Abstract
Background:
There is a gross dearth of correlative data for cardiovascular diseases.
Aim:
We aimed to explore the association of systolic and diastolic blood pressure with anthropometric and biochemical parameters of hypothyroid patients in order to establish any correlation that may exist and be useful in an early diagnosis and management against cardiovascular risk.
Materials and Methods:
The study included 100 healthy controls and 150 newly diagnosed hypothyroid patients. Subjects were evaluated anthropometrically and biochemically for fasting blood sugar, triiodothyronine, thyroxine, thyroid stimulating hormone, Insulin, C–peptide, lipid profile, apo–B and apo–A1. The results were statistically analysed using unpaired t–test and Spearman's coefficient of Correlation.
Results:
The hypothyroids had a female preponderance (73.3%) however; their biochemical profiles were comparable with those of male counterparts. They had raised Body Mass Index, hypertension, hyperinsulinemia, insulin resistance, raised C–peptide, dyslipidaemia with raised apo–B and reduced apo–A1 and strong association of systolic and diastolic blood pressure with insulin, insulin resistance, C–peptide and Total cholesterol/HDLc (TC/HDLc).
Conclusion:
Strong association of hypertension with serum insulin, IR, C–peptide and TC/HDLc hints significant contribution towards cardiovascular risk in hypothyroid adults of Jodhpur.
Keywords: C–peptide, Dyslipidaemia, Hypertension, Hypothyroidism, Hyperinsulinemia, HOMA–IR
Introduction
Cardiovascular diseases (CVDs) such as coronary heart disease and stroke are the largest causes of death in developing countries and one of the main contributors of disease burden.[1,2] Hypertension increases the attendant risks of heart disease stroke and is the major cause of morbidity and mortality due to CVDs.[3] More than a quarter of the world's adult population had hypertension in the year 2000 and is projected to increase by 29% in 2025.[4] Hypothyroidism is a listed cause of secondary hypertension.[5,6] A high prevalence of hypertension was shown in hypothyroid patients above 50 years of age.[7] However, hypothyroidism as a cause of hypertension has often been overlooked, with the prevalence ranging from 0-50% owing to the variation in ethnicities, age groups, different ranges of hypothyroidism, the range of systolic blood pressure (SBP) and diastolic blood pressure (DBP) considered as criteria.[8] Using the World Health Organization's (WHO) criteria for hypertension, namely blood pressure (BP) greater than 160/95mm Hg; the prevalence of hypertension in the hypothyroidism is 3 times that of age–matched euthyroid patients.[7]
Epidemiological studies in India have shown that hypertension is present in at least 25% of urban and 10% of rural adult populations.[9] Some recent studies from Jaipur reported a 36.9% prevalence of hypertension in the urban population of Jaipur.[10] However, there is gross dearth of epidemiological, cross–sectional and correlative data for non–communicable diseases like hypertension, diabetes mellitus (DM) etc., from Jodhpur region, the second most populous town of Rajasthan. The prevalence of various forms of secondary hypertension depends upon the nature of the population studied and how extensive the evaluation is. There are no available data to define the frequency of secondary hypertension in the general population, although in middle aged males it has been reported to be 6%.[11] Despite the widespread efforts to improve education and enhance public awareness, about 33% of the persons with hypertension are still undiagnosed and about 50% of those diagnosed are adequately controlled.[12] Thus, intensive efforts are needed by the public health planners to diagnose the hypertensive patients at an early stage and initiate treatment especially in diseases like hypothyroidism, which are important causes of undiagnosed hypertension.
Identifying novel biomarkers would help an early diagnosis of hypertensive patients and prove to be important in patient care, management and prevent severe clinical outcomes like coronary artery disease (CAD), CVDs, stroke etc.
An ever increasing frequency of hypertensive hypothyroid patients reporting to our outpatient clinics implored us to explore the biochemical frontiers that may associate with the raised BP in these patients as an easy, non invasive way to manage the patients early on.
Materials and Methods
The current study was conducted as a part of three year Ph. D research program. The study protocol was approved by the institutional ethics committee. We planned to screen the newly diagnosed hypothyroid patients reporting to the outpatient endocrinal clinics, with biochemical parameters that are important independent markers of CVD risk and to correlate them with SBP and DBP for a possible risk of CVD due to hypertension. We included 250 age matched (age – 47 ± 12.5 years) subjects (100 healthy controls [HC]–50 females and 50 males, 150 hypothyroid–110 females and 40 males) [Table 1 and Figure 1].
Table 1.
Age distribution of healthy controls and hypothyroid patients
Figure 1.
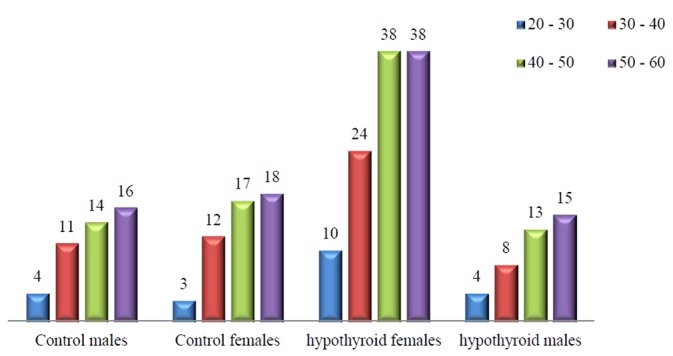
Age-wise distribution of healthy controls and hypothyroid patients
Selection of HC
We selected the HC from outdoor patients who reported with complains like pain abdomen, fever, general seasonal illnesses but were found to have no disease and did not have hypertension, DM, CVDs etc., Such patients were informed of the type of study and they volunteered for it. These subjects were screened for thyroid function and were euthyroid.
Selection of hypothyroid patients
The hypothyroid patients were selected randomly from those reporting to the endocrine clinics during the study duration with complains of weight gain and hypertension. Such patients were screened for thyroid function and on finding to be hypothyroid were selected for the study and the remaining parameters done with them. There were a higher percentage of females reporting with hypothyroidism than males. The hypothyroid patients selected in the study were age matched with the HC [Table 1 and Figure 1]
Inclusion criteria
Patients should be:
Age matched
Have typical hypothyroid symptomatology, confirmed by evaluating triiodothyronine (T3), thyroxine (T4), thyroid stimulating hormone (TSH).
Overt hypothyroid (TSH > 10 μIU/ml)
Exclusion criteria
Patients should not be:
Pre diagnosed hypothyroid to rule out the effect of drugs.
Diagnosed diabetics taking drugs or insulin.
Taking anti-hypertensive/diuretics/hypolipidemic drugs.
Have any other secondary cause of hypertension.
Should be non–smoker and non–tobacco chewer.
The weight of subjects was taken in light clothing and height without shoes for calculating body mass index (BMI). Clinical examination of patients was done and SBP – DBP recorded in sitting position by auscultatory method. Patients were considered hypertensive based on the Joint National Committee (JNC – 7) on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (as > 139/89 mm of Hg.) Biochemical analysis of fasting blood samples was done using fully automated analyser (Chemwell, Ark Co.)
Fasting blood sugar (FBS) – enzymatically
Thyroid function tests (TFT) (T3, T4, TSH) – by solid phase sandwich ELISA.
The detection limit of the Biomerica TSH ELISA -0.03μIU/ml; sensitivity of T4 –0.05μg/dl and T3 0.2 ng/ml.
Insulin – by ELISA (solid phase sandwich ELISA – Magiwel Co. sensitivity < 1.5 μIU/ml and minimal detectable concentration - 0.5 μIU/ml.
C –peptide – by ELISA by solid phase sandwich ELISA – Magiwel Co. sensitivity < 0.3 ng/ml.
Insulin resistance (IR) was calculated by using the Homeostasis model assessment insulin resistance (HOMA – IR) formula –

[Fasting glucose in mg/dl is converted into mmol/l by multiplying with conversion factor of 0.05551]
Lipid profile – enzymatically.
Apo proteins apo –B and apo–A1 –Immunoturbidimetrically (DAIICHI)
Statistical analysis
The data were expressed as mean ± SD. Statistical analysis was done using the unpaired t–test to find out the significance and compare the mean values of male and female and HC and hypothyroid patients. The Spearman's coefficient of correlation assessed the significant [S] or non – significant correlation [NS] of the various biochemical parameters with hypertension in the HC and hypothyroid adults. The statistical evaluations were done using computer software from www.graphpad.com. P < 0.05 were considered as [S] and the P > 0.05 were considered as [NS].
Results
There was a female preponderance (73.3%) among the hypothyroid patients. BMI of hypothyroid female patients was significantly raised as compared with the males. [Table 2] Of the total hypothyroid patients, 43.99% were hypertensive in the current study; with diastolic hypertension as compared to the HC [Table 3].
Table 2.
BMI of healthy controls and hypothyroid patients
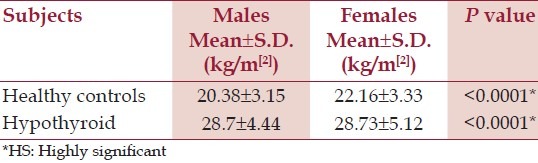
Table 3.
Blood pressure of healthy controls and hypothyroid patients
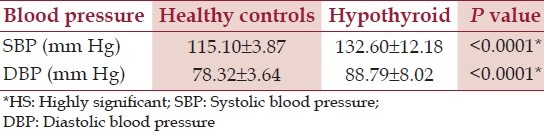
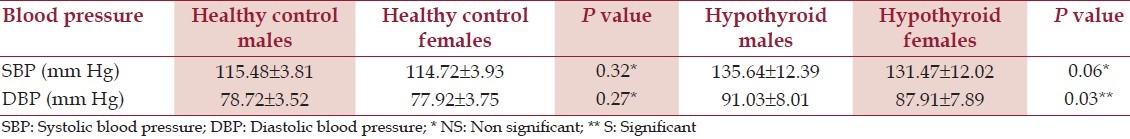
The gender based analysis of hypertension has shown a [NS] difference amongst the male and female counterparts in both HC and hypothyroid patients (with an exception of higher DBP in the hypothyroid males than in females) [Table 4].
Table 4.
Blood pressure of healthy controls and hypothyroid patients based on sex
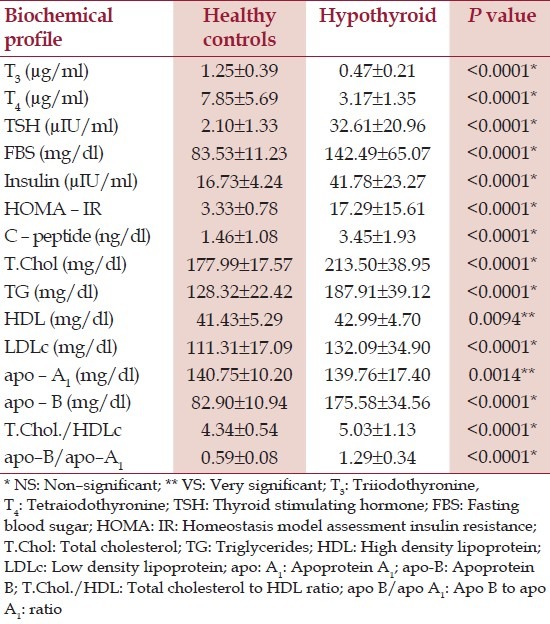
Furthermore, the biochemical parameters of the hypothyroid patients showed grossly deranged TFT, hyperglycaemia, hyperinsulinemia and raised C – peptide levels [Table 5]. The hypothyroid patients were observed to be highly insulin resistant. There was a deranged lipid profile in the hypothyroid patients, with hypercholesterolemia characterised by a significantly raised levels of cholesterol and cholesterol rich lipoproteins and raised atherogenic apo –B levels and reduced cardio – protective apo – A1. T he CVD risk of hypothyroid patients was higher than HC as the TC/HDLc and apo – B/apo –A1 ratios were significantly raised.
Table 5.
Biochemical profile of healthy controls and hypothyroid patients
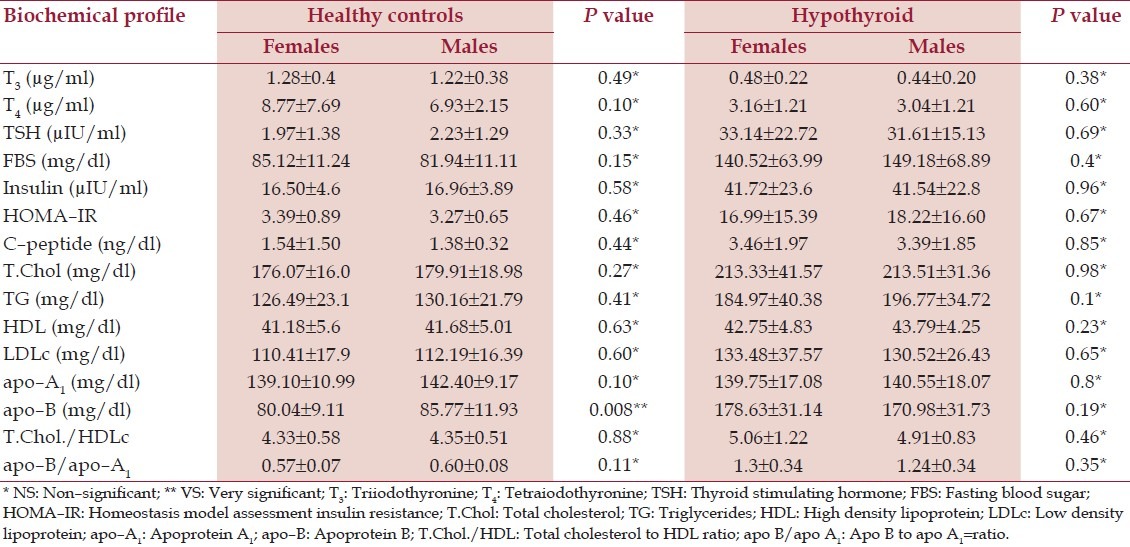
The gender based biochemical profiles helped rule out the effect of female gender preponderance as there was [NS] difference in all the biochemical parameters. Thus, the females, though higher in number, did not influence the overall results owing to comparable biochemical profiles [Table 6].
Table 6.
Biochemical profile based on sex of healthy controls and hypothyroid patients
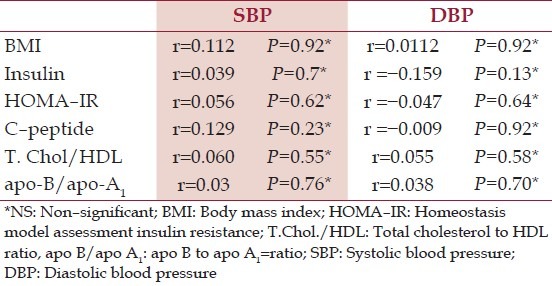
Correlative analysis
There was a [NS] association of SBP and DBP with various CVD risk factors in HC [Table 7]. However, in the hypothyroid patients there was a strong association of SBP and DBP with – serum insulin, HOMA – IR, C – peptide and with TC/HDLc. There was a [NS] and negative association of BMI with SBP and DBP of hypothyroid patients and a [NS] but positive association of CVD risk ratio apo –B/apo –A1 with SBP and DBP [Table 8].
Table 7.
Correlative analysis of systolic blood pressure and diastolic blood pressure with potential cardiovascular diseases risk factors in hypothyroid patients
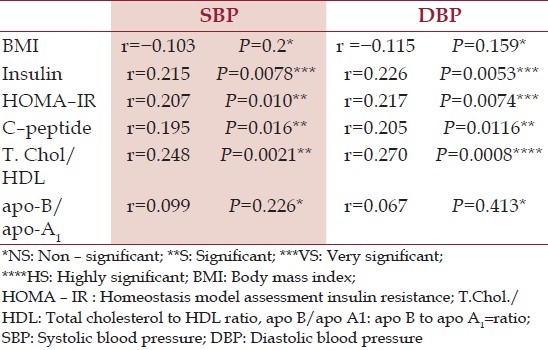
Table 8.
Correlative analysis of systolic blood pressure and diastolic blood pressure with potential cardiovascular diseases risk factors in healthy controls
There was an associated rise of BP and other CVD risk parameters with the severity of hypothyroidism as shown by linear regression of serum TSH with SBP, DBP, HOMA – IR, TC/HDLc and apo – B/apo – A1 [Figure 2a–e].
Figure 2.
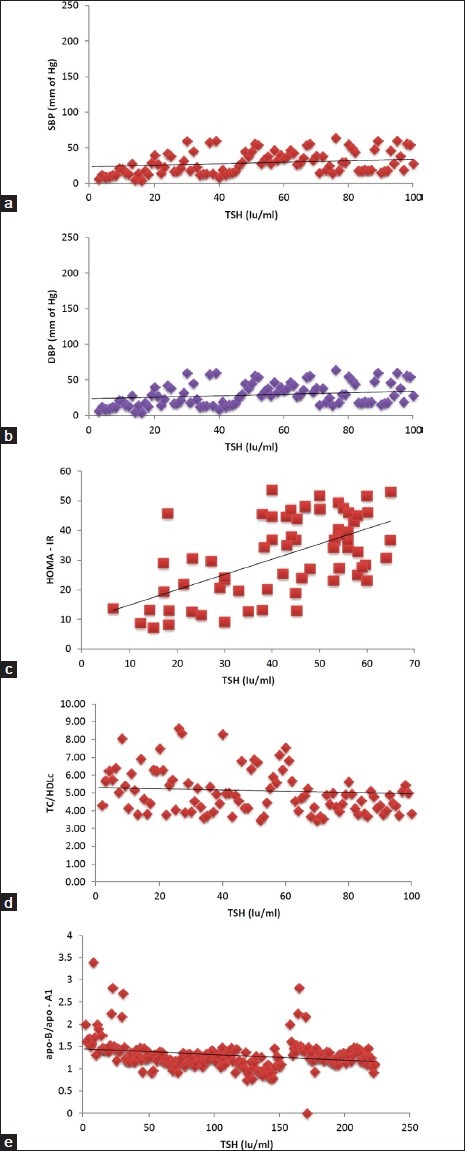
(a) Correlation of serum TSH with SBP in Hypothyroid patients. (b) Correlation of serum TSH with DBP in Hypothyroid patients. (c) Correlation of serum TSH with HOMA – IR in hypothyroid patients. (d) Correlation of serum TSH with TC/HDLc in hypothyroid. (e) Correlation of serum TSH with apo – B/ apo – A1 in hypothyroid patients
Discussion
Hypertension is a major risk factor for CVDs and on better control can lead to prevention of 300,000 of the 1.5 million annual deaths from CVDs in India.[11] Our observations in the current study of hypothyroid female preponderance and a typically raised BMI (more in females than males) than the HC is in-sync with many workers.[13,14] Raised BMI in itself is an independent cause of hypertension[15] and CVDs. A higher percentage of hypothyroid patients presented specially with diastolic hypertension.[7] Biochemically, these patients showed fasting hyperglycaemia, hyperinsulinemia, raised HOMA – IR, C – peptide, grossly atherogenic dyslipidaemia with the raised levels of TC, triglycerides (TG), low density lipoprotein (LDLc), high density lipoprotein (HDLc) and apo – B and reduced apo – A1.[16–20]
Hyperinsulinemia, as observed in the current study, is an independent cause of CVDs because raised insulin levels:
Are unable to stimulate release of nitric oxide (NO) from endothelial cells which causes hypertension.
Have been shown to enhance the sympathetic activity.
Have been shown to cause sodium retention.
According to an estimate, approximately 25–47% of patients with hypertension present with IR or impaired glucose tolerance (IGT). A number of putative mechanisms[21] have been proposed, that IR or reactive hyperinsulinemia, or both, actually cause hypertension and the recent observations that insulin sensitizing agents attenuate the development of hypertension lend credence to this hypothesis.[22] Furthermore, the significantly raised values of C–peptide in the hypothyroid patients observed in current study and several others[23–28] suggests a compensating state for the hyperglycemia, achieved by an increased conversion of proinsulin to Insulin and C–peptide. The raised C–peptide in hypothyroid patients has been shown to enhance the turnover of serum TSH,[29] deteriorating the hypothyroid state further.
Hypothyroidism has been generally considered as CVD risk factor in majority of studies, mainly because of its association with elevated serum TC and LDLc as observed in current study. Dyslipidaemia in hypothyroidism probably results from reduced catabolism of lipoproteins, a phenomenon that may be explained by a decreased expression of lipoprotein receptors.[30,31]
The dyslipidaemia in the hypothyroid patients worsens by an associated IR,[21] and IR is the major abnormality driving CVDs owing to the fact that insulin resistant fat cells do not store TGs and thus initiate dyslipidaemia. Furthermore, the IR at the level of adipose tissue leads to a high rate of exchange of TGs between very low density lipoprotein (VLDL) and HDLc, such that the TG rich HDLc particles are unable to retain their apo -A1 and clears via the renal tubules, reducing the levels of apo –A1 and the reverse cholesterol transport. Also the cholesterol esters (CEs) rich VLDL particle gradually transform to the small dense LDL particles, which are richer in cholesterol and more prone to oxidation posing a greater threat of CVDs.
A strong association of HOMA-IR in hypothyroidism with serum lipids like TC, TG, LDLc, HDLc and apo – B in the hypothyroid patients adds up to their risk of CVDs.[21,23]
The correlation of SBP and DBP with insulin and IR in the hypothyroid patients of the current study suggests a rise in both, with the deterioration of the IR state in hypothyroidism, probably due to the volume – dependent hypertension.[22]
An association of SBP and DBP with C–peptide in these hypothyroid patients further indicates rise of both SBP and DBP with progressing hypothyroidism and emphasizes the enhanced risk of CVD due to IR with a deteriorating state of hypothyroidism. The strong association of the SBP and DBP with the CVD risk ratio TC/HDLc of the hypothyroid patients’ hints raised CVD risk in the hypothyroid patients with a worsening of hypertensive state.
Conclusion
An enhanced CVD risk in the newly diagnosed hypothyroid patients of Jodhpur region was due to the strong association of SBP and DBP with CVD risk factors. The study suggested that a routine screening of the newly diagnosed hypothyroid patients, not only traditional lipid profile but also FBS, insulin, IR, C – peptide, apo – B and A1 as important markers of CVD risk for managing the hypothyroid patients early and preventing progression into high risk category for CVDs.
Limitation
The study provides important correlative data of various biochemical parameters and hypertension in the hypothyroid patients; however we included only the overt hypothyroid patients and left out subclinical hypothyroids (SCHs), which form a very important chunk of the thyroid disorder patients. Thus, further studies including the SCHs should be performed. Besides the study is an observational study and not a controlled study therefore further studies designed as controlled studies maybe performed for standardizing the correlative analysis observed in the current study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.WHO, Preventing chronic diseases: a vital investment. Geneva: WHO; 2005. [Google Scholar]
- 2.Gaziano T, Reddy KS, Paccaud F, Horton S, Chaturvedi V. CVD. In: Jamison DT, Berman J, Measham A, Alleyne G, Claeson M, Evans D, et al., editors. Disease Control Priorities in Developing World. Oxford: Oxford University Press; 2006. pp. 645–62. [Google Scholar]
- 3.Singh VB, Nayak KC, Kala A, Tundwal V. Prevalence of hypertension in geriatric population – A community based study in north-west Rajasthan. Indian J Gerontol. 2005;9:135–46. [Google Scholar]
- 4.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 5.Strong CG, Northcut RC, Sheps SG. In: Clinical examination and investigation of the hypertensive patients in Hypertension. Gnest J, Koiw E, Kuchel O, editors. New York: McGraw Hill Book Company; 1977. p. 659. [Google Scholar]
- 6.Kaplan NM. In: Hypothyroidism in Clinical Hypertension. Kaplan NM, editor. Baltimore: The Williams and Wilkins Company; 1978. p. 362. [Google Scholar]
- 7.Saito I, Ito K, Saruta T. Hypothyroidism as a cause of hypertension. Hypertension. 1983;5:112–5. doi: 10.1161/01.hyp.5.1.112. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher AK, Weetman AP. Hypertension and hypothyroidism. J Hum Hypertens. 1998;12:79–82. doi: 10.1038/sj.jhh.1000574. [DOI] [PubMed] [Google Scholar]
- 9.Nair KG, Shaikh I, Iyer S, Ananthan Al. Trends in hypertension: Role of beta blockers. Hansa Med Cell. 2007:5–6. [Google Scholar]
- 10.Gupta R, Gupta VP. Hypertension epidemiology in India: Lessons learnt from JHW. Curr Sci. 2009;97:349–55. [Google Scholar]
- 11.Fischer ND, Williams GH. Medicine. 16th ed. 2006. Hypertensive vascular diseases. Harrison's Int; pp. 1463–79. 16th. [Google Scholar]
- 12.Springer International+Bussiness Media, Inc. (First Indian Reprint 2006) Delhi: Rajkamal Electric Press; 2005. Hypertension. Brutnton SA. Taylor's CVDs: A Handbook. [Google Scholar]
- 13.Corbetta S, Englaro P, Giambona S, Persani L, Blum WF, Beck-Peccoz P. Lack of effects of circulating thyroid hormone levels on serum leptin concentrations. Eur J Endocrinol. 1997;137:659–63. doi: 10.1530/eje.0.1370659. [DOI] [PubMed] [Google Scholar]
- 14.Mehta S, Mathur D, Chaturvedi M, Devpura G, Jat VS. Thyroid hormone profile in obese subjects–a clinical study. J Indian Med Assoc. 2001;99:260–1. [PubMed] [Google Scholar]
- 15.Ekizie J, Anyanwu EG, Danborno B, Anthony U. Impact of Urbanisation, obesity, antropometric profiles and blood pressure in the Igbos of Nigeria. N Am J Med Sci. 2011;3:242–6. doi: 10.4297/najms.2011.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Triguero ML, Hernández-Mijares A, Nguyen TT, Muñoz ML, Peña H, Morillas C, et al. Effect of thyroid hormone replacement on Lp (a), lipids and apolipoprotein in subjects with hypothyroidism. Mayo Clin Proc. 1998;73:837–41. doi: 10.4065/73.9.837. [DOI] [PubMed] [Google Scholar]
- 17.Muls E, Blaton V, Rosseneu M, Lesaffre E, Lamberigts G, Demoor P. Serum lipids and apolipoproteins A1, A2 and B in hypothyroidism before treatment and after treatment. Eur J Clin Invest. 1984;14:12–5. doi: 10.1111/j.1365-2362.1984.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 18.Muls E, Rosseneu M, Lamberigts G, De Moor P. Changes in the distribution and composition of HDL in primary hypothyroidism. Metabolism. 1985;34:345–53. doi: 10.1016/0026-0495(85)90224-0. [DOI] [PubMed] [Google Scholar]
- 19.Tan KC, Shiu SW, Kung AW. Effect of thyroid dysfunction on HDL subfraction metabolism: roles of HL and CETP. J Clin Endocrinol Metabol. 1998;83:2921–4. doi: 10.1210/jcem.83.8.4938. [DOI] [PubMed] [Google Scholar]
- 20.Kung AW, Pang RW, Lauder I, Lam KS, Janus ED. Changes in serum Lp (a) and lipids during treatment of hyperthyroidism. Clin Chem. 1995;41:226–31. [PubMed] [Google Scholar]
- 21.Purohit P, Mathur R. High CVD risk in hypothyroid females of Jodhpur region: A strong association of HOMA – IR with Lipid profile and Apo – proteins. Proceedings of 38th Annual ACBICON. 2011;26:S115–6. [Google Scholar]
- 22.Ginsberg H. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–8. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purohit P. Estimation of serum insulin, HOMA-IR and C-peptide can help identify possible cardiovascular disease risk in thyroid disorder patients. Indian J Endocrinol Metab. 2012;7:S93–103. doi: 10.4103/2230-8210.94263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purohit P, Mathur R. Role of C – peptide in hypothyroid patients for early diagnosis of Type – 2 DM. Proceedings of 38th Annual ACBICON. IJCM. 2011;(Suppl 26):S42. [Google Scholar]
- 25.Kotchen TA. Attenuation of hypertension by insulin-sensitizing agents. Hypertension. 1996;28:219–23. doi: 10.1161/01.hyp.28.2.219. [DOI] [PubMed] [Google Scholar]
- 26.Reutrakul S, Hathout EH, Janner D, Hara M, Donfack J, Bass J, et al. Familial juvenile autoimmune hypothyroidism, pituitary enlargement, obesity and insulin resistance. Thyroid. 2004;14:311–9. doi: 10.1089/105072504323030988. [DOI] [PubMed] [Google Scholar]
- 27.Chandrashekara WM, Balaguruswamy S, Furlong N, McNulty S. Severe insulin resistance in hypothyroidism: A case report. Endocr Abstr. 2010;21:110. [Google Scholar]
- 28.Yang JK, Liu W, Shi J, Li YB. An association between sub–clinical hypothyroidism and sight threatening diabetic retinopathy in type–2 diabetic patients. Diabetes Care. 2010;33:1018–20. doi: 10.2337/dc09-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udiong CE, Udoh AE, Etukudoh ME. Evaluation of thyroid function in DM in Calabar, Nigeria. Indian J Clin Biochem. 2007;22:74–8. doi: 10.1007/BF02913318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien T, Dinneen SF, O’Brien PC, Palumbo PJ. Hyperlipidemia in patients with primary and secondary hypothyroidism. Mayo Clin Proc. 1993;68:860–6. doi: 10.1016/s0025-6196(12)60694-6. [DOI] [PubMed] [Google Scholar]
- 31.Liberopoulos EN, Elisaf MS. Dyslipidemia in patients with thyroid disorders. Hormones. 2002;1:218–23. doi: 10.14310/horm.2002.1170. [DOI] [PubMed] [Google Scholar]


