a biologic pathway that suggests that, for people who have the variant, diet and exercise alone may not be effective in reducing future risks associated with heart disease. Further, a specific class of cholesterol-lowering drug might be indicated.
This kind of finding was viewed more positively; again excluding chairs who already indicated that the researcher “definitely should” offer results (n=24), over half (53%) said they would be more likely to favor disclosure.
Taken together, these results again highlight the importance chairs assigned to information conveyed during the consent process for the original study, this time about disclosure of results. They also suggest that, at a minimum, the clinical validity of the results from the original study will be a significant factor in the minds of many chairs when considering whether such results should be offered in the context of genotype-driven recruitment. Several chairs offered comments at the end of our survey reflecting this opinion:
In general I favor personal autonomy, but I draw the line at informing people about findings whose significance is not clear even to the researchers. It is bad enough that we burden patients with information we believe to be true that later turns out to be wrong. We should not load them with information whose significance is unclear even to us.
I am very reluctant, whether one is dealing with a medical device, a new assay, or genetic test results, to allow the results to enter into real-time decision making… The subject could make decisions based on the results that could place them at risk for additional negative consequences—all because they agreed to participate in a research protocol. Placing a subject at avoidable risk ‘because they agreed to receive the results’ is insufficient. The risk versus benefit ratio determination is independent of whether the volunteer states they want to assume the risk, in my view. ‘Do no harm’ and ‘autonomy’ are obviously in tension here. I will always give ‘do no harm’ the right of way.
One chair’s comment serves as a reminder that how results are communicated can be as important as the content of those results:
One concern is whether the participants who are re-contacted and given some form of individual genetic information will be able to comprehend the information accurately. If it is technically precise it may not be comprehensible. If it is stated in layman’s terms, it may be so inaccurate as to give rise to unfounded anxiety. So, I would pay close attention to the manner in which individual genetic information is presented to participants.
Ethical Dilemmas: Weighing the Issues
We concluded our survey by asking directly about the ethical dilemmas involved in genotype-driven research recruitment (Table 2). With regard to whether researchers should be allowed to contact eligible participants in one study about taking part in a second study, we asked chairs to weigh the importance of protecting participants from unwelcome contact versus providing participants the opportunity to hear about more research. Slightly more than half (51%) decided in favor of avoiding unwelcome contact.
Table 2.
Weighing ethical dilemmas
| n | (%) * | |
|---|---|---|
| When thinking about whether researchers should be allowed to contact eligible participants in the first study about taking part in the second study, which of the following considerations should be given more weight (recognizing that both may be important)? | ||
| Protecting participants from unwelcome contact from researchers | 103 | (51) |
| Providing participants the opportunity to hear about research they might like to participate in | 60 | (30) |
| Unsure/Don’t know | 23 | (11) |
| When thinking about whether participants should be offered their individual genetic results from the first study when contacted about taking part in the second study, which of the following considerations should be given more weight (recognizing that both may be important)? | ||
| Avoiding the disclosure of unwanted genetic information | 98 | (49) |
| Avoiding leaving participants uninformed about why they are eligible for the second study | 72 | (36) |
| Unsure/Don’t know | 16 | (8) |
| Again when thinking about whether participants should be offered their individual genetic results from the first study when contacted about taking part in the second study, which of the following considerations should be given more weight (recognizing that both may be important)? | ||
| Avoiding the disclosure of genetic information that has uncertain clinical utility | 93 | (46) |
| Promoting participant autonomy to decide for themselves what kind of information they find useful | 79 | (39) |
| Unsure/Don’t know | 12 | (6) |
May not sum to 100% due to missing data
With regard to whether participants should be offered their individual genetic results from the first study when contacted about taking part in the second study, more chairs (49%) prioritized avoiding disclosure of unwanted genetic information over avoiding leaving participants uninformed about why they are eligible for the second study (36%).
Finally, in a second dilemma we posed about offering individual genetic results, more chairs (46%) chose avoiding disclosure of genetic information with uncertain clinical utility over promoting participants’ autonomy to make their own determinations about the usefulness of the information (39%).
DISCUSSION
Identifying and contacting individuals about their interest in research participation must take place within the context of well-established requirements for ethically responsible research.9 Even so, research recruitment is typically considered less risky than research participation. When contacted by a researcher, individuals have a number of options, including not responding, expressing disinterest at the outset, or learning more about the research and then making an informed decision about whether to take part.10
Certain approaches to research recruitment, however, involve risks that can rise to the level of harm. Genotype-driven recruitment is one such approach, where potential harms associated with the use and disclosure of genetic information are linked to the offer to participate in research. At the same time, genotype-driven recruitment could significantly advance the pace of research on the functional significance of human genetic variation, and speed progress toward the ultimate goal of benefitting human health.11
As a first step toward developing guidelines on ethical approaches to genotype-driven recruitment, we gathered empirical data from one stakeholder group, IRB chairs, about the acceptability of recontact for further research recruitment and the disclosure of individual genetic research results during the recruitment process. The distribution of responses we received to general questions on both of these topics was highly variable and seemed to suggest that the answer may often be “it depends.” This conclusion is further reinforced by the statistically significant differences we found between responses to our general versus scenario-specific questions. A major consequence of these findings is that it is unlikely that there will be a “one-size-fits-all” solution, but rather several approaches to genotype-driven recruitment that may be ethically acceptable depending on a variety of context-dependent factors. Examples of such factors include whether the genotype-driven study focuses on the same medical condition as the study in which participants were originally enrolled, whether it involves the same researchers and/or institution, whether it involves the recruitment of family members, and how and by whom prospective participants are contacted.
Two context-dependent factors in particular generated strong agreement among our survey respondents: First, disclosures made during the informed consent process for the original study are key. Addressing the possibility of future research contact and disclosure of individual results during the consent process—and potentially offering participants choices about these—may help mitigate some of the ethical dilemmas involved in genotype-driven recruitment. Specifically, incorporating these topics into the original consent process is one way of addressing the considerations that IRB chairs selected in our survey as deserving more weight: avoiding unwelcome researcher contact and avoiding disclosure of unwanted genetic information. However, there are several ethical and logistical challenges involved in offering and honoring choices on consent forms,12 and attention is also needed to developing appropriate and easy-to-understand consent language.
The second area of strong agreement that emerged concerned the importance of the clinical validity (and, to a slightly lesser degree, the clinical utility) of the information when deciding whether individual genetic results should be offered during the recruitment process. Issues surrounding the uncertainty and usefulness of genetic research results, together with ethical arguments based on respect for persons, beneficence, paternalism, reciprocity, and the boundaries between research and clinical practice,13 fuel the continuing debate over the general issue of whether or not individual genetic research results should be disclosed to research participants.14 Clinical utility has been the most frequently recommended standard,15 but will likely be difficult to reach in the context of genotype-driven recruitment, where further research is needed specifically because more must be learned to decipher the meaning of genetic research results in terms of risk, inheritance, diagnosis, prognosis, and treatment.16 When choosing the appropriate threshold for disclosure in genotype-driven recruitment, the counterbalancing concern about evasiveness when explaining why prospective participants are eligible must be addressed: If individuals are not offered their individual results, what should they be told about why they are being recontacted? No matter what approach is taken, researchers must be fully prepared to communicate and answer questions in clear, lay language about what is known and not known about the role of genetics in their proposed area of research.
In this study, we collected data from a key stakeholder group to inform policy development on a rapidly emerging issue. Other studies of IRB professionals’ views on issues arising in genomic research—such as what constitutes human subjects research, the need to re-consent participants for new uses of biospecimens, the disclosure of individual genetic research results, and the risks associated with large-scale data sharing—have similarly reported a wide range of opinions.17 This diversity of views may be due in part to a lack of federal regulatory guidance and established IRB policies on some of these new and unresolved questions.18 To help address the ethical challenges in the swiftly evolving field of genomics, it may be especially important for IRBs to have access to a variety of resources, including input from scientific colleagues, individuals who can articulate the perspective of research participants, and experts in research ethics.19
Our national sampling frame and good response rate are important strengths of this work. Genotype-driven recruitment is a complex topic that we knew would be novel to most respondents, and we developed our survey instrument through multiple iterations and detailed cognitive interviews to help ensure that questions would be comprehended as intended and to identify answer options that should be revised or added. Several factors, however, may limit the interpretation of our results. First, we do not have data about the characteristics of chairs who did not respond to our survey and thus cannot assess potential response bias; in general, the demographic characteristics of our respondents were similar to those found in surveys of IRBs on other topics.20 Second, because our survey comprised primarily closed-ended questions and was completed online, we were unable to probe for even more nuanced views or to explore other factors that chairs themselves might have identified as influencing their opinions. Third, to constrain the survey to a reasonable length, we did not include questions covering every possible issue (e.g., whether genetic research results are produced in a CLIA-certified laboratory and the attendant implications for disclosure to participants21). Thus, further research, perhaps involving semi-structured interviews, among IRB leaders is warranted. Input is also needed from other stakeholders, such as researchers, research participants, and treating physicians, for the development of sound recruitment policies that protect participants, yet avoid excessive restrictions that have a chilling effect on beneficial research.22
Supplementary Material
Acknowledgments
The project described was supported by Award Number RC1HG005787 from the National Human Genome Research Institute (NHGRI). The content is solely the responsibility of the authors and does not necessarily represent the official views of NHGRI or the National Institutes of Health.
We would like to thank our colleagues Gail Henderson, Ben Wilfond, and Jean Cadigan for their input. Thanks also to Cristina Kapustij for her assistance with pilot testing and assembling the survey sample, and to Alexandra Fox for her technical assistance with implementing the survey.
Footnotes
Human subjects protection statement: The Duke University Health System IRB determined that this study was exempt under 45 CFR 46.101(b)(2) and served as the IRB of record for the University of North Carolina at Chapel Hill.
None of the authors has any conflict, financial or otherwise, to declare.
Contributor Information
Laura M. Beskow, Assistant Research Professor, Duke Institute for Genome Sciences and Policy, Duke University, Durham, NC.
Emily E. Namey, Clinical Research Coordinator, Duke Institute for Genome Sciences and Policy, Duke University, Durham, NC.
Patrick R. Miller, Post-Doctoral Fellow, Social Science Research Institute, Duke University, Durham, NC.
Daniel K. Nelson, Director, Office of Human Research Ethics and Professor of Social Medicine and Adjunct Professor of Pediatrics, University of North Carolina, Chapel Hill, NC;.
Alexandra Cooper, Associate Director for Education & Training, Social Science Research Institute, Duke University, Durham, NC.
References
- 1.McGuire SE, McGuire AL. Don’t throw the baby out with the bathwater: enabling a bottom-up approach in genome-wide association studies. Genome Res. 2008;18:1683–1685. doi: 10.1101/gr.083584.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beskow LM, Linney KN, Radtke RA, et al. Ethical challenges in genotype-driven research recruitment. Genome Res. 2010;20:705–709. doi: 10.1101/gr.104455.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chulada PC, Vahdat HL, Sharp RR, et al. The Environmental Polymorphisms Registry: a DNA resource to study genetic susceptibility loci. Hum Genet. 2008;123:207–214. doi: 10.1007/s00439-007-0457-5. [DOI] [PubMed] [Google Scholar]
- 4.See ref. 2.
- 5.See ref. 3
- 6.See ref. 1
- 7.See ref. 2.
- 8.See ref. 2
- 9.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington DC: US Government Printing Office; 1979. [PubMed] [Google Scholar]
- 10.Beskow LM, Botkin JR, Daly M, et al. Ethical issues in identifying and recruiting participants for familial genetic research. Am J Med Genet A. 2004;130A:424–431. doi: 10.1002/ajmg.a.30234. [DOI] [PubMed] [Google Scholar]; Beskow LM, Sandler RS, Weinberger M. Research recruitment through US central cancer registries: balancing privacy and scientific issues. Am J Public Health. 2006;96:1920–1926. doi: 10.2105/AJPH.2004.061556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.See ref. 1
- 12.National Cancer Institute. Best Practices for Biospecimen Resources. 2007 http://biospecimens.cancer.gov/practices/default.asp.; Ram N. Tiered consent and the tyranny of choice. Jurimetrics. 2008;48:253–284. [Google Scholar]; Beskow LM, Burke W. Offering individual genetic research results: context matters. Sci Transl Med. 2010;2(38):38cm20. doi: 10.1126/scitranslmed.3000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haga SB, Beskow LM. Ethical, legal, and social implications of biobanks for genetics research. Adv Genet. 2008;60:505–544. doi: 10.1016/S0065-2660(07)00418-X. [DOI] [PubMed] [Google Scholar]; Dressler LG. Disclosure of research results from cancer genomic studies: state of the science. Clin Cancer Res. 2009;15:4270–4276. doi: 10.1158/1078-0432.CCR-08-3067. [DOI] [PubMed] [Google Scholar]
- 14.See ref. 12 Beskow & Burke 2010 Kohane IS, Taylor PL. Multidimensional results reporting to participants in genomic studies: getting it right. Sci Transl Med. 2010;2(37):37cm19. doi: 10.1126/scitranslmed.3000809.Fernandez C. Public expectations for return of results--time to stop being paternalistic? Am J Bioeth. 2008;8:46–48. doi: 10.1080/15265160802513127.Miller FA, Christensen R, Giacomini M, Robert JS. Duty to disclose what? Querying the putative obligation to return research results to participants. J Med Ethics. 2008;34:210–213. doi: 10.1136/jme.2006.020289.Clayton EW, Ross LF. Implications of disclosing individual results of clinical research. JAMA. 2006;295:37. doi: 10.1001/jama.295.1.37-a.Meltzer LA. Undesirable implications of disclosing individual genetic results to research participants. Am J Bioeth. 2006;6:28–30. doi: 10.1080/15265160600935811.Parker LS. Rethinking respect for persons enrolled in research. ASBH Exchange. 2006;9:1,6–7.Sharp RR, Foster MW. Clinical utility and full disclosure of genetic results to research participants. Am J Bioeth. 2006;6:42–44. doi: 10.1080/15265160600938443.Shalowitz DI, Miller FG. Disclosing individual results of clinical research: implications of respect for participants. JAMA. 2005;294:737–740. doi: 10.1001/jama.294.6.737.
- 15.Fabsitz RR, McGuire A, Sharp RR, et al. Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung, and Blood Institute working group. Circ Cardiovasc Genet. 2010;3:574–580. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bookman EB, Langehorne AA, Eckfeldt JH, et al. Reporting genetic results in research studies: summary and recommendations of an NHLBI working group. Am J Med Genet A. 2006;140:1033–1040. doi: 10.1002/ajmg.a.31195. [DOI] [PMC free article] [PubMed] [Google Scholar]; National Bioethics Advisory Commission. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance, Volume 1. Rockville, MD: US Government Printing Office; 1999. [Google Scholar]
- 16.See ref. 2
- 17.Wolf LE, Catania JA, Dolcini MM, et al. IRB chairs’ perspectives on genomics research involving stored biological materials: ethical concerns and proposed solutions. J Empir Res Hum Res Ethics. 2008;3:99–111. doi: 10.1525/jer.2008.3.4.99. [DOI] [PubMed] [Google Scholar]; Lemke AA, Trinidad SB, Edwards KL, et al. Attitudes toward genetic research review: results from a national survey of professionals involved in human subjects protection. J Empir Res Hum Res Ethics. 2010;5:83–91. doi: 10.1525/jer.2010.5.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lemke AA, Smith ME, Wolf WA, Trinidad SB. Broad data sharing in genetic research: views of institutional review board professionals. IRB. 2011;33:1–5. [PMC free article] [PubMed] [Google Scholar]
- 18.See ref. 17, Lemke et al 2011 Kozanczyn C, Collins K, Fernandez CV, et al. Offering results to research subjects: U.S. Institutional Review Board policy. Account Res. 2007;14:255–267. doi: 10.1080/08989620701670179.
- 19.See ref. 17 Lemke et al. 2010 Sirotin N, Wolf LE, Pollack LM, et al. IRBs and ethically challenging protocols: views of IRB chairs about useful resources. IRB. 2010;32:10–19.
- 20.Campbell EG, Weissman JS, Vogeli C, et al. Financial relationships between institutional review board members and industry. N Engl J Med. 2006;355:2321–2329. doi: 10.1056/NEJMsa061457. [DOI] [PubMed] [Google Scholar]; Catania JA, Lo B, Wolf LE, et al. Survey of U.S. boards that review mental health-related research. J Empir Res Hum Res Ethics. 2008;3:71–79. doi: 10.1525/jer.2008.3.4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.See ref. 15, Fabsitz et al. 2010.
- 22.See ref. 2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


