Abstract
Emerging evidence suggests that there are IgM-autoantibodies that may play protective roles in SLE. While IgM are often considered polyreactive, we postulate that there are distinct sets of IgM-autoantibodies of defined autoreactive specificities relevant to different features of SLE. We examined the relationships between levels of IgM natural autoantibodies (NAbs) to apoptosis-associated phosphorylcholine (PC) or malondialdehyde (MDA) antigens, with lupus-associated autoantibodies and features of disease, in 120 SLE patients. IgM anti-PC was significantly higher in patients with low disease activity and less organ damage determined by the SELENA-SLEDAI, the physician's evaluation and the SLICC damage score. Furthermore, IgM anti-PC was significantly higher in patients without cardiovascular events. In contrast, IgM anti-cardiolipin and IgM anti-dsDNA were significantly higher in patients without renal disease. These results support the hypothesis that some IgM autoantibodies are part of a natural immune repertoire that provide homeostatic functions and protection from certain clinical lupus features.
Keywords: Systemic lupus erythematosus, Natural antibodies, IgM, Cardiovascular disease, Renal disease
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease characterized by a hyperactive immune system and the production of pathogenic autoantibodies with formation of immune complexes, leading to diverse clinical features and multi-organ damage [1]. Pathogenesis may be triggered by a complex interplay of genetic and environmental contributors that likely differ between patients [1]. In particular, a preponderance of evidence indicates that clinical disease may arise in settings associated with impaired phagocytic clearance of apoptotic cells (ACs), which may result in secondary necrosis, release of inflammatory cellular contents and autoantigens, and expanding breaches of immunologic tolerance [2,3].
Due to the fundamental importance of the phagocytic clearance of ACs, the immune system has developed a primordial and specialized process that ensures efficient AC recognition and phagocytosis [4]. We and others have recently reported that this fundamental process may be augmented by a class of IgM natural autoantibodies (NAbs), which spontaneously arise in the perinatal period without known antigenic challenge, and that can discriminate ACs from healthy cells [5–7]. These IgM-NAbs recognize immuno-dominant oxidation-specific AC-membrane associated phosphorylcholine (PC) and malondialdehyde (MDA) containing determinants, and NAb levels that can be greatly elevated by intravenous AC immunization [5]. Such IgM antibodies can affect fundamental functions of innate immune cells to enhance the phagocytic clearance of ACs, as well as to suppress inflammatory responses, and can block the development of inflammatory autoimmune disease in experimental models [5,8].
In humans, natural IgM-antibodies to these AC-associated oxidation-specific epitopes are present in both healthy adults and in neonates [9,10]. Multiplex autoantibody micro-array studies have also demonstrated that these NAbs to PC and MDA epitopes are amongst the most prevalent and highly expressed of all antibody specificities in clinical SLE [11]. In addition, our surveys of disease-discordant lupus twins appeared to support the notion of protective roles of these NAbs [11] .Yet, other disease-associated autoantibodies have been reported to also recognize determinants on ACs [12] that can affect the efficiency of AC phagocytosis [13].
In the current studies, we surveyed SLE patients for expression levels of NAbs to PC and MDA as well as lupus-associated autoantibodies to beta 2-glycoprotein I (β2-GPI), cardiolipin (CL), phosphatidylserine/prothrombin (PS/PT), dsDNA, chromatin, and C1q, and looked for correlations with overall clinical disease activity and organ-specific involvement. Our findings highlight important immunologic and clinical distinctions between the different types of IgM-autoantibodies and co-expressed IgG-autoantibodies, and support the hypothesis that some IgM-autoantibodies may affect certain disease manifestations.
2. Methods
2.1. Subjects
Plasma samples were obtained with written informed consent from 120 patients from the Hopkins’ Lupus Cohort, who satisfied the revised ACR criteria for the classification of SLE [14], under IRB approved protocols at Johns Hopkins University School of Medicine and UCSD. At the time of blood drawing, clinical status was assessed with the SELENA revision of the SLE disease activity index (SLEDAI) [15], and for the Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index [16]. Furthermore, patients were tested at the Hopkins clinical laboratory for lupus anticoagulant (by Russell Viper venom time, RVVT, with confirmatory tests), C3, and C4 levels (upper limits of normal: C3, 79 mg/dl; C4, 12 mg/dl), at time of visit. For each patient, records were reviewed for history of deep venous thrombosis (DVT), renal disease and for atherosclerotic cardiovascular disease (ASCVD) events: myocardial infarctions (MI) and cerebrovascular accidents (CVA). Renal disease associated with lupus was identified by the level of proteinuria, and confirmed by renal biopsy. CVA had to have resulted in permanent neurological deficit, and did not include transient ischemic attacks. DVT was defined as lower extremity deep venous thrombosis or pulmonary embolus. Events could occur at any time point in the clinical history of the patient, DVT on average occurred 8 years before sample collection, ASCVD event 5 years before, and renal disease was confirmed by biopsy on average 5 years earlier. The demographic and clinical characteristics of patients with SLE are presented in Table 1. Our analyses included 28 healthy adult samples from the San Diego Blood Bank, and previously described SLE samples [11].
Table 1.
Characteristics of SLE patients.
| Characteristic | |
|---|---|
| Age, average | 44 (25–79) years |
| SLEDAI, average | 3.3 (0–31) |
| SLICC, average | 3.1 (0–12) |
| Phys. estimate of disease activity, average | 0.8 (0–3) |
| Number of patients (n = 120) | |
| Gender | |
| Male | 7 (5.8%) |
| Female | 113 (94%) |
| Ethnicity | |
| African-American | 51 (43%) |
| Asian | 3 (2%) |
| Caucasian | 65 (54%) |
| Other | 1 (1%) |
| Clinical history | |
| CVA and/or MI | 25 (20%) |
| CVA | 19 (16%) |
| MI | 7 (6%) |
| DVT | 19 (16%) |
| Renal disease | 27 (23%) |
SLEDAI: SELENA revision of the SLE disease activity index.
SLICC: Systemic Lupus International Collaborating Clinics damage index.
CVA: cerebrovascular accident.
MI: myocardial infarction.
DVT: deep venous thrombosis.
2.2. Autoantibody assays
Levels of IgM and IgG anti-β2-GPI, and anti-CL, as well as IgG anti-PS/PT, anti-C1q and anti-chromatin, were evaluated by commercial ELISA (QuantaLite assay, INOVA Diagnostics, Inc., San Diego, CA, USA). Samples positive for IgG anti-β2-GPI or IgG anti-CL (13 and 19 samples, respectively) were then further analyzed for IgG-subclass reactivity using biotinylated anti-IgG-subclass mAbs (Invitrogen, Carlsbad, CA, USA), and poly HRP-conjugated streptavidin (RDI, Fitzgerald, Acton, MA, USA), with subclasses specificities confirmed with monoclonal IgG (data not shown).
For IgM anti-dsDNA antibodies, modified commercial assays were used (INOVA Diagnostics), developed with μ-specific goat anti-IgM HRP (Southern Biotech, Birmingham, AL, USA) using reference samples. For anti-PC and anti-MDA antibodies, ELISA wells were coated with MDA-BSA (Academic Bio-medical Co., Houston, TX, USA) or PC6-BSA (Bio-search Technologies Inc., Novato, CA, USA) and blocked with 3% BSA-PBS. All samples were evaluated at 1:200 and 1:1000 dilutions in 1% BSA-PBS in duplicate, and detected with goat anti-IgG-HRP, γ-specific (Jackson Immunoresearch, West Grove, PA, USA) or goat anti-IgM-HRP, μ-specific(Southern Biotech, Birmingham, AL, USA) and TMB substrate (KPL, Gaithersburg, MD, USA). IgG-subclass reactivities were detected, as described above. All plates contained a standard curve from a separate SLE pool, with activity presented in relative units/ml. Total IgM and IgG were similarly measured.
2.3. Statistical analyses
We first characterized the patients with respect to demographic and clinical characteristics. We explored data graphically and numerically to assess the distributions of measurements and to detect outliers. The Kolmogorov–Smirnov test was utilized to test the normality of the variables. Antibody levels and non-normal clinical variables were dichotomized by median or determined as continuous variables as indicated. The association between auto-antibodies and clinical factors (disease activity) was first assessed by univariate analysis, and then by multivariate analysis. Correlations between numerical variables were determined using Spearman's correlation coefficient and a χ2 test or, in some cases, Fisher's exact test was used for categorical variables. Dichotomized data are expressed as odds ratios (OR) with 95% confidence intervals. Finally, multivariate (logistic) analysis was performed in order to assess for the influence of age, sex and race. p Values less than 0.05 were considered significant. A two-tailed p-value<0.05 was considered as significant. R statistical package (www.r-project.org) was used for the statistical analyses.
3. Results
3.1. Correlations between autoantibodies and clinical disease activity and organ damage
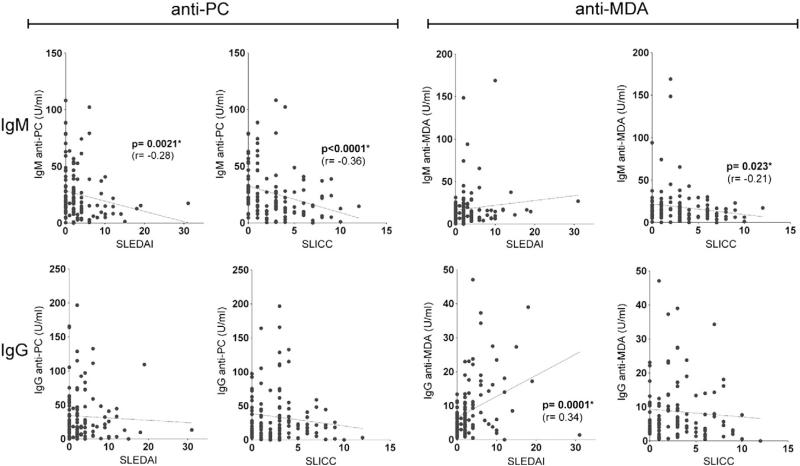
To look for evidence that certain types of IgM autoantibodies can affect SLE pathogenesis, we first evaluated for associations with overall disease activity. Univariate correlation analysis showed that IgM anti-PC levels were inversely associated with the risk of higher disease activity by SELENA-SLEDAI and with less organ damage by the SLICC/ACR Damage Index (correlation coefficient=–0.28, p-value=0.021, correlation coefficient=–0.36, p-value<0.001) (Fig. 1). Categorical analysis (dichotomized by median) confirmed this negative association that higher IgM anti-PC group was associated with 76 and 63 percent decreases in risk of higher SLEDAI activity group (odds ratio (OR)=0.24, p-value=0.0007) and SLICC (OR=0.37, p-value=0.014) (Table 2). We found a similar correlation for higher IgM anti-PC values with lower activity using a second disease activity measure, the physicians estimate of lupus activity, at the time of the visit (OR=0.47, p=0.046) when dichotomized data were compared.
Figure 1.
Correlations between anti-PC and anti-MDA autoantibody levels with the SLEDAI or SLICC scores. Analyses were performed by Spearman's rank correlations, and significant associations are indicated.
Table 2.
Associations between IgM antibody levels and disease activity and damage indices.
| IgM anti- |
SLEDAIa |
Phys. est.b |
SLICC |
|||
|---|---|---|---|---|---|---|
| (≥ median) | Odds ratio (95% CI) | p-Values | Odds ratio (95% CI) | p-Values | Odds ratio (95% CI) | p-Values |
| PC | 0.24 (0.15, 0.55) | 0.0007* | 0.47 (0.23, 0.99) | 0.046* | 0.37 (0.17, 0.82) | 0.014* |
| MDA | 1.00 (0.47, 2.1) | 1.00 | 1.22 (0.60, 2.50) | 0.58 | 0.51 (0.24, 1.10) | 0.085 |
| β2-GPI | 1.00 (0.47, 2.1) | 1.00 | 0.55 (0.26, 1.13) | 0.10 | 0.44 (0.20, 0.95) | 0.036* |
| CL | 0.64 (0.24, 1.68) | 0.36 | 0.87 (0.38, 1.98) | 0.74 | 0.84 (0.35, 2.04) | 0.70 |
| dsDNA | 1.30 (0.50, 3.39) | 0.59 | 0.73 (0.32, 1.66) | 0.45 | 1.26 (0.52, 3.05) | 0.61 |
Odds ratios and p-values presented from Fisher's exact test comparing autoantibody levels dichotomized on median level of IgM antibody (anti-PC = 18.6; anti-MDA=13.6; anti-β2-GPI=3.8; anti-CL=7.9; anti-dsDNA=227) with SLEDAI dichotomized on 4, physician's estimate of disease dichotomized on median of 0.5, or SLICC dichotomized on median of 3.
Some samples were insufficient for all tests. IgM anti-CL and anti-dsDNA: n=95; all other: n=120.
SLEDAI includes IgG anti-dsDNA positive.
Phys. est., physician's estimate of lupus activity index, 1–3.
Statistically significant.
While we also examined several other types of IgM auto-antibodies, only IgM anti-MDA showed a weaker inverse relationship with the SLICC/ACR Damage Index by both Spearman correlation (r=–0.21, p=0.023) and categorical analysis (OR=0.51, p-value=0.085). Higher levels of the IgM anti-phospholipid antibody to β2-GPI also displayed an inverse relationship to dichotomized values of the SLICC/ACR Damage Index (Table 2, OR=0.44, p=0.036) only when dichotomized data were compared. These correlations retained significance in multivariate models adjusting for sex, age, and race (anti-PC vs. SLEDAI p=0.0001; IgM anti-PC vs. SLICC p=0.02; IgM anti-β2-GPI vs. SLICC p=0.037).
In studies of IgG-autoantibodies, we did not find that IgG anti-PC had the same strong protective correlations with SLEDAI and SLICC indices, although higher levels of IgG anti-PC significantly correlated with lower disease activity assessed by the Physicians Estimate in univariant analysis (p=0.018) as well as in multivariate model when adjusted for sex, age and race (p=0.0075) (see Supplemental Table 1). In contrast, several of the other IgG antibodies instead showed direct correlation with higher antibody levels correlating with higher SLEDAI (i.e., anti-MDA, p=0.0077; anti-chromatin, p=0.0048; anti-dsDNA, p=0.05; and anti-C1q, p=0.05) (Supplemental Table 1). Taken together, these data indicate that the levels of many, but not all, types of IgG-autoantibodies have direct correlations with greater overall disease activity, while IgM anti-PC, and to a lesser extent IgM anti-MDA and IgM anti-β2-GPI, displayed a different pattern that may provide evidence of protective properties.
3.2. Correlations with complement levels
As consumption of serum complement can also reflect lupus disease activity, we also assessed levels of the complement factors, C3 and C4, as reported from the clinical laboratory. In Spearman correlation analyses, we found that most of the IgM autoantibodies had significant inverse correlation with levels of C3 and C4, such that higher IgM autoantibody levels were associated with depressed complement levels (Table 3). IgM anti-MDA also showed a significant correlation with lower levels of C3 (p=0.0086) (Table 3). The corresponding IgG auto-antibodies also showed similar inverse correlations (see Supplemental Table 2). In contrast, neither IgM anti-PC nor IgG anti-PC showed any correlation with depressed complement levels. When adjusted for sex, age and race, all associations were confirmed.
Table 3.
Correlations between IgM antibody levels and complement factors.
| IgM anti- | C3 |
C4 |
||
|---|---|---|---|---|
| r | p | r | p | |
| PC | 0.031 | 0.7364 | 0.065 | 0.4851 |
| MDA | –0.24 | 0.0086* | –0.14 | 0.13 |
| β2-GPI | –0.22 | 0.015* | –0.31 | 0.0007* |
| CL | –0.27 | 0.0078* | –0.41 | <0.0001* |
| dsDNA | –0.19 | 0.068 | –0.23 | 0.025* |
r-Values and p-values are presented from Spearman (rank-based) analysis on logarithmic transformed data.
Some samples were insufficient for all tests. Some patients did not have laboratory values for C3 and C4 available. IgM anti-CL, anti-dsDNA: n=93; all other: n = 118.
Statistical significant correlation.
3.3. IgM antibodies and renal disease
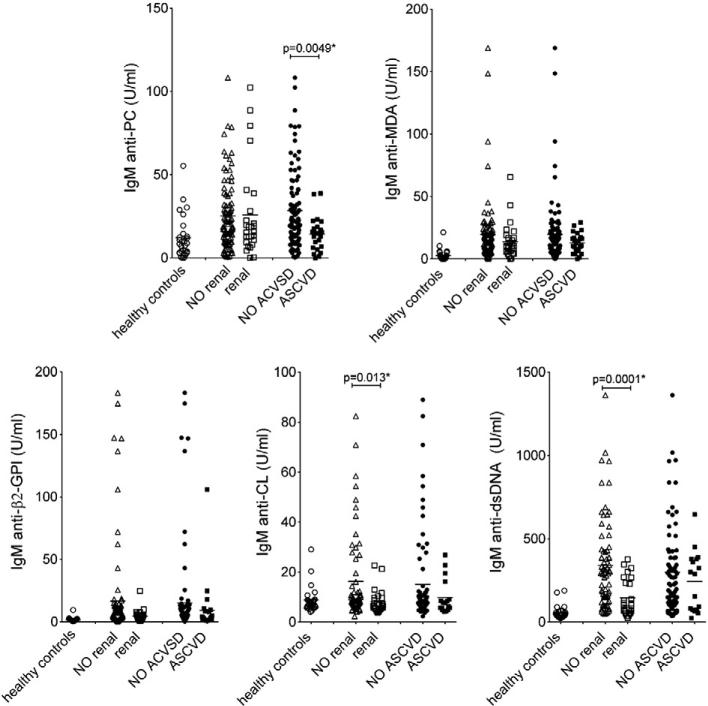
Lupus nephritis is one of the most common causes of morbidity and mortality in SLE patients, and we therefore evaluated for differences in IgM antibody levels between patients with or without renal disease. The association between IgM antibodies and incident renal disease was determined by calculation of odds ratios and 95% confidence intervals (CI) using logistic regression. In studies of dichotomized values, we found that patients with higher levels of IgM antibodies to CL and dsDNA had significantly lower risk of developing biopsy-proven renal disease (Table 4; OR=0.37, p=0.046 and OR=0.37, p=0.046; respectively). The same patterns were seen in comparisons of antibody levels in patients with and without documented lupus nephritis (Fig. 2; p=0.013 and p=0.0001 by Mann–Whitney). In contrast, no such associations were demonstrated for anti-PC IgM antibody levels. Due to the discordance between the different distinct IgM specificities, it is unlikely that the lower levels of IgM anti-CL and -dsDNA are due to increased urine protein secretion in the patients with a history of renal disease. However, these correlations did not reach statistical significance in multivariate analysis adjusting for sex, age and race, indicating some dependence of the demographic factors. No significant correlation with renal disease could be seen for the studied IgG autoantibodies (Supplemental Table 3).
Table 4.
Associations between IgM antibody levels and prevalence of renal disease or cardiovascular disease.
| IgM anti- |
Renal disease |
ASCVD events |
||
|---|---|---|---|---|
| (≥ median) | Odds ratio (95% CI) | p-Values | Odds ratio (95% CI) | p-Values |
| PC | 0.75 (0.32, 1.77) | 0.51 | 0.24 (0.09, 0.65) | 0.0053* |
| MDA | 0.41 (0.17, 1.01) | 0.053 | 0.90 (0.37, 2.18) | 0.82 |
| β2-GPI | 0.62 (0.26, 1.47) | 0.27 | 0.49 (0.20, 1.21) | 0.12 |
| CL | 0.37 (0.14, 0.98) | 0.046* | 0.67 (0.23, 1.93) | 0.45 |
| dsDNA | 0.37 (0.14, 0.98) | 0.046* | 1.18 (0.41, 3.39) | 0.75 |
Odds ratios and p-values presented Fisher's exact test analysis on autoantibody levels dichotomized on median values indicated for Table 2. Renal disease confirmed by biopsy. Atherosclerotic cardiovascular disease (ASCVD) events: cerebrovascular accident or myocardial infarction.
The sizes of some samples were inadequate for all tests. IgM anti-CL, anti-dsDNA: n=95; all other: n = 120.
Statistically significant.
Figure 2.
IgM-autoantibody levels and the presence or the absence of renal involvement or ASCVD events. Healthy n=28, anti-PC, anti-MDA patients with renal disease n=27, patients without renal disease n=93, patients with ASCVD event n=25, patients without ASCVD event n=95. For anti-β2-GPI, -CL, and anti-dsDNA: patients with renal disease n=25, patients without renal disease n=70, patients with ASCVD event n=23, patients without ASCVD n=78. In some cases the amount of sample was not adequate for all tests. p-Values were determined with two-sided Mann–Whitney non-parametric testing, with significant values indicated.
3.4. IgM anti-PC and cardiovascular disease
As SLE patients are at risk for developing accelerated atherosclerosis syndromes [17], we also investigated whether differences in autoantibody levels correlated with a clinical history of MI and/or CVA events. Importantly, we found that patients with higher levels of IgM anti-PC had a significantly lower risk of having a history of an ASCVD event (odds ratio=0.2, p=0.005; Table 4) and higher levels of IgM anti-PC were also found in comparisons between patient groups with and without these clinical features (p=0.005; Fig. 2). Multivariate logistic regression analysis adjusting for sex, age and race confirmed this association (p=0.0078). Furthermore, the correlation was not influenced by traditional ASCVD risk factors hypertension, diabetes, cigarette smoking, obesity or high cholesterol, as we found no differences between patients with high (highest quartile) and low levels of IgM anti-PC (Supplemental Table 4). No significant differences between the patient groups were detected for the other IgM-autoantibodies. Moreover, whereas no protective correlations were found with any type of IgG-antibody (Supplemental Table 3), in contrast, higher levels of IgG anti-CL were instead found in patients with a clinical history of a cardiovascular event (odds ratio 4.2, p=0.02), which has previously been reported [18]. These results clearly demonstrated very different associations for the specific types of IgM autoantibodies in patients with documented ASCVD events compared to those with renal disease.
3.5. PC and MDA reactive Abs are not related to anti-phospholipid Abs
The presence of antibodies that bind to CL and β2-GPI, together with a lupus anticoagulant, is a hallmark of the Antiphospholipid Syndrome (APS), a condition associated with clinical episodes of thromboembolic disease [19,20]. Although not currently part of accepted criteria, anti-PS/PT antibodies have also recently been identified as a possible serologic marker for APS [21]. Indeed, our studies confirmed that higher levels of IgM anti-β2-GPI, IgM anti-CL or IgG anti-CL each significantly correlated with a higher risk of positive lupus anticoagulant test (Supplemental Table 5), as expected [22]. Strikingly, although these autoantibodies also recognize phospholipid-related determinants on cells, we found that the higher levels of both IgG and IgM to PC and MDA were not associated with DVT. In fact, patients with higher levels of IgG anti-PC levels appeared to have a lower risk for a positive lupus anticoagulant test (OR=0.41, p=0.023) (Supplemental Table 5). In conclusion, the results further support the notion that autoantibodies to PC and MDA have very different immunobiologic properties than classic autoantibodies implicated in APS.
3.6. Clinical features and autoantibody levels
To understand the expression of these autoantibodies, we performed comparative surveys in patients with SLE and in healthy adult controls (Supplemental Table 6). While healthy adult controls commonly expressed detectable IgM anti-PC, other types of autoantibodies were uncommon or not detectable in our assays. However, in the studies of the SLE patients, all antibody levels in relative units were considered continuous variables independent of the cut-off being positive or negative. As expected, these autoantibody surveys also demonstrated that the presence of high IgM-reactivity in a subject significantly correlated with high IgG-reactivity for the same antigen (Supplemental Table 7). Interestingly, while there were correlations between levels of several different IgM specificities, there was no similar correlation with the natural antibody IgM anti-PC (Supplemental Table 7). In general, IgG anti-PC also did not correlate with other tested autoantibody reactivities. Furthermore, none of the levels of specific IgG-autoantibodies showed correlations with total serum IgG levels (data not shown).
We also examined IgG anti-PC subclass expression and found that SLE patients most commonly expressed IgG2 subclass responses, although IgG1 and IgG3 were also detected, which is the same pattern seen in healthy individuals (data not shown) [23]. IgG anti-β2-GPI and IgG anti-CL were also predominantly of the IgG2 subclass (data not shown), as previously reported [24]. Notably, IgG2 is a subclass with low inflammatory potential, due to limited ability to mediate complement fixation and with low affinity for activating IgG Fcγ receptors. In contrast, IgG anti-MDA in SLE patients was dominated by IgG1 and IgG3 subclass usage, which instead are better able to trigger activating FcγR and the complement cascade.
To understand how the level of an antibody may change over time during the course of the disease, we performed longitudinal surveys of 18 lupus patients, with 4–6 separate sampling over 15 years. Over this period, we found that levels of IgM anti-PC and IgM anti-MDA were generally higher at earlier time points and then decreased significantly, with ~50% mean decreases (p=0.0099, p=0.038), while levels of IgM anti-β2-GPI or total IgM levels did not significantly change over time (Supplemental Fig. 1).
4. Discussion
In the present study, we performed the first SLE survey designed to better understand the clinical relevance of a panel of IgM autoantibodies to defined antigen-specificities with emphasis on natural antibodies to the AC membrane-associated determinants, PC and MDA. In murine studies, these antibodies protect from the development of autoimmune and inflammatory conditions [8] (reviewed in [25]). We confirmed that IgM anti-PC and IgM anti-MDA were commonly expressed in healthy individuals as previously shown [11], while levels of these antibodies were significantly higher in the lupus cohort compared to the healthy adult controls.
We found that higher levels of IgM anti-PC correlated with less long-term organ damage, as defined by the SLICC/ACR Damage Index score, as well as lower disease activity as assessed by the SELENA-SLEDAI at the time of visit. IgM anti-PC levels also correlated with an absence of cardiovascular events, while there were no associations with renal disease. These results are consistent with a previous report from a smaller cohort of Swedish patients [26] and with studies showing that lower IgM anti-PC levels are associated with more frequent cardiovascular events in non-autoimmune patients [27,28].
Unexpectedly, anti-PC and anti-MDA IgM, which both bind to neo-determinants on apoptotic cells, showed significant differences in their associations with lupus clinical manifestations, as IgM anti-MDA showed only weak inverse correlations with the SLICC/ACR Damage Index but not the SELENA SLEDAI score. There were no significant associations with renal disease or cardiovascular events.
We also found that higher levels of the IgM antibody to β2-GPI correlated with less organ damage by SLICC/ACR Damage Index. Furthermore, patients without renal disease had higher levels of IgM anti-CL, and IgM anti-dsDNA. This may indicate that higher levels of some IgM antibodies may protect some patients from kidney disease, as suggested in an earlier and more focused report [29].
Our studies refute the notion that circulating IgM autoantibodies are inherently polyreactive and without true antigen binding specificity. While Mohan and colleagues have reported that protection from lupus nephritis correlated with higher levels of polyreactive IgM [30], our studies documented that the absence of renal involvement correlated with higher levels of specific types of IgM-autoantibodies (i.e., anti-CL and anti-dsDNA), while the distinct set of IgM anti-PC antibodies in contrast correlated with protection from cardiovascular events. Our data therefore strongly argue that the antigen specificity of the IgM determines whether there is an association with protection from certain lupus disease features.
The expression patterns of IgG anti-PC were also different from all other types of IgG-antibodies, as anti-PC was the only IgG antibody that neither showed a direct correlation with disease activity measurements nor with standard clinical inflammatory markers or disease manifestations. In contrast to anti-PC, we found that higher levels of IgG anti-MDA, similar to the lupus-associated antibodies, anti-dsDNA, anti-chromatin and anti-C1q, were strongly associated with increased lupus disease activity based on the SELENA-SLEDAI score. In fact, within our panel, IgG anti-MDA was the best biomarker for high disease activity, and showed most correlation with depressed levels of C3 and C4 levels as well as strong correlation with increased erythrocyte sedimentation rate (data not shown). These findings are therefore consistent with a report that levels of MDA, a marker of systemic oxidative stress, and IgG anti-MDA can be significantly increased in lupus patients with more active disease [31].
In general, our investigations evaluated antibody levels at single time points, and looked for correlations with overall disease activity at the time of visit, organ-specific disease, and history of prior cardiovascular events and renal disease. While the disease activity was determined at the time of visit and sample collection, the clinical events typically occurred several years earlier. It is therefore possible that disease duration and treatment may affect antibody levels. While we could not adequately control for the effects of medication, we did perform a series of longitudinal surveys, which showed that levels of the natural IgM antibodies (anti-PC and anti-MDA) were often at highest levels at first sampling and then decreased over time, while others were more stable. In our statistical models we also adjusted for age, but found that major findings for IgM anti-PC were unaffected. Further longitudinal studies with a larger cohort will be needed to determine whether changes in levels of a specific autoantibody can truly be a predictor increased risk of a clinical event.
The potential mechanisms of action of IgM anti-PC antibodies have been examined in the mouse, and appear to involve the formation of complexes with apoptotic cell membranes, which have immunomodulatory activity [5]. Such properties may be amplified by effector functions of the IgM to enhance recruitment of mannose binding lectin and C1q, which serve as “eat me” signals for AC phagocytosis, and which also enhance IgM mediated suppression of inflammatory activity [5]. As IgM are predominantly intravascular molecules, these properties may be especially relevant to the inhibition of atherogenesis, as well as potentially global SLE activity, which is suggested here. The antigens, cardiolipin, dsDNA and β2-GPI, can be present on apoptotic cells and could potentially mediate equivalent homeostatic properties. However, IgM antibodies to CL and dsDNA instead had a different clinical correlation, with renal protection. These protective properties are currently unexplained.
The differential expression of these very distinct autoantibodies may provide important clues to their roles in pathogenesis as well their cellular origins within the B-cell compartment. We speculate that IgM-NAbs to AC-membranes naturally arise in health and may be further raised in autoimmune disease-predisposed individuals before overt clinical manifestations are present, as suggested by patterns seen in lupus twins [11]. In mice, natural antibodies, such as anti-PC, are predominantly produced by innate-like B-1 cells without the need for foreign antigen stimulation, and these are commonly germline-encoded and autoreactive. This repertoire has been suggested to be positively selected for self-reactivity and to have important functions in tissue homeostasis (reviewed in [32]). Interestingly, in mice natural antibodies may represent more than 80% of the circulating IgM [33] and in both human and mice these predominantly recognize oxidation-specific epitopes [10] that could be present on dying cells. Recent studies have reported that human IgM antibodies to PC predominantly arise from CD43+ CD27+ B-1 cell analogs, which may also be the predominant source of constitutively produced IgM and which can also respond to antigenic challenge [34]. IgM antibodies to nuclear components such as dsDNA and chromatin are not present in healthy individuals and only appear during early pathogenesis before overt organ damage [35]. We speculate that in predisposed individuals and during early stages of pathogenesis the differential expression of specific IgM NAbs may protect organs from certain aspects of disease progression.
In conclusion, our survey of autoantibodies in SLE patients demonstrates that higher levels of several types of IgM autoantibodies that recognize epitopes on dead and dying cells, such as anti-PC, correlate with lower individual susceptibility or even protection from distinct disease manifestations. The results of our studies may lead to the development of novel prognostic test panels. Such an approach may enable better predictions of the likelihood for the development of renal involvement, and accelerated cardiovascular disease, and thereby improve our understanding of the potential benefits of therapeutic interventions in each patient afflicted by SLE.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH; R01AI090118, R01 AI068063 and ARRA supplement, R01AI090118, and from the ACR REF Within Our Reach campaign, the Alliance for Lupus Research, the Arthritis Foundation, and the P. Robert Majumder Charitable Trust. The Hopkins Lupus Cohort is supported by NIH AR 43727 and the Institute for Clinical and Translational Research ULIRR025005.
Footnotes
Conflict of interest statement
Dr. Burlingame is employed by INOVA Diagnostics, Inc. The other authors declare that they have no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10.1016/j.clim.2012.01.002.
References
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 3.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J. Clin. Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J. Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 7.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur. J. Immunol. 2005;35:252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, Gronwall C, Vas J, Boyle DL, Corr M, Kono DH, Silverman GJ. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J. Immunol. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binder CJ. Natural IgM antibodies against oxidation-specific epitopes. J. Clin. Immunol. 2010;30(Suppl. 1):S56–S60. doi: 10.1007/s10875-010-9396-3. [DOI] [PubMed] [Google Scholar]
- 10.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman GJ, Srikrishnan R, Germar K, Goodyear CS, Andrews KA, Ginzler EM, Tsao BP. Genetic imprinting of autoantibody repertoires in systemic lupus erythematosus patients. Clin. Exp. Immunol. 2008;153:102–116. doi: 10.1111/j.1365-2249.2008.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J. Immunol. 2002;169:159–166. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- 13.Rovere P, Manfredi AA, Vallinoto C, Zimmermann VS, Fascio U, Balestrieri G, Ricciardi-Castagnoli P, Rugarli C, Tincani A, Sabbadini MG. Dendritic cells preferentially internalize apoptotic cells opsonized by anti-beta2-glycoprotein I antibodies. J. Autoimmun. 1998;11:403–411. doi: 10.1006/jaut.1998.0224. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 15.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, Lockshin M, Merrill JT, Belmont HM, Askanase AD, McCune WJ, Hearth-Holmes M, Dooley MA, Von Feldt J, Friedman A, Tan M, Davis J, Cronin M, Diamond B, Mackay M, Sigler L, Fillius M, Rupel A, Licciardi F, Buyon JP. Combined oral contraceptives in women with systemic lupus erythematosus. N. Engl. J. Med. 2005;353:2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 16.Dayal NA, Gordon C, Tucker L, Isenberg DA. The SLICC damage index: past, present and future. Lupus. 2002;11:261–265. doi: 10.1191/0961203302lu190sa. [DOI] [PubMed] [Google Scholar]
- 17.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger T.A. Jr., Jansen-McWilliams L, D'Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 18.Bili A, Moss AJ, Francis CW, Zareba W, Watelet LF, Sanz I. Anticardiolipin antibodies and recurrent coronary events: a prospective study of 1150 patients. Thrombogenic Factors, and Recurrent Coronary Events Investigators. Circulation. 2000;102:1258–1263. doi: 10.1161/01.cir.102.11.1258. [DOI] [PubMed] [Google Scholar]
- 19.Cohen D, Berger SP, Steup-Beekman GM, Bloemenkamp KW, Bajema IM. Diagnosis and management of the antiphospholipid syndrome. BMJ. 2010;340:c2541. doi: 10.1136/bmj.c2541. [DOI] [PubMed] [Google Scholar]
- 20.George D, Erkan D. Antiphospholipid syndrome. Prog. Cardiovasc. Dis. 2009;52:115–125. doi: 10.1016/j.pcad.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Amengual O, Atsumi T, Koike T. Specificities, properties, and clinical significance of antiprothrombin antibodies. Arthritis Rheum. 2003;48:886–895. doi: 10.1002/art.10831. [DOI] [PubMed] [Google Scholar]
- 22.Petri M. Update on anti-phospholipid antibodies in SLE: the Hopkins’ Lupus Cohort. Lupus. 2010;19:419–423. doi: 10.1177/0961203309360541. [DOI] [PubMed] [Google Scholar]
- 23.Brown M, Schiffman G, Rittenberg MB. Subpopulations of antibodies to phosphocholine in human serum. J. Immunol. 1984;132:1323–1328. [PubMed] [Google Scholar]
- 24.Guerin J, Casey E, Feighery C, Jackson J. Anti-Beta 2-glycoprotein I antibody isotype and IgG subclass in antiphospholipid syndrome patients. Autoimmunity. 1999;31:109–116. doi: 10.3109/08916939908994054. [DOI] [PubMed] [Google Scholar]
- 25.Silverman GJ, Gronwall C, Vas J, Chen Y. Natural autoanti-bodies to apoptotic cell membranes regulate fundamental innate immune functions and suppress inflammation. Discov. Med. 2009;8:151–156. [PubMed] [Google Scholar]
- 26.Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegard J. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008;47:1144–1150. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 27.de Faire U, Su J, Hua X, Frostegard A, Halldin M, Hellenius ML, Wikstrom M, Dahlbom I, Gronlund H, Frostegard J. Low levels of IgM antibodies to phosphorylcholine predict cardiovascular disease in 60-year old men: effects on uptake of oxidized LDL in macrophages as a potential mechanism. J. Autoimmun. 2010;34:73–79. doi: 10.1016/j.jaut.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Fiskesund R, Stegmayr B, Hallmans G, Vikstrom M, Weinehall L, de Faire U, Frostegard J. Low levels of antibodies against phosphorylcholine predict development of stroke in a population-based study from northern Sweden. Stroke. 2010;41:607–612. doi: 10.1161/STROKEAHA.109.558742. [DOI] [PubMed] [Google Scholar]
- 29.Mehrani T, Petri M. IgM anti-beta2 glycoprotein I is protective against lupus nephritis and renal damage in systemic lupus erythematosus. J. Rheumatol. 2011;38:450–453. doi: 10.3899/jrheum.100650. [DOI] [PubMed] [Google Scholar]
- 30.Li QZ, Xie C, Wu T, Mackay M, Aranow C, Putterman C, Mohan C. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J. Clin. Invest. 2005;115:3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum. 2010;62:2064–2072. doi: 10.1002/art.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 33.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20(+)CD27(+)TD43(+)CD70(−) (vol 208, pg 67, 2011) J. Exp. Med. 2011;208 doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burlingame RW, Rubin RL, Balderas RS, Theofilopoulos AN. Genesis and evolution of antichromatin autoantibodies in murine lupus implicates T-dependent immunization with self antigen. J. Clin. Invest. 1993;91:1687–1696. doi: 10.1172/JCI116378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.