Abstract
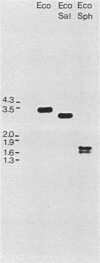
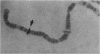
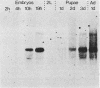
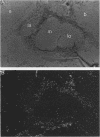
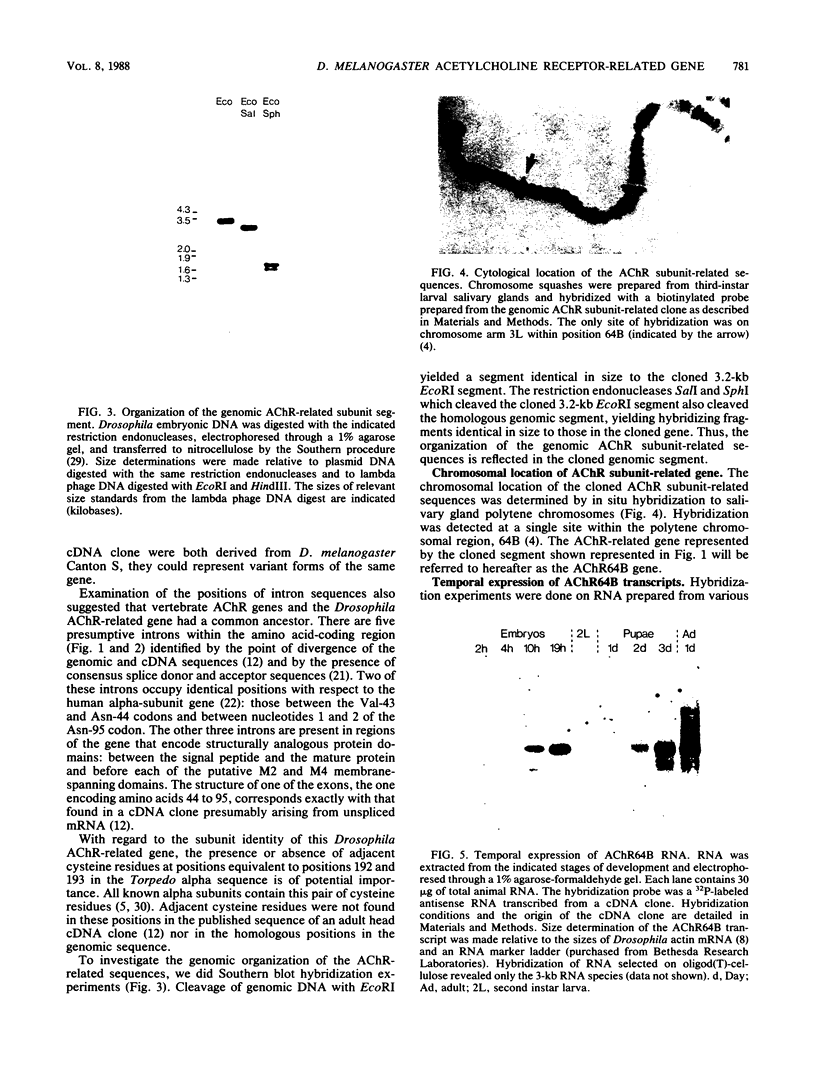
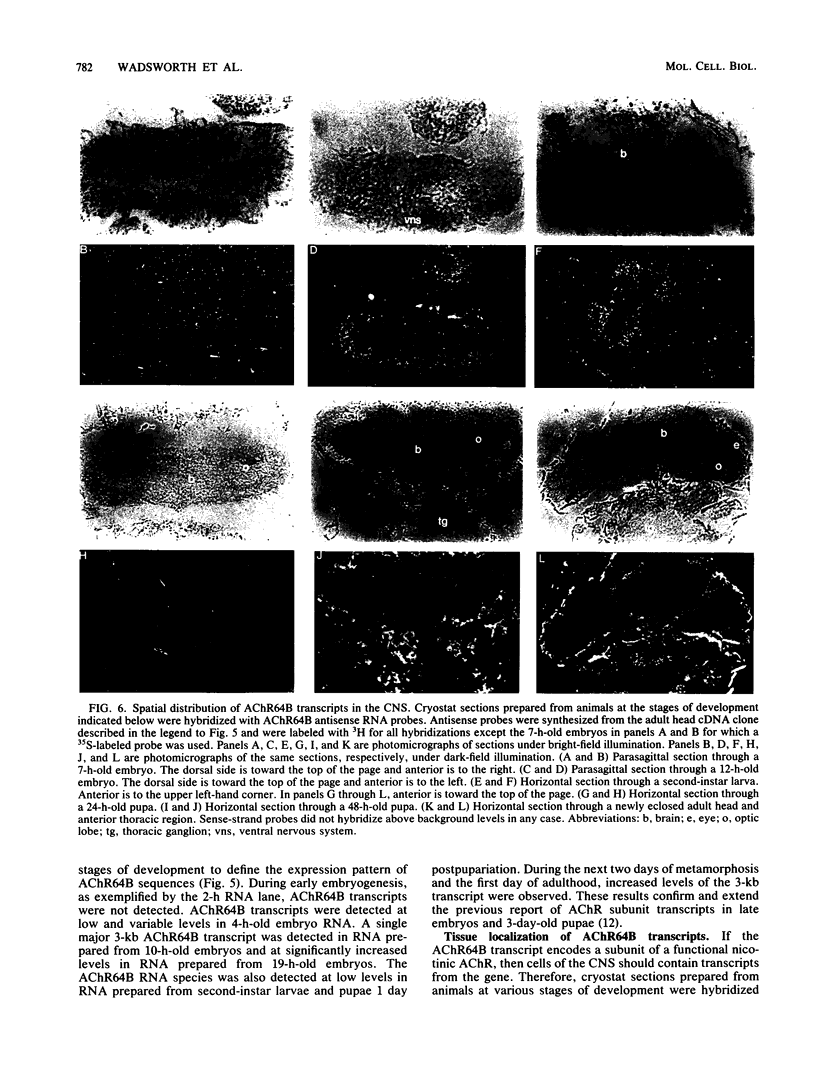
We isolated Drosophila melanogaster genomic sequences with nucleotide and amino acid sequence homology to subunits of vertebrate acetylcholine receptor by hybridization with a Torpedo acetylcholine receptor subunit cDNA probe. Five introns are present in the portion of the Drosophila gene encoding the unprocessed protein and are positionally conserved relative to the human acetylcholine receptor alpha-subunit gene. The Drosophila genomic clone hybridized to salivary gland polytene chromosome 3L within region 64B and was termed AChR64B. A 3-kilobase poly(A)-containing transcript complementary to the AChR64B clone was readily detectable by RNA blot hybridizations during midembryogenesis, during metamorphosis, and in newly enclosed adults. AChR64B transcripts were localized to the cellular regions of the central nervous system during embryonic, larval, pupal, and adult stages of development. During metamorphosis, a temporal relationship between the morphogenesis of the optic lobe and expression of AChR64B transcripts was observed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Breer H., Kleene R., Hinz G. Molecular forms and subunit structure of the acetylcholine receptor in the central nervous system of insects. J Neurosci. 1985 Dec;5(12):3386–3392. doi: 10.1523/JNEUROSCI.05-12-03386.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Goldman D., Simmons D., Swanson L. W., Patrick J., Heinemann S. Mapping of brain areas expressing RNA homologous to two different acetylcholine receptor alpha-subunit cDNAs. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4076–4080. doi: 10.1073/pnas.83.11.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. J., Kammermeyer K. L., Vincent W. S., 3rd, Wadsworth S. G. Primary sequence and developmental expression of a novel Drosophila melanogaster src gene. Mol Cell Biol. 1987 Jun;7(6):2119–2127. doi: 10.1128/mcb.7.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans-Borgmeyer I., Zopf D., Ryseck R. P., Hovemann B., Betz H., Gundelfinger E. D. Primary structure of a developmentally regulated nicotinic acetylcholine receptor protein from Drosophila. EMBO J. 1986 Jul;5(7):1503–1508. doi: 10.1002/j.1460-2075.1986.tb04389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Slemmon J. R., Hawke D. H., Williamson R., Morita E., Itakura K., Roberts E., Shively J. E., Crawford G. D., Salvaterra P. M. Cloning of Drosophila choline acetyltransferase cDNA. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4081–4085. doi: 10.1073/pnas.83.11.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeyer K. L., Wadsworth S. C. Expression of Drosophila epidermal growth factor receptor homologue in mitotic cell populations. Development. 1987 Jun;100(2):201–210. doi: 10.1242/dev.100.2.201. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Sedat J. Localization of antigenic determinants in whole Drosophila embryos. Dev Biol. 1983 Sep;99(1):261–264. doi: 10.1016/0012-1606(83)90275-0. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Patrick J., Ballivet M., Boas L., Claudio T., Forrest J., Ingraham H., Mason P., Stengelin S., Ueno S., Heinemann S. Molecular cloning of the acetylcholine receptor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):71–78. doi: 10.1101/sqb.1983.048.01.009. [DOI] [PubMed] [Google Scholar]
- Rudloff E. Acetylcholine receptors in the central nervous system of Drosophila melanogaster. Exp Cell Res. 1978 Jan;111(1):185–190. doi: 10.1016/0014-4827(78)90248-3. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Prescott D. J., Hildebrand J. G. Cholinergic neurochemical development of normal and deafferented antennal lobes during metamorphosis of the moth, Manduca sexta. Brain Res. 1977 Jan 7;119(2):389–402. doi: 10.1016/0006-8993(77)90318-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Nielsen B. K., Gepner J. I., Teng N. N., Hall L. M. Characterization of an alpha-bungarotoxin binding component from Drosophila melanogaster. J Neurochem. 1977 Dec;29(6):1013–1029. doi: 10.1111/j.1471-4159.1977.tb06505.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Finer-Moore J. Acetylcholine receptor structure, function, and evolution. Annu Rev Cell Biol. 1985;1:317–351. doi: 10.1146/annurev.cb.01.110185.001533. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth S. C., Madhavan K., Bilodeau-Wentworth D. Maternal inheritance of transcripts from three Drosophila src-related genes. Nucleic Acids Res. 1985 Mar 25;13(6):2153–2170. doi: 10.1093/nar/13.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K., Kankel D. R. Patterns of cell division and cell movement in the formation of the imaginal nervous system in Drosophila melanogaster. Dev Biol. 1978 Aug;65(2):296–321. doi: 10.1016/0012-1606(78)90029-5. [DOI] [PubMed] [Google Scholar]
- Wu C. F., Suzuki N., Poo M. M. Dissociated neurons from normal and mutant Drosophila larval central nervous system in cell culture. J Neurosci. 1983 Sep;3(9):1888–1899. doi: 10.1523/JNEUROSCI.03-09-01888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]