Abstract
Background:
Garlic is known as a medicinal herb with broad therapeutic properties ranging from antibacterial to anticancer and even anticoagulant.
Aim:
Current study was designed to evaluate antitumor effects of aged garlic extract (AGE) on fibrosarcoma tumor in BALB/c mice.
Materials and Methods:
WEHI-164 fibrosarcoma cells were implanted subcutaneously on day zero into right flank of 40 BALB/c mice aged eight weeks. Mice were randomly categorized in two separate groups: 1st received AGE (100 mg/kg, intraperitoneally), 2nd group as control received phosphate buffered saline, (PBS). Treatments were done three times per week. Tumor growth was measured and morbidity was recorded. Subpopulations of CD4+/CD8+ T cells were determined using flow cytometry. WEHI-164 cell specific cytotoxicity of splenocytes and in vitro production of gamma-interferon, (IFN-γ) and Interleukin-4, (IL-4) cytokines were measured.
Results:
The mice received AGE had significantly longer survival time compared to control mice. The inhibitory effect on tumor growth was seen in AGE treated mice. The CD4+/CD8+ ratio and in vitro IFN-γ production of splenocytes were significantly increased in AGE group.
Conclusions:
Administration of AGE resulted in improved immune responses against experimentally implanted fibrosarcoma tumors in BALB/c mice. AGE showed significant effects on inhibition of tumor growth and longevity of survival times.
Keywords: Aged garlic extract, BALB/c mice, Fibrosarcoma, Immune responses, Tumor therapy
Introduction
Cancer is accounted for 7.6 million deaths globally in 2008, and it is estimated to continue to rise to over 11 millions in 2030. Different therapeutic formulations are available for different cancers based on the types and stages of the cancer and the tissue involved. While common therapies, like chemotherapy, are accompanied with numerous side effects,[1,2] supplement therapy of cancers using dietetic ingredients, like alpha lipoic acid[3,4] or medicinal herbs, like aged garlic extract (AGE)[5] may results in enhanced efficacy of the generic therapies and improved life quality for cancer patients. In addition, aged garlic can reduce the negative side effects of chemotherapy agents.
Garlic, Allium sativum, is a species of the onion family, Alliaceae. It has been extensively used throughout the history for its prophylactic and therapeutic effects. Its immunomodulatory and antitumor effects have also been demonstrated by in vitro and in vivo experiments. Garlic extract contains more than 200 chemicals with diverse properties. Different chemicals were detected in garlic using different extraction procedures. Garlic also shifts polarization of CD4+ T cells towards Th1,[6,7] increases frequency and function of natural killer cells,[6,8,9] enhances frequency and proliferation potential of lymphocytes,[10,11] improves CD4+/CD8+ T cell ratio,[12] has anti proliferative and anti angiogenesis effects on tumor cells,[1,13,14] modifies monocyte/macrophage function upon endotoxin stimulation through involving in the tool like receptor (TLR) signal transduction events,[15] has anti apoptotic properties,[2,16,17] and demonstrates high antioxidant activities.[18] Several purified chemicals from garlic or its crude extracts have been demonstrated to have obvious antitumor activities.[19,20] Direct pre-exposure of tumor cells with garlic extracts resulted in complete growth inhibition of implanted tumor cells.[21] It is believed that malignancies are accompanied with increased production and release of potentially harmful free reactive oxygen species (ROS) and AGE has antioxidant potential to scavenging the ROS. Furthermore, AGE acts as an enhancer of cellular antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase activities, and also increases glutathione levels in the cells.[22] Antioxidant constituents of garlic result in enhanced antioxidant capacity of the body, improved immunity and effective scavenging of free radicals.[23] Increased nitric oxide synthase activities of endothelial cells and neutrophiles[22,24] and also anti-teratogenic properties of garlic have been demonstrated.[23] Golovchenko and coworkers studied the mechanisms contributing to antiangiogenic properties of garlic in 2003. Methylallyl thiosulfinate, a constituent of garlic extract, was shown to have inhibitory effect on platelet-derived growth factor (PDGF)-induced vascular smooth muscle cell migration, via inhibition of geranylgeranyl transferase I.[23]
Present study investigated effects of administration of AGE on experimental fibrosarcoma cell tumors in BALB/c mice. Survival times, tumor volumes, CD4+/CD8+ T cell ratios in spleen, tumor specific cytotoxicity of splenocytes and in vitro production of IFN-γ and IL-4 cytokines were assayed and compared.
Materials and Methods
All procedures were done in accordance with laboratory animal guidelines of Pasteur Institute of Iran.
Animals
Female inbred BALB/c mice and WEHI-164 BALB/c mouse fibrosarcoma cell line were purchased from Pasteur Institute, Tehran, Iran. All experimental procedures were in accordance with the animal care guidelines outlined by the Animal Care Committee of the Pasteur Institute of Iran (Tehran, Iran). Other reagents used for in vitro measurements were as follows: Fluorescein Isothiocyanate (FITC)-conjugated anti-CD4 (Serotech), Phycoerythrin (PE)-conjugated anti-CD8 (Serotech), Lactate dehydrogenase (LDH) release kit (Roche-Applied), Interleukin-4 and Interferon-γ cytokines kits (Quantikine).
Aged garlic extract
Garlic bulbs were peeled and minced in an aqueous-alcoholic solution and kept under anaerobic conditions for 18 months. Aged garlic was crushed using mortar and pestle and homogenized in distilled water. The homogenized preparation was filtered through Whatman paper No. 1 and the filtrate was centrifuged at 4500 g for 30 minutes. The cleared supernatant was collected and used. The extract, (containing 0.4 g of garlic materials per ml) was diluted in distilled water and 100 mg/kg of the preparation was administered intraperitoneally to each mouse.
Experimental tumor model
WEHI-164 cell line was used for generation of tumor in mice and as a target cell for cytotoxicity (cytotoxic T lymphocyte, CTL) assay. The cells were cultured using Roswell Park Memorial Institute 1640 (RPMI) (GIBCO) media, supplemented with 10% heat inactivated fetal bovine serum (FBS, GIBCO), 100 μg/ml streptomycin, and 100 U/ml penicillin (GIBCO), and incubated in 37°C in a humidified, 5% CO2 atmosphere. The cells in logarithmic growth phase were used to establish tumor model by subcutaneously implanting of 1 × 106 cells/200 μl into the right flank of mouse. Tumor dimension was measured on 7 days intervals using Vernier caliper. Tumor volume (mm3) was calculated by the formula: Length × Width2 × π/6.
Experimental design
A total number of 40 BALB/c mice at 6-8 week of age went under generation of experimental tumor by injecting of WEHI-164 cells obtained from logarithmic phase of the cell line culture on day zero. Tumorized mice were randomly divided into two groups, 1st received AGE (100 mg/kg, i.p.), 2nd group, as control, received only PBS. All injections were given on alternate days intervals (three times per week) until the time for mouse killing (for in vitro study) or dying (for survival analysis). Half of the mice in each treated group were considered for survival study and tumor volume measurements and the others were euthanized on day 28 (contemporary with the first mortality in PBS group) for in vitro studies of immunologic parameters.
Measurements
For survival analysis, mortalities of tumor bearing mice were recorded with respect to time. Tumor volumes were also recorded within weekly intervals.
For in vitro studies, mice were euthanized on day 28 and the splenocytes were isolated as a single-cell suspension. Erythrocytes were then lysed at room temperature using Ammonium-Chloride-Potassium (ACK) lysis buffer (NH4Cl, KHCO3, Na2EDTA). The isolated splenocytes were used for in vitro measurements after three steps of washing with PBS. The CD4+ and CD8+ T cells percentages, specific cytotoxicity of splenocytes against WEHI-164 cells, and in vitro production of both IL-4 and IFN-γ cytokines were determined.
Flow cytometric analysis of T CD4+ and T CD8+cells in spleen
The percentages of T CD4+ and T CD8+ cells of freshly isolated splenocytes were determined. Viability of isolated cells was determined by trypan blue exclusion method. The cells were resuspended in RPMI 1640 (GIBCO) supplemented with 10% FCS (fetal calf serum, GIBCO). The freshly prepared cells were analyzed by flow cytometry after immunostaining with two fluorochrome labeled antibodies (FITC-conjugated anti-CD4 and PE-conjugated anti-CD8 antibodies, both from Serotech). Each sample was immunostained with the antibodies for 45 min at 4°C. The cells were washed in washing buffer and fixed with 2% paraformaldehyde. The percentage of CD4+ and CD8+ T cells was determined by flow cytometric analysis of immunostained cells using an EPICS flow cytometer (Coulter).
In vitro cytotoxic activity of splenocytes
The cytolytic activity of splenocytes was measured by LDH release assay. Single cell suspension of splenocytes was prepared and used as effector cells. WEHI-164 cells (1 × 104 cells/100 μl) as target cells were incubated with 100 μl effector cells suspension at effector/target ratios of 1:10, 1:25 or 1:50. LDH release assaying kit was used (according to the instruction of manufacturer, Roche-Applied) to measure LDH enzymes released from lysed cells. For low and high control wells, (spontaneous release and maximum release, respectively) 100 μl of assay medium or 2% Triton ×100 in assay medium was added. All experiments were done in triplicates and percentage of specific cytotoxicity was determined by the following formula:
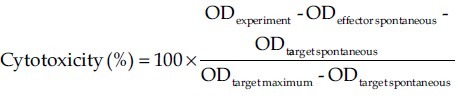
In vitro cytokine production of stimulated splenocytes
Cytokine releases after in vitro stimulation of splenocytes with tumor cell lysate were measured using standard Enzyme-linked immunosorbent assay (ELISA) kits. Splenocytes at the concentration of 1 × 106 cells/well were cultured in the 24 well plates in the presence of 50 μg/ml WEHI-164 cell lysate (prepared by sonication of the cells), 8 μg/ml PHA or complete media, in the total volume of 1 ml. RPMI-1640 supplemented with 10% heat inactivated FBS, 100 μg/ml streptomycin, 100 U/ml penicillin was used. The plates were incubated for 48 h at 37°C in a humidified 5% CO2 atmosphere. Supernatants were collected after centrifugation, IFN-γ and IL-4 concentrations were determined using commercial sandwich ELISA assay kits (Quantikine) according to the manufacturer's instructions.
Statistical analysis
Results from triplicate measurements were averaged. Statistical analysis was performed using the Student's t-test. Kaplan-Meier analysis was applied to compare survival time between groups and log-rank test was used to pairwise comparisons. A value of P < 0.05 was considered to be statistically significant. All statistical analyses were conducted with Statistical Product and Service Solutions (SPSS) 16 software.
Results
Only tumor-bearing mice were entered in the study. Environmental conditions were the same for all mice. The day of tumor implantation was considered as zero for survival time records and treatment programs. One day after tumor implantation animals were randomly assigned to treatment groups (two groups each 2 × 10 mice), and treatments were initiated. Survival analysis was conducted in parallel with in vitro study. Survival records were terminated when the last living mouse was lost. The in vitro study was begun at day 28 when the first mouse died of tumor.
Survival analysis
Survival analysis was done for tumor bearing mice had been treated using two different treatment protocols. Dying of each mouse as a terminal outcome was recorded with respect to time. Kaplan-Meier analysis and simultaneous log-rank test were applied for total and pair-wise comparisons. The increase in life span (ILS) of the treated groups was expressed as a percentage (ILS%). Median Survival Times (MST) was determined, and the percentage of increase in life span was calculated as: ILS% = ((MST (days) of treated mice/MST (days) of control mice) – 1) ×100. Garlic treated group had increased ILS (60.6%). Mean ± SE of survival times (day) in AGE treated group was 52.4 ± 2.1 and in control group was 35 ± 1.8, (P < 0.01).
Tumor volume following treatments
Tumor volume was calculated as Mean ± SE for all mice and expressed in mm3. All mice in control group had palpable tumors on day 14 post implantation, while the percent of tumor bearing mice in AGE was 2%. Garlic exerted inhibitory effects on tumor growth [Figure 1].
Figure 1.
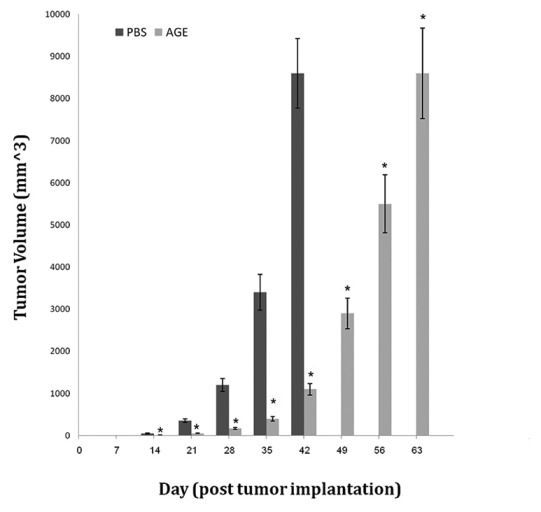
Tumor volume (mm3) with respect of time (day) stratified by treatment. *Significant difference in comparison to PBS group (P < 0.01)
Flow cytometric analysis of T CD4+ and T CD8+ cells in spleen
Tumor volume was calculated as Mean ± SE for all mice and expressed in mm3. All mice in control group had palpable tumors on day 14 post implantation, while the percent of tumor bearing mice in AGE was 2%. Garlic exerted inhibitory effects on tumor growth [Figure 2].
Figure 2.
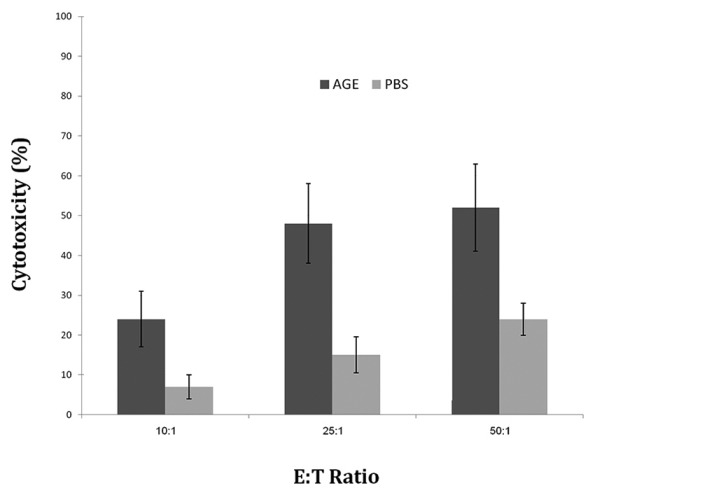
Percent cytotoxicity of splenocytes against WEHI-164 cells at 3 E:T ratio
In vitro cytokine production of stimulated splenocytes
The splenocytes from the mice were analyzed for in vitro production of IFN-γ and IL-4 cytokines due to stimulation by exposure to WEHI-164 cell lysate. The splenocytes of AGE receiving group showed significant P < 0.05) increase in the level of IFN-γ comparing to control group [Figure 3]. There were no noticeable differences between groups considering the in vitro IL-4 production level [Figure 4].
Figure 3.
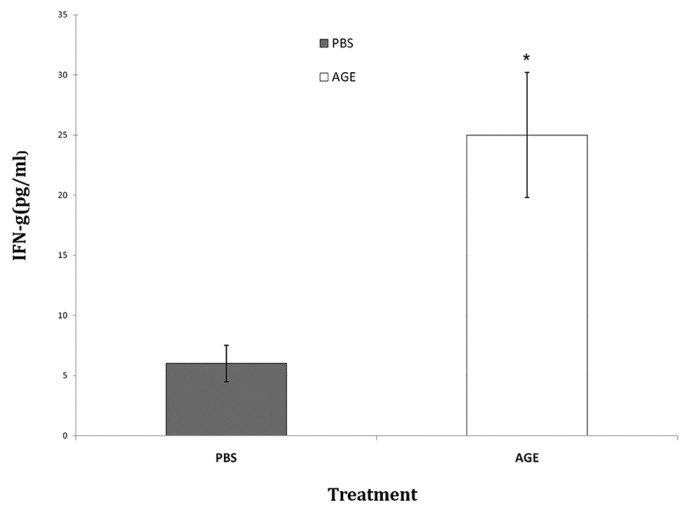
In vitro IFN-γ production of splenocytes after exposure to WEHI-164 cell lysate. *Significant difference in comparison to PBS group (P < 0.05)
Figure 4.
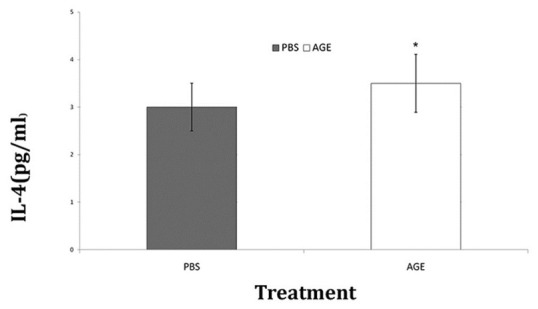
In vitro IL-4 production of splenocytes after exposure to WEHI-164 cell lysate
Discussion
Cancer is the second leading cause of death in the United States. It is estimated to be the leading cause of death within the next decade. Selective therapies for cancer patients depend on the type of cancer, its stage, and its proximity to other organs. Garlic has well-known anti cancer properties.
Chronic inflammation and continuous oxidative stress may result in genetically modified cells that may finally transform to cancerous cells. Various cancers also result in depletion of body's antioxidant resources and lead to unresolved oxidative stress that can further damage cells and tissues and help the existing tumor readily survive. In diseases other than cancers, which accompany with chronic oxidative stress, the incidences of cancer are also noticeably high. In other words, proper facing on production and release of free radicals and their harmful products, like nitrosamines, is the central step in fighting against cancer.[24–26]
Herbals and plant materials are rich source of antioxidants and other therapeutic agents.[27,28] Garlic resolves the oxidative stress firstly by its antioxidant ingredients, and secondly, by enhancing pre-existing antioxidant systems of the body.
Hodge has reported in 2002 that application of fresh garlic extract by the in vitro culture methods resulted in inhibition of Th1 and inflammatory cytokines production by peripheral blood mononuclear cells while the production of Th2 cytokine, IL-10, was up regulated.[29] Augmenting of Th2 cytokines is probably because of the fresh garlic application in the study. Fresh garlic preparations have usually few antioxidants and make of different ingredients compared to AGE.[24] Kasuga also mentioned in his report in 2001 that different types of garlic preparations have different pharmacologic properties, and among the four garlic preparations studied, AGE could be the most useful garlic preparation.[30]
Upon occurrence of any mutated and uncontrolled cells in the body, this is the commitment of cells contributing in immune system responses to recognize and efficiently destroy it. Garlic modulates the immune responses by different pathways. Garlic increases antioxidant capacity of the cells and prepare improved circumstances to proper deciding and acting against threats. In this way, garlic exerts its modulatory effects on innate and adaptive immunity.[31] Intraperitoneal administration of AGE has led to increased macrophage counts and enhanced killing activities of the cells.[32] Dietary administration of garlic has led to elevation of white blood cell counts[31] and enhanced natural killer (NK) activity in peripheral bloods of animal models. Garlic also modifies productions of cytokines by immune cells.[15,33,34]
In present study, AGE was used to inhibit fibrosarcoma tumor growth in BALB/c mice. AGE receiving group showed significantly retardation in tumor appearance. All of PBS treated control mice showed visible tumors (100%) 14 days post-implantation. Administration of 100 mg/kg of AGE efficiently inhibited tumor growth and significantly increased life spans of the mice in comparison to control mice.
Von Euler et al., have reported for the first time in 1947, growth-inhibiting activity of pure allicin, an active component of garlic, on various tumors, with inconsistent complete inhibition in rats of Jensen sarcoma and of a benzpyrene-induced sarcoma. In 1967, Fujiwara has tried to induce tumor immunity using tumor cells treated with extract of garlic.[35] Slight delay in tumor appearance and animal death through administration of garlic extract has also been reported by Aboul-Enein in 1986.[36] It has also been shown by Hu, et al., used an in vitro assessment, in 2002, that direct exposure of tumor cells with AGE resulted in suppression of tumor cell growth and inhibition of the cell migration. They concluded that garlic, as a natural plant may play a role in fighting against cancer without significant side effects.[21] Present study with AGE (i.p.) administered 1 day after tumor implantation also showed significant retardation in tumor appearance and increase in life span (60.6% compared to control group).
For preliminary elucidation of the mechanisms involving in mouse resistance against the tumor, we assayed several immune parameters using splenocytes. There were no significant differences between AGE and control groups (P > 0.05). Low ratio was seen in AGE treated mice probably was the result of increases in both CD4+ and CD8+ T cells, thus the ratio undergo minimum changes. Present study showed increased cytotoxic activity of splenocytes from AGE treated mice but the differences were not statistically significant.
INF-γ secretion by CD4+ Th1 cells, CD8 cells, gamma/delta T cells and activated NK cells plays an important role in activating lymphocytes to enhance anti-microbial and anti-tumor effects. We found significant increase in IFN-γ production of splenocytes from AGE treated mice. It seems garlic administration has profound effect on IFN-γ production of splenocytes.
Conclusion
Our findings showed that administration of AGE induced effective immune responses against fibrosarcoma tumor in BALB/c mice and led to significant inhibition of tumor growth and enhanced survival times of mice.
Footnotes
Source of Support: Department of Immunology, Tehran Medical University, Tehran, Iran.
Conflict of Interest: None declared.
References
- 1.Alkreathy H, Damanhouri ZA, Ahmed N, Slevin M, Ali SS, Osman AM. Aged garlic extract protects against doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol. 2010;48:951–6. doi: 10.1016/j.fct.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Horie T, Li T, Ito K, Sumi S, Fuwa T. Aged garlic extract protects against methotrexate-induced apoptotic cell injury of IEC-6 cells. J Nutr. 2006;136:861S–3. doi: 10.1093/jn/136.3.861S. [DOI] [PubMed] [Google Scholar]
- 3.Berkson BM, Rubin DM, Berkson AJ. The long-term survival of a patient with pancreatic cancer with metastases to the liver after treatment with the intravenous alpha-lipoic acid/low-dose naltrexone protocol. Integr Cancer Ther. 2006;5:83–9. doi: 10.1177/1534735405285901. [DOI] [PubMed] [Google Scholar]
- 4.Berkson BM, Rubin DM, Berkson AJ. Revisiting the ALA/N (alpha-lipoic acid/low-dose naltrexone) protocol for people with metastatic and nonmetastatic pancreatic cancer: A report of 3 new cases. Integr Cancer Ther. 2009;8:416–22. doi: 10.1177/1534735409352082. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S, Haruma K, Yoshihara M, Kajiyama G, Kira K, Amagase H, et al. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J Nutr. 2006;136:821S–6. doi: 10.1093/jn/136.3.821S. [DOI] [PubMed] [Google Scholar]
- 6.Lamm DL, Riggs DR. The potential application of Allium sativum (garlic) for the treatment of bladder cancer. Urol Clin North Am. 2000;27:157–62. doi: 10.1016/s0094-0143(05)70243-3. [DOI] [PubMed] [Google Scholar]
- 7.Zare A, Farzaneh P, Pourpak Z, Zahedi F, Moin M, Shahabi S, et al. Purified aged garlic extract modulates allergic airway inflammation in BALB/c mice. Iran J Allergy Asthma Immunol. 2008;7:133–41. [PubMed] [Google Scholar]
- 8.Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, et al. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr. 2006;136:816S–20. doi: 10.1093/jn/136.3.816S. [DOI] [PubMed] [Google Scholar]
- 9.Morioka N, Sze LL, Morton DL, Irie RF. A protein fraction from aged garlic extract enhances cytotoxicity and proliferation of human lymphocytes mediated by interleukin-2 and concanavalin A. Cancer Immunol Immunother. 1993;37:316–22. doi: 10.1007/BF01518454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zagon IS, McLaughlin PJ. Opioid antagonist (naltrexone) stimulation of cell proliferation in human and animal neuroblastoma and human fibrosarcoma cells in culture. Neuroscience. 1990;37:223–6. doi: 10.1016/0306-4522(90)90207-k. [DOI] [PubMed] [Google Scholar]
- 11.Cheng F, McLaughlin PJ, Banks WA, Zagon IS. Passive diffusion of naltrexone into human and animal cells and upregulation of cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R844–52. doi: 10.1152/ajpregu.00311.2009. [DOI] [PubMed] [Google Scholar]
- 12.Scifo R, Cioni M, Nicolosi A, Batticane N, Tirolo C, Testa N, et al. Opioid-immune interactions in autism: Behavioural and immunological assessment during a double-blind treatment with naltrexone. Ann Ist Super Sanita. 1996;32:351–9. [PubMed] [Google Scholar]
- 13.Matsuura N, Miyamae Y, Yamane K, Nagao Y, Hamada Y, Kawaguchi N, et al. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr. 2006;136:842S–6. doi: 10.1093/jn/136.3.842S. [DOI] [PubMed] [Google Scholar]
- 14.Golovchenko I, Yang CH, Goalstone ML, Draznin B. Garlic extract methylallyl thiosulfinate blocks insulin potentiation of platelet-derived growth factor-stimulated migration of vascular smooth muscle cells. Metabolism. 2003;52:254–9. doi: 10.1053/meta.2003.50042. [DOI] [PubMed] [Google Scholar]
- 15.Youn HS, Lim HJ, Lee HJ, Hwang D, Yang M, Jeon R, et al. Garlic (Allium sativum) extract inhibits lipopolysaccharide-induced Toll-like receptor 4 dimerization. Biosci Biotechnol Biochem. 2008;72:368–75. doi: 10.1271/bbb.70434. [DOI] [PubMed] [Google Scholar]
- 16.Li T, Ito K, Sumi S, Fuwa T, Horie T. Protective effect of aged garlic extract (AGE) on the apoptosis of intestinal epithelial cells caused by methotrexate. Cancer Chemother Pharmacol. 2009;63:873–80. doi: 10.1007/s00280-008-0809-4. [DOI] [PubMed] [Google Scholar]
- 17.Yüncü M, Eralp A, Celik A. Effect of aged garlic extract against methotrexate-induced damage to the small intestine in rats. Phytother Res. 2006;20:504–10. doi: 10.1002/ptr.1896. [DOI] [PubMed] [Google Scholar]
- 18.Abdalla FH, Bellé LP, De Bona KS, Bitencourt PE, Pigatto AS, Moretto MB. Allium sativum L.extract prevents methyl mercury-induced cytotoxicity in peripheral blood leukocytes (LS) Food Chem Toxicol. 2010;48:417–21. doi: 10.1016/j.fct.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Wargovich MJ. Diallyl sulfide, a flavor component of garlic (Allium sativum), inhibits dimethylhydrazine-induced colon cancer. Carcinogenesis. 1987;8:487–9. doi: 10.1093/carcin/8.3.487. [DOI] [PubMed] [Google Scholar]
- 20.Amagase H, Milner JA. Impact of various sources of garlic and their constituents on 7,12-dimethylbenz[a] anthracene binding to mammary cell DNA. Carcinogenesis. 1993;14:1627–31. doi: 10.1093/carcin/14.8.1627. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Cao BN, Hu G, He J, Yang DQ, Wan YS. Attenuation of cell migration and induction of cell death by aged garlic extract in rat sarcoma cells. Int J Mol Med. 2002;9:641–3. doi: 10.3892/ijmm.9.6.641. [DOI] [PubMed] [Google Scholar]
- 22.Morihara N, Sumioka I, Moriguchi T, Uda N, Kyo E. Aged garlic extract enhances production of nitric oxide. Life Sci. 2002;71:509–17. doi: 10.1016/s0024-3205(02)01706-x. [DOI] [PubMed] [Google Scholar]
- 23.Assayed ME, Khalaf AA, Salem HA. Protective effects of garlic extract and vitamin C against in vivo cypermethrin-induced teratogenic effects in rat offspring. Food Chem Toxicol. 2010;48:3153–8. doi: 10.1016/j.fct.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Wargovich MJ, Woods C, Eng VW, Stephens LC, Gray K. Chemoprevention of N-nitrosomethylbenzylamine-induced esophageal cancer in rats by the naturally occurring thioether, diallyl sulfide. Cancer Res. 1988;48:6872–5. [PubMed] [Google Scholar]
- 25.Schaffer EM, Liu JZ, Green J, Dangler CA, Milner JA. Garlic and associated allyl sulfur components inhibit N-methyl-N-nitrosourea induced rat mammary carcinogenesis. Cancer Lett. 1996;102:199–204. doi: 10.1016/0304-3835(96)04160-2. [DOI] [PubMed] [Google Scholar]
- 26.Samaranayake MD, Wickramasinghe SM, Angunawela P, Jayasekera S, Iwai S, Fukushima S. Inhibition of chemically induced liver carcinogenesis in Wistar rats by garlic (Allium sativum) Phytother Res. 2000;14:564–7. doi: 10.1002/1099-1573(200011)14:7<564::aid-ptr664>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.Ogbuewu IP, Unamba-Oparah IC, Odoemenam VU, Etuk IF, Okoli IC. The potentiality of medicinal plants as the source of new contraceptive principles in males. N Am J Med Sci. 2011;3:255–63. doi: 10.4297/najms.2011.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barakat LA, Mahmoud RH. The antiatherogenic, renal protective and immunomodulatory effects of purslane, pumpkin and flax seeds on hypercholesterolemic rats. N Am J Med Sci. 2011;3:411–7. doi: 10.4297/najms.2011.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48:209–15. doi: 10.1002/cyto.10133. [DOI] [PubMed] [Google Scholar]
- 30.Kasuga S, Uda N, Kyo E, Ushijima M, Morihara N, Itakura Y. Pharmacologic activities of aged garlic extract in comparison with other garlic preparations. J Nutr. 2001;131:1080S–4. doi: 10.1093/jn/131.3.1080S. [DOI] [PubMed] [Google Scholar]
- 31.Bogdan C, Röllinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 32.Ghazanfari T, Hassan ZM, Khamesipour A. Enhancement of peritoneal macrophage phagocytic activity against Leishmania major by garlic (Allium sativum) treatment. J Ethnopharmacol. 2006;103:333–7. doi: 10.1016/j.jep.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Ali M. Mechanism by which garlic (Allium sativum) inhibits cyclooxygenase activity.Effect of raw versus boiled garlic extract on the synthesis of prostanoids. Prostaglandins Leukot Essent Fatty Acids. 1995;53:397–400. doi: 10.1016/0952-3278(95)90102-7. [DOI] [PubMed] [Google Scholar]
- 34.Ali M, Angelo-Khattar M, Farid A, Hassan RA, Thulesius O. Aqueous extracts of garlic (Allium sativum) inhibit prostaglandin synthesis in the ovine ureter. Prostaglandins Leukot Essent Fatty Acids. 1993;49:855–9. doi: 10.1016/0952-3278(93)90210-n. [DOI] [PubMed] [Google Scholar]
- 35.Fujiwara M, Natata T. Induction of tumour immunity with tumour cells treated with extract of garlic (Allium sativum) Nature. 1967;216:83–4. doi: 10.1038/216083b0. [DOI] [PubMed] [Google Scholar]
- 36.Aboul-Enein AM. Inhibition of tumor growth with possible immunity by Egyptian garlic extracts. Nahrung. 1986;30:161–9. doi: 10.1002/food.19860300216. [DOI] [PubMed] [Google Scholar]


