Abstract
Background
Barrett’s esophagus (BE) is a premalignant lesion that predisposes to esophageal adenocarcinoma. However, the reported incidence of esophageal adenocarcinoma in patients with BE varies widely. We examined the risk of malignant progression in patients with BE using data from the Northern Ireland Barrett’s esophagus Register (NIBR), one of the largest population-based registries of BE worldwide, which includes every adult diagnosed with BE in Northern Ireland between 1993 and 2005.
Subjects and Methods
We followed 8522 patients with BE, defined as columnar lined epithelium of the esophagus with or without specialized intestinal metaplasia (SIM), until the end of 2008. Patients with incident adenocarcinomas of the esophagus or gastric cardia or with high-grade dysplasia of the esophagus were identified by matching the NIBR with the Northern Ireland Cancer Registry, and deaths were identified by matching with records from the Registrar General’s Office. Incidence of cancer outcomes or high-grade dysplasia was calculated as events per 100 person-years (% per year) of follow-up, and Cox proportional hazard models were used to determine incidence by age, sex, length of BE segment, presence of SIM, macroscopic BE, or low-grade dysplasia. All P values were from two-sided tests.
Results
After a mean of 7.0 years of follow-up, 79 patients were diagnosed with esophageal cancer, 16 with cancer of the gastric cardia, and 36 with high-grade dysplasia. In the entire cohort, incidence of esophageal or gastric cardia cancer or high-grade dysplasia combined was 0.22% per year (95% confidence interval [CI] = 0.19% to 0.26%). SIM was found in 46.0% of patients. In patients with SIM, the combined incidence was 0.38% per year (95% CI = 0.31 to 0.46%). The risk of cancer was statistically significantly elevated in patients with vs without SIM at index biopsy (0.38% per year vs 0.07% per year; hazard ratio [HR] = 3.54, 95% CI = 2.09 to 6.00, P < .001), in men compared with women (0.28% per year vs 0.13% per year; HR = 2.11, 95% CI = 1.41 to 3.16, P < .001), and in patients with low-grade dysplasia compared with no dysplasia (1.40% per year vs 0.17% per year; HR = 5.67, 95% CI = 3.77 to 8.53, P < .001).
Conclusion
We found the risk of malignant progression among patients with BE to be lower than previously reported, suggesting that currently recommended surveillance strategies may not be cost-effective.
CONTEXT AND CAVEATS
Prior Knowledge
Reports have varied regarding the risk of developing esophageal adenocarcinoma in patients with Barrett’s esophagus.
Study Design
Cancer risk among 8522 patients with Barrett's esophagus, defined as columnar esophageal epithelium with or without specialized intestinal metaplasia (SIM), was examined using data from the Northern Ireland Barrett’s esophagus Register for 1993–2005 matched with cancer registry records. Patients were followed to the end of 2008 (mean follow-up = 7.0 years). Incidence of cancer or high-grade dysplasia of the esophagus or gastric cardia cancer was calculated as the number of events per 100 person-years of follow-up.
Contribution
The incidence of cancer or high-grade dysplasia in this population-based study was 0.22% per year; it was 0.38% per year among those with SIM at index biopsy. Cancer risk was higher among patients with SIM, patients with low-grade dysplasia, and male patients.
Implications
Risk of cancer among patients with Barrett's esophagus was lower in this study than in previous studies, so current surveillance strategies might not be optimal.
Limitations
Standardized biopsy protocols or pathological reporting were not used. Reasons for endoscopy and complete data on segment length were not available.
From the Editors
The incidence of esophageal adenocarcinoma is rising in the United States and Europe (1,2). Despite general improvements in cancer survival in most countries, patients with esophageal adenocarcinoma have a poor prognosis, with fewer than 20% surviving for 5 years (3,4). Barrett’s esophagus (BE) is the metaplastic transformation of the native esophageal squamous epithelium into columnar epithelium in response to gastroesophageal reflux. Patients with BE, a known precursor to esophageal adenocarcinoma, are estimated to carry a 30- to 60-fold increased risk of developing esophageal adenocarcinoma (5).
Endoscopic surveillance of BE is the currently accepted standard of care and aims to reduce morbidity and mortality through early detection of dysplasia or cancer (6,7). The cost-effectiveness of surveillance is dependent on the risk of progression of BE to cancer (8–10). However, a wide variation in the incidence of esophageal adenocarcinoma in BE has been observed, ranging from 0% to 3.5% per annum (11,12). Also, it is not currently known whether the rate of progression of BE to esophageal adenocarcinoma varies with time from diagnosis of BE. Change in risk over time has implications regarding both the need for, and the frequency of, endoscopic surveillance.
The aim of this study was to examine the risk of adenocarcinoma or high-grade dysplasia in a large cohort of unselected BE patients. The risk of cancer or high-grade dysplasia was examined using both the British definition of BE, that is, columnar lined epithelium of the esophagus (CLE) and the American definition of BE, that is, specialized intestinal metaplasia (SIM). Debate continues as to whether the definition of BE should include SIM, which is usually defined by the histological confirmation of goblet cells within the columnar mucosa (13). Lack of a consensus definition has led to differing recommendations for patient entry into endoscopic surveillance programs (6,7).
In a previous publication, we presented follow-up data until 1999 on 2969 patients from this cohort (14). The current study includes 5553 additional patients, better identifies and classifies relevant outcomes, and extends follow-up until December 2008.
Subjects and Methods
The Northern Ireland Barrett’s esophagus Register (NIBR) includes every adult identified with BE, defined as CLE, within Northern Ireland (population 1.7 million) between 1993 and 2005 and now includes 9334 patients. We constructed the register by examining all pathology reports from esophageal biopsies obtained by endoscopy in Northern Ireland over this period, irrespective of the diagnosis made by the reporting pathologist. We examined the clinical summaries for the biopsies to exclude from our study the pathology report of any biopsy that was taken at the esophagogastric junction. Pathology reports relating to all esophageal biopsies were examined further by applying a standardized set of rules that were drawn up in consultation with gastroenterologists and pathologists. Only those biopsies that were documented to show CLE were included in the study. The date of the earliest (index) biopsy showing CLE was taken as the date of entry into the register. A statement in the clinical summary that Barrett’s mucosa was seen or suspected at endoscopy was used to further classify the biopsies as “macroscopic BE.” Details on the length of the Barrett’s mucosal region were also noted where available. Mucosal areas of columnar metaplasia that were of 3 cm or more in length were classified as long-segment Barrett’s esophagus (LSBE), and such areas that were less than 3 cm long were classified as short-segment Barrett’s esophagus (SSBE). All esophageal biopsies with BE histology were further subdivided according to whether the pathologist specifically stated that SIM was present or absent in the biopsy specimen. If an esophageal biopsy showed evidence of malignancy, it was excluded from the study.
Patients were considered to have no dysplasia if the pathologist reported “no dysplasia”; low-grade dysplasia, if the pathologist reported the presence of “low-grade,” “mild,” “moderate,” or “indefinite”dysplasia; and high-grade dysplasia, if the pathologist reported “high-grade” or “severe” dysplasia. Otherwise, patients were classified as having unknown dysplasia status.
All 9334 patients in the NIBR were followed for deaths, incident esophageal and gastric cardia malignancies, and high-grade dysplasia of the esophagus until December 31, 2008. Patients who were diagnosed with high-grade dysplasia or cancer within 1 year of index biopsy were considered to have prevalent disease and were excluded from the analysis; patients with less than 12 months follow-up since their initial diagnosis of BE were also excluded. Patients with incident esophageal adenocarcinoma and histologically unspecified esophageal malignancies were identified by matching the cohort with the Northern Ireland Cancer Registry (NICR) database. Patients who developed other histological subtypes of esophageal cancer, including squamous cell carcinomas, were excluded. The NICR records all cancers diagnosed within Northern Ireland. Incident gastric cardia adenocarcinomas and histologically unspecified carcinomas of the gastric cardia were identified within the cohort in a similar fashion. Classification of tumors of the esophagogastric junction is known to be difficult (15,16), so adenocarcinoma of the gastric cardia was included as an outcome because it is likely that these tumors in a patient with a history of BE are esophageal in origin. BE patients with high-grade dysplasia were identified by examining all esophageal pathology reports from Northern Ireland for the period 1993–2008. Patients were considered to have high-grade dysplasia if it was diagnosed twice within a 1-year period or in two subsequent biopsies, even if the duration between them was more than 1 year, or if high-grade dysplasia was present in a single biopsy and the duration of available follow-up after the development of high-grade dysplasia was less than 1 year. High-grade dysplasia that occurred in squamous epithelium was not included as an outcome. Patients who were initially diagnosed with high-grade dysplasia but then diagnosed as having esophageal adenocarcinoma on subsequent biopsy were classified as having esophageal adenocarcinomas for the purposes of analysis. Deaths were identified by matching with death files from the Northern Ireland Registrar General’s office using the patient’s surname, first name, and date of birth. Socioeconomic status of patients was estimated by deriving income deprivation quintiles from patient address data.
Statistical Methods
In this study, the primary outcomes of interest were diagnoses of esophageal cancer, cancer of the gastric cardia, or high-grade dysplasia of the esophagus that occurred at least 12 months after a patient's first biopsy showed the presence of BE. Person-years of follow-up were calculated for each member of the cohort with censoring on the date of death, on the date on which cancer was diagnosed, on the date on which high-grade dysplasia was diagnosed if cancer did not occur subsequently, or at the end of follow-up (December 31, 2008). Incidence of the primary outcomes was calculated as the number of events divided by the person-years of follow-up and was expressed as events per 100 person-years (% per year) of follow-up.
The data were analyzed for cancer outcomes and for the combined outcome of cancer or high-grade dysplasia; the analysis was repeated for those with SIM at index biopsy. We examined incidence of cancer or high-grade dysplasia according to factors that included age, sex, segment length, presence of SIM at index biopsy, and low-grade dysplasia at index biopsy. We analyzed data from biopsies after the index biopsy to assess the risk of malignant progression in patients who developed low-grade dysplasia after their index biopsy. We also performed sensitivity analyses that included additional patients with at least 6 months of follow-up time and events occurring after 6 months. However, the analyses including patients with at least 6 months follow-up showed similar incidence rates to those seen in the main analysis (data not shown).
We used survival analysis to examine the risk of progression to cancer over time in BE patients. We used Cox proportional hazards modeling to compare the incidence of cancer by variables including age group (<50, 50–59, 60–69, 70–79, or ≥80 years), sex (male or female), length of BE segment (long, short, or unknown), presence of SIM at index biopsy (present, absent, or unknown), presence of visible segment of BE (yes or unknown), and presence of low-grade dysplasia at index biopsy (present, absent, or unknown). All variables were treated as categorical for the Cox proportional hazards model and were adjusted for each other within the model. Kaplan–Meier curves were plotted for each variable to examine the assumption of proportional hazards. Hazard ratios (HRs) and 95% confidence intervals (CI) were calculated. P values that were less than .05 were considered to be statistically significant. All P values were from two-sided tests. The χ2 and independent samples t tests were used to compare demographic data between groups. All statistical analysis was conducted using SPSS version 15 (SPSS Inc, Chicago, IL).
Results
Patient Characteristics
A total of 9334 patients were diagnosed with CLE in Northern Ireland between 1993 and 2005 (Table 1). Of this cohort, 5437 (58.2%) were men and 3897 (41.8%) were women, with a mean age of 60.9 years. Men were younger than women at the time of BE diagnosis (mean age = 58.6 vs 64 years; P < .001). Income deprivation quintiles as a marker of socioeconomic status were available for 83.5% of the NIBR cohort. Statistically significantly more BE patients were from the lowest socioeconomic group than the highest (18.2% vs 14.6%, P < .001). A total of 96 patients were excluded from the analysis because they had high-grade dysplasia at baseline, and 189 additional patients were excluded from analysis because, having been diagnosed with high-grade dysplasia or cancer within 1 year of index biopsy, they were classified as having prevalent disease. Furthermore, 527 patients were excluded because they had less than 1 year of follow-up. This left 8522 patients for analysis, who were followed for up to 16 years. Of these 8522 patients, 2840 (33.3%) were noted to have an endoscopy with biopsy subsequent to the index biopsy. The average number of endoscopies with biopsies for the whole cohort was 1.8 per person; 1538 patients (18%) had a total of three or more endoscopies with biopsies.
Table 1.
Characteristics of patients in the Northern Ireland Barrett’s esophagus register
| Characteristic | No. (%), n = 9334 |
| Sex | |
| Female | 3897 (41.8) |
| Male | 5437 (58.2) |
| Mean age (SD, y) | 60.9 (15.5) |
| Age group, y | |
| <50 | 2336 (25.0) |
| 50–59 | 1973 (21.1) |
| 60–69 | 2065 (22.1) |
| 70–79 | 1917 (20.5) |
| ≥80 | 1043 (11.2) |
| Specialized intestinal metaplasia at index biopsy | |
| Absent | 3388 (36.3) |
| Present | 4307 (46.1) |
| Unknown | 1639 (17.6) |
| Dysplasia at index biopsy | |
| No dysplasia | 8775 (94.0) |
| Low-grade dysplasia | 374 (4.0) |
| High-grade dysplasia | 96 (1.0) |
| Socioeconomic status (income deprivation quintile) | |
| Most-deprived quintile | 1701 (18.2) |
| Quintile 2 | 1650 (17.7) |
| Quintile 3 | 1552 (16.6) |
| Quintile 4 | 1473 (15.8) |
| Least-deprived quintile | 1360 (14.6) |
| Unknown | 1598 (17.1) |
SIM was documented in almost half of the patients (3917 of 8522: 46.0%), as judged from their index biopsies (Table 2). Patients with SIM were more likely to be men (59.1% vs 49.8%, P < .001) and older (mean age 62.0 vs 57.0 years, P < .001) than those whose biopsies showed no evidence of SIM (data not shown). For 3462 (40.6%) of the 8522 patients, the clinical summary recorded that Barrett’s mucosa was seen or suspected at endoscopy (Table 2). The length of the BE segment was poorly documented within the clinical summaries for most patients, with the majority (6894 of 8522; 81.0%) listed as unknown; 947 (11.1%) of 8522 of patients were recorded as having LSBE and 681 (8.0%) of 8522 as having SSBE. The majority of patients in the cohort (8128 of 8522; 95.4%) did not have dysplasia at index biopsy.
Table 2.
Incidence of esophageal adenocarcinoma, gastric cardia adenocarcinoma, and combined events among patients with Barrett’s esophagus (BE)
| Characteristic | Patients, No. | Person-years of follow-up | High-grade dysplasia, No. | Esophageal adenocarcinoma, No. | Gastric cardia adenocarcinoma, No. | Incidence of esophageal cancer, % per year (95% Confidence Interval [CI]) | Incidence of cancer of esophagus and gastric cardia, % per year (95% CI) | Incidence of combined cancer or high-grade dysplasia, % per year (95% CI) | Combined events hazard ratio* (95% CI) |
| Total | 8522 | 59 784 | 36 | 79 | 16 | 0.13 (0.10 to 0.16) | 0.16 (0.13 to 0.20) | 0.22 (0.19 to 0.26) | NA |
| Sex | |||||||||
| Female | 3586 | 25 272.7 | 9 | 19 | 5 | 0.08 (0.05 to 0.12) | 0.09 (0.06 to 0.14) | 0.13 (0.09 to 0.18) | 1.00 (referent) |
| Male | 4936 | 34 493.3 | 27 | 60 | 11 | 0.17 (0.13 to 0.22) | 0.21 (0.17 to 0.26) | 0.28 (0.23 to 0.34) | 2.11 (1.41 to 3.16) |
| Age, y | |||||||||
| <50 | 2292 | 17 365.5 | 8 | 9 | 3 | 0.05 (0.03 to 0.1) | 0.07 (0.04 to 0.12) | 0.12 (0.08 to 0.18) | 1.00 (referent) |
| 50–59 | 1882 | 13 894.3 | 8 | 19 | 4 | 0.14 (0.09 to 0.22) | 0.17 (0.11 to 0.25) | 0.22 (0.15 to 0.31) | 1.62 (0.92 to 2.85) |
| 60–69 | 1903 | 13 707.1 | 13 | 24 | 8 | 0.18 (0.12 to 0.27) | 0.23 (0.16 to 0.33) | 0.33 (0.25 to 0.44) | 2.43 (1.43 to 4.13) |
| 70–79 | 1649 | 10 810.9 | 6 | 21 | 1 | 0.19 (0.12 to 0.29) | 0.20 (0.13 to 0.30) | 0.26 (0.18 to 0.38) | 2.02 (1.13 to 3.63) |
| ≥80 | 796 | 4006.7 | 1 | 6 | 0 | 0.15 (0.07 to 0.33) | 0.15 (0.07 to 0.33) | 0.17 (0.08 to 0.35) | 1.59 (0.67 to 3.82) |
| Specialized intestinal metaplasia at index biopsy | |||||||||
| Absent | 3179 | 23 416.5 | 4 | 9 | 4 | 0.04 (0.02 to 0.08) | 0.06 (0.04 to 0.10) | 0.07 (0.04 to 0.11) | 1.00 (referent) |
| Present | 3917 | 28 323.1 | 31 | 66 | 10 | 0.23 (0.18 to 0.29) | 0.27 (0.22 to 0.34) | 0.38 (0.31 to 0.46) | 3.54 (2.09 to 6.00) |
| Unknown | 1426 | 8045.0 | 1 | 4 | 2 | 0.05 (0.02 to 0.13) | 0.07 (0.03 to 0.16) | 0.09 (0.04 to 0.18) | 1.05 (0.43 to 2.54) |
| Visible segment seen | |||||||||
| Unknown | 5060 | 38 502.4 | 17 | 53 | 11 | 0.14 (0.11 to 0.18) | 0.17 (0.13 to 0.22) | 0.21 (0.17 to 0.26) | 1.00 (referent) |
| Yes | 3462 | 21 282.1 | 19 | 26 | 5 | 0.12 (0.08 to 0.18) | 0.15 (0.11 to 0.21) | 0.23 (0.17 to 0.30) | 0.62 (0.36 to 1.07) |
| Length of BE segment | |||||||||
| Short | 681 | 4395.8 | 2 | 3 | 0 | 0.07 (0.02 to 0.20) | 0.07 (0.02 to 0.20) | 0.11 (0.05 to 0.26) | 1.00 (referent) |
| Long | 947 | 6451.0 | 12 | 14 | 3 | 0.22 (0.13 to 0.37) | 0.26 (0.16 to 0.42) | 0.45 (0.31 to 0.65) | 2.31 (0.89 to 6.01) |
| Unknown | 6894 | 48 937.8 | 22 | 62 | 13 | 0.13 (0.10 to 0.17) | 0.15 (0.12 to 0.19) | 0.20 (0.16 to 0.24) | 1.08 (0.40 to 2.99) |
| Dysplasia at index biopsy | |||||||||
| No | 8128 | 56 989.7 | 26 | 58 | 14 | 0.10 (0.08 to 0.13) | 0.13 (0.10 to 0.16) | 0.17 (0.14 to 0.21) | 1.00 (referent) |
| Low grade | 323 | 2282.7 | 9 | 21 | 2 | 0.92 (0.60 to 1.40) | 1.01 (0.67 to 1.51) | 1.40 (0.99 to 1.97) | 5.67 (3.77 to 8.53) |
| Unknown | 71 | 512.2 | 1 | 0 | 0 | 0 | 0 | 0.20 (0.04 to 1.11) | 0.90 (0.13 to 6.49) |
| Well-defined BE† | |||||||||
| Total | 1990 | 12 322.8 | 16 | 22 | 3 | 0.18 (0.12 to 0.27) | 0.20 (0.14 to 0.30) | 0.33 (0.24 to 0.45) | NA |
Adjusted for sex, age category, index SIM status, presence of visible segment, length of BE and presence of index low-grade dysplasia.
Well-defined BE was defined as both the presence of SIM at index biopsy and a visible segment seen.
The mean period of follow-up for the 8522 patients included for analysis was 7.0 years, and the total number of person-years of follow-up within the study period was 59 784. There were 79 patients diagnosed with esophageal cancer at least 12 months after diagnosis of CLE (62 with esophageal adenocarcinoma, one with esophageal adenosquamous carcinoma, and 16 with histologically unspecified carcinomas of the esophagus). Sixteen additional patients were diagnosed with adenocarcinoma of the gastric cardia, and 36 patients developed high-grade dysplasia during follow-up. The mean age at diagnosis was 63.3 years (SD = 11.8) for esophageal adenocarcinoma and adenocarcinoma of the gastric cardia, and 59.8 years (SD = 12.1) for high-grade dysplasia.
Incidence of Cancers and Combined Events in BE
The incidence of cancers in the whole cohort was 0.16% per year (95% CI = 0.13% to 0.20% per year; Table 2). This estimate increased when the rate of the combined events (cancers or high-grade dysplasia) was examined: 0.22% per year (95% CI = 0.19% to 0.26% per year).
Incidence of Cancers and Combined Events in Different Subgroups
We categorized the rates of cancers and combined events according to sex, age category, SIM status, macroscopic BE, segment length, and presence of low-grade dysplasia (Table 2). Analysis by sex showed that men were statistically significantly more likely to progress to malignancy than women; the risk of combined cancer or high-grade dysplasia was 0.28% per year in men compared with 0.13% per year in women (adjusted HR for combined events = 2.11, 95% CI = 1.41 to 3.16, P < .001). The relationship between age and risk of neoplastic progression was complex. When initially examined as a continuous variable, age was statistically significantly associated with the risk of neoplastic progression (adjusted HR = 1.02, 95% CI = 1.01 to 1.03, P < .05). However, on closer inspection of the data, risk of progression did not uniformly increase with age. To provide further insight into the relationship, categorical analysis was also conducted. When analyzed by age category, the highest risk of progression appeared in the 60- to 69-year age category (0.33% per year), and the lowest risk in patients younger than 50 years (0.12% per year). The group of patients who were older than 80 years showed a low risk of progression, 0.17% per year, although this category had the shortest follow-up (4006 person-years). Incidence with age by sex is shown in graphical form (Figure 1). Statistical analysis did not reveal an interaction between incidence with age (categorical) and sex (P = .55). The incidence of combined events did not vary by socioeconomic status (data not shown).
Figure 1.
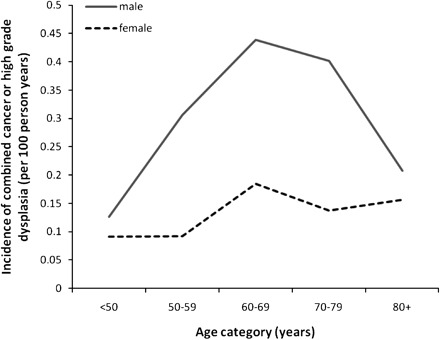
Incidence of combined cancer or high-grade dysplasia of the esophagus in patients with Barrett’s esophagus, by sex and age category.
The incidence in patients with a visible BE segment at endoscopy was 0.15% per year (95% CI = 0.11% to 0.21% per year) for cancer and 0.23% per year (95% CI = 0.17% to 0.30% per year) for combined events. No statistically significant difference was detected between this rate and that seen in patients with unknown visible segment status.
Presence of low-grade dysplasia at index biopsy was associated with the greatest risk of progression to cancer (combined events’ risk = 1.40% per year, 95% CI = 0.99% to 1.97% per year); patients with low-grade dysplasia were at much greater risk than those with no dysplasia at index biopsy (1.40% vs 0.17% per year, adjusted HR = 5.67, 95% CI = 3.77 to 8.53, P < .001). A similar risk of progression was seen among the 288 patients who were classified as having no dysplasia at index biopsy but were diagnosed with low-grade dysplasia at subsequent biopsy (combined event’s risk = 1.37% per year, 95% CI = 0.99% to 1.90% per year).
A subset of 1990 patients with “well-defined” BE (both SIM and visible segment present) had a mean follow-up of 6.2 years and a total follow-up of 12 312 person-years. The incidence of combined events in this group was 0.33% per year (95% CI = 0.24% to 0.45% per year). This incidence is not significantly different from the incidence seen in the SIM subgroup, although it is higher than the rate seen in the whole cohort.
Subgroup Analysis by SIM Status
The incidence of cancers in patients with SIM at index biopsy was 0.27% per year (95% CI = 0.22% to 0.34% per year), and the incidence of combined events in this group was 0.38% per year (95% CI = 0.31% to 0.46% per year). Adjusting for other factors, the risk of cancer was statistically significantly higher in patients with SIM than in patients whose biopsies did not show SIM at first biopsy (0.38% vs 0.07% per year; HR = 3.54, 95% CI = 2.09 to 6.00, P < .001; Table 2). Further examination of the 13 patients without index SIM who developed cancer or high-grade dysplasia determined that 10 of these patients had interval biopsies before developing cancer and that seven of the 10 biopsies showed SIM.
We next compared the risk of progression to cancer by SIM status at index biopsy (Table 3). The incidence rates of combined events were statistically significantly lower across most subgroups analyzed when we compared patients without SIM at index biopsy to those with SIM.
Table 3.
Comparison of combined outcomes (adenocarcinomas of the esophagus or gastric cardia or high-grade dysplasia) among Barrett’s esophagus patients with columnar lined epithelium of the esophagus (CLE) with or without specialized intestinal metaplasia (SIM)
| Characteristic | CLE without SIM, incidence: % per year (95% Confidence Interval [CI]) | CLE with SIM, incidence: % per year (95% CI) | Hazard ratio (95% CI) | P* |
| Sex | ||||
| Female | 0.05 (0.02 to 0.11) | 0.26 (0.18 to 0.38) | 5.65 (2.18 to 14.68) | <.001 |
| Male | 0.10 (0.06 to 0.17) | 0.45 (0.36 to 0.56) | 4.63 (2.52 to 8.49) | <.001 |
| Age category, y | ||||
| <50 | 0.03 (0.01 to 0.10) | 0.25 (0.15 to 0.41) | 7.02 (2.05 to 24.09) | .001 |
| 50–59 | 0.10 (0.04 to 0.23) | 0.37 (0.25 to 0.54) | 3.78 (1.45 to 9.87) | .002 |
| 60–69 | 0.11 (0.05 to 0.26) | 0.49 (0.35 to 0.68) | 4.46 (1.75 to 11.35) | .001 |
| 70–79 | 0.08 (0.03 to 0.23) | 0.43 (0.29 to 0.64) | 5.45 (1.64 to 18.09) | .001 |
| ≥80 | 0.08 (0.01 to 0.44) | 0.29 (0.13 to 0.63) | 3.39 (0.41 to 28.25) | .135 |
| Visible segment seen | ||||
| Unknown | 0.07 (0.04 to 0.12) | 0.41(0.32 to 0.52) | 6.27 (3.31 to 11.87) | <.001 |
| Yes | 0.09 (0.04 to 0.20) | 0.33 (0.24 to 0.45) | 3.72 (1.58 to 8.77) | .001 |
| Segment length† | ||||
| Short | 0.05 (0.01 to 0.28) | 0.15 (0.05 to 0.44) | 2.92 (0.31 to 28.76) | .47 |
| Long | 0.23 (0.08 to 0.67) | 0.50 (0.34 to 0.74) | 2.19 (0.66 to 7.29) | .36 |
| Unknown | 0.06 (0.03 to 0.10) | 0.37 (0.30 to 0.46) | 5.72 (3.18 to 10.28) | <.01 |
| Index dysplasia status | ||||
| No dysplasia | 0.06 (0.04 to 0.10) | 0.31 (0.25 to 0.38) | 5.04 (2.86 to 8.89) | <.001 |
| Low grade | 0.91 (0.31 to 2.64) | 1.45 (0.98 to 2.13) | 1.53 (0.47 to 5.10) | .75 |
| Unknown | 0 | 0.33 (0.06 to 1.85) | Not calculable | NA |
P values were from two-sided tests, using Cox proportional hazards modeling to estimate hazard ratios.
Segment length was categorized as short (<3cm), long (>3cm), or unknown.
Rate of Progression to Cancer Over Time
The Kaplan–Meier plot of progression to cancer over time for the entire NIBR cohort (Figure 2) illustrates a uniform rate of progression to cancer. Risk of progression to cancer in the years 1–6 (0.25% per year, 95% CI = 0.20% to 0.31% per year) following index biopsy was similar to that in the years 6–11 (0.27% per year, 95% CI = 0.20% to 0.37% per year). No statistically significant difference was seen for the years after the 11th year (0.28% per year, 95% CI = 0.14% to 0.58% per year).
Figure 2.
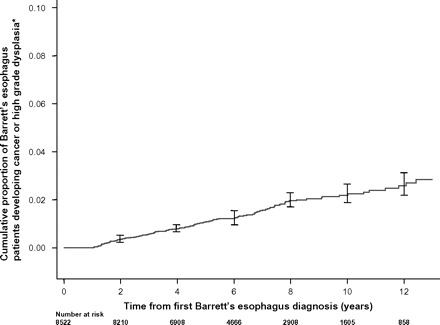
Kaplan–Meier plot showing the proportion of Barrett’s esophagus (BE) patients who developed esophageal or gastric cardia cancer or esophageal high-grade dysplasia relative to time from first diagnosis of BE. The asterisk denotes that individuals who developed cancer or high-grade dysplasia in the first year after diagnosis of BE were presumed to have had prevalent disease and were excluded from the study. In this graph, 95% confidence intervals are shown at biennial intervals.
Discussion
This study is one of the largest population-based studies to date to investigate the risk of esophageal malignancy in patients with BE. Previous work from the NIBR showed a low risk of progression to cancer in CLE patients with SIM (0.4% per year) and without SIM (0.26% per year) (14). Despite the inclusion of adenocarcinoma of the gastric cardia and high-grade dysplasia as outcomes, this larger study, which had much longer follow-up, shows a lower risk of progression and makes it possible to more accurately determine risk in subgroups and to estimate changes in risk of progression over time.
This study found that among all patients with CLE, the incidence of cancer was 0.16% per year, and the incidence of combined cancer or high-grade dysplasia was 0.22% per year. A recent study from the Netherlands reported an incidence of combined cancer or high-grade dysplasia of 0.58% per year in a large cohort of unselected BE patients (17). These incidence rates are lower than those in previously published studies that have reported risks of progression to cancer of up to 3% per year in other populations (11).
Reasons that previous studies may have overestimated the risk of cancer include smaller study size, shorter duration of follow-up, variable definition of BE used, inclusion of patients with early incident cancer, and inclusion of patients with high-grade dysplasia at baseline (11,12). As such, the low incidence of cancer in this study may in part be accounted for by the larger study size, the rigid exclusion of baseline high-grade dysplasia and early incident cancer, and the nature of an unselected non–referral-based cohort. The inclusion of patients with intestinal metaplasia from the esophagogastric junction could lead to an underestimate of the risk of cancer. Jung et al. (18) found that patients with intestinal metaplasia of this area are at a lower risk of cancer than those with BE. This study specifically excluded biopsies that were stated to have come from the esophagogastric junction; however, it is still possible that some biopsies were from this area.
Previous studies have used different definitions for the malignancies that develop from BE. In this study, adenocarcinoma of the gastric cardia was included as an outcome because tumors of the esophagogastric junction are likely to be esophageal in origin in patients with a history of BE. Chandrasoma et al. (16) found that most adenocarcinomas of the gastric cardia were esophageal adenocarcinomas if the esophagogastric junction was defined as the proximal limit of the gastric mucosa.
A change in risk of progression to esophageal cancer with age among BE patients has been reported in some studies (19,20) but not in others (21–23). Data from the NIBR showed that the rate of progression to cancer varied with age category, with peak cancer incidence in the 60- to 69-year age group, and a reduction in incidence if BE was first diagnosed when patients were older than 70 years. Possible reasons for this variation in cancer incidence by age group may include differences in the indication for the index endoscopy, lower malignant potential in BE that presents at later age, or differences in mortality rates between the age groups. Some patients who are diagnosed with BE at an older age may not survive long enough to develop BE-related cancer.
Differences in cancer risk between the sexes have also been variably reported (20,23,24). In our entire cohort, men were more than twice as likely to develop high-grade dysplasia or cancer compared with women. An apparent lag between women and men developing cancer is also seen in the NIBR cohort (Figure 1). The higher male to female ratio of patients diagnosed with esophageal adenocarcinoma may be explained by the finding that men develop BE at younger ages compared with women (25). Women appear to develop BE up to 20 years later than men (26). This pattern, combined with the latent period between development of BE and progression to cancer, may mean that fewer women than men have BE for sufficiently long periods to develop esophageal adenocarcinoma.
It is generally believed that the risk of esophageal adenocarcinoma among patients with BE is confined to patients with SIM (27–29). Findings from the current study largely support this premise, because the hazard ratio of combined events was over three times greater in those with SIM at index biopsy than in those without SIM. However, patients with absence of SIM are not entirely without risk of esophageal adenocarcinoma. Among the 3179 patients who did not have SIM in their index esophageal biopsies, four developed high-grade dysplasia and 13 developed cancers. When the data for those patients were examined further, 10 of the patients had had interval biopsies before developing cancer or high-grade dysplasia, of which seven showed SIM. Therefore, cancer that occurs in CLE without SIM may actually be occurring in SIM that was not diagnosed because of sampling error or pathologist misclassification. Sampling error has been shown to be a major limitation in the diagnosis of BE (13). SIM may also have developed over time, so cancer risk in patients without SIM cannot be completely ignored. Misclassification of index SIM means that a small proportion of those that were classified as SIM-negative will have a higher risk of malignant progression than the rest. In this study, the hazard ratio for malignant progression comparing index SIM–positive patients with index SIM–negative patients may therefore be an underestimate. This finding also provides a reason to consider all patients with CLE, regardless of SIM status, for entry into surveillance, as is currently recommended by British guidelines. However, clinicians need to balance this consideration with the disadvantages of entering all patients in this lower-risk group into surveillance. Research into more accurate histological classification of index SIM status, or using the index biopsy for risk stratification, for example, through tissue biomarkers, may resolve this issue. This study demonstrates a very low incidence of cancer (0.06% per year) in patients who have columnar epithelium in their esophagus without SIM at index biopsy. The value of routine endoscopic surveillance must be questioned in these patients, on both clinical and economic grounds.
Considerable debate exists over the relationship between the length of Barrett's segment and risk of cancer (30–33). Analysis of unadjusted data from the NIBR initially indicated that patients with LSBE have a statistically significantly higher risk of progression to cancer than patients with SSBE; however, no statistically significant difference was found following adjustment for other variables. The finding that BE length is not a statistically significant determinant of cancer risk is in keeping with evidence from a systematic review (12); however, the results from the NIBR should be interpreted with caution because data regarding segment length were available for fewer than 20% of the patients in the cohort.
Patients in the NIBR cohort who had low-grade dysplasia had a higher risk of progression to cancer than those without dysplasia. Their risk for developing high-grade dysplasia or cancer was 1.4% per year, with a hazard ratio 5.7 times that of patients who had BE but no dysplasia. A similar risk (1.28% per year) was seen in a study of 575 BE patients in California (34). Other studies (35–37) demonstrated that about one quarter of patients with low-grade dysplasia progressed to cancer, however these studies were based on small numbers of patients (n = 20–34) and included cancers that occurred during the first 12 months after BE diagnosis.
It is clear that the cost-effectiveness of a BE surveillance program is dependent on the incidence of cancer in the group under surveillance. The risk of malignancy and high-grade dysplasia that was seen among patients with SIM in this study (0.38% per year) is similar to the estimate used by Provenzale et al. (10) in their modeling of the cost-effectiveness of endoscopic surveillance in BE. They concluded that, with this risk of cancer, 5-year surveillance was the only viable strategy, because endoscopic and surgical complications result in a lower gain in quality-adjusted life expectancy with shorter surveillance intervals. Another study (9) that modeled surveillance from the perspective of the UK health-care system concluded that at a cancer risk equivalent to 0.5% per year, it costs less and results in a better quality of life to do no surveillance than to do surveillance at any surveillance interval. A lower estimate of cancer incidence, such as the one that is identified in our study, would further reduce the cost-effectiveness of surveillance in this model and would call the cost-effectiveness of the current surveillance program in the United Kingdom into question.
Surveillance of patients with a cancer risk of 1% per year or higher is possibly cost-effective (10,38–40). In the NIBR cohort, a cancer risk of this magnitude is limited to patients with low-grade dysplasia. According to our study, limiting surveillance to BE patients with low-grade dysplasia in their index biopsy would reduce the number of patients requiring surveillance by 95%, but importantly, only 24% of incident cancers or high-grade dysplasia occurred within this group.
A further consideration is that current surveillance protocols assume a constant risk of progression to cancer over time. To date, only one study (17) has reported on the relationship between the duration of BE and patients’ risk of progression to cancer. That study showed an increase in cancer risk with increased duration of BE. Results from the NIBR demonstrate a constant risk of progression to cancer over time, suggesting that patients require a uniform frequency and interval between endoscopies within any surveillance program.
Advantages of this study include the large study size and the avoidance of referral bias to tertiary care centers because everyone identified as having BE within a recognized geographic boundary was potentially included in the study. Identification of incident malignancies using a cancer registry that covers the identical geographic area has minimized loss to follow-up, which may be substantial in hospital-based surveillance cohorts (41). A further strength of the study is that endoscopic ablation of BE was not routinely undertaken in Northern Ireland during the study period, which minimizes alteration of the natural history of BE. Specifically excluding outcomes that were diagnosed within 12 months of the BE diagnosis minimized the capture of prevalent tumor data that would have falsely elevated the risk of progression to malignancy in BE.
There are some limitations with this study. It is a pragmatic study that utilizes routine UK National Health Service data, and our classification of BE is unlikely to be as robust as that of other studies that were undertaken in specialist referral centers, where specific esophageal biopsy protocols are used. However, this study reflects standard clinical practice, and its findings may be more widely applicable within standard health care. This study specifically excluded biopsies that were reported to be taken from the esophagogastric junction; however it is possible that some biopsies were taken from this junction. A recent study has shown that intestinal metaplasia in this area has a substantially lower risk of progression to esophageal cancer than from BE found in the esophagus (18). Therefore, our study may underestimate the risk of malignant progression. There is the potential for loss of follow-up of those patients who have migrated from Northern Ireland. However, data from the Northern Ireland Statistics and Research Agency (42) indicate that there is a low rate of emigration from Northern Ireland among people who are older than 60 years, at between 0.2% and 0.3% per year A further limitation is the availability of clinical information for individual biopsies such as the reason for endoscopy. The NIBR is a pathology-based register, and data from the clinical summary in pathology reports are not uniformly recorded. However, the majority of patients are likely to have been investigated due to symptoms because the index endoscopy was used for creation of the NIBR database. Limited availability of clinical data has restricted the precision with which incidence of cancer or high-grade dysplasia can be calculated according to these factors.
In summary, this study has shown a lower incidence of cancer among patients with BE than what was previously reported. Current recommendations for surveillance are based on higher estimates of cancer risk among patients with BE than were seen in this study and therefore, they may not be justified. We eagerly await further research into tissue biomarkers, optical recognition of dysplasia, and nonsurgical therapies for dysplasia and neoplasia. Such research may enable targeted surveillance of those BE patients who are at greatest risk of neoplastic progression with the ultimate aim of reducing morbidity and mortality from esophageal adenocarcinoma.
Funding
This work was supported by funding from the Ulster Cancer Foundation and the Health and Social Care Research and Development Office, Northern Ireland. The Northern Ireland Cancer Registry is funded by the Public Health Agency for Northern Ireland.
Footnotes
The study sponsors provided funding for the study but had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the article, or the decision to submit the article for publication.
References
- 1.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer. 2009;101(5):855–859. doi: 10.1038/sj.bjc.6605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steevens J, Botterweck AA, Dirx MJ, van den Brandt PA, Schouten LJ. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol. 2010;22(6):669–678. doi: 10.1097/MEG.0b013e32832ca091. [DOI] [PubMed] [Google Scholar]
- 3.Eloubeidi MA, Mason AC, Desmond RA, El-Serag HB. Temporal trends (1973-1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol. 2003;98(7):1627–1633. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 4.US National Cancer Institute. SEER Stat Fact Sheets: Esophagus. 2010. http://seer.cancer.gov/statfacts/html/esoph.html - survival. Accessed October 5, 2010. [Google Scholar]
- 5.Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54(suppl 1):i1–i5. doi: 10.1136/gut.2004.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.British Society of Gastroenterology. Guidelines for the Diagnosis and Management of Barrett’s columnar-lined oesophagus. 2010. http://www.bsg.org.uk/clinical-guidelines/oesophageal/guidelines-for-the-diagnosis-and-management-of-barrett-s-columnar-lined-oesophagus.html. Accessed October 5, 2010. [Google Scholar]
- 7.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103(3):788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 8.Somerville M, Pitt M. Surveillance of Barrett’s oesophagus: do we yet know whether it is worthwhile? Frontline Gastroenterol. 2010;1(2):88–93. doi: 10.1136/fg.2009.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N. Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling. Health Technol Assess. 2006;10(8):1–142. doi: 10.3310/hta10080. iii–iv. [DOI] [PubMed] [Google Scholar]
- 10.Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94(8):2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 11.Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology. 2000;119(2):333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 12.Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(3):237–249. doi: 10.1093/aje/kwn121. [DOI] [PubMed] [Google Scholar]
- 13.Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink—is intestinal metaplasia dead? Am J Gastroenterol. 2009;104(10):2588–2594. doi: 10.1038/ajg.2009.390. [DOI] [PubMed] [Google Scholar]
- 14.Murray L, Watson P, Johnston B, Sloan J, Mainie IM, Gavin A. Risk of adenocarcinoma in Barrett’s oesophagus: population based study. BMJ. 2003;327(7414):534–535. doi: 10.1136/bmj.327.7414.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsman WA, Tytgat GN, ten Kate FJ, van Lanschot JJ. Differences and similarities of adenocarcinomas of the esophagus and esophagogastric junction. J Surg Oncol. 2005;92(3):160–168. doi: 10.1002/jso.20358. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasoma P, Wickramasinghe K, Ma Y, DeMeester T. Adenocarcinomas of the distal esophagus and “gastric cardia” are predominantly esophageal carcinomas. Am J Surg Pathol. 2007;31(4):569–575. doi: 10.1097/01.pas.0000213394.34451.d2. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge PJ, van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett’s oesophagus: a Dutch nationwide cohort study. Gut. 2010;59(8):1030–1036. doi: 10.1136/gut.2009.176701. [DOI] [PubMed] [Google Scholar]
- 18.Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population-based study [published online ahead of print April 12, 2011] Am J Gastroenterol. 2011 doi: 10.1038/ajg.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopal DV, Lieberman DA, Magaret N, et al. Risk factors for dysplasia in patients with Barrett’s esophagus (BE): results from a multicenter consortium. Dig Dis Sci. 2003;48(8):1537–1541. doi: 10.1023/a:1024715824149. [DOI] [PubMed] [Google Scholar]
- 20.Gatenby PA, Caygill CP, Ramus JR, Charlett A, Watson A. Barrett’s columnar-lined oesophagus: demographic and lifestyle associations and adenocarcinoma risk. Dig Dis Sci. 2008;53(5):1175–1185. doi: 10.1007/s10620-007-0023-y. [DOI] [PubMed] [Google Scholar]
- 21.de Jonge PJ, Steyerberg EW, Kuipers EJ, et al. Risk factors for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2006;101(7):1421–1429. doi: 10.1111/j.1572-0241.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 22.Avidan B, Sonnenberg A, Schnell TG, Chejfec G, Metz A, Sontag SJ. Hiatal hernia size, Barrett’s length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am J Gastroenterol. 2002;97(8):1930–1936. doi: 10.1111/j.1572-0241.2002.05902.x. [DOI] [PubMed] [Google Scholar]
- 23.Oberg S, Wenner J, Johansson J, Walther B, Willen R. Barrett esophagus: risk factors for progression to dysplasia and adenocarcinoma. Ann Surg. 2005;242(1):49–54. doi: 10.1097/01.sla.0000167864.46462.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bani-Hani K, Sue-Ling H, Johnston D, Axon AT, Martin IG. Barrett’s oesophagus: results from a 13-year surveillance programme. Eur J Gastroenterol Hepatol. 2000;12(6):649–654. [PubMed] [Google Scholar]
- 25.van Soest EM, Dieleman JP, Siersema PD, Sturkenboom MCJM, Kuipers EJ. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54(8):1062–1066. doi: 10.1136/gut.2004.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol. 2005;100(3):568–576. doi: 10.1111/j.1572-0241.2005.40187.x. [DOI] [PubMed] [Google Scholar]
- 27.Spechler SJ, Goyal RK. The columnar-lined esophagus, intestinal metaplasia, and Norman Barrett. Gastroenterology. 1996;110(2):614–621. doi: 10.1053/gast.1996.v110.agast960614. [DOI] [PubMed] [Google Scholar]
- 28.McGarrity TJ. Barrett’s oesophagus: the continuing conundrum. BMJ. 2000;321(7271):1238–1239. doi: 10.1136/bmj.321.7271.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandrasoma P, Wickramasinghe K, Ma Y, DeMeester T. Is intestinal metaplasia a necessary precursor lesion for adenocarcinomas of the distal esophagus, gastroesophageal junction and gastric cardia? Dis Esophagus. 2007;20(1):36–41. doi: 10.1111/j.1442-2050.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 30.Sharma P, Weston AP, Morales T, Topalovski M, Mayo MS, Sampliner RE. Relative risk of dysplasia for patients with intestinal metaplasia in the distal oesophagus and in the gastric cardia. Gut. 2000;46(1):9–13. doi: 10.1136/gut.46.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weston AP, Sharma P, Mathur S, et al. Risk stratification of Barrett’s esophagus: updated prospective multivariate analysis. Am J Gastroenterol. 2004;99(9):1657–1666. doi: 10.1111/j.1572-0241.2004.30426.x. [DOI] [PubMed] [Google Scholar]
- 32.Conio M, Cameron AJ, Romero Y, et al. Secular trends in the epidemiology and outcome of Barrett’s oesophagus in Olmsted County, Minnesota. Gut. 2001;48(3):304–309. doi: 10.1136/gut.48.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatenby PA, Caygill CP, Ramus JR, Charlett A, Fitzgerald RC, Watson A. Short segment columnar-lined oesophagus: an underestimated cancer risk? A large cohort study of the relationship between Barrett’s columnar-lined oesophagus segment length and adenocarcinoma risk. Eur J Gastroenterol Hepatol. 2007;19(11):969–975. doi: 10.1097/MEG.0b013e3282c3aa14. [DOI] [PubMed] [Google Scholar]
- 34.Dulai GS, Shekelle PG, Jensen DM, et al. Dysplasia and risk of further neoplastic progression in a regional Veterans Administration Barrett’s cohort. Am J Gastroenterol. 2005;100(4):775–783. doi: 10.1111/j.1572-0241.2005.41300.x. [DOI] [PubMed] [Google Scholar]
- 35.Skacel M, Petras RE, Gramlich TL, Sigel JE, Richter JE, Goldblum JR. The diagnosis of low-grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol. 2000;95(12):3383–3387. doi: 10.1111/j.1572-0241.2000.03348.x. [DOI] [PubMed] [Google Scholar]
- 36.Hage M, Siersema PD, van Dekken H, Steyerberg EW, Dees J, Kuipers EJ. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol. 2004;39(12):1175–1179. doi: 10.1080/00365520410003524. [DOI] [PubMed] [Google Scholar]
- 37.Lim CH, Treanor D, Dixon MF, Axon AT. Low-grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy. 2007;39(7):581–587. doi: 10.1055/s-2007-966592. [DOI] [PubMed] [Google Scholar]
- 38.Wright TA, Gray MR, Morris AI, et al. Cost effectiveness of detecting Barrett’s cancer. Gut. 1996;39(4):574–579. doi: 10.1136/gut.39.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streitz JM, Jr., Ellis FH, Jr., Tilden RL, Erickson RV. Endoscopic surveillance of Barrett’s esophagus: a cost-effectiveness comparison with mammographic surveillance for breast cancer. Am J Gastroenterol. 1998;93(6):911–915. doi: 10.1111/j.1572-0241.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson J, Skobe V, Johansson J, Willen R, Johnsson F. Screening for oesophageal adenocarcinoma: an evaluation of a surveillance program for columnar metaplasia of the oesophagus. Scand J Gastroenterol. 2000;35(1):10–16. [PubMed] [Google Scholar]
- 41.Macdonald CE, Wicks AC, Playford RJ. Final results from 10 year cohort of patients undergoing surveillance for Barrett’s oesophagus: observational study. BMJ. 2000;321(7271):1252–1255. doi: 10.1136/bmj.321.7271.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Northern Ireland Statistics and Research Agency. Migration Statistics. 2010. http://www.nisra.gov.uk/demography/default.asp18.htmAccessed August 18, 2010. [Google Scholar]


