Abstract
Hypothesis
Acoustically evoked neural and hair cell potentials can be measured from the round window (RW) intraoperatively in the general population of cochlear implant recipients.
Background
Cochlear implant performance varies greatly among patients. Improved methods to assess and monitor functional hair cell and neural substrate prior to and during implantation could potentially aid in enhanced non-traumatic intracochlear electrode placement and subsequent improved outcomes.
Methods
Subjects (1–80 years) undergoing cochlear implantation were included. A monopolar probe was placed at the RW after surgical access was obtained. The cochlear microphonic (CM), summating potential (SP), compound action potential (CAP), and auditory nerve neurophonic (ANN) were recorded in response to tone bursts at frequencies of 0.25 – 4 kHz at various levels.
Results
Measurable hair cell/neural potentials were detected to one or more frequencies in 23 of 25 subjects. The greatest proportion and magnitude of cochlear responses were to low frequencies (<1000 Hz). At these low frequencies the ANN, when present, contributed to the ongoing response at the stimulus frequency. In many subjects the ANN was small or absent while hair cell responses remained.
Conclusions
In cochlear implant recipients, acoustically evoked cochlear potentials are detectable even if hearing is extremely limited. Sensitive measures of cochlear and neural status can characterize the state of hair cell and neural function prior to implantation. Whether this information correlates with speech performance outcomes, or can help in tailoring electrode type, placement or audiometric fitting, can be determined in future studies.
Keywords: Cochlear Implant, Cochlear Electrophysiology, Hearing Preservation, Electrocochleography, Intraoperative monitoring, Auditory Nerve Neurophonic, Cochlear Microphonic
INTRODUCTION
Speech recognition with cochlear implants remains quite variable among patients and different factors affecting outcome have been postulated (1–3). Examples of relevant factors include pre-implantation residual hearing (4,5), age of onset and duration of hearing loss (4–6), and patterns of intracochlear electrode placement (7–10). A factor often considered important is neural survival. Although not all studies agree, it is widely assumed that cochlear implant performance relies on the number, distribution and viability of neural elements (11–18). Unfortunately, actual nerve survival and function has been difficult to estimate prior to implantation. One possible approach is to use cochlear electrophysiological responses to sound stimuli, called electrocochleography (ECoG) (19–22). Different components of the ECoG waveforms provide information about different cochlear structures. The cochlear microphonic (CM) is primarily an outer hair cell response, and represents the current flow through the mechanoelectric transducer channels in the stereocilia (23,24). The summating potential is generally considered an inner hair cell response at low intensities and a mixed response from inner and outer hair cells at high intensities, and represents the sustained depolarization in the hair cell body during sound presentation (25–28). The compound action potential (CAP) is derived from nerve fibers, and represents the sum of the well-timed responses to the onsets and offsets of sounds (29–32). For this study ECoG potentials were measured from the round window (RW) of subjects at the time of cochlear implantation. There were two goals. One goal was to determine if high-quality measurements of each ECoG potential could be obtained from cochlear implant recipients, so that the differential survival of hair cell and neural elements could be estimated. The second was to introduce an additional ECoG measurement that can provide information about temporal processing capabilities of the auditory nerve. This potential is the auditory nerve neurophonic (ANN), which represents the phase-locked responses of auditory nerve fibers (33). Temporal information derived from phase-locking is used for critical auditory functions such as sound localization based on interaural time differences, noise reduction based on interaural correlation, and pitch perception based on spike intervals. Deficits in temporal processing represent a major category of impairment in auditory neuropathy spectrum disorder.
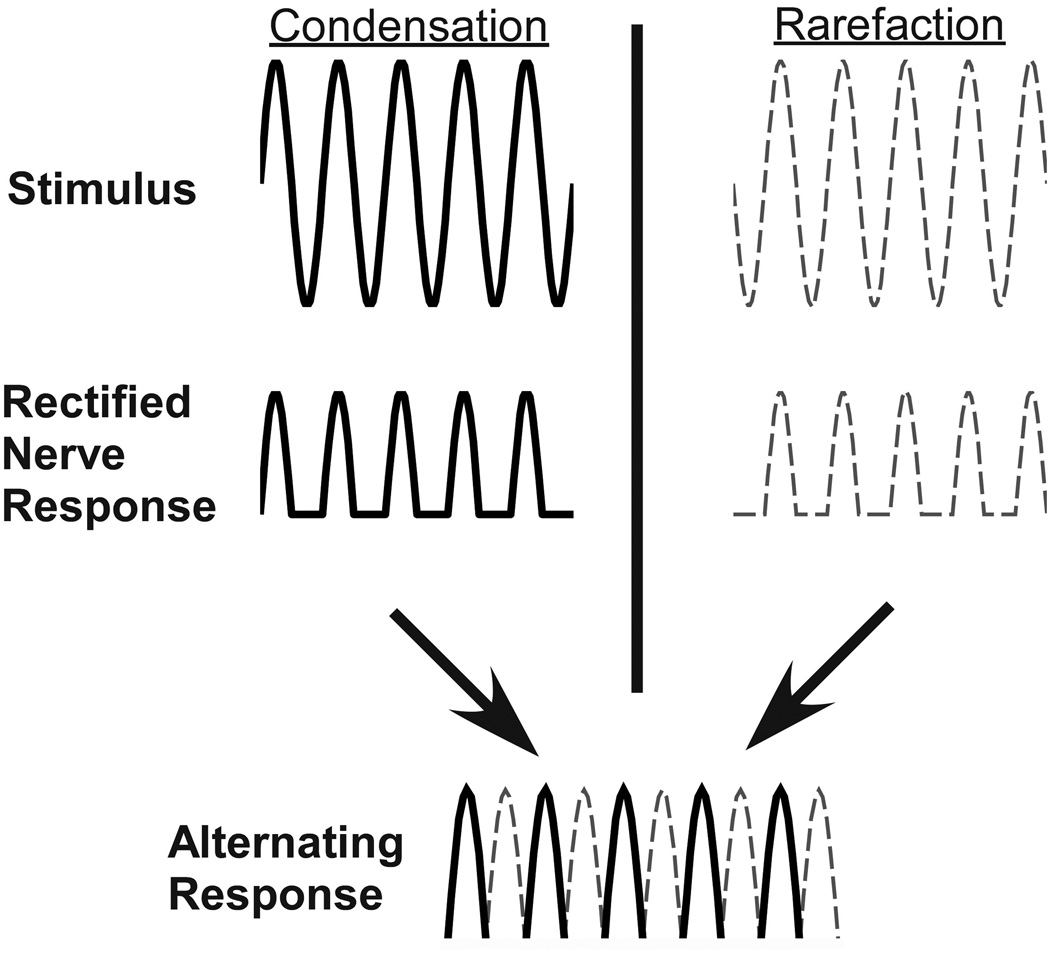
The ANN has been described in animals but has not been used in human ECoG recordings, as far as we are aware. In animals, it has been recorded from the RW (34,35) or from electrodes placed directly on the auditory nerve (33,36,37). It is an ongoing response that follows the waveform of a tonal stimulus, and thus can be separated from the CAP, which occurs only at stimulus onset and offset. It can be isolated from the CM, which is also present while the sound is on, by alternating the phase of stimulus presentation, as shown in Fig. 1. The top row, labeled “Stimulus”, shows the sinusoidal waveforms of a tone burst alternated at 180° (Condensation and Rarefaction). In each case the RW recorded response waveform would consist of both the CM and ANN, since both hair cell and neural responses follow the waveform of the tone. However, the auditory nerve fibers only respond to the positive half of each cycle of the tone, shown in the waveforms in the second row (labeled “Rectified Nerve Response”), and this positive-only rectification causes harmonic distortion not present in the CM. If the responses to both phases are averaged (alternating phase response) the CM is removed, but because of the positive only rectification, the ANN is present at twice the stimulus frequency, as shown in the bottom row (labeled “Alternating Response”) (34). The ANN is the auditory nerve analogue of the frequency following response (FFR), which is the phase locked activity of brainstem nuclei (38). For this study, we measured ECoG potentials, including the ANN, intraoperatively from the RW of cochlear implant patients to tone bursts of varying frequency.
Figure 1.
Schematic showing the origin of the auditory nerve neurophonic (ANN) in the alternating response waveform. First row, labeled “Stimulus” shows a sinusoidal tone burst in two phases with a difference of 180 degrees (Condensation and Rarefaction). Second row, labeled “Rectified Nerve Response”, shows the ANN component of the response to each phase of the sinusoidal stimulus. A positive going response to each positive going portion of the sinusoidal stimulus is seen (1/2 wave rectification). The last row, labeled “Alternating Response” shows the combination of the ANN response to each phase, which results in a waveform that is twice the original stimulus sinusoidal wave frequency. This response is the alternating phase response, which isolates the neural response.
MATERIALS AND METHODS
The procedure was to record responses from the RW of all cochlear implant recipients to acoustic stimuli intraoperatively prior to electrode array insertion. Twenty-five subjects were included. The procedures were in accordance with the ethical standards of the institution’s IRB and informed consent was obtained for all subjects enrolled (Protocol number 05-2616).
Human Subject Inclusion Criteria
All pediatric and adult subjects undergoing cochlear implantation were potential participants. The IRB for this study did not permit consent through an interpreter; so non-English speaking patients were excluded, as were patients without an external auditory canal, those undergoing revision surgery, or those with malformed cochlear anatomy.
Surgery and Recording Set-up
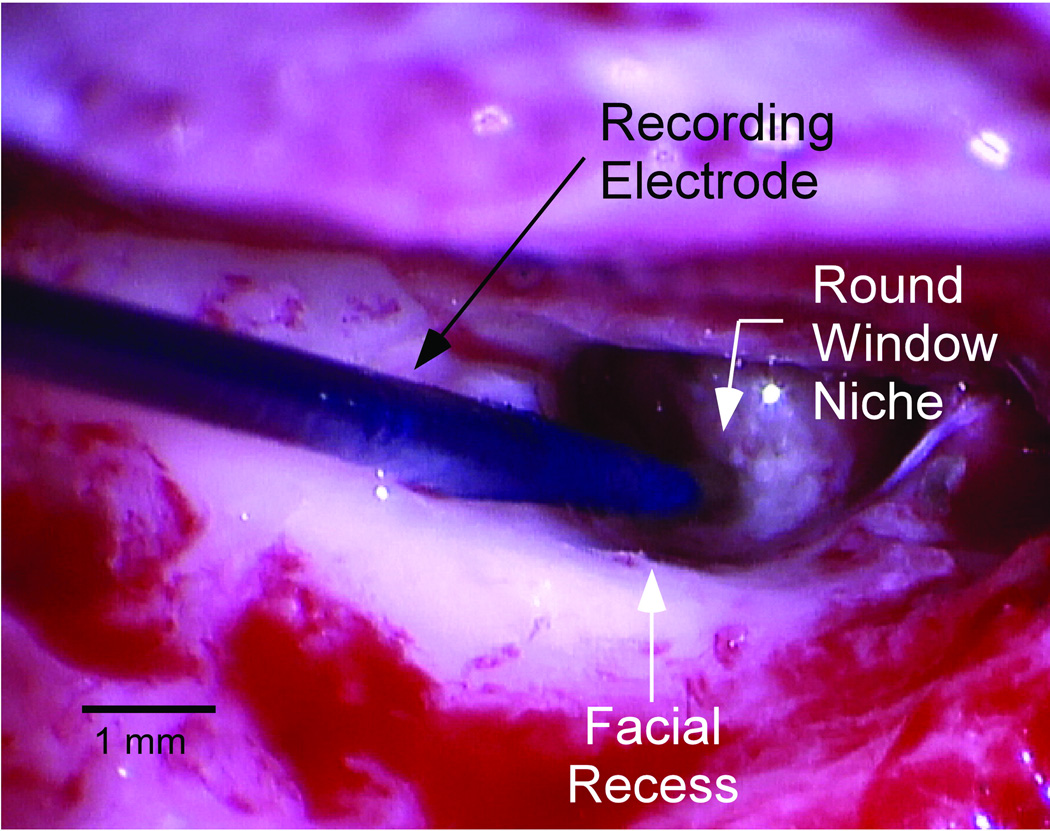
After induction, a foam insert attached to a sound tube was placed in the external auditory canal of the ear being implanted. A sterile disposable monopolar probe (Neurosign, Magstim Co., Wales, UK) served as the active input for recordings, a surface electrode on the contralateral mastoid was the return and the common was a surface electrode on the glabella. A standard transmastoid facial recess approach was used to access the promontory. The bony overhang was removed and the monopolar probe was placed on the membranous portion of the window. Figure 2 is an intraoperative photograph demonstrating recording electrode placement. The Bio-logic Navigator Pro (Natus Medical Inc., San Carlos, CA) was used to generate acoustic stimuli and make all recordings using the AEP hearing diagnostics software. Impedance measurements were obtained and saline was added if impedance was greater than 16k Ohms.
Figure 2.
A photograph taken through the operating microscope of the intraoperative recording setup showing the sterile monopolar probe placed in the round window niche after surgical access was gained.
Sound Stimulation and Evoked Potential Recording
Recordings from the RW in response to 500 repetitions of alternating phase tone bursts were obtained. Tone bursts had 1–4 ms rise and fall times shaped by a Blackman window and a 10 ms plateau for the higher frequencies of 1, 2 and 4 kHz, and 28 ms for the lower frequencies of 250, 500 and 750 Hz. Sound was delivered through Etymotic speakers calibrated to normal hearing level (nHL). Typically, the series of frequencies was first tested at 90 dB nHL. Then, a frequency with a good response (typically 500 Hz) was used for a level series (decreasing from 90 dB nHL in 5 – 10 dB steps). Recordings with the sound tube crimped were taken at 90 dB nHL at each frequency to estimate electrical artifact, if any. A stimulating rate of 23.3 Hz was used for the lower frequency tones and a rate of 29.3 Hz was used for the higher frequencies. Recordings were started 4 ms prior to stimulus onset and the recording epoch was 32 ms for each stimulus. The sampling rate was 8,000 Hz for the 0.25–1 kHz and 16,000 Hz for the 2 and 4 kHz stimuli. Filters were high-pass at 10 and low-pass at 3,000 Hz or 5,000 Hz. Artifact rejection was set to 47.50 µV and the acoustic delay accounted for was 0.8 ms. It routinely took less than 10 minutes to administer the intraoperative recording protocol used in the study.
Data Analysis
The data from condensation and rarefaction phases was stored separately. A difference curve was determined by subtracting the response to the condensation from the rarefaction phase, and an “alternating” curve was determined from the average. The spectrum of each signal was obtained from the fast Fourier transform (FFT). To avoid contributions from the CAP, windowing was used prior to calculating the FFT (5–25 ms for low frequencies and 5–15 ms for high). A response at the signal frequency or one of its harmonics was considered significant if the magnitude of the peak in question exceeded the noise level by three standard deviations. The noise and its variance were determined from six bins, three on each side of the signal starting two bins away from the peak.
RESULTS
Measurable potentials to sound were obtained in 23 of 25 subjects. The two without measurable potentials, and six subjects excluded for clear technical reasons for unsuccessful recordings, will be described later. Information about the 25 subjects is given in Table 1. Figure 3 shows a typical recording to a 500 Hz tone at 90 dB nHL. At this intensity there is considerable distortion in waveforms, also visible as additional peaks in the spectrum (Fig. 3B). The spectrum shows that the distortion appears at 1 and 2 kHz, the first two even harmonics of the stimulus frequency (500 Hz). Taking the difference between the two waveforms (Fig. 3C) removes most of the distortion yielding a single peak at the stimulus frequency in the spectrum (Fig. 3D). Averaging the waveforms (Fig. 3E, called alternating) removes the energy at the stimulus frequency as the alternating phase makes this energy sum to zero when added, yielding a residual response at twice the stimulus frequency. This residual response is labeled the ANN, because our interpretation is that its represents the rectified phase-locking to low frequencies from the auditory nerve (Fig. 1). Evidence that the distortion is due to asymmetry in the waveform, such as can be caused by rectification, is that only even harmonics are present (39,40).
TABLE 1. Age, sex, frequency with lowest audiogram threshold (TH), and etiology of hearing loss of each subject tested.
Summary of subjects tested. Subject number, age, sex, lowest threshold (TH) per preoperative audiogram, frequency of lowest TH, and etiology of hearing loss are listed.
| Subject | Age | Sex | Lowest TH per Preoperative Audiogram (dB HL) |
Frequency of Lowest TH per Preoperative Audiogram (Hz) |
Etiology of Hearing Loss |
|---|---|---|---|---|---|
| 1 | 12 mon | M | 90 | 250 | unknown/possibly genetic (sibling with AN) |
| 2 | 13 mon | F | 70 | 250 | unknown infection with antibiotics administered |
| 3 | 21 mon | M | >100 | all frequencies | Waardenburg syndrome |
| 4 | 2 yr | F | >100 | all frequencies | unknown/connexin 26 variant of unknown clinical significance present |
| 5 | 2 yr | M | 80 | 250 | unknown etiology but likely genetic |
| 6 | 2 yr | F | 90 | 250 | unknown |
| 7 | 3 yr | M | >100 | all frequencies | Congenital CMV infection |
| 8 | 7 yr | F | 15 | 250 | unknown/onset age 4 |
| 9 | 8 yr | F | >100 | all frequencies | Congenital CMV infection |
| 10 | 10 yr | F | 55 | 250 | 1555A>G mutation in MT-RNR1 gene |
| 11 | 18 yr | M | >100 | all frequencies | Progressive since kindergarden, asymmetric |
| 12 | 39 yr | M | 75 | 250 | sickle cell disease |
| 13 | 41 yr | F | 70 | 2000 | pontine infarction |
| 14 | 42 yr | F | 5 | 250 | NIHL/Presbycusis |
| 15 | 47 yr | F | 60 | 250 | unknown/onset age 5 - progressive |
| 16 | 48 yr | M | 100 | 500 | Radiation |
| 17 | 49 yr | F | 70 | 4000 | sudden HL at age 34, antibiotic use |
| 18 | 66 yr | M | 40 | 250 | NIHL/Presbycusis |
| 19 | 66 yr | F | 100 | 250, 4000 | NIHL/Presbycusis |
| 20 | 69 yr | F | 35 | 250 | NIHL/Presbycusis |
| 21 | 70 yr | F | 80 | 250 | NIHL/Presbycusis |
| 22 | 71 yr | M | 15 | 250 | NIHL/Presbycusis |
| 23 | 75 yr | M | 50 | 250 | NIHL/Presbycusis |
| 24 | 78 yr | F | >100 | all frequencies | onset age 5 after measles/Presbycusis |
| 25 | 80 yr | F | 45 | 250 | NIHL/Presbycusis |
AN = Auditory Neuropathy; CMV = Cytomegalovirus; NIHL = Noise Induced Hearing Loss; HL = Hearing Loss.
Figure 3.
Example of a typical response seen to a 500 Hz tone at 90 dB nHL. A. The averaged waveforms for both the condensation and rarefaction phases show distortion compared to the tone stimuli. B. Fast Fourier Transform (FFT) spectral analysis of 2A, shows that the distortion is harmonically related to the stimulus frequency, appearing at 1 and 2 kHz. C. The difference between the two waveforms in 2A. D. The subtraction removes most of the distortion yielding a single peak at the stimulus frequency in the spectrum. Open circle depicts calculated noise floor plus three standard deviations of the noise. A peak larger than this was determined to be significant. E. The averages of the waveforms in 2A, called alternating. The averaging removes the energy at the stimulus frequency and yields a residual response at twice the stimulus frequency. The compound action potential (CAP) is seen towards the beginning of the stimulus, and the auditory nerve neurophonic (ANN), phase locked neural response, is shown lasting the duration of the stimulus. F. The FFT retains only the energy of the harmonic distortion, with the largest peak at twice the stimulus frequency. Again, the open circle represents the calculated noise floor plus three standard deviations of the noise.
Responses Across Frequency
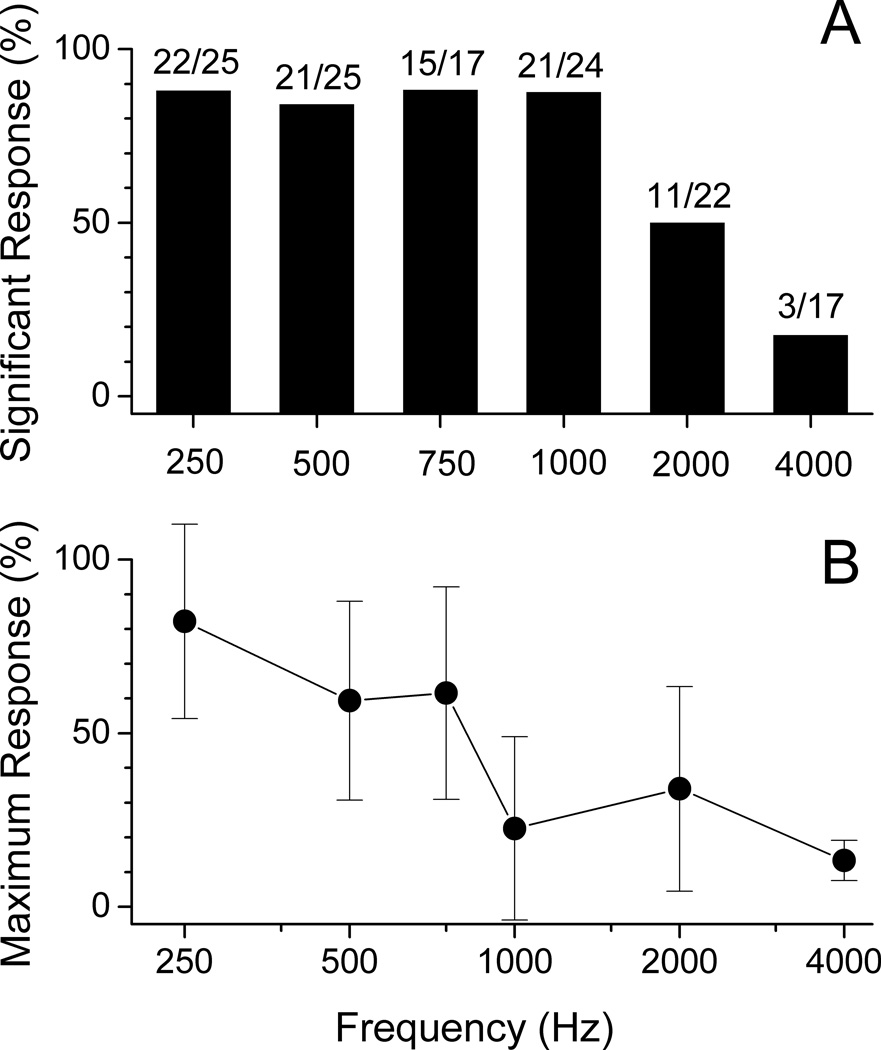
The magnitude of response at the stimulus frequency was obtained from the FFT analysis of the difference plot (e.g., Fig. 3D). This energy contains the entirety of the CM and that proportion of the ANN that is at the stimulus frequency. Note that the ANN can either add to or subtract from the size of the difference peak depending on if the two sources are in or out of phase. Figure 4A shows the percent of subjects that had a significant peak (see Methods) in the FFT of the difference curve to each frequency. A greater percentage of subjects had significant responses at 250–1,000 Hz compared to 2,000–4,000 Hz. Also, most subjects showed the largest response to lower frequencies. The magnitudes of the response at each frequency, normalized to the maximum for each subject, declined over the frequency range (Fig. 4B). Some subjects did have maximal responses to frequencies other than 250 Hz - four showed the maximal response to 500 Hz, three to 750 Hz, and one to 2,000 Hz.
Figure 4.
A. Summary of the percent of subjects that had a significant response at each frequency. A greater percentage of subjects were noted to have significant responses (defined as responses greater than the noise level plus three times the standard deviation in the six bins used for the noise measurements) at 250–1,000 Hz compared to 2,000–4,000 Hz. B. Depicts the magnitudes of the response at each frequency across subjects, normalized to the maximum for each subject. The bars are standard error of the mean. Most subjects showed the largest responses to lower frequencies, the magnitudes of the response declined over the frequency range.
Response Thresholds
For most subjects (n=21) we obtained an intraoperative threshold for the difference and alternating curves from a level series at a single frequency. In Fig. 5, we compare these intraoperative thresholds to the preoperative audiogram threshold at the frequency used for the level series. For the thresholds from the difference curves (Fig. 5A), six of the subjects had RW thresholds within 10 dB of the audiogram threshold (dashed lines) and nine of the patients had difference thresholds >10 dB higher than the behavioral audiogram. Somewhat surprisingly, six had difference thresholds that were >10 dB lower than those in the audiogram.
Figure 5.

Comparisons of intraoperative threshold recordings at a single frequency and preoperative audiogram threshold at the same frequency. A. Shows comparison for difference waveform response. The arrow highlights one of the cases where the physiological threshold was less than behavioral threshold. B. Compares preoperative audiogram threshold with threshold from twice the stimulus frequency using the alternating waveform. The arrow points to the same subject as in 4A, which now shows a better match to the behavioral threshold. Asterisks represent subjects that did not show a response in the alternating waveform at the highest level tested (90 dB nHL).
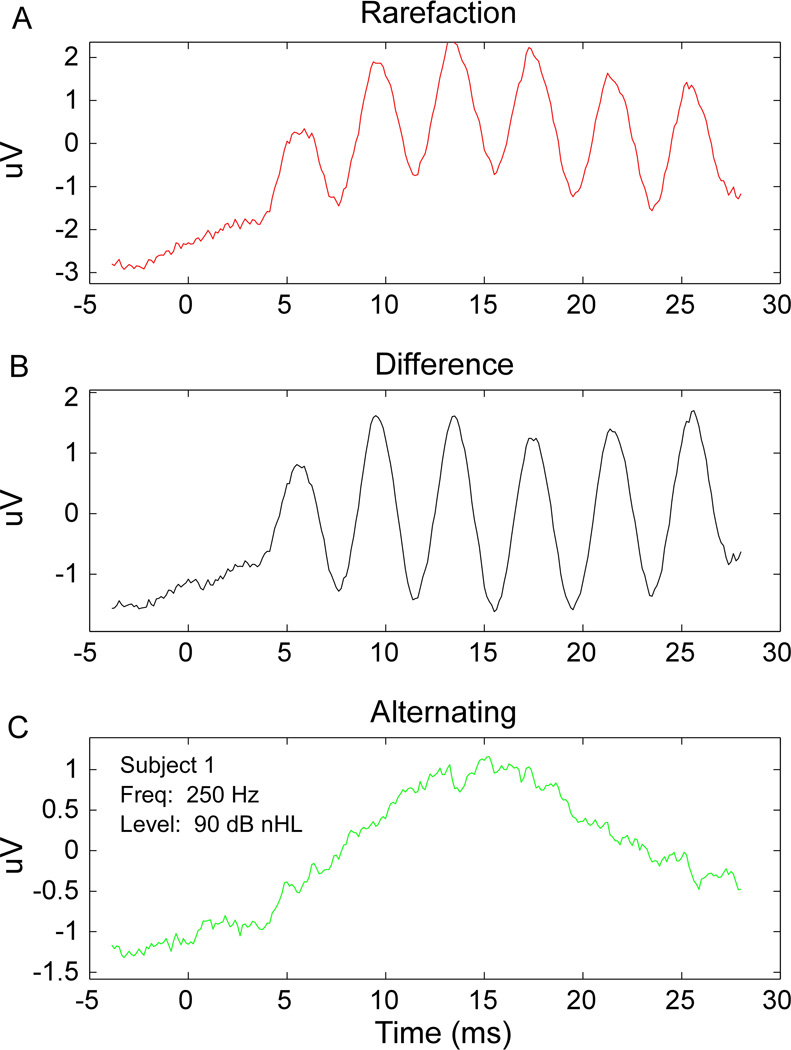
Figure 5B compares intraoperative thresholds from the alternated curves with the preoperative audiogram thresholds. Of the 21 subjects, all but one had intraoperative thresholds that were the same or >10 dB higher than that determined by preoperative audiogram. There were four subjects that had no detectable ANN to the highest level tested, 90 dB nHL (asterisks), though each of these four did have significant responses in the difference curves (Fig.5A). Of these four, three had thresholds >100 dB HL in the audiogram. Thus, the thresholds from the alternating curves were in general less sensitive to the audiogram threshold than were thresholds from the difference signal. These results mean that for some cases the sensitivity of intraoperative measures of hair cell function was greater than the behavioral sensitivity, suggesting the presence of surviving hair cells not connected to auditory nerve fibers, which can be interpreted as a form of auditory neuropathy. An example from one case where the difference response had lower threshold than the ANN is indicated by the arrows in Figs. 5A and B, and further illustrated in Fig. 6. In Fig. 6, a robust response in the rarefaction and difference curves was evident, but there was no response in the alternating curve. In addition, there was no CAP in the alternating curve. The CAP was small or absent at other frequencies as well, as was the ANN. Thus, in this subject, as was true in other cases where the difference curve showed a lower threshold than the audiogram, the neural potentials were attenuated compared to the hair cell potentials, which may be indicative of pathology in the synapses onto the auditory nerve dendrites or of the auditory nerve itself. This condition would be similar to an auditory neuropathy (see Discussion). There is one final case that is also interesting. In this subject, the RW responses were significant only to higher frequencies and the level series was taken at 2 kHz. There was no ANN, but a large CAP was present. This combination indicates the presence of neural responses that could support the hearing threshold, but the neural responses are to frequencies above the phase-locking range of the auditory nerve and therefore no ongoing neural response is seen.
Figure 6.
The rarefaction, A, difference, B, and alternating, C, waveforms are shown for Subject 1. Given this stimulus (250 Hz tone at 90 dB nHL) a robust response in the rarefaction and difference waveform is evident, with no distortion in the rarefaction waveform. No response is noted in the alternating waveform. In addition, there is no CAP, indicating a differential hair cell and neural response to the stimulus.
In general, the CAP was small or absent to the 250 Hz stimulus, which was typically the frequency with the best response. When present, the CAP thresholds were typically 10 dB or more higher than the ANN thresholds.
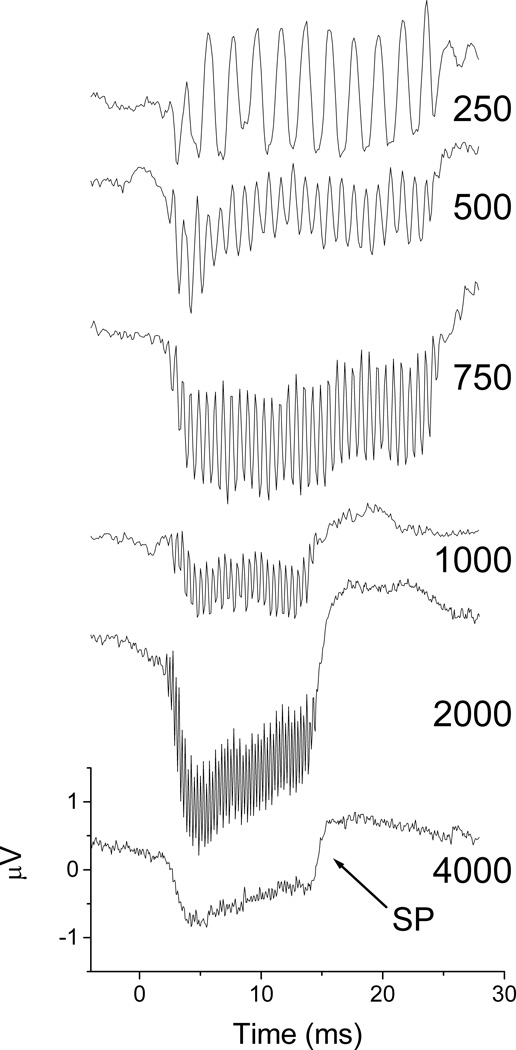
Summating Potential
A summating potential (SP), or envelope response, was often present in the alternating curves. An example of a subject with a strong SP is shown in Figure 7. The SP began at 500 Hz and was present at all higher frequencies. The ANN was attenuated at 2,000 Hz, and absent at 4000 Hz.
Figure 7.
Alternating responses across frequencies at 90 dB nHL for Subject 15 that showed a large summating potential (SP). A SP was present at 500 Hz and increased at higher frequencies. The auditory nerve neurophonic (ANN) represents phase-locking of neural elements, and is a ½-wave rectified version of the original signal. The phase-locked response was present until 2 kHz.
Additional subject information
Table 1 summarizes the age, sex, frequency with lowest behavioral threshold prior to implantation, and hearing loss etiology of the 25 subjects included in this study. The two subjects that did not show a measureable response are depicted in italics. One of these underwent radiation therapy for a nasopharyngeal carcinoma, and a total lack of cochlear function was expected. The other was a case with sickle cell disease, and since some hearing was detected in the preoperative audiogram, responses were expected from the recording. The reason for no response in this case is not clear. Six additional subjects had clear technical reasons for a lack of response and were not included. One of these had a surface electrode dislodge, two had large amounts of artifact that were consistently found in a particular operating room, two others were due to kinking of the sound tube resulting in no sound delivery, and one was excluded because the cochleostomy was made prior to recording, a breach in the protocol.
DISCUSSION
The major results were 1) measureable cochlear potentials were found in 23 out of 25 tested subjects, 2) most subjects showed the largest potentials to low frequencies (<1000 Hz), 3) along with the CM, SP and CAP, the ANN is a component of ECoG potentials at low frequencies, and 4) many subjects showed evidence of differential neural and hair cell pathology.
Prevalence of Cochlear Potentials in Cochlear Implant Subjects
The aim of the study was to generate ECoG data from recordings at the RW of the general population of cochlear implant recipients, in order to determine the presence and characteristics of acoustically evoked potentials, if any. The hypothesis was that because most patients have at least some residual hearing, potentials would be present. However, the percentage of patients undergoing cochlear implantation that would have measurable potentials was unknown. Obtaining a measurable ECoG potential in 23 of 25 subjects to at least one frequency was an unexpected and interesting result given that the preoperative audiograms of many subjects showed severe-profound hearing loss at all frequencies. Even subjects with audiogram thresholds >100 dB HL typically showed measurable responses (CM and ANN) at 90 dB nHL. In a recent study, Harris et al. (21) reported measurable ECoGs in 11 of 16 cases, excluding five cases that failed for technical reasons. Similarly, six of six patients showed ECoG responses pre-implantation in a study by Radeloff et al. (20). Taken together, these results show that the functional status of remaining hair cells and nerve fibers in individual patients is measureable, which may in turn be useful for determining cochlear trauma during surgery, predicting the success or failure of electrical hearing outcomes, and audiometric fitting. Whether the degree of functional connections between hair cells and neural elements is proportional to the survival of neural elements that can be electrically stimulated is a hypothesis that may prove true for at least some etiologies of hearing loss, as can be determined from further studies.
Hair Cell and Neural Potentials
The ECoG is typically considered to consist of the CM, CAP and SP, which can be separated through windowing and filtering the raw signal. However, animal studies have shown that the RW response to low frequencies is a mixture of the CM and ANN (34,35,41,42). In this study, we have shown that the same is true in human subjects. The presence of the ANN results in one source of the distorted ECoG waveform compared to the tone burst stimulus. The distortion arises from the auditory nerve contribution to the recorded response, which is a rectified version of the stimulus waveform (Fig. 1). The presence of predominantly even harmonics of the stimulus frequency indicates that rectification is responsible for the distortion (39). In animals, evidence that the ANN is due to neural responses is that the distortion largely disappears after administration of drugs that block sodium channels (34) and it is subject to forward masking (35), which the CM is not. Thus, the presence of mixed hair cell and neural sources in RW responses to low frequencies seems clear.
The ANN provides useful information about temporal processing as will be further discussed below. However, its presence represents challenges in analyses of human ECoG potentials that have not been previously considered. When a pure tone is synthesized and rectified, there is energy at the stimulus frequency as well as at the second harmonic. Thus, the estimate of the ANN magnitude from the alternating stimulus, which isolates the distortion, only captures a portion of the total energy from the neural response. Determining the actual magnitude is not straightforward because the relative phases of the CM and ANN will affect the measured magnitude of the potentials. While the phases of all the signals are measureable, because these are evoked potentials the sources can vary in location with frequency, so the analysis is more complicated than can be considered here. Chimento and Schreiner (1990) used forward masking to separate the CM from the FFR (40). The FFR is similar to the ANN but its source is phase-locking in the brainstem rather than the auditory nerve. Complicating this procedure is that the intensity where the neural response can be fully masked is difficult to know in cases of hearing loss, and intraoperative recording time does not permit testing at many different frequency/intensity combinations.
A possible further confound to the analysis is rectification that occurs in the CM. At high stimulus levels, the CM can rectify because the resting position of the hair bundle is displaced in the direction of closed rather than open channels (43). Still, a wide range of motion is available to the hair bundles, so this rectification is less complete than is the case with the auditory nerve. In many subjects the ANN was present at low intensities where hair cell rectification is unlikely, so this confound is not likely to be large. In addition, in several cases the responses were large and there was no distortion at the highest level tested, 90 dB nHL (as in Fig. 6A and B). Thus, hair cell rectification is unlikely to be major contaminant in the current recordings, but they may play a larger role in ECoGs derived from normal hearing subjects.
Other than the CM and ANN, the ECoG also contains the CAP and SP. The CAP is purely neural in origin, while the SP is purely a hair cell potential. Thus, these potentials provide additional clues about the status of the different elements. At the low frequencies where the majority of significant potentials at the stimulus frequency occurred, the presence of the ANN was more reliable than the presence of a CAP. That is, there were numerous cases to 250 and 500 Hz where there was an ANN response in the alternating response with no CAP, but not the reverse. The SP is thought to be due to the build-up of depolarization inside hair cells, predominantly inner hair cells (25–28). The SP could be prominent (e.g., Fig. 7) but was not seen in all cases. When it was prominent, it was absent to 250 Hz, first appeared to 500 Hz, and increased in magnitude to higher frequencies (Fig. 7). This pattern is similar to that reported for the intracellular depolarization during tone bursts in inner hair cells (44), and has implications for the low pass filter that is a prominent feature of cochlear models (e.g.,45).
Evidence of Auditory Neuropathy and other Cochlear and Neural Pathology
In many cases there was a large signal with little distortion and little or no ANN (e.g., Fig. 6). This result indicates functioning hair cells producing a CM but a limited auditory nerve response, i.e., an auditory neuropathy. The threshold of the difference response can determine the threshold of the CM, if it is an undistorted response (which was usually the case at threshold intensities). The fact that it was at times lower than the audiogram threshold also indicates that hair cell responses can occur in the absence of connections to auditory nerve fibers. Recent work in animals has shown that excitotoxicity can cause the synapse to retract with subsequent fiber and ganglion cell loss. The lack of hair cell loss in these cases overturns the previous view that hair cells were the most vulnerable site for hearing loss in the cochlea (46,47). The presence of hair cell responses without neural responses is evidence that the hair cell-nerve fiber synapse is also a particularly vulnerable site in humans.
Measurable responses (CM and ANN) were detected a greater percentage of the time to frequencies of 1000 Hz or less than to 2000 and 4000 Hz. and the responses to lower frequencies were also larger in magnitude. Given that the majority of the subjects had a progressive high frequency sloping hearing loss pattern, the results are consistent with this pattern of pathology. However, a few subjects had the largest responses to frequencies other than the lowest tested. Too few cases have been obtained to determine if these have a specific etiology.
It is important to note that a precise etiology is not required for a determination that an implant will be beneficial; instead this determination is made based on the hearing condition itself. Thus, one future aim of these recordings is to assess whether particular suspected etiologies correlate with a particular cochlear state. Another aim is to correlate the state of cochlear survival of hearing elements with speech outcomes. A reasonable hypothesis would be that patients with the best-preserved functional neural elements would have the best outcomes. A correlate to this hypothesis is that if functional hair cells and neural elements are well-preserved, the patient should do well with the implant, and if functional hair cell and neural elements are not preserved, the patient may not perform well. These considerations could affect fitting strategies and efforts. Finally the recordings themselves can be extended and used during insertion to determine cochlear trauma due to electrode placement, which may also correlate with hearing outcomes.
Acknowledgments
Support: This work has been supported by the University of North Carolina Translational and Clinical Studies Institute and by NIH, NIDCD, Project #: 5T32DC005360-08.
Conflict of Interest: Dr. Oliver Adunka is a consultant for MED-EL Corporation and receives research support. Dr. Douglas Fitzpatrick receives research support from MED-EL Corporation. Dr. Craig Buchman serves as a consultant for Advanced Bionics, Cochlear Corp., and MED-EL Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation: This manuscript will be presented at the 47th Annual Spring Meeting of the American Neurotology Society, April 18-22, 2012, San Diego, CA
IRB Approval: All procedures were in accordance with the ethical standards of the University of North Carolina at Chapel Hill IRB and with the Helsinki Declaration. Informed consent was obtained for all subjects enrolled in the study. Protocol number 05-2616.
REFERENCES
- 1.Firszt JB, Holden LK, Skinner MW, et al. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear and hearing. 2004;25:375–387. doi: 10.1097/01.aud.0000134552.22205.ee. [DOI] [PubMed] [Google Scholar]
- 2.Gomaa NA, Rubinstein JT, Lowder MW, et al. Residual speech perception and cochlear implant performance in postlingually deafened adults. Ear and hearing. 2003;24:539–544. doi: 10.1097/01.AUD.0000100208.26628.2D. [DOI] [PubMed] [Google Scholar]
- 3.Francis HW, Niparko JK. Cochlear implantation update. Pediatric clinics of North America. 2003;50:341–361. doi: 10.1016/s0031-3955(03)00034-8. viii. [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein JT, Parkinson WS, Tyler RS, et al. Residual speech recognition and cochlear implant performance: effects of implantation criteria. The American journal of otology. 1999;20:445–452. [PubMed] [Google Scholar]
- 5.Friedland DR, Venick HS, Niparko JK. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2003;24:582–589. doi: 10.1097/00129492-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiology & neuro-otology. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- 7.Aschendorff A, Kromeier J, Klenzner T, et al. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear and hearing. 2007;28:75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 8.Aschendorff A, Kubalek R, Turowski B, et al. Quality control after cochlear implant surgery by means of rotational tomography. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2005;26:34–37. doi: 10.1097/00129492-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008;29:920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adunka OF, Pillsbury HC, Buchman CA. Minimizing intracochlear trauma during cochlear implantation. Advances in oto-rhino-laryngology. 2010;67:96–107. doi: 10.1159/000262601. [DOI] [PubMed] [Google Scholar]
- 11.Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. The Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 12.Nadol JB, Jr, Shiao JY, Burgess BJ, et al. Histopathology of cochlear implants in humans. The Annals of otology, rhinology, and laryngology. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- 13.Khan AM, Handzel O, Burgess BJ, et al. Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? The Laryngoscope. 2005;115:672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- 14.Kawano A, Seldon HL, Clark GM, et al. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta oto-laryngologica. 1998;118:313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- 15.Smith L, Simmons FB. Estimating eighth nerve survival by electrical stimulation. The Annals of otology, rhinology, and laryngology. 1983;92:19–23. doi: 10.1177/000348948309200105. [DOI] [PubMed] [Google Scholar]
- 16.Hall RD. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hearing research. 1990;49:155–168. doi: 10.1016/0378-5955(90)90102-u. [DOI] [PubMed] [Google Scholar]
- 17.Khan AM, Whiten DM, Nadol JB, Jr, et al. Histopathology of human cochlear implants: correlation of psychophysical and anatomical measures. Hearing research. 2005;205:83–93. doi: 10.1016/j.heares.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Kim JR, Abbas PJ, Brown CJ, et al. The relationship between electrically evoked compound action potential and speech perception: a study in cochlear implant users with short electrode array. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2010;31:1041–1048. doi: 10.1097/MAO.0b013e3181ec1d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandala M, Colletti L, Tonoli G, et al. Electrocochleography during Cochlear Implantation for Hearing Preservation. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012 doi: 10.1177/0194599811435895. [DOI] [PubMed] [Google Scholar]
- 20.Radeloff A, Shehata-Dieler W, Scherzed A, et al. Intraoperative monitoring using cochlear microphonics in cochlear implant patients with residual hearing. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012;33:348–354. doi: 10.1097/MAO.0b013e318248ea86. [DOI] [PubMed] [Google Scholar]
- 21.Harris R, Cruise A, Gibson W, et al. Preliminary results and technique for electrophysiological intra-operative monitoring of residual hearing during cochlear implantation. Cochlear implants international. 2011;12:209–215. doi: 10.1179/146701011X12950038111657. [DOI] [PubMed] [Google Scholar]
- 22.Santarelli R, Starr A, Michalewski HJ, et al. Neural and receptor cochlear potentials obtained by transtympanic electrocochleography in auditory neuropathy. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2008;119:1028–1041. doi: 10.1016/j.clinph.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Verpy E, Weil D, Leibovici M, et al. Stereocilin-deficient mice reveal the origin of cochlear waveform distortions. Nature. 2008;456:255–258. doi: 10.1038/nature07380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patuzzi RB, Yates GK, Johnstone BM. Outer hair cell receptor current and sensorineural hearing loss. Hearing research. 1989;42:47–72. doi: 10.1016/0378-5955(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 25.Santarelli R, Del Castillo I, Rodriguez-Ballesteros M, et al. Abnormal cochlear potentials from deaf patients with mutations in the otoferlin gene. Journal of the Association for Research in Otolaryngology : JARO. 2009;10:545–556. doi: 10.1007/s10162-009-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dallos P, Wang CY. Bioelectric correlates of kanamycin intoxication. Audiology : official organ of the International Society of Audiology. 1974;13:277–289. doi: 10.3109/00206097409071685. [DOI] [PubMed] [Google Scholar]
- 27.Durrant JD, Wang J, Ding DL, et al. Are inner or outer hair cells the source of summating potentials recorded from the round window? The Journal of the Acoustical Society of America. 1998;104:370–377. doi: 10.1121/1.423293. [DOI] [PubMed] [Google Scholar]
- 28.Zheng XY, Ding DL, McFadden SL, et al. Evidence that inner hair cells are the major source of cochlear summating potentials. Hearing research. 1997;113:76–88. doi: 10.1016/s0378-5955(97)00127-5. [DOI] [PubMed] [Google Scholar]
- 29.Ruth RA, Lambert PR, Ferraro JA. Electrocochleography: methods and clinical applications. The American journal of otology. 1988;9 Suppl:1–11. [PubMed] [Google Scholar]
- 30.Portmann M, Harrison RV, Negrevergne M, et al. Electrocochleographic measures of cochlear frequency selectivity in hearing loss of cochlear origin. Acta oto-laryngologica. 1983;95:657–663. doi: 10.3109/00016488309139459. [DOI] [PubMed] [Google Scholar]
- 31.Eggermont JJ. Analysis of compound action potential responses to tone bursts in the human and guinea pig cochlea. The Journal of the Acoustical Society of America. 1976;60:1132–1139. doi: 10.1121/1.381214. [DOI] [PubMed] [Google Scholar]
- 32.Schoonhoven R, Fabius MA, Grote JJ. Input/output curves to tone bursts and clicks in extratympanic and transtympanic electrocochleography. Ear and hearing. 1995;16:619–630. doi: 10.1097/00003446-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Snyder RL, Schreiner CE. The auditory neurophonic: basic properties. Hearing research. 1984;15:261–280. doi: 10.1016/0378-5955(84)90033-9. [DOI] [PubMed] [Google Scholar]
- 34.Henry KR. Auditory nerve neurophonic recorded from the round window of the Mongolian gerbil. Hearing research. 1995;90:176–184. doi: 10.1016/0378-5955(95)00162-6. [DOI] [PubMed] [Google Scholar]
- 35.Henry KR. Auditory nerve neurophonic tuning curves produced by masking of round window responses. Hearing research. 1997;104:167–176. doi: 10.1016/s0378-5955(96)00195-5. [DOI] [PubMed] [Google Scholar]
- 36.Snyder RL, Schreiner CE. Forward masking of the auditory nerve neurophonic (ANN) and the frequency following response (FFR) Hearing research. 1985;20:45–62. doi: 10.1016/0378-5955(85)90058-9. [DOI] [PubMed] [Google Scholar]
- 37.Chimento TC, Schreiner CE. Adaptation and recovery from adaptation of the auditory nerve neurophonic (ANN) using long duration tones. Hearing research. 1992;62:131–141. doi: 10.1016/0378-5955(92)90178-p. [DOI] [PubMed] [Google Scholar]
- 38.Marsh JT, Worden FG, Smith JC. Auditory frequency-following response: neural or artifact? Science. 1970;169:1222–1223. doi: 10.1126/science.169.3951.1222. [DOI] [PubMed] [Google Scholar]
- 39.Rizzoni G. Fundamentals of electrical engineering. Dubuque, IA: McGraw-Hill; 2009. p. 726. viii. [Google Scholar]
- 40.Chimento TC, Schreiner CE. Selectively eliminating cochlear microphonic contamination from the frequency-following response. Electroencephalography and clinical neurophysiology. 1990;75:88–96. doi: 10.1016/0013-4694(90)90156-e. [DOI] [PubMed] [Google Scholar]
- 41.Henry KR. Tuning curves of the difference tone auditory nerve neurophonic. Hearing research. 1996;99:160–167. doi: 10.1016/s0378-5955(96)00097-4. [DOI] [PubMed] [Google Scholar]
- 42.Henry KR. Auditory nerve neurophonic produced by the frequency difference of two simultaneously presented tones. Hearing research. 1996;99:151–159. doi: 10.1016/s0378-5955(96)00096-2. [DOI] [PubMed] [Google Scholar]
- 43.Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer AR, Russell IJ. Phase-locking in the cochlear nerve of the guinea-pig and its relation to the receptor potential of inner hair-cells. Hearing research. 1986;24:1–15. doi: 10.1016/0378-5955(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 45.Meddis R, O'Mard LP, Lopez-Poveda EA. A computational algorithm for computing nonlinear auditory frequency selectivity. The Journal of the Acoustical Society of America. 2001;109:2852–2861. doi: 10.1121/1.1370357. [DOI] [PubMed] [Google Scholar]
- 46.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin HW, Furman AC, Kujawa SG, et al. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. Journal of the Association for Research in Otolaryngology : JARO. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]