Abstract
Periodontitis is an infectious inflammatory disease that results in attachment loss and bone loss. Regeneration of the periodontal tissues entails de novo formation of cementum, periodontal ligament, and alveolar bone. Several different approaches are currently being explored to achieve complete, reliable, and reproducible regeneration of periodontal tissues. The therapeutic management of new bone formation is one of the key issues in successful periodontal regeneration. Bone morphogenetic proteins form a unique group of proteins within the transforming growth factor superfamily of genes and have a vital role in the regulation in the bone induction and maintenance. The activity of bone morphogenetic proteins was first identified in the 1960s, but the proteins responsible for bone induction were unknown until the purification and cloning of human bone morphogenetic proteins in the 1980s, because of their osteoinductive potential. Bone morphogenetic proteins have gained a lot of interest as therapeutic agents for treating periodontal defects. A systematic search for data related to the use of bone morphogenetic proteins for the regeneration of periodontal defects was performed to recognize studies on animals and human (PUBMED, MEDLINE, COCHRANE, and Google search). All the studies included showed noticeable regeneration of periodontal tissues with the use of BMP.
Keywords: Bone, Bone morphogenetic proteins, Gene therapy, Osteogenic protein, Periodontal regeneration
Introduction
Periodontitis is an inflammatory disease characterized by destruction of the alveolar bone, cementum, periodontal ligament, and gingiva as a response to insults elicited by microbial accumulations. The characteristics of periodontal defects may be seen as suprabony, infra bony, or furcation defect or the combination of defects. Regeneration is defined as reconstitution of a lost or injured part, with form and function of lost structures restored.[1] Periodontal Regeneration comprises de novo cementogenesis, osteogenesis, and generation of functionally oriented periodontal fibers into both newly formed cementum and alveolar bone.[2] Regeneration of periodontal structures constitute a complex multifactor process regulated by interaction among cells, hormones, growth factors, and extra cellular matrices.[3] A great amount of knowledge has now been gained regarding the molecular signals that determine the emergence of complex tissue morphologies during regeneration of the periodontal tissues.[4,5] Initiation and promotion of osteogenesis are the problems central to periodontal regeneration. Research in molecular biology lead to identification of initiators of bone differentiation called bone morphogenetic proteins (BMPs) that regulate cartilage and bone differentiation.[6] A systematic search of literature was carried out to identify relevant studies (original article and controlled trials) by using keywords like bone morphogenetic proteins, periodontal defects, periodontitis, and periodontal regeneration in PUBMED, MEDLINE, COCHRANE, and Google databases. Animal models as well as human trails were included in this search. In addition, we searched the reference list of all relevant articles. Initial screening of titles and abstracts was performed. Articles were considered for inclusion in this review based on the regenerative outcome with the use of BMP. Studies reported with regeneration of periodontal defects using BMP were evaluated thoroughly and integrated. The aim of this review is to assess the potential role of BMPs, mechanism of action, delivery systems, and their application in the regeneration of periodontal defects.
Bone morphogenetic proteins
From the times of Hippocrates it has been known that bone has considerable potential for regeneration and repair. Several decades ago Urist reported the discovery that BMPs induce cartilage, bone formation when implanted intramuscularly in a rodent model.[7,8] The responsible proteins, BMPs, were identified after extensive purification and cloning.[9] Bone morphogenetic proteins form a unique family within the transforming growth factor beta (TGF-β) superfamily of proteins that play essential role in regulation of bone formation and repair. Even though BMPs are frequently referred to as growth factors, they are now regarded as differentiation factors, because BMPs are involved in morphogenesis and organogenesis.[3] The structure of most of the BMPs comprising three portions, i.e., signal peptide, propeptide, and a mature region [Figure 1] The propeptide and mature region contain seven conserved cysteine residues characteristic of TGF-β superfamily.[10] BMPs are synthesized inside the cell in a precursor form with a hydrophobicsecretory leader and a propeptide sequences joined to the mature region. Proteolytic cleavage frees the mature region which can then dimerize with other BMPs. Dimeric molecules can be either homodimers, when both subunits are the same/or heterodimers consisting of two different subunits. Structural and chemical differences between the homodimeric and heterodimeric forms may be responsible for variations of their biologic potential and binding characteristics.[11] The influence of BMP may begin early and continue throughout postfetal life in embryonic bone formation.[12] BMPs act as growth and differentiation factors and chemotactic agents. They stimulate angiogenesis and migration, proliferation and differentiation of mesenchymal stem cells into cartilage and bone forming cells. More than 20 BMP-related proteins have been identified, several of which induce bone formation.[13] The BMPs can be broadly classified into three subfamilies.[14–16] Both BMP-2 and BMP-4 have 80% amino acid sequence homology of molecules. In the second group, consisting of BMP-5, -6, and -7, the mean is 78% amino acid sequence homology whereas the third group, composed solely of BMP-3, is significantly different from the other members of the BMP family and generally stands alone. It is of interest that there is a substantial homology between decapentaplegic peptide and BMP-2 and BMP-4 subfamily, implying that these two BMPs are equivalents of decapentaplegic peptide gene products [for the list of BMPs refer to Table 1]. The function of BMP is most commonly associated with chondrogenesis and osteogenesis. In addition to postfetal osteogenesis, BMP-3 may play a role in embryonic skeletogenesis.[17]
Figure 1.
Structure of the bone morphogenetic protein
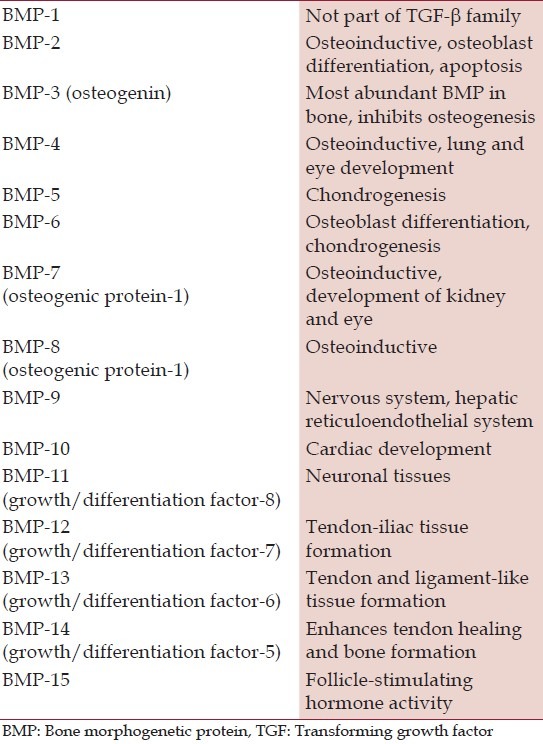
Table 1.
Classification of bone morphogenetic proteins
Furthermore the temporally and spatially distinct patterns of BMP-2,3,4 and BMP-6 mRNA expression in different developing systems suggest that BMPS are also involved in inductive events unrelated to bone induction that control pattern formation during embryonic development.[18–21] Most of the biological action of BMPs are mediated through the BMP receptors which initiate signaling from the cell surface when bind to two distinct type I and II serine/threonine kinase receptors, required for signal transduction.[22,23] BMP receptors are composed of three parts: a short extracellular domain, a single membrane-spanning domain, and an intracellular domain with active serine/threonine region.[24] The type II receptor is the primary binding site of the ligand and upon its activation, phosporylation of type I receptor occurs.[25] It is the type I receptor (or activin receptor-like kinases) that determines the nature of biologic response. Once activated it associates with various specific receptors regulated Smad {human homologous of mothers against decapentaplegic (dpp)} proteins that link the ligand receptors signals to transcription control. Thus, these cytoplasmic Smad proteins associate with specific DNA-binding proteins in the nucleus in order to generate transcriptional complexes [Figure 2].[26]
Figure 2.
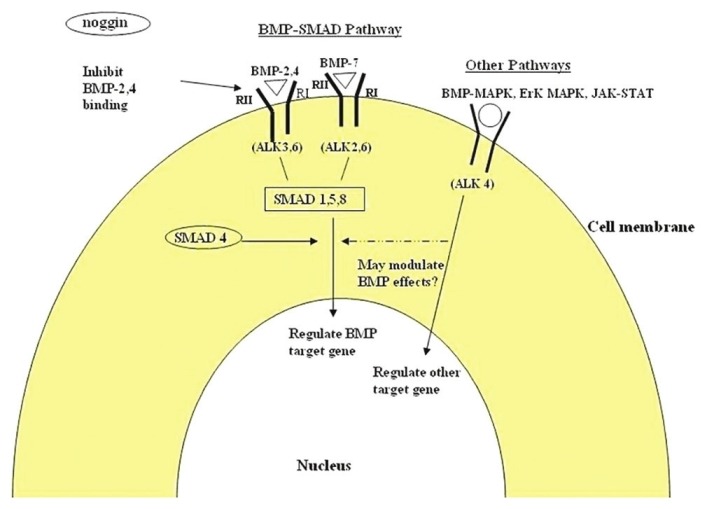
Signaling mechanism of the bone morphogenetic protein-activin receptor-like kinase (ALK), bone morphogenetic protein (BMP), type I and type II receptors (R I and R II), mitogen-activated protein kinase (MAPK), extracellular signal-related kinases (ErK), Janus kinase (JAK), signal transducers, and activators of transcription (STAT)
Other pathways that could further modulate the effect of BMPs include BMP-MAPK, ERK-MAPK, and Wnt signaling.[27] Sonic hedgehog (Shh) is another growth-factor-like protein which binds to a specific cell-surface receptor and promotes osteoblast differentiation, suggesting that Shh is an upstream regulator of BMP production.[28] BMPs has the ability to induce production of osterix (runt-related transcription factor) which is required for differentiation of mesenchymal cells to osteoblasts. It is likely that many inductive pathways have multiple additional levels of regulation. Certain binding proteins prevent receptor activation. These inhibitory binding proteins could temporarily inhibit differentiation. One such particular binding protein is Noggin, which has high affinity for BMP-2 and -4.[29] Therefore the variety of BMP receptors and numerous pathways they regulate suggest that they may bring about synergistic, negative or additive effects. Although BMP is one among the growth factors, it is unique. It is the only morphogen of all known growth factors that has the ability to transform connective tissue cell into osteoprogenitor cells; thus, it is not only a mitogen stimulating the multiplication of connective tissue cells but also can be a morphogen, which is able to transform connective tissue cells into osteoprogenitor cells.
BMPs in bone induction
Bone formation can take place by an intramembraneous (direct) or endochondrol (indirect) process. Endochondral bone formation involves the formation of an intermediate cartilage that eventually becomes ossified that contains all the cellular components of mature bone.[30] In both mechanisms, the induction of bone and cartilage occurs through an epithelial-mesenchymal interaction that initiates specific cell differentiation.[31] Depending on the concentration gradient BMPs can attract various types of cells and can act as chemotactic, mitogenic/or differentiating agent.[32] BMPs can induce differentiation of mesenchymal progenitor cells into various cell types including chondroblasts and osteoblasts.[33] This suggests that BMPs may be able to influence both direct and indirect bone formation. Wide spectra of cells that are sensitive to BMP action include fibroblasts, mesenchymal connective tissue cells, muscle-derived connective tissue cells, astroglial lineage, and many more.[19] Bone marrow stromal cells form an important source of mesenchymal pluripotent progenitors that are capable of differentiating into various cell lineages under appropriate conditions. Osteoblast and chondroblast originate from a common precursor which is a bipotential mesenchymal progenitor called osteo--chondro progenitor.[34] Evidence supports the hypothesis that BMPs act on the skeletal progenitor cells and induce differentiation of both osteoblast and chondroblast. Some of the factors that influence BMPs ability in bone induction include amount, qualitative composition, and possible presence of inhibitors, processing, and storage. In addition, dose, concentration, and also time of BMP action are important parameters of the inductive outcome.[11]
BMPs – recombinant technologies
Identification of osteogenic proteins in bone matrix has been difficult to obtain due to small quantities of proteins tightly bound to organic and inorganic components of the extracellular matrix of bone; hence, recombinant technologies have been used to produce BMP for therapeutic evaluation. Because the structures of several human BMPs have been identified, it is possible to use DNA probes to obtain human complimentary DNA sequence. The human cDNA is cloned and spliced into a viral expression vector. Chinese hamster ovary cells and E. coli transfected to become carriers have been used to produce BMPs in large quantities for preclinical and clinical evaluation.[35,36] Therefore rh-BMP (recombinant human – rh) produced provides optimum capability for clinical applications. In 2002, The US Food and Drug Administration (FDA) approved BMP-2 and BMP-7 for use in bone regeneration.[10]
BMP – delivery systems
Several matrices and delivery systems have been used and evaluated for their efficacy and biocompatibility as carrier for BMPs. Three major strategies for growth factor delivery: gene therapy, cell therapy, and protein therapy. Gene therapy and stem cell-based therapy represent the major advance, however, presently are still in their infancy regarding safety and efficacy in human.[37] Protein therapy, on the other hand, has demonstrated the most practical promise, mainly incorporating osteoinductive morphogens (BMPs) even so with some limitations. It was suggested that the clinical efficacy of rhBMPs will depend upon the carrier system, for effective delivery of adequate protein concentrations to the desired site.[38] One of the carrier functions is to maintain the factor at the site of implantation and thus enhance its local concentration. Ideal qualities[39] of the delivery system or carrier include biocompatibility, biodegradability, structural integrity, absence of immunogenecity, absorption, rate of release, cost, and handling. Properties of the best carrier may vary depending on the specific implantation site and the intended therapeutic outcome. Carriers can be of different types such as solids, gels or combinations [Table 2]. Moreover BMPs preparations have been used in conjunction with occlusive or porous resorbable or non-resorbable space providing devices for guided bone regeneration.[10] BMPs are soluble and if delivered in a buffer solution, there clearance is rapid. Less than 5% dose remains at the application site, whereas combinations of the proteins with gelatin foam or collagen showed increased retention.[40] An absorbable collagen sponge (ACS) was the first BMP carrier technology to be approved by the US Food and Drug Administration (FDA). The absorbable collagen sponge is a bovine type I collagen matrix that is soak loaded with a BMP solution before surgical implantation. The rhBMP/ACS construct has shown the clinical efficacy for a number of indications; however, it is vulnerable to tissue compression.[41] The collagen matrix retains 65% of the BMPs during initial impregnation and releases it in two phases an initial phase within hours of implantation and a second phase that depends on nature and geometrical characteristics.[42] BMPs combined with porous particles of hydroxyapatite or fibrous collagen membrane lead to intramembranous ossification, whereas fibrous glass membrane or insoluble bone matrix support indirect bone formation via a cartilaginous intermediate.[43] There are some disadvantages associated with the carriers such as lack of bone induction with BMPs combined with hydroxyapatite alone – probably as a result of the lack of resorption of hydroxyapatite and the tight binding affinity between BMPs and hydroxyapatite; moreover, immunogenecity and risk of disease transmission with the use of demineralized bone matrix and acidic breakdown products of synthetic polymers which might prove detrimental to wound healing. The degradation rate of the carrier matrix should be in synch with the rate of bone regeneration. If the matrix degrades too slowly it will inhibit bone growth and retard the remodeling process. However, if the carrier matrix degrades too quickly, the risk is that the defect shape will no longer be detected by the matrix, which often serves as a template for new tissue. A further complicating factor is that different anatomical sites might require different kinetics of release for optimal performance.[44] A major problem with delivery of growth factor proteins is the limited bioactivity (half-life) of proteins due to degradation and difficulty in achieving a controlled release. Therefore localized growth factor delivery remains a problem in clinical practice. Use of the gene therapy approaches as one of the methods to address the problems associated with traditional protein delivery.[45]
Table 2.
Types of carrier systems for the delivery of bone morphogenetic proteins

Periodontal regeneration and BMPs
BMPs play an important role in the process of bone modeling and remodeling through chemotatic, mitogenic or differentiating mechanism.[11] Earlier studies have shown that there is a homology of osteogenic proteins among mammals and that bovine BMPs, in conjunction with baboon collagenous matrix, induce bone differentiation in extra skeletal sites of the baboon.[46,47] In previous experiments, the efficacy of bone-derived BMPs (BMP-2, osteogenin, osteoprotein-1) for regeneration in surgically created large furcation defects in the mandibular first and second molar was investigated in adult male baboons (Papio ursinus).[48] Histological analysis showed that BMPs, in conjunction with the collagenous matrix, induced cementum, periodontal ligament, and alveolar bone regeneration. Another study reported that partially purified osteogenin, isolated from human bone matrix, when reconstituted with allogenic freeze dried deminerlized bone matrix, enhanced new connective tissue attachment, and alveolar bone regeneration in a root submerged environment in a series of human biopsies.[49] A study where rhBMP-2 was used in a prepared periodontal defect in beagle dogs showed significant regeneration of the periodontal tissues.[50] The effect of rhBMP-2 was evaluated in the surgically created critical size, supra alveolar periodontal defects in mandibular premolar teeth in beagle dogs which were implanted with rhBMP-2/ACS at different concentrations. Extensive alveolar regeneration and limited cementum regeneration were observed. However, ankylosis was observed in all teeth receiving rhBMP-2/ACS without apparent correlation with rhBMP-2 concentration or dose. The ankylotic union was observed in the coronal aspect of supra alveolar defects.[51] Other studies using rhBMP-2[52,53] or rhOP-1[54] in various carriers also provide evidence of ankylosis in large experimental periodontal defects in rodent, canine, and nonhuman primate models. Given the unique action of BMPs on mineralized tissue formation, obliteration of periodontal ligament space and ankylosis are a potential complication for the use of BMPs in the periodontium. The cause of ankylosis is, however, not clearly understood, but may be related to the perturbation of the homeostatic mechanism within the periodontium.[55] In a study BMP-6 (0.13 and 10 μg) in a type 1 collagen sponge carrier was applied into periodontal fenestration defects in rats. Complete osseous healing occurred in BMP-6-treated animals following a 4-week healing interval.[56] Osteogenetic protein-1 (BMP-7) has been evaluated for periodontal wound healing regeneration using surgically induced mandibular molar class II furcation defects in baboons. Defects implanted with rhOP-1 at 0, 100, and 500 μg/g bovine bone insoluble collagen matrix were subject to histometric analysis following an 8-week healing interval. Sites receiving rhOP-1 showed significant cementogenesis, including inserting sharpey fibers.[57] A similar study using a 24-week healing interval showed that rhOP-1 at 0.5 and 2.5 mg/g collagen matrix induced significantly greater periodontal ligament (PDL) and alveolar bone formation.[58] These observations demonstrate beneficial effects of OP-1 as a candidate therapeutic agent for periodontal wound healing/regeneration. In a pilot study, the potential of growth and differentiation factor-7 (GDF-7)/BMP-12 to stimulate PDL formation was evaluated in a supra alveolar periodontal defect model. This study suggested that GDF-7 has a significant potential to support regeneration of PDL.[59] The effect of BMP-14 (GDF-5) on periodontal wound healing/regeneration has been evaluated using an established canine defect model.[60] In a randomized control trial 20 patients with intra-bony defects treated with rhGDF-5 showed a significant clinical attachment gain, favorable bone, and periodontal regeneration.[61] In a study 30 intra-bony periodontal defects were created in 15 Wister rats to evaluate the regenerative potential of injectable macroporous calcium phosphate cement (CaP) in combination with bone morphogenetic protein-2 (BMP-2). Animals were euthanized after 12 weeks and processed for histology and histomorphometry. CaP + BMP-2 showed a significant 2.4-fold increase in bone healing.[62] Periodontal regeneration reaches the next level of predictability by developing gene therapy techniques. Understanding of the molecular pathways underlying the regeneration is increasing; however, translation of this knowledge into regenerative strategies remains in its early stages.
Gene therapy for bone regeneration
Gene therapy is defined as the treatment of disease by transferring genetic materials to induce specific genes that direct an individual's own cells to produce therapeutic agent.[63] In gene therapy, it is critical to establish effective carrier (vectors) systems that facilitate gene transfer to targeted cells. Vectors for gene delivery: (a) Viral vectors such as adenovirus, adeno-associated virus, and retrovirus. (b) Nonviral vectors such as liposomes, polymers, and electroporation and ultrasound. The major advantage of viral vectors is their high transduction efficiency. The main disadvantage of viral vectors is their immunogenic potential.[45] The application of growth factors by gene transfer provides a greater sustainability than that of single protein application. Gene therapy may achieve greater bioavailability of growth factors within periodontal wounds, which may provide greater regenerative potential. Also it decreases technical challenges related to ex vivo protein expression and purificaton[64] Gene transfer is accomplished through the use of viral and non-viral vectors. In an early approach to regenerate alveolar bone in an animal model, the ex vivo delivery of Ad-encoding (Ad-adenovirus) murine BMP-7 was found to promote periodontal tissue regeneration in large mandibular periodontal bone defects. BMP-7 gene transfer not only enhanced alveolar bone repair but also stimulated cementogenesis and PDL fiber formation.[65] It has been reported that LIM domain mineralization protein 3 (LMP3), a transcription variant of LIM domain mineralization protein (LMP) lacking LIM domains, can induce osteogenesis in vitro and in vivo. The study result showed that adenoviral-mediated gene transfer of LMP3 (AdLMP3) significantly up-regulated ALP (alkaline phosphatase), BSP (bone sialoprotein) and BMP2 gene expression and increased in vitro matrix mineralization in human PDL. Although AdLMP3 gene delivery to PDL cells did not induce ectopic bone formation in vivo, it has been found that AdLMP3 augments new bone formation, which co-delivered with AdBMP-7 gene transfer. This study provides the evidence that there is a synergistic effect between LMP3 and BMP-7 in vivo, suggesting that LMP3 delivery may be used to augment BMP-mediated osteogenesis. LMP3 and BMP-7 combinatory gene therapy may also have specific applications for oral and periodontal regeneration.[66] In spite of many of the positive results using BMPs for periodontal regeneration, several limitations persist such as relative short half-life, inductive activity of rhBMP-2 is 10 times less than that of purified BMPs, varying degrees of ankylosis associated with periodontal regeneration. It is unclear why the impressive and convincing results seen in vitro and in animal models are difficult to reproduce in humans. Unfavorable release kinetics, insufficient mechanical stability, and porosity to allow blood vessel infiltration into the carrier and inflammatory tissue reactions are few reasons.[39] Despite a lack of complete understanding of BMP cellular pathways, addition of BMPs remains the growth factor of choice to induce mesenchymal stem cell differentiation to osteoblasts to induce bone formation.[67] However, to date, sufficient human studies with BMPs in periodontal defects are lacking.
Conclusion
Periodontal tissue regeneration entails the induction of periodontal ligament, cementum, and alveolar bone. Although, several studies have shown significant regeneration of the periodontal tissues with the use of BMP, it is important to understand the biologic processes of periodontal wound healing and the effects of these biologic processes on BMP activity. Further studies are needed for the development of delivery systems that have mechanical and surgical properties appropriate for controlled release of bone morphogenetic proteins and identifying optimal condition for the use of BMPs for periodontal regeneration.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Glossary of periodontal terms. 3rd ed. Chicago (IL): American Academy of Periodontology; 1992. American Academy of Periodontology. [Google Scholar]
- 2.Melcher AH. On the repair potential of the periodontal tissues. J Periodontol. 1976;47:256–60. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 3.Jaebum L, Andreas S, Cristiano S, Wikesjo ME. Periodontal Regeneration: Focus on Growth and Differentiation Factors. Dent Clin N Am. 2010;54:93–111. doi: 10.1016/j.cden.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Bartold PM, McCulloch AG, Narayanan AS, Pitarus Tissue engineering: A new paradigm for periodontal regeneration based on molecular cell biology. Periodontology 2000. 2000;24:253–69. doi: 10.1034/j.1600-0757.2000.2240113.x. [DOI] [PubMed] [Google Scholar]
- 5.Ripamonti U, Reddi AH. Tissue engineering, morphogenesis and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit Rev Oral Biol Med. 1997;8:154–63. doi: 10.1177/10454411970080020401. [DOI] [PubMed] [Google Scholar]
- 6.Ripamonti U, Reddi AH. Periodontal regeneration: Potential role of bone morphogenetic proteins. J Periodont Res. 1994;29:225–35. doi: 10.1111/j.1600-0765.1994.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 7.Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 8.Urist MR, Strates Bone Morphogenetic proteins. J Dent Res. 1971;50:S1392–406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- 9.Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, et al. Identification of transforming growth factor-B family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci USA. 1990;87:9843–7. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YH, Polimeni G, Qahash M, Wikesjo ME. Bone morphogenetic proteins and osseointegration: Current knowledge-future possibilities. Periodontology 2000. 2008;47:206–23. doi: 10.1111/j.1600-0757.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- 11.Sykaras N, Opperman L. Bone Morphogenetic proteins (BMPs): How do they function and what can they offer the clinician? J Oral Sci. 2003;45:57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 12.Urist MR. Substratum for bone morphogenesis. Symp Soc Dev Biol. 1970;4:125–63. [PubMed] [Google Scholar]
- 13.Wikesjo UM, Huang YH, Polimeni G, Qahash M. Bone morphogenetic proteins: A realistic alternative to bone grafting for alveolar reconstruction. Oral Maxillofacial Surg Clin N Am. 2007;19:535–51. doi: 10.1016/j.coms.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Sakou T. Bone morphogenetic proteins: From basic studies to clinical approaches. Bone. 1998;22:591–603. doi: 10.1016/s8756-3282(98)00053-2. [DOI] [PubMed] [Google Scholar]
- 15.Schaub RG, Wozney J. Novel agents that promote bone regeneration. Curr Opin Biotechnol. 1991;2:868–71. doi: 10.1016/s0958-1669(05)80123-5. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt JM, Hwang K, Winn SR, Hollinger JO. Bone morphogenetic proteins: An update on basic biology and clinical relevance. J Orthop Res. 1999;17:269–78. doi: 10.1002/jor.1100170217. [DOI] [PubMed] [Google Scholar]
- 17.Vukicevic S, Paralkar VM, Cunningham NS, Gutkind JS, Reddi AH. Autoradiographic localization of osteogenin binding sites in cartilage and bone during rat embryonic development. Dev Biol. 1990;140:209–14. doi: 10.1016/0012-1606(90)90068-t. [DOI] [PubMed] [Google Scholar]
- 18.Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-β like genes co-ordinately regulate aspects of embryonic development. Genes Dev. 1989;3:1657–68. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- 19.Lyons KM, Pelton RW, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109:833–44. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- 20.Jones CM, Lyons KM, Hogan BL. Involvement of bone morphogenetic protein-4(BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development. 1991;111:531–42. doi: 10.1242/dev.111.2.531. [DOI] [PubMed] [Google Scholar]
- 21.Wall NA, Blessing M, Wright CV, Hogan BL. Biosynthesis and in vivo localization of the decapentaplegic-Vg-related protein, DVR-6 (bone morphogenetic protein-6) J Cell Biol. 1993;120:493–502. doi: 10.1083/jcb.120.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massgue J, Weis-Garcia F. Serine/threonine kinase receptors: Mediators of transforming growth factor beta family signals. Cancer Surv. 1996;27:41–64. [PubMed] [Google Scholar]
- 23.Attisano L, Wrana JL, Lopez-Casillas F, Massgue J. TGF-beta receptors and actions. Biochim Biophys Acta. 1994;1222:71–80. doi: 10.1016/0167-4889(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 24.Lin HY, Wang XF, Ng-Eaton E, Weinberg RA, Lodish HF. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase Cell. 1992;68:775–85. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita H, Ten Dijke P, Heldin CH, Miyazono K. Bone morphgenetic protein receptors. Bone. 1996;19:569–74. doi: 10.1016/s8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- 26.Massgue J. TGF-beta signal transduction. Ann Rev Biochem. 1998;106:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 27.Von Bubnoff A, Cho KW. Intracellular BMP signalling regulation in vertebrates: Pathway or network? Dev Biol. 2001;239:1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- 28.Kawai S, Sugiura T. Characterization of human bone morphgenetic protein (BMP)-4 and -7 gene promoters: Activation of BMP promoters by Gli, a Sonic hedgehog mediator. Bone. 2001;29:54–61. doi: 10.1016/s8756-3282(01)00470-7. [DOI] [PubMed] [Google Scholar]
- 29.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–7. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 30.Carrinton JL, Reddi AH. Parallels between development of embryonic and matrix induced endochondral bone. Bioessays. 1991;13:403–8. doi: 10.1002/bies.950130807. [DOI] [PubMed] [Google Scholar]
- 31.Hall BK. Cellular interactions during cartilage and bone development. J Craniofac Genet Dev Biol. 1991;11:238–50. [PubMed] [Google Scholar]
- 32.Reddi AH. Bone and cartilage differentiation. Curr Opin Genet Dev. 1994;4:737–44. doi: 10.1016/0959-437x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- 33.Wozney JM. The bone morphogenetic family and osteogenesis. Mol Reprood Dev. 1992;32:160–7. doi: 10.1002/mrd.1080320212. [DOI] [PubMed] [Google Scholar]
- 34.Boyan BD, Caplan AL, Heckman JD, Lennon DP, Ehler W, Schwartz Z. Osteochondral progenitor cells in acute and chronic canine nonunions. J Orthop Res. 1999;17:246–55. doi: 10.1002/jor.1100170214. [DOI] [PubMed] [Google Scholar]
- 35.Israel DI, Nove J, Kerns KM, Moutsatsos IK, Kaufman RJ. Expression and characterization of Bone morphogenetic protein-2 in Chinese hamster ovary cells. Growth Factors. 1992;7:139–50. doi: 10.3109/08977199209046403. [DOI] [PubMed] [Google Scholar]
- 36.Zhao M, Wang H, Zhou T. Expression of recombinant mature peptide of human bone morphogenetic protein-2 in Escherichia coli and its activity in bone formation. Chin Biochem J. 1994;10:319–24. [Google Scholar]
- 37.Kimelman N, Pelled G, Helm GA, Huard J, Schwarz EM, Gazit D. Review: Gene and stem cell-based therapeutics for bone regeneration and repair. Tissue Eng. 2007;13:1135–50. doi: 10.1089/ten.2007.0096. [DOI] [PubMed] [Google Scholar]
- 38.Mont MA, Ragland PS, Biggins B, Friedlaender G, Patel T, Cook S, et al. Use of bone morphogenetic proteins for musculoskeletal applications. An overview. J Bone Joint Surg. 2004;86:41–55. doi: 10.2106/00004623-200412002-00008. [DOI] [PubMed] [Google Scholar]
- 39.Haider ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: current challenges in BMP delivery. Biotechnol Lett. 2009;31:1817–24. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 40.Holinger JO, Schmitt JM, Buck DC, Shannon R, Joh SP, Zegzula HD, et al. Recombinant human bone morphogenetic protein -2 and collagen for bone regeneration. J Biomed Mater Res. 1998;43:356–64. doi: 10.1002/(sici)1097-4636(199824)43:4<356::aid-jbm3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Huang YH, Polimeni G, Qahash M, Wikesjo UM. Bone morphogenetic proteins and osseointegration: current knowledge- future possibilities. Periodontol 2000. 2008;47:206–23. doi: 10.1111/j.1600-0757.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- 42.Uludag H, Augusta D, Palmer R, Timony G, Wozney J. Characterization of rhBMP-2 pharmakokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46:193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 43.Murata M, Inoue M, Arisue M, Kuboki Y, Nagai N. Carrier-dependency of cellular differentiation induced by bone morphogenetic protein in ectopic sites. Int J Oral Maxillofac Surg. 1998;27:391–6. doi: 10.1016/s0901-5027(98)80071-4. [DOI] [PubMed] [Google Scholar]
- 44.Li RH, Wozney JM. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001;19:255–65. doi: 10.1016/s0167-7799(01)01665-1. [DOI] [PubMed] [Google Scholar]
- 45.Yamano S, Haku K, Ishioka M, Lin TY, Hanatani S. The potential of tissue engineering and regeneration for craniofacial bone. Dentistry. 2012;2:136. [Google Scholar]
- 46.Sampath TK, Reddi AH. Homology of bone – inductive proteins from human, monkey, bovine and rat extra cellular matrix. Proc Natl Acad Sci USA. 1983;80:6591–5. doi: 10.1073/pnas.80.21.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ripamonti U, Ma S, Cunningham N, Yeates L, Reddi AH. Initiation of bone regeneration in adult baboons by osteogenin, a bone morphogenetic protein. Matrix. 1992;12:369–80. doi: 10.1016/s0934-8832(11)80033-8. [DOI] [PubMed] [Google Scholar]
- 48.Ripamonti U, Heliotis M, van den Heever B, Reddi AH. Bone morphogenetic proteins induce periodontal regenerationin the baboon (Papio Ursinus) J Periodont Res. 1994;29:439–45. doi: 10.1111/j.1600-0765.1994.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 49.Bowers G, Felton F, Middleton C, Glynn D, Sharp S, Mellonig J, et al. Histologic comparision of regeneration in human intrabony defects when osteogenin is combined with demineralised freeze dried bone allograft and with purified bovine collagen. J Periodontol. 1991;62:690–702. doi: 10.1902/jop.1991.62.11.690. [DOI] [PubMed] [Google Scholar]
- 50.Sigurdsson TJ, Nygaard L, Tatakis DN, Fu E, Turek TJ, Jin L, et al. Periodontal repair in dogs.Evaluation of rhBMP/2 carriers. Int J Periodont Restor Dent. 1996;16:524–37. [PubMed] [Google Scholar]
- 51.Wikesjö UM, Guglielmoni P, Promsudthi A, Cho KS, Trombelli L, Selvig KA, et al. Peridontal repair in dogs: Effect of rhBMP-2 concentration on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol. 1999;26:392–400. doi: 10.1034/j.1600-051x.1999.260610.x. [DOI] [PubMed] [Google Scholar]
- 52.Ishikawa I, Kinoshita A, Oda S, Roongruangphol T. Regenerative therapy in periodontal disease: Histological observations after implantation of rhBMP-2 in surgically created periodontal defects in adult dogs. Dent Jpn. 1994;31:141–6. [Google Scholar]
- 53.King GN, King N, Churchley AT, Wozney JM, Hughes FJ. Recombinant human bone morphogenetic protein-2 promotes wound healing in rat periodontal fenestration defects. J Dent Res. 1997;76:1460–70. doi: 10.1177/00220345970760080801. [DOI] [PubMed] [Google Scholar]
- 54.Giannobile WV, Ryan S, Shih MS, Su DL, Kaplan PL, Chan TC. Recombinant osteogenic protein-1(OP-1) stimulates periodontal wound healing in class III furcation defects. J Periodontal. 1998;69:129–37. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- 55.King GN, Cochran DL. Factors that modulate the effects of bone morphogenetic protein- induced periodontal regeneration: A critical review. J Periodontol. 2002;73:925–36. doi: 10.1902/jop.2002.73.8.925. [DOI] [PubMed] [Google Scholar]
- 56.Huang KK, Shen C, Chiang CY, Hsieh YD, Fu E. Effects of bone morphogenetic proteins-6 on periodontal wound healing in a fenestration defect of rats. J Periodont Res. 2005;40:1–10. doi: 10.1111/j.1600-0765.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 57.Ripamonti U, Heliotis M, Rueger DC, Sampath TK. Induction of cementogenesis by recombinant human osteogenic protein-1 (hOP-1/BMP-7) in the baboon (Papio ursinus) Arch Oral Biol. 1996;41:121–6. doi: 10.1016/0003-9969(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 58.Ripamonti U, Crooks J, Teare J, Petit JC, Rugger DC. Periodontal tissue regeneration by recombinant human osteogenic protein-1 in periodontally – induced furcation defects in primate Papio ursinus. S Afr J Sci. 2002;98:361–8. [Google Scholar]
- 59.Wikesjö UM, Sorensen RG, Kinoshita A, Jian Li X, Wozney JM. Periodontal repair in dogs: effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J Clin Periodontal. 2004;31:662–70. doi: 10.1111/j.1600-051X.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- 60.Kim CS, Choi SH, Chai JK, Cho KS, Moon IS, Wikesjö UM, et al. Periodontal repair in surgically created intrabony defects in dogs: Influence of the number of bone walls on healing response. J Periodontol. 2004;75:229–35. doi: 10.1902/jop.2004.75.2.229. [DOI] [PubMed] [Google Scholar]
- 61.Stavropoulos A, Windisch P, Gera I, Capsius B, Sculean A, Wikesjo UM. A phase IIa randomized controlled clinical and histological pilot study evaluating rhGDF-5/b-TCP for periodontal regeneration. J Clin Periodontol. 2011;38:1044–54. doi: 10.1111/j.1600-051X.2011.01778.x. [DOI] [PubMed] [Google Scholar]
- 62.Oortgiesen DA, Walboomers XF, Brockners AL, Meijer GJ, Jansen JA. Periodontal regeneration using injectable bone cement combined with BMP-2 or FGF-2. J Tissue Eng Regen Med. 2012 May 2; doi: 10.1002/term.1514. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Ditto AJ, Shah PN, Yun YH. Non-viral gene delivery using nanoparticles. Expert Opin Drug Deliv. 2009;6:1149–60. doi: 10.1517/17425240903241796. [DOI] [PubMed] [Google Scholar]
- 64.Rios HF, Lin Z, Oh B, Park CH, Giannobile WV. Cell- and gene – based therapeutic strategies for periodontal regenerative medicine. J Periodontol. 2011;82:1223–37. doi: 10.1902/jop.2011.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. 2003;74:202–13. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin Z, Rios HF, Park CH, Taut AD, Jin Q, Sugai JV, et al. LIM domain protein-3 (LMP3) cooperates with BMP7 to promote tissue regeneration by ligament progenitor cells. Gene Ther. 2012;20:1–6. doi: 10.1038/gt.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miron RJ, Zhang YF. Osteoinduction: A review of old concepts with new standards. J Dent Res. 2012;91:736–44. doi: 10.1177/0022034511435260. [DOI] [PubMed] [Google Scholar]


