Abstract
The rising prevalence of obesity has reached pandemic proportions, with an associated cost estimated at up to 7% of health expenditures worldwide. Bariatric surgery is currently the only effective long-term treatment for obesity and obesity-related co-morbidities in clinically severely obese patients. However, the precise physiological mechanisms underlying the postsurgical reductions in caloric intake and body weight are poorly comprehended. It has been suggested that changes in hormones involved in hunger, food intake and satiety via the neurohormonal network may contribute to the efficacy of bariatric procedures. In this review, we consider how gastrointestinal hormone concentrations, involved in appetite and body weight regulation via the gut–brain axis, are altered by different bariatric procedures. Special emphasis is placed on neurohormonal changes following Roux-en-Y gastric bypass surgery, which is the most common and effective procedure used today.
Keywords: brain, hormone, RYGB, ghrelin, GLP-1, PYY
Introduction
Obesity continues to increase in prevalence globally and is associated with the metabolic syndrome as well as chronic diseases, such as diabetes, hypertension and heart disease.1 The etiology of obesity is multifactorial, and levels of appetite-related gut peptides have been shown to be related to body weight.2 With the increase in obesity and the associated morbidity and mortality, research into the contribution of hormones involved in energy homeostasis and metabolism has also increased in recent years. As the number of bariatric procedures has risen concurrently with the rise in severe obesity, greater attention is being paid to how such procedures may affect appetite-related hormones, which is the focus of this review.
Appetite control and feeding behavior are regulated in part by hormones released from the gut that activate areas of the brain primarily located within the limbic and mesolimbic systems.2 Along with other areas within the dopaminergic reward pathway, the hypothalamus has been extensively linked to the control of food intake and energy homeostasis.3 The hormonal signaling network, which provides information to the brain (primarily the hypothalamus) about energy stores and metabolic status includes leptin from fat stores and insulin from the pancreas as well as cholecystokinin (CCK), glucagon-like peptide-l (GLP-1), peptide YY3–36 (PYY3–36) and ghrelin from the gastrointestinal (GI) tract. Ghrelin is known to stimulate appetite whereas cholecystokinin, GLP-1 and PYY3–36 promote satiety. Adipose tissue provides hormonal signals via leptin and insulin to the brain about energy stores, and likely from adiponectin and resistin.4 Enterokines from the gastrointestinal tract and adipokines from fat work together to regulate short- and long-term food intake, respectively.
Surgical intervention for weight loss
Relative to behavioral interventions, surgical interventions produce greater weight loss in both the short and long term.5 The currently employed surgical interventions for obesity all contain a restrictive component, limiting the amount of food that can enter the stomach pouch. Several procedures, most notably Roux-en-Y gastric bypass (RYGB), also contain a malabsorptive component, in which the bowel length is shortened, decreasing nutrient and calorie absorption. However, there is some question as to the degree and durability of postsurgical malabsorption associated with these procedures.6 A number of studies have attempted to assess the mechanisms that lead to postsurgical reductions in body weight and associated medical comorbidities, which can occur before significant weight loss. The majority of these studies implicate postsurgical changes in appetite-related hormone levels.5
The reduction in caloric intake seen following bariatric surgery is likely due to more than just the physical changes made to the gastrointestinal tract.7 However, the precise mechanisms of action are not well understood, particularly with RYGB.8 An increasing number of studies suggest that postsurgical changes within the neurohormonal system may account for a proportion of postsurgical weight loss.9 Gastrointestinal hormone levels are often altered following bariatric procedures and may contribute to postsurgical reductions in caloric intake and body weight. For example, postsurgical reductions in ghrelin, and earlier and enhanced postprandial elevations of PYY and GLP-1, may reduce hunger and promote satiety.10 Recent evidence also suggests that postsurgical changes in such hormones may lead to changes in brain activation in response to appetitive cues.11
Surgical techniques
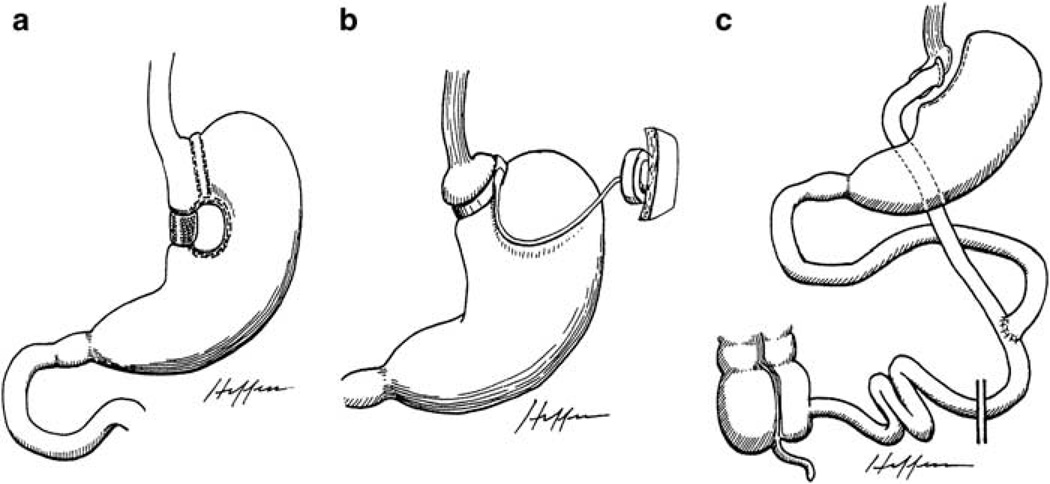
Most purely restrictive procedures create a small gastric pouch with a narrow outlet, limiting the intake of food without disruption of the absorptive function of the small intestine. Vertical banded gastroplasty (VBG) and adjustable gastric banding (AGB) are examples of purely restrictive procedures. In VBG, the cardia of the stomach is sectioned off by a longitudinal staple line with a tight outlet wrapped by a band or mesh (Figure 1a). Adjustable gastric banding, on the other hand, partitions the upper stomach using a tight, adjustable, prosthetic band (Figure 1b). Laparoscopic adjustable gastric banding (LAGB) has progressively replaced VBG as the most commonly performed purely restrictive bariatric procedure due to its simplicity and lower complication rate.12 Other restrictive procedures include sleeve gastrectomy, intragastric balloon, and endoluminal gastroplasty.
Figure 1.
Illustrations of restrictive procedures and Roux-en-Y gastric bypass. Reproduced with permission from Dr Edward C Mun.173 (a) Vertical-banded gastroplasty; (b) Adjustable gastric banding; (c) Roux-en-Y gastric bypass.
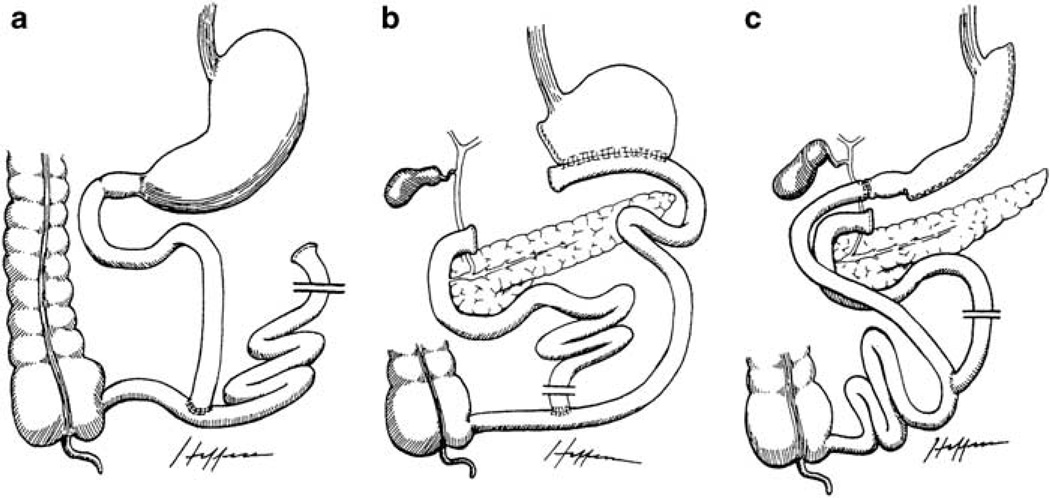
Malabsorptive procedures are primarily designed to bypass a portion of the small intestine, reducing the efficiency of nutrient absorption. The jejunoileal bypass is an example of a purely malabsorptive procedure, which consists of dividing the jejunum near the ligament of Treitz and reconnecting it near the ileocecal valve, bypassing a long small bowel segment (Figure 2a). However, this procedure is no longer performed due to significant complications and relatively greater need for revision surgeries.13
Figure 2.
Illustrations of malabsorptive procedures. Reproduced with permission from Dr Edward C Mun.173 (a) Jejunoileal bypass; (b) Biliopancreatic diversion; (c) Biliopancreatic diversion with duodenal switch.
A combination of restrictive and malabsorptive techniques is employed in several procedures, including the biliopancreatic diversion (BPD), biliopancreatic diversion with duodenal switch (BPD-DS) and RYGB. With the BPD procedure, there is a partial gastrectomy with a gastroileostomy or gastrojejunostomy, where a short bowel channel is attached to a long Roux-Y limb for nutrients and biliopancreatic secretions to be absorbed (Figure 2b). The BPD is also limited in use due to adverse health outcomes related to essential nutrient malabsorption.14 The BPD-DS is a partial sleeve gastrectomy with an intact pylorus and a Roux limb with short bowel channel (Figure 2c). This procedure may be attractive to super-obese patients (BMI>50 kg m−2), as it typically leads to relatively large postsurgical weight loss; however, it is not commonly performed due to adverse health outcomes similar to those seen in BPD.13 Lastly, RYGB surgery is the most common bariatric procedure performed today, accounting for approximately 65% of all procedures worldwide.15 With this operation, a small gastric pouch is created and connected to a short Roux-en-Y alimentary limb of distal small bowel, which is anastomosed to the jejunum, bypassing the duodenum and proximal jejunum (Figure 1c).
Gut and peripheral hormones as key appetite regulators
Hunger and satiety are mediated through a complex interplay of neurological and hormonal signals.2,3 The hypothalamus processes many of these signals in relation to nutrient and energy availability.3 Neural communication takes place between the hypothalamus and other brain regions (including cortical areas), which send effector responses to regulate food intake according to caloric need.3,11,16 There are three different sets of signals from the periphery responsible for providing this information: one from adipose tissue that exerts long-term regulatory mechanisms on food intake, and the other two from the GI tract, with orexigenic as well as anorexigenic properties that exert primarily short-term effects on food intake.17 Afferent signals can also result from direct mechanical stimulation of the GI tract, such as gastric distension due to stretch and pressure in the stomach.16,18
Ghrelin is an orexigenic peptide that can send signals to the hypothalamus via blood circulation as an endocrine hormone, through vagal afferents containing ghrelin receptors, or via release within the hypothalamus.19 Neuropeptide Y (NPY) and agouti-related protein-producing neurons in the arcuate nucleus of the hypothalamus are stimulated by ghrelin to increase food intake.17 Other peripheral hormones have been shown to induce satiety signals that can act directly on the brain, indirectly via the vagus nerve, or by slowing gastric emptying. These satiety hormones include CCK, GLP-1 and PYY, which rise after meals, and can suppress food intake when administered peripherally or centrally.17
Gut and peripheral hormones in relation to bariatric surgery
The seeming inability of the rearrangement of gut anatomy to fully explain the sustained reductions in body weight and medical comorbidities seen following bariatric surgery has inspired a body of literature on postsurgical changes in appetite-related hormones. Gut peptides known to cross the blood–brain barrier and induce changes in neural activation are likely candidates to account for the currently unexplained effects of bariatric surgery.8,9 Ghrelin, PYY, GLP-1, CCK, insulin and leptin are released in the periphery and act indirectly on the vagus nerve and/or directly on target areas of the hypothalamus.20 Thus, this review focuses on recent literature reporting postsurgical changes in appetite hormones that have been linked to hypothalamic targets. Bariatric surgery can also alter the concentrations of other gut hormones such as gastrin, gastric inhibitory polypeptide, serotonin, neurotensin and vasoactive intestinal peptide. However, these hormones do not have substantiated effects on food intake and will not be discussed.
Search/inclusion criteria for studies of gut hormones following bariatric surgery
A literature search was conducted between February 2009 and July 2010. Articles were collected from Medline, PubMed, PsychINFO and TRIP databases. Articles were also identified from UpToDate Inc. published research and reviews. Because the primary aim of this review was to examine changes observed in gut hormones from before to after bariatric surgery, only articles that included measures of gut peptides involved in appetite control were included. No restrictions in terms of participant randomization or blinding were placed on included studies, and no restrictions were placed on the year of publication; however, articles published after July 2010 were not included. Literature searches were conducted using various combinations of the following key words: adiponectin, amylin, appetite, appetite centers, appetite control, bariatric surgery, BPD, BPD-DS, body weight, CCK, duodenal jejunal bypass (DJB), food intake, gastric banding, gastric bypass, gastrointestinal hormones, GLP-1, ghrelin, gut hormones, hypothalamus, insulin, LAGB, leptin, metabolic surgery, neuroendocrine peptides, neuronal activation, obesity, oxyntomodulin, PYY, resistin, RYGB, and weight loss.
Ghrelin
Ghrelin is a potent appetite stimulator and an endogenous ligand for the growth hormone secretagogue receptor. It is mainly synthesized by the gastric antrum and fundus. Injection of ghrelin centrally in animals stimulates the release of the orexigenic neuropeptides NPY and agouti-related protein-producing neurons, most notably in the arcuate nucleus of the hypothalamus.21 Ghrelin enhances gut motility and speeds gastric emptying.22 Ghrelin concentrations peak before meals and fall sharply postprandially, and some data in humans implicate ghrelin’s involvement in pre-meal hunger and meal initiation. Higher ghrelin concentrations are noted during fasting, hunger or negative energy balance states such as short-term starvation, cancer or anorexia.22 Sustained ghrelin levels by infusion can induce adiposity in animals23 and, thus, ghrelin may also have a role in the long-term regulation of body weight.
Reduced ghrelin levels are observed after feeding, during hyperglycemia, and in obesity.24 Fasting ghrelin has been found to be 27% lower in obese as compared to normal-weight individuals,25 and ghrelin concentrations rise following weight loss.26 Despite having lower ghrelin levels, overweight, obese and insulin-resistant individuals often continue to gain weight. The lower fasting levels in obesity suggests downregulation of ghrelin in response to overeating or excess body weight.
In purely restrictive operations, the upper portion of the stomach is reduced, with varied effects on ghrelin levels depending on the type of procedure. Bohdjalian and colleagues27 prospectively studied 26 patients who had sleeve gastrectomy and showed that ghrelin concentrations were reduced 12 months post-operatively and remained low during a 5-year follow-up. A reduction in fasting ghrelin was found in other studies after laparoscopic sleeve gastrectomy;28–31 however, increases in ghrelin following LAGB have been reported.28,32–34 Similar variations in results were noted after AGB and VBG (Table 1). The majority of studies report an increase in ghrelin following both AGB28,32–35 and VGB.34,36–38 However, nearly as many studies report no change following either procedure,39–43 and two cross-sectional studies have reported lower ghrelin concentrations following AGB relative to BMI-matched controls.44,45
Table 1.
Summary of GI hormone changes after restrictive surgical procedures
| Hormone | Type of restrictive surgery |
||
|---|---|---|---|
| AGBc | VBGd | SGe | |
| Ghrelina | ↑28,32–35 | ↑34,36–38 | ↓27–31 |
| ↔39–41 | ↔42,43 | ||
| PYY3–36b | ↑45,193 | ↑91 | ↑92,126 |
| GLP-1b | ↔41,51 | ↑101 | ↑126 |
| CCKb | ↑112 | ↔126 | |
| ↔111 | |||
| Leptina | ↓34,35,39,41,125 | ↓34,37,42 | ↓126 |
| Insulina | ↓34,41,137 | ↓34,42 | ↔126 |
| ↔39 | |||
| PPa | ↔39 | ↓152 | ↔126 |
| ↔153 | |||
| OXMb | ↔111 | ||
| Adiponectina | ↑125,128,194 | ↑37,42,196 | ↔126 |
| ↔195 | ↔195 | ||
| Resistina | ↑195 | ↑195 | ↓126 |
| Amylinb | ↔51 | ↔126 | |
↑ = postsurgical increase. ↓ = postsurgical decrease. ↔ = no significant postsurgical change.
Fasting (vs postprandial based on relevance to peptide and majority of available studies).
Postprandial (vs fasting).
AGB = adjustable gastric banding.
VBG = vertical banded gastroplasty.
SG = sleeve gastrectomy.
Inconsistent postsurgical changes in ghrelin have also been found in malabsorptive procedures (Table 2).
Table 2.
Summary of GI hormone changes after malabsorptive surgical procedures
| Hormone | Type of malabsorptive surgery |
||
|---|---|---|---|
| GBc (JIBd, RYGBe, DJBf) | BPDg | BPD-DSh | |
| Ghrelina | ↑4,48–51 | ↑25,59,60 | ↓37,130 |
| ↓30,38,42,43,47,197 | ↓42 | ||
| ↔31,35,41,57 | ↔50,58 | ||
| PYY3–36b | ↑30,31,41,51,57,92,94,102,103 | ||
| GLP-1b | ↑30,51,57,94,103,106,167,193,198–200 | ↑101,140,199 | |
| CCKb | ↔111,129 | ||
| Leptina | ↓4,35,41,42,49,57,62,128,129 | ↓25,42,59 | ↓37,130 |
| Insulina | ↓4,41,42,57,62,106,127,129,137,141,197 | ↓42,140 | |
| ↔57,201 | |||
| PPa | ↔48,150,151 | ||
| OXMb | ↑57,111,151,161,162 | ||
| Adiponectina | ↑4,42,62,106 | ↑42,130 | ↑37,130 |
| Resistina | ↔4,166 | ||
| Amylinb | ↓51 | ||
↑ = postsurgical increase. ↓ = postsurgical decrease. ↔ = no significant postsurgical change.
Fasting (vs postprandial based on relevance to peptide and majority of available studies).
Postprandial (vs fasting).
GB = gastric bypass.
JIB = jejeunoileal bypass.
RYGB = Roux-en-Y gastric bypass.
DJB = duodenal-jejunal.
BPD = biliopancreatic diversion.
BPD = biliopancreatic diversion. hBPD-DS = biliopancreatic diversion -duodenal switch.
The majority of studies examining changes in ghrelin after RYGB report a decrease in postsurgical circulating ghrelin levels.4,30,38,42,43,46–51 In a cross-sectional comparison, Cummings and colleagues52 found that ghrelin levels were markedly reduced in post RYGB participants, as compared to both obese and normal weight control participants.
They also reported that obese participants who had lost weight by dieting had higher levels of ghrelin than they did before dieting,52 suggesting that ghrelin may have a role in the adaptive response that limits the amount of weight lost by dieting and increases the likelihood of weight regain. Subsequent to Cumming and colleagues52 findings, others have also reported significantly lower levels of ghrelin in patients who lost weight from RYGB in both cross-sectional and prospective studies.30,38,42–44,46,47,53–56 Decreased ghrelin levels were also present within the first year following BPD in two reports.42,44 These studies suggest that a postsurgical reduction of ghrelin may contribute to the sustained weight loss noted in obese patients following gastric bypass. However, a number of researchers have found no significant change in ghrelin levels following gastric bypass31,35,41,57 and BPD,50,58 and higher ghrelin concentrations have also been reported following both RYGB4,48–50 and BPD.25,59,60
Variation in study results of ghrelin levels may be at least in part explained by differences in the comparison groups selected. Holdstock and colleagues61 prospectively studied the effect of RYGB and found that levels of ghrelin increased at 12 months and were similar to BMI-matched controls. These RYGB patients underwent significant weight loss at 12 months, which would be expected to lead to a rise in ghrelin levels. Had these operative patients been compared to BMI-matched controls that had lost weight conventionally, one might have expected a relatively lower ghrelin level in the postsurgical patients. In a prospective study by Faraj and colleagues,62 there was also a rise in ghrelin levels in patients following RYGB undergoing active weight-loss. However, there were no control participants, and, despite the increase in ghrelin levels observed in the surgical patients, they were still lower than levels reported in normal weight or comparably obese participants from other studies.52,63
Cummings and colleagues64 suggest that the variance across findings may also be related to the integrity of autonomic vagal innervation. Vagal innervation affects ghrelin levels,19,65–67 and the degree to which the innervation is left intact is likely to differ between surgeons. Despite the inconsistencies, several key trends are apparent. First, the type of surgical procedure seems to have a major influence on ghrelin levels. The majority of studies examining changes in ghrelin levels following RYGB report a postsurgical decrease, whereas the majority of studies following AGB report an increase (Tables 1 and 2). In RYGB, the stomach antrum, fundus and duodenum, where most of the production of ghrelin occurs,68,69 are largely excluded. Thus, ingested nutrients have significantly less contact with ghrelin-producing cells in the stomach and duodenum, which may lead to an inhibition of ghrelin release. In contrast AGB, which results in little or no reduction in ghrelin (Table 1), does not exclude the fundus or duodenum from contact with nutrients. This explanatory hypothesis is consistent with Fruhbeck and colleagues54 who showed decreased fasting concentrations after RYGB and an increase after AGB as well as following conventional comparable weight loss by diet in obese patients. The reduction in postsurgical ghrelin levels in gastric bypass may contribute to the greater weight loss relative to other procedures.3,16
It should be noted that although the majority of studies refer to total ghrelin, as described above, ghrelin has two major molecular forms: acylated ghrelin and des-acylated ghrelin. Acylated ghrelin, which induces a positive energy balance and is suppressed post-prandially and by pharmacological hyperinsulinemia, was previously presumed to be the only active form in terms of endocrine function. However, des-acylated ghrelin makes up the vast majority of total ghrelin,70 and there is increasing evidence in both animals71 and humans72,73 that des-acylated ghrelin may exert effects in opposition to those exerted by acylated ghrelin. In addition, hyperinsulinemic and hyperinsulinemic–hyperlipidemic clamp studies show suppression of des-acylated ghrelin, but no change in acylated ghrelin, suggesting that insulin regulation of ghrelin may be specific to des-acylated ghrelin.74 Finally, recent evidence suggests that des-acylated ghrelin binds specifically to HDL whereas acylated ghrelin binds equally to all lipoproteins.75 Precisely how these two distinct forms of the same peptide interact in the regulation of energy balance remains under investigation, but illustrate the need to examine all forms of appetite-related hormones in the body.
Peptide YY
Although ghrelin has received the majority of the attention in surgically induced weight loss studies, there has been a shift in focus toward other hormones, such as PYY and GLP-1. In contrast to ghrelin, which is an appetite-stimulating hormone, PYY is a lower gut-derived hormone with anorectic effects.17 It is secreted from intestinal L-cells in amounts that generally correspond to the energy ingested; however, the amount secreted may vary depending on the macronutrient content of the ingested energy.76,77 PYY circulates in two forms: PYY1–36 (total) and PYY3–36 (referred to as ‘active’), with the latter being the major subtype found in the circulation.78 PYY1–36 binds to Y1–Y5 receptors,79 and there is contradictory evidence on the effect of PYY1–36 on food intake.80–82 However, administration of PYY3–36 reduces food intake over the short term in both animals78,83 and humans.84 PYY3–36 likely reduces food intake by acting on Y2 receptors on vagal afferents, which results in increased activity in the arcuate nucleus of the hypothalamus to inhibit NPY activation.85 Appetite suppression by PYY3–36 may also result from slowing of gastric emptying (ileal brake mechanism).86
Levels of PYY3–36 are low during fasting and peak 1–2 h following food intake, with high fat foods resulting in the greatest release of PYY3–36.87 Batterham and colleagues84 demonstrated lower premeal PYY3–36 levels in 12 obese as compared to 12 lean participants, as well as a smaller postprandial rise, suggesting that obesity may be associated with a PYY3–36 deficiency. However, Pfluger and colleagues found no significant difference in fasting PYY levels between 66 lean and 63 obese subjects.88 Nevertheless, obese participants remain sensitive to the anorectic effects of exogenously administered PYY3–36.89
The majority of evidence suggests that restrictive procedures lead to a rise in fasting and postprandial PYY.29–31,90–92 Fasting and postprandial PYY levels in clinically severely obese surgical patients were comparable to non-obese controls, following VBG in cross-sectional studies at 6 months and remained relatively constant at 12 months post-surgery.91 Two studies have reported similar postprandial PYY3–36 levels in post AGB patients and lean controls.45,93
Malabsorptive operations consistently demonstrate a post-surgical increase in fasting and postprandial PYY levels.30,31,41,51,57,90,92,94 In a cross-sectional study at 15–17 months post-RYGB, Korner and colleagues95 found an early postprandial rise in PYY concentrations in 12 patients. In a longitudinal study,41 PYY levels were significantly greater in RYGB patients than in LAGB patients after 52 weeks, despite little difference in BMI’s between the two post surgical groups. The mechanism of this early and exaggerated response may be due to the stomach and pylorus being bypassed, which likely leads to faster transit to the lower gut. Garcia-Fuentes and colleagues50 found that BPD produced an even greater rise in PYY levels than RYGB.
An increase in postprandial PYY concentrations alone may result in an early sense of satiety and reduced meal size, and the combined effect of increased PYY and reduced ghrelin (Tables 1 and 2) may contribute further to weight loss.31,55 PYY suppresses a high proportion of ghrelin-sensitive neurons in the arcuate nucleus of the hypothalamus in a dose-dependent manner.96 A shift in the ghrelin/PYY ratio in favor of PYY after bariatric surgery may result in reduced appetite. Further longitudinal investigations pre and post surgery and across different operations are needed to clarify this point.
Glucagon-like peptide 1
Glucagon-like peptide-l is a key incretin hormone co-released with PYY from the distal intestinal L-cells of the gut after a meal. It is secreted in two equally potent forms, GLP-1 (7–37) and GLP-1 (7–36).97 The primary functions of GLP-1 include the potentiation of glucose-stimulated insulin secretion, enhancement of β-cell growth and survival, inhibition of glucagon release, and control of food intake.98 Following peripheral administration of GLP-1, most studies in humans report decreased food intake and increased fullness.99 GLP-1 acts as an ileal brake for the upper GI tract and reduces food intake in part by slowing gastric emptying, resulting in greater gastric distension. Plasma levels of GLP-1 are higher both before and after food intake in lean as compared to obese individuals, who have lower fasting GLP-1 and an attenuated postprandial release.100 Relatively few studies have examined changes in GLP-1 concentrations in obese patients after restrictive bariatric procedures. With respect to AGB, two studies have reported no postsurgical change in fasting GLP-1.39,41 However, Reinehr and colleagues90 found that fasting GLP-1 was reduced in AGB patients at 2-year post-surgery. Conversely, an increase in fasting and postprandial GLP-1 has been reported in one study29 following sleeve gastrectomy. Other investigators showed that GLP-1 levels during an oral glucose tolerance test were increased in VBG and BPD, with a greater increase in BPD relative to VBG.101 GLP-1 is secreted from the distal small bowel; therefore restriction of the stomach would not be expected to have a major impact on circulating levels of GLP-1.
Postsurgical increases in postprandial GLP-1 have been documented following malabsorptive operations.30,31, 41,51,57,94,102,103 Morinigo and colleagues94 found that RYGB leads to a significant increase in postprandial GLP-1 levels 6 weeks postoperatively, when participants were still markedly obese. Elevated levels of GLP-1 may contribute to the sustained efficacy of RYGB as well as improve and resolve diabetes, consistent with the mechanisms underlying this incretin’s effect on weight and glucose metabolism.104 RYGB reduces the size of the stomach and bypasses the duodenum, which allows for faster delivery of food contents through the gut,105 enhancing GLP-1’s effect. Dramatic increases in GLP levels have been observed immediately after RYGB,94 which may be due to foregut exclusion and/or rapid hindgut delivery.104 Long term follow-up with bariatric surgical patients may be informative about whether treatment with GLP-1 analogs for diabetes is sustainable. As with PYY3–36, it has been suggested that increased hypothalamic satiety signals resulting from increases in postprandial GLP-1 may contribute to some of the postsurgical weight loss following malabsorptive procedures.94,106
Cholecystokinin
Cholecystokinin, an endogenous peptide hormone present in the gut and the brain, helps control appetite, ingestive behavior, and gastric emptying via both peripheral and central mechanisms. CCK is also known to have a number of effects on physiological processes including anxiety, sexual behavior, sleep, memory and intestinal inflammation.107 CCK is actually a collection of hormones labeled according to number of amino acids (for example, CCK 8 in the brain, CCK 33 and CCK 36 in the gut); however, differential effects on human energy balance have not been well established. Therefore, in keeping with convention, we refer to CCK in the singular. CCK originating from the gut is rapidly released from the duodenal and jejunal mucosa in response to nutrients, peaks at about 15–30 min and remains elevated for up to 5 h postprandially.108 It is a potent stimulator of pancreatic digestive enzymes and bile from the gall bladder.17 It delays gastric emptying and promotes intestinal motility. As a neuropeptide, CCK activates receptors on vagal afferent neurons, which transmit satiety signals to the dorsomedial hypothalamus. This action suppresses NPY and provides feedback to reduce meal size and meal duration.109
Studies of postsurgical changes in CCK are sparse, and the interpretation of early studies is somewhat hampered by difficulties associated with previous assay techniques due to low plasma concentrations, extensive molecular heterogeneity and close homology of CCK to gastrin, which circulates in higher concentrations.110 Reported changes following bariatric surgery are variable in both restrictive and malabsorptive procedures (Tables 1 and 2). One study compared CCK levels after a glucose or protein meal before and after RYBG and VBG, and the CCK response was not affected by either procedure.111 However in another study, Foschi and colleagues112 compared patients before and after VBG surgery with healthy lean volunteer controls and found that post-VBG patients had a higher peak CCK response to an acidified meal known to increase CCK production113 and a faster time to the peak than controls, without differences between baseline CCK concentrations.112 In rats, CCK was not significantly altered after RYGB-induced weight loss.109 However, Baldinger and colleagues105 found a greater increase in CCK following RYGB in humans, as well faster emptying which are consistent as nutrients reaching the gut stimulate CCK. Although a reduction in CCK following RYGB might be expected due to the diversion of ingested food away from the upper part of the small intestine (the duodenum), the jejunum also releases CCK.114
In contrast to leptin and insulin,115 CCK does not appear to have an independent role in the long-term regulation of energy balance and body weight,116 but rather a primary role in short term control of appetite and satiety.117 CCK can work synergistically with leptin to enhance short-term reduction of food intake in mice.118 There is also novel work indicating that high insulin levels may increase circulating CCK via insulin-induced suppression of free fatty acids, with lipid infusion abolishing these effects.119 As such, changes in macronutrient absorption after bariatric surgery affecting glucose- and protein-induced insulin secretion may contribute to altered circulating CCK levels, with potential effects on short-term satiety and gastric emptying. However, CCK’s precise role in human obesity remains somewhat unclear, and more work is needed in examining changes in CCK following bariatric surgery.
Leptin
Leptin is produced primarily in the adipose tissue. It is categorized as an adipokine and plays a large role in the regulation of energy balance. Leptin produced from adipocytes sends signals about energy status from the periphery to hypothalamic regulatory centers.17 In humans, serum leptin levels rise or fall in response to acute caloric surplus or deficits, respectively. Leptin administration has anorexigenic effects in both animals and humans,17,20 although much less effective in humans.120 Leptin also helps control adipose metabolism in the body by stimulation of lipolysis and suppression of lipogenesis.121 Fasting serum leptin is higher in the obese due to the presence of more body fat, the main source of leptin.121 Consistent with this, leptin decreases with weight and fat loss.122 Following meals, leptin increases slowly and may make only a small contribution to short-term satiety, but a larger one to long-term body weight regulation.123 Nevertheless, leptin injections in obese humans have not been efficacious in reducing food intake and body weight, likely due to the development of leptin resistance.123 It should be noted that leptin has also been found to be secreted from the gastric mucosa, but in much lesser amounts than from adipose tissue.124 Although leptin secreted from adipocytes acts primarily on the hypothalamus for long-term regulation of food intake, gastric leptin is involved in the short-term regulation of digestion, including the delay of gastric emptying, absorption of nutrients by the intestinal wall and, the secretion of gastric, intestinal, and pancreatic hormones.124
As expected, fasting leptin levels consistently decrease57 following bariatric surgery in relation to fat loss, irrespective of procedure.4,25,34,35,37,39,41,42,59,62,125–130 Relative to pre-surgical levels, lower postprandial leptin levels have also been reported in obese patients after VBG.131 A similar reduction was found at 2 and 12 months post BPD as compared to pre-surgery.59 Plasma leptin concentrations were also lower in clinically severely obese patients who underwent BPD-DS.37,130 Finally, Rubino and colleagues129 found that leptin levels were reduced following gastric bypass as with non-surgical weight loss.37 Recent evidence suggests that leptin-replacement therapy may aid in weight loss maintenance.132,133
Insulin
Insulin is a pancreatic hormone that maintains glucose homeostasis and was the first identified adiposity signal. Insulin levels rise after a meal to optimize glucose use for energy. The excess glucose is converted and stored in the liver and muscle as glycogen, and as fat in adipose tissue. Insulin concentrations vary directly with adiposity, and visceral fat is negatively correlated with insulin sensitivity.134 Fasting and postprandial insulin are higher in obese than in lean individuals.135 Insulin can penetrate the blood–brain barrier and binds to receptors in the arcuate nucleus to decrease food intake.136
In addition to its interactive effects with other hormones mentioned above, insulin itself is a long-term regulator of body weight, and, in the majority of restrictive bariatric operations, insulin tends to fall in post-surgical obese patients.34,41,42,126,137 Reductions in postsurgical levels of circulating insulin were maintained at 2-year post GB and VBG,34 and obese patients had lower insulin levels after LAGB than BMI-matched controls.138 Weight loss, secondary to gastric bypass and BPD, improves insulin resistance.42,62,137,139,140 However, Korner and colleagues95 showed that insulin levels were decreased in surgically treated obese women with RYGB in comparison to BMI-matched obese counterparts. Insulin levels and resistance were also significantly lowered in obese individuals with and without Night Eating Syndrome 5months after RYGB.141 These operations are being further investigated as a potential treatment for diabetes as an alternative to pharmacological agents.142
Other gut hormones
Other gut signals that regulate body weight through stimulation of hypothalamic regions include but are not limited to pancreatic polypeptide,143 oxyntomodulin,144 adiponectin,145 resistin,146 and amylin.147 Pancreatic polypeptide has structural similarities with PYY and NPY. It is secreted from pancreatic cells in relation to caloric ingestion and can remain in the bloodstream for up to 6 h postprandially. 148 It is also involved in gallbladder relaxation and inhibition of pancreatic secretion. Once secreted, the binding action of this enteroendocrine hormone to Y4 receptors in the arcuate nucleus of the hypothalamus has been implicated in the suppression of food intake in mice.143 Few studies have looked at pancreatic polypeptide following obesity surgery, but most show that bariatric surgery has only minimal influence.29,39,48,149–154
Oxyntomodulin (OXM) is co-secreted with GLP-1 from the enteroendocrine L cells to suppress the acid-producing oxyntic glands of the stomach.155 Central injection of OXM reduces food intake and weight gain in rodents and has been shown to reduce hunger and food intake in humans.156 Oxyntomodulin also has an incretin effect following glucose intake similar to GLP-1.157 Central intravenous OXM infusions in the rat hypothalamus reduced food intake,158 and intraperitoneal administration of OXM in rodents suppressed fast-induced and dark-phase food intake.159 In one study, an increase in OXM precursor gene (pre-proglucagon) expression was observed after an ileal transposition in a ratmodel.160 Levels of OXM increased in the majority of bypass operations, 57,111,161,162 whereas no significant changes in OXM levels were observed following VBG.111
Adiponectin is a peptide produced and released exclusively by adipose tissue, in this respect similar to leptin. However, plasma levels of adiponectin remain relatively constant throughout the day and are not affected by food intake.17 Furthermore, there is a negative correlation between BMI and plasma levels of adiponectin.4 Obese individuals with diabetes have even lower plasma levels of adiponectin than non-diabetic obese individuals,4,42 which suggests that diminished adiponectin may contribute to insulin resistance. A dramatic increase has been found in adiponectin levels after RYGB in obese patients.4,42,62,106,163 Adiponectin levels also increased after weight loss following a BPD-DS procedure.37,130
Resistin, also known as adipose tissue-specific secretory factor, is another adipokine hormone that acts on skeletal muscle myocytes, hepatocytes, and adipocytes. Opposite in effects to adiponectin, higher resistin may contribute to insulin resistance.4 Resistin is positively correlated with obesity in animal studies,164,165 but there is contradictory evidence about its role after weight loss induced by diet or surgery in humans.4,163,166 Amylin, which is co-secreted with insulin from the pancreas, is considered a major satiety peptide, and was recently found to be decreased after a 12 kg weight loss following gastric bypass surgery in obese individuals.51
Discussion
Similar postsurgical changes have been found between restrictive and malabsorptive procedures in levels of leptin, insulin, and adiponectin, suggesting that these hormonal changes may result primarily from the associated weight loss.41,42 Differences between these procedures in their effect on other appetite-related hormone levels that may contribute to the generally superior effectiveness of combination procedures over purely restrictive procedures are more difficult to assess, but in general show differences between procedural types on changes in ghrelin and GLP-1. Most studies show a postsurgical decrease in levels of the orexigenic hormone ghrelin following gastric bypass procedures,38,42,43,46,47 but a postsurgical increase in ghrelin levels following gastric banding.32,34,36–38,46 In addition, most studies of the anorexigenic hormone, GLP-1, reveal significant increases following bypass procedures 51,94,106,167,168 but no change following banding.39,41,51 With regard to PYY, most studies show a postsurgical increase in postprandial PYY in malabsorptive31,41,90,94 and some restrictive (VGB, sleeve gastrectomy)29,91 procedures; however, it remains unclear whether AGB has any significant effect on postprandial PYY levels.41
These general findings suggest potential mechanisms by which bypass patients would experience less hunger, as well as greater and sooner postprandial fullness as compared to banding, thus contributing to greater weight loss. The bypassing of the stomach and upper intestine may promote faster gastric emptying. More rapid transit of nutrients through the lower gut may stimulate a faster and enhanced postprandial release of gut peptides, and enhance the effect of the ileal break mechanism.7
Roux-en-Y gastric bypass remains the most commonly performed and effective bariatric procedure used today; however, its mechanisms may be the least well understood. Recent evidence suggests that the restrictive and malabsorptive components alone are insufficient to account for the resulting weight loss.7,8,169,170 Currently, sufficient data are not available to quantify the individual contributions to postsurgical weight loss of the restrictive and malabsorptive components of RYGB surgery. Through comparisons with VGB, it may be possible to crudely estimate the magnitude of postsurgical weight loss not accounted for by the restrictive mechanism in RYGB. The comparison with VGB was chosen over AGB, as VGB involves sectioning of the stomach (as opposed to banding) and the level of restriction in VGB may better approximate that of RYGB.52,171
In prospective randomized trials, 50–80% loss of excess body weight was seen 1–2 years following RYGB, as opposed to only 30–50% 1 to 2 years after VBG, suggesting that 0–50% (Low end point of range (0%) determined by subtracting the highest % excess body weight loss following VGB from the lowest % excess body weight loss following RYGB (50%−50%=0%). High end point of range (50%) determined by subtracting the lowest % excess body weight loss following VGB from the highest % excess body weight loss following RYGB (80%−30%=50%)) of the weight loss seen following RYGB may be left unexplained by the restrictive component. A meta-analysis comparing RYGB to VGB confirms that the short-term (1–2 years) disparity between procedures is approximately 25%,172 suggesting that the restrictive component accounts for up to 75% of post-RYGB weight loss. However, longer-term data suggests a greater disparity between these procedures.173 A nearly 80% failure rate (failure to maintain the loss of at least half of excess body weight) has been reported after 10 years with VGB,12 and both cross-sectional and prospective studies suggest that the disparity between RYGB and VGB may increase over time due to the superior weight loss maintenance following RYGB.169,171,174–176 In addition, it is important to note that estimating the effect of gastric restriction itself by comparisons with VGB would hold true only if weight loss seen following VGB were achieved independent of changes in gut peptides. However, several postsurgical changes in gut peptides have been noted following VGB, such as increases in postprandial PYY3–36 91 and GLP-1,101 and may account for a proportion of postsurgical weight loss. Therefore, we feel it reasonable to estimate that the restrictive component may account for 50–75% of post-RYGB weight loss.
Although a clear effect of malabsorption can be seen in weight loss resulting from procedures such as jejunoileal bypass and BPD, clinically significant malabsorption, measured by indices such as albumin, prealbumin and fecal fat, is not observed after the standard proximal RYGB.7,177–180 In addition, several animal studies181–184 have shown that sleeve gastrectomy with ileal transposition, a new procedure designed to combine gastric restriction with intentional changes in gut peptide profile (earlier and exaggerated release of GLP-1 & PYY, lower ghrelin, etc.) while avoiding nutrient malabsorption, shows weight loss equal to that seen following RYGB. Initial studies in humans suggest similar findings with sleeve gastrectomy with ileal transposition. For example, Gagner et al.185 reported that individuals undergoing sleeve gastrectomy with ileal transposition as a revision surgery of BPD-DS showed completely restored gut absorptive function while maintaining weight loss.
Rubino and Marescaux186 found no reduction in food intake or body weight in rats undergoing gastrojejunal bypass, which involves a bypass of approximately the same amount of intestinal foregut as is excluded in RYGB but spares the stomach, as compared to sham-operated rats. Finally, malabsorptive effects of only 4% have been shown in animal models187 and similarly modest effects have been postulated in humans.7,35,181,188 Thus, a rough estimate of 5% of weight loss attributable to the malabsorptive component of RYGB may be reasonable.
Together, the estimated percentages of post-RYGB weight loss attributable to gastric restriction (50–75%) and malabsorption (B5%) suggest that the restrictive and malabsorptive components combined account for approximately 55–80% of weight lost through RYGB. Thus, approximately 20–45% of post-RYGB weight loss may be currently unexplained. Increases in resting energy expenditure have been raised as a potential contributing mechanism.187,189 However, evidence appears to indicate the REE decreases postsurgically in proportion to fat loss.190,191 Similarly, dumping syndrome was proposed as an additional potential mechanism; however, severity of dumping syndrome correlates poorly with weight loss,7 rendering it unlikely to play a significant role in the efficacy of RYGB. Therefore, an estimated 20–45% of weight loss secondary to RYGB surgery could be explained by other factors,192 a large percentage of which may be attributable to the associated neurohormonal changes discussed in this review, leaving the potential open for substantial and sustainable weight loss effects if these neurohormonal effects can be identified and replicated pharmacologically.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 2008;16(Suppl 3):S11–S22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 4.Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and metaanalysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 6.Brolin RE, LaMarca LB, Kenler HA, Cody RP. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6:195–203. doi: 10.1016/s1091-255x(01)00022-1. discussion 204-5. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 8.Tadross JA, le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes (London) 2009;33(Suppl 1):S28–S32. doi: 10.1038/ijo.2009.14. [DOI] [PubMed] [Google Scholar]
- 9.Orlando FA, Goncalves CG, George ZM, Halverson JD, Cunningham PR, Meguid MM. Neurohormonal pathways regulating food intake and changes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:486–495. doi: 10.1016/j.soard.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Doucet E, Cameron J. Appetite control after weight loss: what is the role of bloodborne peptides? Appl Physiol Nutr Metab. 2007;32:523–532. doi: 10.1139/H07-019. [DOI] [PubMed] [Google Scholar]
- 11.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 12.Balsiger BM, Poggio JL, Mai J, Kelly KA, Sarr MG. Ten and more years after vertical banded gastroplasty as primary operation for morbid obesity. J Gastrointest Surg. 2000;4:598–605. doi: 10.1016/s1091-255x(00)80108-0. [DOI] [PubMed] [Google Scholar]
- 13.Bult MJ, van Dalen T, Muller AF. Surgical treatment of obesity. Eur J Endocrinol. 2008;158:135–145. doi: 10.1530/EJE-07-0145. [DOI] [PubMed] [Google Scholar]
- 14.Marceau P, Hould FS, Simard S, Lebel S, Bourque RA, Potvin M, et al. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22:947–954. doi: 10.1007/s002689900498. [DOI] [PubMed] [Google Scholar]
- 15.Poves I, Cabrera M, Maristany C, Coma A, Ballesta-Lopez C. Gastrointestinal quality of life after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16:19–23. doi: 10.1381/096089206775222032. [DOI] [PubMed] [Google Scholar]
- 16.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 17.Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity—review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Geliebter A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol Behav. 1988;44:665–668. doi: 10.1016/0031-9384(88)90333-2. [DOI] [PubMed] [Google Scholar]
- 19.Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin—hormone with multiple functions. Front Neuroendocrinol. 2004;25:27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24:597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 22.Bloomgarden ZT. Gut hormones, obesity, polycystic ovarian syndrome, malignancy, and lipodystrophy syndromes. Diabetes Care. 2007;30:1934–1939. doi: 10.2337/dc07-zb07. [DOI] [PubMed] [Google Scholar]
- 23.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 24.Nonogaki K. Ghrelin and feedback systems. Vitam Horm. 2008;77:149–170. doi: 10.1016/S0083-6729(06)77007-8. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Unzueta MT, Fernandez-Santiago R, Dominguez-Diez A, Vazquez-Salvi L, Fernandez-Escalante JC, Amado JA. Fasting plasma ghrelin levels increase progressively after biliopancreatic diversion: one-year follow-up. Obes Surg. 2005;15:187–190. doi: 10.1381/0960892053268453. [DOI] [PubMed] [Google Scholar]
- 26.Geloneze B, Tambascia MA, Pilla VF, Geloneze SR, Repetto EM, Pareja JC. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg. 2003;13:17–22. doi: 10.1381/096089203321136539. [DOI] [PubMed] [Google Scholar]
- 27.Bohdjalian A, Langer FB, Shakeri-Leidenmuhler S, Gfrerer L, Ludvik B, Zacherl J, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20:535–540. doi: 10.1007/s11695-009-0066-6. [DOI] [PubMed] [Google Scholar]
- 28.Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–1029. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 29.Depaula AL, Macedo AL, Schraibman V, Mota BR, Vencio S. Hormonal evaluation following laparoscopic treatment of type 2 diabetes mellitus patients with BMI 20–34. Surg Endosc. 2008;23:1724–1732. doi: 10.1007/s00464-008-0168-6. [DOI] [PubMed] [Google Scholar]
- 30.Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 31.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 32.Schindler K, Prager G, Ballaban T, Kretschmer S, Riener R, Buranyi B, et al. Impact of laparoscopic adjustable gastric banding on plasma ghrelin, eating behaviour and body weight. Eur J Clin Invest. 2004;34:549–554. doi: 10.1111/j.1365-2362.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 33.Uzzan B, Catheline JM, Lagorce C, Airinei G, Bon C, Cohen R, et al. Expression of ghrelin in fundus is increased after gastric banding in morbidly obese patients. Obes Surg. 2007;17:1159–1164. doi: 10.1007/s11695-007-9197-9. [DOI] [PubMed] [Google Scholar]
- 34.Nijhuis J, van Dielen FM, Buurman WA, Greve JW. Ghrelin, leptin and insulin levels after restrictive surgery: a 2-year follow-up study. Obes Surg. 2004;14:783–787. doi: 10.1381/0960892041590980. [DOI] [PubMed] [Google Scholar]
- 35.Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346–350. doi: 10.1038/oby.2004.43. [DOI] [PubMed] [Google Scholar]
- 36.Foschi D, Corsi F, Rizzi A, Asti E, Carsenzuola V, Vago T, et al. Vertical banded gastroplasty modifies plasma ghrelin secretion in obese patients. Obes Surg. 2005;15:1129–1132. doi: 10.1381/0960892055002338. [DOI] [PubMed] [Google Scholar]
- 37.Kotidis EV, Koliakos GG, Baltzopoulos VG, Ioannidis KN, Yovos JG, Papavramidis ST. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment—prospective study. Obes Surg. 2006;16:1425–1432. doi: 10.1381/096089206778870058. [DOI] [PubMed] [Google Scholar]
- 38.Foschi D, Corsi F, Colombo F, Vago T, Bevilaqua M, Rizzi A, et al. Different effects of vertical banded gastroplasty and Roux-en-Y gastric bypass on meal inhibition of ghrelin secretion in morbidly obese patients. J Invest Surg. 2008;21:77–81. doi: 10.1080/08941930701883624. [DOI] [PubMed] [Google Scholar]
- 39.Shak JR, Roper J, Perez-Perez GI, Tseng CH, Francois F, Gamagaris Z, et al. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes Surg. 2008;18:1089–1096. doi: 10.1007/s11695-008-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ram E, Vishne T, Diker D, Gal-Ad I, Maayan R, Lerner I, et al. Impact of gastric banding on plasma ghrelin, growth hormone, cortisol, DHEA and DHEA-S levels. Obes Surg. 2005;15:1118–1123. doi: 10.1381/0960892055002329. [DOI] [PubMed] [Google Scholar]
- 41.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (London) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia de la Torre N, Rubio MA, Bordiu E, Cabrerizo L, Aparicio E, Hernandez C, et al. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J Clin Endocrinol Metab. 2008;93:4276–4281. doi: 10.1210/jc.2007-1370. [DOI] [PubMed] [Google Scholar]
- 43.Lin E, Gletsu N, Fugate K, McClusky D, Gu LH, Zhu JL, et al. The effects of gastric surgery on systemic ghrelin levels in the morbidly obese. Arch Surg. 2004;139:780–784. doi: 10.1001/archsurg.139.7.780. [DOI] [PubMed] [Google Scholar]
- 44.Fruhbeck G, Diez-Caballero A, Gil MJ, Montero I, Gomez-Ambrosi J, Salvador J, et al. The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes Surg. 2004;14:606–612. doi: 10.1381/096089204323093363. [DOI] [PubMed] [Google Scholar]
- 45.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 46.Fruhbeck G, Rotellar F, Hernandez-Lizoain JL, Gil MJ, Gomez-Ambrosi J, Salvador J, et al. Fasting plasma ghrelin concentrations 6 months after gastric bypass are not determined by weight loss or changes in insulinemia. Obes Surg. 2004;14:1208–1215. doi: 10.1381/0960892042386904. [DOI] [PubMed] [Google Scholar]
- 47.Morinigo R, Casamitjana R, Moize V, Lacy AM, Delgado S, Gomis R, et al. Short-term effects of gastric bypass surgery on circulating ghrelin levels. Obes Res. 2004;12:1108–1116. doi: 10.1038/oby.2004.139. [DOI] [PubMed] [Google Scholar]
- 48.Sundbom M, Holdstock C, Engstrom BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 49.Pardina E, Lopez-Tejero MD, Llamas R, Catalan R, Galard R, Allende H, et al. Ghrelin and apolipoprotein AIV levels show opposite trends to leptin levels during weight loss in morbidly obese patients. Obes Surg. 2009;19:1414–1423. doi: 10.1007/s11695-008-9793-3. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, Garcia-Arnes J, Gallego-Perales JL, Rivas-Marin J, et al. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes Surg. 2008;18:1424–1429. doi: 10.1007/s11695-008-9560-5. [DOI] [PubMed] [Google Scholar]
- 51.Bose M, Machineni S, Olivan B, Teixeira J, McGinty JJ, Bawa B, et al. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring) 2010;18:1085–1091. doi: 10.1038/oby.2009.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 53.Tritos NA, Mun E, Bertkau A, Grayson R, Maratos-Flier E, Goldfine A. Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes Res. 2003;11:919–924. doi: 10.1038/oby.2003.126. [DOI] [PubMed] [Google Scholar]
- 54.Fruhbeck G, Diez Caballero A, Gil MJ. Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med. 2004;350:308–309. doi: 10.1056/NEJM200401153500323. [DOI] [PubMed] [Google Scholar]
- 55.Chan JL, Mun EC, Stoyneva V, Mantzoros CS, Goldfine AB. Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring) 2006;14:194–198. doi: 10.1038/oby.2006.25. [DOI] [PubMed] [Google Scholar]
- 56.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 57.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Rouxen-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 58.Adami GF, Cordera R, Marinari G, Lamerini G, Andraghetti G, Scopinaro N. Plasma ghrelin concentratin in the short-term following biliopancreatic diversion. Obes Surg. 2003;13:889–892. doi: 10.1381/096089203322618713. [DOI] [PubMed] [Google Scholar]
- 59.Adami GF, Cordera R, Andraghetti G, Camerini GB, Marinari GM, Scopinaro N. Changes in serum ghrelin concentration following biliopancreatic diversion for obesity. Obes Res. 2004;12:684–687. doi: 10.1038/oby.2004.79. [DOI] [PubMed] [Google Scholar]
- 60.Valera Mora ME, Manco M, Capristo E, Guidone C, Iaconelli A, Gniuli D, et al. Growth hormone and ghrelin secretion in severely obese women before and after bariatric surgery. Obesity (Silver Spring) 2007;15:2012–2018. doi: 10.1038/oby.2007.240. [DOI] [PubMed] [Google Scholar]
- 61.Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–3183. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 62.Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 63.Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8:643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 64.Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–2525. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 65.le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab. 2005;90:4521–4524. doi: 10.1210/jc.2004-2537. [DOI] [PubMed] [Google Scholar]
- 66.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 67.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–5187. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- 68.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 69.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 70.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and desacyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 71.Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, et al. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004;89:3062–3065. doi: 10.1210/jc.2003-031964. [DOI] [PubMed] [Google Scholar]
- 73.Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, et al. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab. 2005;90:1055–1060. doi: 10.1210/jc.2004-1069. [DOI] [PubMed] [Google Scholar]
- 74.Weickert MO, Loeffelholz CV, Arafat AM, Schofl C, Otto B, Spranger J, et al. Euglycemic hyperinsulinemia differentially modulates circulating total and acylated-ghrelin in humans. J Endocrinol Invest. 2008;31:119–124. doi: 10.1007/BF03345577. [DOI] [PubMed] [Google Scholar]
- 75.Holmes E, Davies I, Lowe G, Ranganath LR. Circulating ghrelin exists in both lipoprotein bound and free forms. Ann Clin Biochem. 2009;46:514–516. doi: 10.1258/acb.2009.008254. [DOI] [PubMed] [Google Scholar]
- 76.Weickert MO, Spranger J, Holst JJ, Otto B, Koebnick C, Mohlig M, et al. Wheat-fibre-induced changes of postprandial peptide YY and ghrelin responses are not associated with acute alterations of satiety. Br J Nutr. 2006;96:795–798. doi: 10.1017/bjn20061902. [DOI] [PubMed] [Google Scholar]
- 77.Lomenick JP, Melguizo MS, Mitchell SL, Summar ML, Anderson JW. Effects of meals high in carbohydrate, protein, and fat on ghrelin and peptide YY secretion in prepubertal children. J Clin Endocrinol Metab. 2009;94:4463–4471. doi: 10.1210/jc.2009-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 79.Ballantyne GH. Peptide YY(1–36) and peptide YY(3–36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 80.Unniappan S, McIntosh CH, Demuth HU, Heiser U, Wolf R, Kieffer TJ. Effects of dipeptidyl peptidase IV on the satiety actions of peptide YY. Diabetologia. 2006;49:1915–1923. doi: 10.1007/s00125-006-0310-8. [DOI] [PubMed] [Google Scholar]
- 81.Chelikani PK, Haver AC, Reidelberger RD. Comparison of the inhibitory effects of PYY(3–36) and PYY(1–36) on gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1064–R1070. doi: 10.1152/ajpregu.00376.2004. [DOI] [PubMed] [Google Scholar]
- 82.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292:E1062–E1068. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 83.Challis BG, Pinnock SB, Coll AP, Carter RN, Dickson SL, O’Rahilly S. Acute effects of PYY3–36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem Biophys Res Commun. 2003;311:915–919. doi: 10.1016/j.bbrc.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 84.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 85.le Roux CW, Bloom SR. Peptide YY, appetite and food intake. Proc Nutr Soc. 2005;64:213–216. doi: 10.1079/pns2005427. [DOI] [PubMed] [Google Scholar]
- 86.Adrian TE, Savage AP, Sagor GR, Allen JM, Bacarese-Hamilton AJ, Tatemoto K, et al. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology. 1985;89:494–499. doi: 10.1016/0016-5085(85)90442-1. [DOI] [PubMed] [Google Scholar]
- 87.Lin HC, Chey WY. Cholecystokinin and peptide YY are released by fat in either proximal or distal small intestine in dogs. Regul Pept. 2003;114:131–135. doi: 10.1016/s0167-0115(03)00115-0. [DOI] [PubMed] [Google Scholar]
- 88.Pfluger PT, Kampe J, Castaneda TR, Vahl T, D’Alessio DA, Kruthaupt T, et al. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3–36. J Clin Endocrinol Metab. 2007;92:583–588. doi: 10.1210/jc.2006-1425. [DOI] [PubMed] [Google Scholar]
- 89.Wynne K, Bloom SR. The role of oxyntomodulin and peptide tyrosine-tyrosine (PYY) in appetite control. Nat Clin Pract Endocrinol Metab. 2006;2:612–620. doi: 10.1038/ncpendmet0318. [DOI] [PubMed] [Google Scholar]
- 90.Reinehr T, Roth CL, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg. 2007;17:1571–1577. doi: 10.1007/s11695-007-9323-8. [DOI] [PubMed] [Google Scholar]
- 91.Alvarez Bartolome M, Borque M, Martinez-Sarmiento J, Aparicio E, Hernandez C, Cabrerizo L, et al. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002;12:324–327. doi: 10.1381/096089202321088084. [DOI] [PubMed] [Google Scholar]
- 92.Valderas JP, Irribarra V, Boza C, de la Cruz R, Liberona Y, Acosta AM, et al. Medical and surgical treatments for obesity have opposite effects on peptide YY and appetite: a prospective study controlled for weight loss. J Clin Endocrinol Metab. 2010;95:1069–1075. doi: 10.1210/jc.2009-0983. [DOI] [PubMed] [Google Scholar]
- 93.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 95.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 96.Riediger T, Bothe C, Becskei C, Lutz TA. Peptide YY directly inhibits ghrelin-activated neurons of the arcuate nucleus and reverses fasting-induced c-Fos expression. Neuroendocrinology. 2004;79:317–326. doi: 10.1159/000079842. [DOI] [PubMed] [Google Scholar]
- 97.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 98.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S42–S47. doi: 10.1038/sj.ijo.0801912. [DOI] [PubMed] [Google Scholar]
- 99.Naslund E, King N, Mansten S, Adner N, Holst JJ, Gutniak M, et al. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr. 2004;91:439–446. doi: 10.1079/BJN20031064. [DOI] [PubMed] [Google Scholar]
- 100.Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satietyFeffect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 101.Valverde I, Puente J, Martin-Duce A, Molina L, Lozano O, Sancho V, et al. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg. 2005;15:387–397. doi: 10.1381/0960892053576613. [DOI] [PubMed] [Google Scholar]
- 102.Morinigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–275. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- 103.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 104.Bose M, Olivan B, Teixeira J, Pi-Sunyer FX, Laferrère B. Do Incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: What are the evidence? Obes Surg. 2009;19:217–229. doi: 10.1007/s11695-008-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baldinger M, Rubin R, Wright N, Flancbaum A, Geliebter A. Change in hunger, fullness, ghrelin, PYY and GLP-1 in relation to a fixed test meal pre and post Rou-en-Y gastric bypass (RYGBS) Appetite. 2007;49:277. (abstract) [Google Scholar]
- 106.de Carvalho CP, Marin DM, de Souza AL, Pareja JC, Chaim EA, de Barros Mazon S, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg. 2009;19:313–320. doi: 10.1007/s11695-008-9678-5. [DOI] [PubMed] [Google Scholar]
- 107.Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2007;14:63–67. doi: 10.1097/MED.0b013e3280122850. [DOI] [PubMed] [Google Scholar]
- 108.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75:1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138:283–290. doi: 10.1016/j.surg.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 110.Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem. 1998;44:991–1001. [PubMed] [Google Scholar]
- 111.Kellum JM, Kuemmerle JF, O’Dorisio TM, Rayford P, Martin D, Engle K, et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763–770. doi: 10.1097/00000658-199006000-00016. discussion 770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Foschi D, Corsi F, Pisoni L, Vago T, Bevilacqua M, Asti E, et al. Plasma cholecystokinin levels after vertical banded gastroplasty: effects of an acidified meal. Obes Surg. 2004;14:644–647. doi: 10.1381/096089204323093426. [DOI] [PubMed] [Google Scholar]
- 113.Konturek JW. Cholecystokinin in the control of gastric acid and plasma gastrin and somatostatin secretion in healthy subjects and duodenal ulcer patients before and after eradication of Helicobacter pylori. J Physiol Pharmacol. 1994;45(4 Suppl 1):3–66. [PubMed] [Google Scholar]
- 114.Katsusuke S, Takeuchi T, Watanabe S, Nishiwaki H. Postprandial plasma cholecystokinin response in patients after gastrectomy and pancreatoduodenectomy. Am J Gastroenterol. 2008;81:1038–1042. [PubMed] [Google Scholar]
- 115.Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–123. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 116.Wang L, Barachina MD, Martinez V, Wei JY, Tache Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul Pept. 2000;92:79–85. doi: 10.1016/s0167-0115(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 117.Chi MM, Fan G, Fox EA. Increased short-term food satiation and sensitivity to cholecystokinin in neurotrophin-4 knock-in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1044–R1053. doi: 10.1152/ajpregu.00420.2004. [DOI] [PubMed] [Google Scholar]
- 118.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weickert MO, Mohlig M, Spranger J, Schofl C, Loeffelholz CV, Riepl RL, et al. Effects of euglycemic hyperinsulinemia and lipid infusion on circulating cholecystokinin. J Clin Endocrinol Metab. 2008;93:2328–2333. doi: 10.1210/jc.2007-2787. [DOI] [PubMed] [Google Scholar]
- 120.Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S37–S50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang MY, Lee Y, Unger RH. Novel form of lipolysis induced by leptin. J Biol Chem. 1999;274:17541–17544. doi: 10.1074/jbc.274.25.17541. [DOI] [PubMed] [Google Scholar]
- 122.Fried SK, Ricci MR, Russell CD, Laferrère B. Regulation of leptin production in humans. J Nutr. 2000;130:3127S–3131S. doi: 10.1093/jn/130.12.3127S. [DOI] [PubMed] [Google Scholar]
- 123.Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26:504–509. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- 124.Cammisotto PG, Bendayan M. Leptin secretion by white adipose tissue and gastric mucosa. Histol Histopathol. 2007;22:199–210. doi: 10.14670/HH-22.199. [DOI] [PubMed] [Google Scholar]
- 125.Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–1581. doi: 10.1210/jc.2005-2248. [DOI] [PubMed] [Google Scholar]
- 126.DePaula AL, Macedo AL, Schraibman V, Mota BR, Vencio S. Hormonal evaluation following laparoscopic treatment of type 2 diabetes mellitus patients with BMI 20–34. Surg Endosc. 2009;23:1724–1732. doi: 10.1007/s00464-008-0168-6. [DOI] [PubMed] [Google Scholar]
- 127.Pardina E, Lopez-Tejero MD, Llamas R, Catalan R, Galard R, Allende H, et al. Ghrelin and apolipoprotein AIV levels show opposite trends to leptin levels during weight loss in morbidly obese patients. Obes Surg. 2009;19:1414–1423. doi: 10.1007/s11695-008-9793-3. [DOI] [PubMed] [Google Scholar]
- 128.Trakhtenbroit MA, Leichman JG, Algahim MF, Miller CC, III, Moody FG, Lux TR, et al. Body weight, insulin resistance, and serum adipokine levels 2 years after 2 types of bariatric surgery. Am J Med. 2009;122:435–442. doi: 10.1016/j.amjmed.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kotidis EV, Koliakos G, Papavramidis TS, Papavramidis ST. The effect of biliopancreatic diversion with pylorus-preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: is there a hormonal contribution to the weight-reducing effect of this procedure? Obes Surg. 2006;16:554–559. doi: 10.1381/096089206776944940. [DOI] [PubMed] [Google Scholar]
- 131.Foschi D, Corsi F, Pisoni L, Vago T, Bevilacqua M, Trabucchi A, et al. Plasma leptin levels after vertical banded gastroplasty for morbid obesity: effects of an acidified meal. Obes Surg. 2003;13:874–878. doi: 10.1381/096089203322618696. [DOI] [PubMed] [Google Scholar]
- 132.Boozer CN, Leibel RL, Love RJ, Cha MC, Aronne LJ. Synergy of sibutramine and low-dose leptin in treatment of diet-induced obesity in rats. Metabolism. 2001;50:889–893. doi: 10.1053/meta.2001.24917. [DOI] [PubMed] [Google Scholar]
- 133.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maffeis C, Manfredi R, Trombetta M, Sordelli S, Storti M, Benuzzi T, et al. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93:2122–2128. doi: 10.1210/jc.2007-2089. [DOI] [PubMed] [Google Scholar]
- 135.Bjorntorp P. Obesity, atherosclerosis and diabetes mellitus. Verh Dtsch Ges Inn Med. 1987;93:443–448. doi: 10.1007/978-3-642-85460-6_107. [DOI] [PubMed] [Google Scholar]
- 136.Rushing PA, Lutz TA, Seeley RJ, Woods SC. Amylin and insulin interact to reduce food intake in rats. Horm Metab Res. 2000;32:62–65. doi: 10.1055/s-2007-978590. [DOI] [PubMed] [Google Scholar]
- 137.Ballantyne GH, Farkas D, Laker S, Wasielewski A. Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16:1189–1197. doi: 10.1381/096089206778392158. [DOI] [PubMed] [Google Scholar]
- 138.Dixon AF, Dixon JB, O’Brien PE. Laparoscopic adjustable gastric banding induces prolonged satiety: a randomized blind crossover study. J Clin Endocrinol Metab. 2005;90:813–819. doi: 10.1210/jc.2004-1546. [DOI] [PubMed] [Google Scholar]
- 139.Lee WJ, Lee YC, Ser KH, Chen JC, Chen SC. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–1125. doi: 10.1007/s11695-008-9457-3. [DOI] [PubMed] [Google Scholar]
- 140.Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 141.Morrow J, Gluck M, Lorence M, Flancbaum L, Geliebter A. Night eating status and influence on body weight, body image, hunger, and cortisol pre- and post-Roux-en-Y Gastric Bypass (RYGB) surgery. Eat Weight Disord. 2008;13:e96–e99. doi: 10.1007/BF03327512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kruszynska YT, Yu JG, Olefsky JM, Sobel BE. Effects of troglitazone on blood concentrations of plasminogen activator inhibitor 1 in patients with type 2 diabetes and in lean and obese normal subjects. Diabetes. 2000;49:633–639. doi: 10.2337/diabetes.49.4.633. [DOI] [PubMed] [Google Scholar]
- 143.Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M, et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124:1325–1336. doi: 10.1016/s0016-5085(03)00216-6. [DOI] [PubMed] [Google Scholar]
- 144.Chaudhri OB, Parkinson JR, Kuo YT, Druce MR, Herlihy AH, Bell JD, et al. Differential hypothalamic neuronal activation following peripheral injection of GLP-1 and oxyntomodulin in mice detected by manganese-enhanced magnetic resonance imaging. Biochem Biophys Res Commun. 2006;350:298–306. doi: 10.1016/j.bbrc.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 145.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 146.Tovar S, Nogueiras R, Tung LY, Castaneda TR, Vazquez MJ, Morris A, et al. Central administration of resistin promotes shortterm satiety in rats. Eur J Endocrinol. 2005;153:R1–R5. doi: 10.1530/eje.1.01999. [DOI] [PubMed] [Google Scholar]
- 147.Lutz TA. Pancreatic amylin as a centrally acting satiating hormone. Curr Drug Targets. 2005;6:181–189. doi: 10.2174/1389450053174596. [DOI] [PubMed] [Google Scholar]
- 148.Adrian TE, Bloom SR, Bryant MG, Polak JM, Heitz PH, Barnes AJ. Distribution and release of human pancreatic polypeptide. Gut. 1976;17:940–944. doi: 10.1136/gut.17.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schrumpf E, Bergan A, Djoseland O, Fausa O, Flaten O, Skagen DW, et al. The effect of gastric bypass operation on glucose tolerance in obesity. Scand J Gastroenterol Suppl. 1985;107:24–31. doi: 10.3109/00365528509099748. [DOI] [PubMed] [Google Scholar]
- 150.Meryn S, Stein D, Straus EW. Fasting- and meal-stimulated peptide hormone concentrations before and after gastric surgery for morbid obesity. Metabolism. 1986;35:798–802. doi: 10.1016/0026-0495(86)90218-0. [DOI] [PubMed] [Google Scholar]
- 151.Meryn S, Stein D, Straus EW. Pancreatic polypeptide, pancreatic glucagon and enteroglucagon in morbid obesity and following gastric bypass operation. Int J Obes. 1986;10:37–42. [PubMed] [Google Scholar]
- 152.Amland PF, Jorde R, Giercksky KE, Burhol PG. Diurnal GIP, PP and insulin levels in morbid obesity before and after stapled gastric partitioning with gastro-gastrostomy. Int J Obes. 1984;8:117–122. [PubMed] [Google Scholar]
- 153.Amland PF, Jorde R, Kildebo S, Burhol PG, Giercksky KE. Effects of a gastric partitioning operation for morbid obesity on the secretion of gastric inhibitory polypeptide and pancreatic polypeptide. Scand J Gastroenterol. 1984;19:857–861. [PubMed] [Google Scholar]
- 154.Civalleri D, Bloom SR, Sarson DL, Gianetta E, Bonalumi U, Friedman D, et al. Behavior of plasma pancreatic polypeptides and motilin in obese patients subjected to biliopancreatic bypass] Boll Soc Ital Biol Sper. 1980;56:1929–1935. [PubMed] [Google Scholar]
- 155.Dubrasquet M, Bataille D, Gespach C. Oxyntomodulin (glucagon-37 or bioactive enteroglucagon): a potent inhibitor of pentagastrin-stimulated acid secretion in rats. Biosci Rep. 1982;2:391–395. doi: 10.1007/BF01119301. [DOI] [PubMed] [Google Scholar]
- 156.Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88:4696–4701. doi: 10.1210/jc.2003-030421. [DOI] [PubMed] [Google Scholar]
- 157.Baldissera FG, Holst JJ, Knuhtsen S, Hilsted L, Nielsen OV. Oxyntomodulin (glicentin-(33–69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul Pept. 1988;21:151–166. doi: 10.1016/0167-0115(88)90099-7. [DOI] [PubMed] [Google Scholar]
- 158.Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142:4244–4250. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 159.Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, et al. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 160.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 161.Barry RE, Barisch J, Bray GA, Sperling MA, Morin RJ, Benfield J. Intestinal adaptation after jejunoileal bypass in man. Am J Clin Nutr. 1977;30:32–42. doi: 10.1093/ajcn/30.1.32. [DOI] [PubMed] [Google Scholar]
- 162.Holst JJ, Sorensen TI, Andersen AN, Stadil F, Andersen B, Lauritsen KB, et al. Plasma enteroglucagon after jejunoileal bypass with 3:1 or 1:3 jejunoileal ratio. Scand J Gastroenterol. 1979;14:205–207. doi: 10.3109/00365527909179871. [DOI] [PubMed] [Google Scholar]
- 163.Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ, et al. Adipokine response in diabetics and nondiabetics following the Roux-en-Y gastric bypass: a preliminary study. J Surg Res. 2007;142:295–300. doi: 10.1016/j.jss.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 164.Rajala MW, Qi Y, Patel HR, Takahashi N, Banerjee R, Pajvani UB, et al. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53:1671–1679. doi: 10.2337/diabetes.53.7.1671. [DOI] [PubMed] [Google Scholar]
- 165.Osawa H, Ochi M, Tabara Y, Kato K, Yamauchi J, Takata Y, et al. Serum resistin is positively correlated with the accumulation of metabolic syndrome factors in type 2 diabetes. Clin Endocrinol (Oxf) 2008;69:74–80. doi: 10.1111/j.1365-2265.2007.03154.x. [DOI] [PubMed] [Google Scholar]
- 166.Gokce N, Vita JA, McDonnell M, Forse AR, Istfan N, Stoeckl M, et al. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol. 2005;95:266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 167.Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ, et al. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res. 2007;141:31–39. doi: 10.1016/j.jss.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 168.Bose M, Teixeira J, Olivan B, Scherer PE, Pi-Sunyer FX, Bawa B, et al. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes. 2010;2:47–55. doi: 10.1111/j.1753-0407.2009.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cummings DE, Shannon MH. Roles for ghrelin in the regulation of appetite and body weight. Arch Surg. 2003;138:389–396. doi: 10.1001/archsurg.138.4.389. [DOI] [PubMed] [Google Scholar]
- 170.Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf) 2008;69:173–179. doi: 10.1111/j.1365-2265.2007.03164.x. [DOI] [PubMed] [Google Scholar]
- 171.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg. 1987;205:613–624. doi: 10.1097/00000658-198706000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 173.Mun EC, Blackburn GL, Matthews JB. Current status of medical and surgical therapy for obesity. Gastroenterology. 2001;120:669–681. doi: 10.1053/gast.2001.22430. [DOI] [PubMed] [Google Scholar]
- 174.Nightengale ML, Sarr MG, Kelly KA, Jensen MD, Zinsmeister AR, Palumbo PJ. Prospective evaluation of vertical banded gastroplasty as the primary operation for morbid obesity. Mayo Clin Proc. 1991;66:773–782. doi: 10.1016/s0025-6196(12)61194-x. [DOI] [PubMed] [Google Scholar]
- 175.Howard L, Malone M, Michalek A, Carter J, Alger S, VanWoert J. Gastric bypass and vertical banded gastroplasty-a prospective randomized comparison and 5-year follow-up. Obes Surg. 1995;5:55–60. doi: 10.1381/096089295765558169. [DOI] [PubMed] [Google Scholar]
- 176.Naslund I, Wickbom G, Christoffersson E, Agren G. A prospective randomized comparison of gastric bypass and gastroplasty. Complications and early results. Acta Chir Scand. 1986;152:681–689. [PubMed] [Google Scholar]
- 177.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–2796. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 178.Faraj M, Jones P, Sniderman AD, Cianflone K. Enhanced dietary fat clearance in postobese women. J Lipid Res. 2001;42:571–580. [PubMed] [Google Scholar]
- 179.MacLean LD, Rhode BM, Nohr CW. Long- or short-limb gastric bypass? J Gastrointest Surg. 2001;5:525–530. doi: 10.1016/s1091-255x(01)80091-3. [DOI] [PubMed] [Google Scholar]
- 180.Naslund I. Gastric bypass versus gastroplasty. A prospective study of differences in two surgical procedures for morbid obesity. Acta Chir Scand Suppl. 1987;536:1–60. [PubMed] [Google Scholar]
- 181.Boza C, Gagner M, Devaud N, Escalona A, Munoz R, Gandarillas M. Laparoscopic sleeve gastrectomy with ileal transposition (SGIT): a new surgical procedure as effective as gastric bypass for weight control in a porcine model. Surg Endosc. 2008;22:1029–1034. doi: 10.1007/s00464-007-9685-y. [DOI] [PubMed] [Google Scholar]
- 182.Koopmans HS, Sclafani A, Fichtner C, Aravich PF. The effects of ileal transposition on food intake and body weight loss in VMH-obese rats. Am J Clin Nutr. 1982;35:284–293. doi: 10.1093/ajcn/35.2.284. [DOI] [PubMed] [Google Scholar]
- 183.Koopmans HS, Ferri GL, Sarson DL, Polak JM, Bloom SR. The effects of ileal transposition and jejunoileal bypass on food intake and GI hormone levels in rats. Physiol Behav. 1984;33:601–609. doi: 10.1016/0031-9384(84)90378-0. [DOI] [PubMed] [Google Scholar]
- 184.de Paula AL, Macedo AL, Prudente AS, Queiroz L, Schraibman V, Pinus J. Laparoscopic sleeve gastrectomy with ileal interposition (‘neuroendocrine brake’)Fpilot study of a new operation. Surg Obes Relat Dis. 2006;2:464–467. doi: 10.1016/j.soard.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 185.Gagner M. La transposition ile’ale avec ou sans gastrectomy par laparoscopie chez l’homme (TIG) J Coeliochirurgie. 2005;54:4–9. [Google Scholar]
- 186.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 2009;17:1839–1847. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Naslund E, Hellstrom PM, Kral JG. The gut and food intake: an update for surgeons. J Gastrointest Surg. 2001;5:556–567. doi: 10.1016/s1091-255x(01)80095-0. [DOI] [PubMed] [Google Scholar]
- 189.Flancbaum L, Choban PS, Bradley LR, Burge JC. Changes in measured resting energy expenditure after Roux-en-Y gastric bypass for clinically severe obesity. Surgery. 1997;122:943–949. doi: 10.1016/s0039-6060(97)90336-6. [DOI] [PubMed] [Google Scholar]
- 190.Carrasco F, Papapietro K, Csendes A, Salazar G, Echenique C, Lisboa C, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 191.de Castro Cesar M, de Lima Montebelo MI, Rasera I, Jr, de Oliveira AV, Jr, Gomes Gonelli PR, Aparecida Cardoso G. Effects of Roux-en-Y gastric bypass on resting energy expenditure in women. Obes Surg. 2008;18:1376–1380. doi: 10.1007/s11695-008-9460-8. [DOI] [PubMed] [Google Scholar]
- 192.Xu Y, Ramos EJ, Middleton F, Romanova I, Quinn R, Chen C, et al. Gene expression profiles post Roux-en-Y gastric bypass. Surgery. 2004;136:246–252. doi: 10.1016/j.surg.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 193.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (London) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Moschen AR, Molnar C, Wolf AM, Weiss H, Graziadei I, Kaser S, et al. Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J Hepatol. 2009;51:765–777. doi: 10.1016/j.jhep.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 195.Nijhuis J, van Dielen FM, Fouraschen SM, van den Broek MA, Rensen SS, Buurman WA, et al. Endothelial activation markers and their key regulators after restrictive bariatric surgery. Obesity (Silver Spring) 2007;15:1395–1399. doi: 10.1038/oby.2007.167. [DOI] [PubMed] [Google Scholar]
- 196.Kopp HP, Krzyzanowska K, Mohlig M, Spranger J, Pfeiffer AF, Schernthaner G. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes (London) 2005;29:766–771. doi: 10.1038/sj.ijo.0802983. [DOI] [PubMed] [Google Scholar]
- 197.Roth CL, Reinehr T, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Ghrelin and obestatin levels in severely obese women before and after weight loss after Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19:29–35. doi: 10.1007/s11695-008-9568-x. [DOI] [PubMed] [Google Scholar]
- 198.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–380. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. discussion 4–5. [PubMed] [Google Scholar]