Abstract
Iron-deficiency anemia (IDA) continues to be the most common single nutrient deficiency in the world. An estimated 20-25% of the world’s infants have IDA, with at least as many having iron deficiency without anemia. Infants are at particular risk due to rapid growth and limited dietary sources of iron. We found that infants with IDA showed different motor activity patterning in all sleep-waking states and several differences in sleep states organization. Sleep alterations were still apparent years after correction of anemia with iron treatment in the absence of subsequent IDA. We suggest that altered sleep patterns may represent an underlying mechanism that interferes with optimal brain functioning during sleep and wakefulness in former IDA children.
Keywords: iron deficiency anemia, infancy, sleep, REM sleep, NREM sleep, childhood
“Gold is for the mistress-silver for the maid-copper for the craftsman cunning at his trade.” “Good!” said the Baron, sitting in his hall, but “Iron-Cold Iron-is master of them all.”
Rudyard Kipling
In memoriam to our colleague and friend John L. Beard who contributed consistently to the science in the iron/brain field.
INTRODUCTION
Iron deficiency is the single most common and highly preventable nutritional deficiency in the world. It is prevalent in most of the developing world and it is probably the only micronutrient deficiency of public health significance in industrialized countries [1-4]. It is also a major cause of anemia in infancy, childhood, and pregnancy, affecting more than 2 billion persons worldwide [2,3]. The prevalence of anemia among children less than 4 years of age is estimated to range between 46 and 66% in developing countries, and half of the anemia is thought to be iron-deficiency anemia [4].
IDA can no longer be considered simply a hematologic alteration, since there are broader systemic effects. The peak period for IDA is 6 to 24 months, when the central nervous system (CNS) is rapidly developing and highly vulnerable. This age period corresponds to the latter part of the brain growth spurt and the development of fundamental mental and motor processes. Numerous studies have shown that IDA is associated with altered behavior of infants in cognitive, motor, and socio-emotional domains, interfering thus with optimal development [5,6]. Despite correction of IDA with iron therapy, IDA in infancy is associated with long-lasting dysfunctions in these domains. Since inadequate sleep is also associated with poorer cognitive and behavioral development [7], the assessment of sleep-wake patterns is particularly relevant to understanding the effects of IDA during early human development. Sleep alterations, if persistent, could also help account for some long-lasting effects of early IDA.
In this context, the Sleep and Functional Neurobiology Laboratory of the Institute of Nutrition and Food Technology (INTA), University of Chile, as part of an ongoing collaborative NIH project with the Center for Human Growth and Development of the University of Michigan in Ann Arbor [8], has been collecting evidence that IDA infants do not follow normal neuromaturational patterns [9-15]. Sleep, in particular, has been assessed by polysomnographic recordings including EEG, cardiac, respiratory, and motor patterns, neuroendocrine profile, 24-h actigraphic recordings performed at home, and a set of questionnaires. Group comparisons (IDA vs. non-anemic controls) have been performed after controlling for demographic characteristics (anthropometric, socioeconomic, maternal characteristics, and home microenvironment). Any background characteristic that was even weakly associated with the variable studied was considered as a covariate in the corresponding analyses. The research protocols have been approved by the Institutional Review Boards of the University of Michigan Medical Center, Ann Arbor, of INTA, University of Chile, Santiago, and of the Office of Protection from Research Risks, NIH. Parental signed informed consent and child assent beginning at 10 years have been obtained.
We will first summarize findings in infancy, then ones observed with advancing ages. We finish with data from animal models that supports, at least in part, our results.
1. IDA-related sleep alterations in infancy
The quality and amount of sleep are increasingly recognized as important factors in human development, with concomitant effects on affective behavior and cognitive performance [16-18]. The organization of sleep depends on various mechanisms involving both neural and humoral processes, several of which are affected by iron deficiency. Since iron deficiency in human infants is most prevalent during the latter part of the brain growth spurt, the normal development of sleep patterns could be particularly affected.
1.1. Sleep spindles
Sleep spindles are one of the most characteristic EEG patterns during sleep and a hallmark of NREM sleep stage 2 appearance, with a known anatomic generator. They are initiated by a deep brain structure, the thalamic reticular nucleus, in connection with principal thalamic nuclei and are synchronized by corticocortical, corticothalamic, and thalamocortical loops [19]. Spindles have been postulated to be a marker of normal brain functional development and integrity, and their absence or abnormality strongly suggests cerebral dysfunction or pathology. Indeed, sleep spindle activity has been identified as abnormal in several pathological conditions, including mental retardation, deafness, hyperkinesis, prenatal or perinatal insults, children suffering from congenital hypothyroidism, PKU children, autistic children, children with generalized spike-wave discharges, and moyamoya disease [see ref 13].
As we have reported elsewhere, infants with IDA at the age of 6 months showed altered sleep spindle patterns [13]. Both NREM stage 2 and SWS were characterized by reduced spindle index, longer inter-spindle interval, and lower spindle frequency compared to non-anemic controls, without affecting spindle duration. Differences between groups could not be attributed to differences in the total amount of NREM sleep stages since they were similar in both groups. Furthermore, IDA infants did not have longer duration of the preceding waking episode. In fact, the opposite was observed. Since sleep restriction/deprivation, perhaps analogous to longer duration of waking, reduces the amount of sleep spindles in the subsequent sleep episode [20], the shorter duration of the waking episode in the IDA group would usually be associated with increased spindles amount, instead of the decreased amount we observed. Furthermore, differences in spindles frequency between groups could not be explained by their prevalence as a function of EEG derivation placement throughout the scalp (i.e., slower in more anterior localizations) [21] since sleep spindles were assessed in the same derivations. Since NREM stage 2 and SWS amounts were similar between groups, the changing prevalence of sleep spindles frequency throughout the NREM sleep episode [21] is also not an apparent explanation for differences between groups.
Sleep spindles, however, appear to do more than reflect network properties and/or promote the formation of thalamocortical networks [19,22]; they also seem to play an important role in (a) memory formation and (b) regulation of motor activity. These aspects are briefly summarized below.
1.1.a. Sleep spindles appear to provide necessary conditions for the plastic modifications underlying memory formation [23,24]. Although the functions of sleep remain largely unknown, one of the most exciting hypotheses is that sleep significantly contributes to processes of memory and brain plasticity [for review see ref. 25,26]. There is evidence that the spindles are markers for ability to learn certain kinds of tasks even during a daytime nap [27]. Moreover, in contrast to previous studies using positron emission tomography that showed decreased regional cerebral blood flow and glucose metabolism during light NREM sleep [28-30] or in relation to sigma power [31], recent results using functional MRI describe transient increased regional brain activity in association with spindles [32].
However, research to date continues to be fragmentary and has been conducted almost exclusively in adults (human or animal). Large amounts of sleep in infancy suggest that sleep may play an important role in brain maturation [33,34]. Sleep state organization, especially quiet sleep-NREM sleep, in early infancy correlates with measures of cognitive functioning and attention in later childhood and early adolescence [35]. A recent report showing a close relationship between spindles density and reading impairment in dyslexic children adds support to the hypothesis of a role for spindles in sleep-related neurocognitive processing [36]. If the connections between sleep spindles and learning also apply in infancy, it is possible that the altered patterns of sleep spindles in IDA infants restrict their cognitive and memory-related abilities and contribute to the poorer developmental outcome that is consistently observed [5,6]. Of note, the slower frequency of sleep spindles in IDA infants might deserve attention while considering recent results that bring additional support for the existence of distinct slow and fast sleep spindles with potentially different functional significance, with fast being involved in processing sensorimotor and mnemonic information [32,37-40].
1.1.b. Sleep spindles have also been associated in the past with suppression of muscle tone and regulation of phasic motor activity [41]. Production of 12-14 Hz activity during waking in specially trained cats correlated behaviorally with the suppression of movements. Facilitation of this rhythm through conditioning during wakefulness selectively increased spindles and decreased motor output during subsequent sleep, thus resulting in longer epochs of undisturbed sleep [41]. Since a stronger decrease in muscle tone implies a corresponding decrease in the degree of contraction of muscle fibers [42], we speculated [13] that the reduced sleep spindles density in IDA infants may underlie a less marked reduction in muscle tone which, in turn, might provide less consistent inhibition of motor activity during sleep [10,11]. In addition to the obvious implications for the developmental field, this aspect should also be of interest to those examining other neurological conditions, especially motor control issues during sleep. The potential relationship between sleep spindles, muscle tone, and motor activity could bring new insights regarding alterations of motor activity regulation during sleep, particularly during NREM sleep.
1.2. Sleep/wake patterns and motor activity organization in IDA infants
To our knowledge, our studies were the first to use objective quantitative methods to assess spontaneous motor activity in the laboratory and also at home for long recording periods in infants with IDA [10,11]. By using actigraphic recording data we found that 6-month-old IDA infants napped longer during the day and were more restless during sleep, with increased time awake and decreased time in QS at night. Furthermore, despite improvements in iron status after iron therapy, some differences in sleep patterns were still statistically significant [11].
A few parent-report studies have also assessed the association between iron deficiency and sleep. A study of Guatemalan toddlers did not find any relationship between anemia and sleep when measured before an intervention to reduce coffee intake [43]. In a more recent study, 6- to 18-mo-old IDA infants in Nepal and Zanzibar were reported to sleep longer and wake up at night more frequently than non-IDA infants [44]. Iron supplementation was associated with longer night-time and total sleep duration [45]. However, since the supplementation also included folic acid, it is not clear whether the reported effects on sleep could be attributed to iron and/or folate status. The results from a study of the relationship between sleep and cognitive development in infancy [46] could be relevant to understanding the impact of the altered sleep patterns observed in IDA infants. Higher motor activity during sleep and more episodes of waking were negatively correlated with both mental and psychomotor developmental indices in 10-month-old infants. Indeed, fragmented sleep patterns could impact cognition, behavior, and emotions in infants and young children, manifested through irritability, hyperactivity, short attention span, and/or low tolerance to frustration [16-18]. Finally, a recent paper based on parental reports in a group of children with attention-deficit/hyperactivity disorder showed a significant relation between plasma ferritin concentration and the score on the sleep wake transition disorders subscale of a sleep disturbance scale for children; children with lower ferritin values showed higher scores [47].
Based on the complex iron-dopamine relationship that appears to be a main factor in restless legs syndrome (RLS) pathogenesis [48], we speculated that the phenomena observed in our studies [10,11] might share similar underlying mechanisms with those responsible for RLS and/or periodic leg movement disorder of sleep (PLMs) seen later in life [49-51]. One of the most explored hypotheses for the prevalence of these motor disorders involves changes in central nervous system iron metabolism and deficits in the dopamine neurotransmitter system. Decreased availability of iron in the brain has been directly tied to decreased function of the dopaminergic system and alterations in dopamine-related behaviors in animal studies [52]. Very recently, a clear indication of dopamine pathology in RLS was revealed in an autopsy study, and the cellular regulation of dopamine production closely matched the data from cellular and animal iron insufficiency models. The findings add support for the hypothesis that a primary iron insufficiency produces a dopaminergic abnormality as part of the RLS pathology [53]. The relationship between sleep and PLMs/RLS and ADHD are covered in separate articles of this issue.
In summary, infants with IDA showed alterations in sleep patterns and motor activity organization, with some differences persisting despite timely correction of anemia with carefully supervised iron therapy [10,11,13-15].
2. PERSISTENT EFFECTS ON SLEEP IN CHILDHOOD
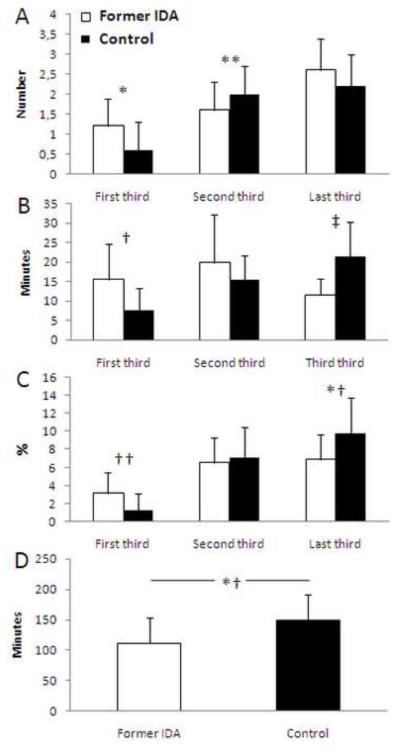
In our study, we found that 4-year-old children who had IDA in infancy showed altered sleep organization throughout the night (Fig. 1), despite adequate iron therapy in infancy [14] and absence of anemia thereafter. The pattern of REM sleep episode duration in controls showed the expected lengthening with advancing thirds of the night, whereas former IDA children did not. Compared to controls, the duration of their REM sleep episodes was longer in the first third and shorter in the last third of the night. The timing of REM sleep episodes also differed between groups. Former IDA children showed a higher number of REM sleep episodes, statistically significant in the first third and a suggestive tendency in the third, whereas they showed fewer REM sleep episodes in the second third of the night. In addition, the first sleep cycle in former IDA children differed markedly relative to controls. The latency to the first REM sleep episode was shorter, the episode tended to be longer, and the episodes of NREM2 and SWS were shorter.
Figure 1.
REM sleep characteristics for the successive thirds of the nighttime in healthy 4-year-old former iron-deficient anemic (Former IDA, white columns) and non-anemic controls (Control, black columns) children: (A) Number of REM sleep episodes, (B) Duration of REM sleep episodes, and (C) REM sleep as percentage of total sleep time. Finally, in (D) is presented the first REM sleep episode latency for both groups. Values are means ± SD; *p<0.01, **p<0.05, †p<0.007, ‡p<0.0001, †† p<0.006, *†p<0.002.
Differences in the patterning of sleep organization did not relate to the amount of sleep stages, since their percentages for the whole night were similar in both groups. Although it could be argued that daytime sleep or naps may relate to REM sleep latency, daytime differences did not appear to explain our findings. First, the longer duration of the prior waking episode in the former IDA group would usually be associated with increased SWS amount at the onset of the sleep episode, instead of the decreased SWS we observed. Indeed, SWS appears to be preferentially recovered on recovery nights following a period of sleep restriction in human subjects [54,55]. Second, daytime sleep exerts a strong inhibitory effect on the expression of SWS in the subsequent night [56,57], but the presence of naps was equally represented in the groups. Compared with children without daytime sleep, those who napped showed shorter REM sleep latency, regardless of whether they were control or former IDA children. Therefore, it is unlikely that daytime sleep played a relevant role in the differences between groups in nighttime sleep organization.
Since former IDA children did not appear to have difficulty in achieving or maintaining REM sleep, the altered REM sleep features in formerly IDA children might be an expression of a delayed developmental profile [58,59]. One possible explanation for their REM sleep features may be related to the developmental nature of IDA. For instance, in the rat it is now known that the brain region responsible for controlling sleep stages and stage shifts (the pedunculopontine of the reticular activating system) undergoes a massive neuronal reorganization during the first postnatal weeks [for review, see reference 58]. Of note, the postnatal increased amount of REM sleep has to be suppressed in favour of non-REM sleep to enable sufficient brain maturation [59]. Non-REM sleep and REM latency increase, whereas tonic and phasic REM sleep and both the number and mean duration of REM sleep episodes decrease. Indeed, a common developmental REM sleep-inhibitory process appears to be necessary to guarantee normal maturation. An incomplete development of this inhibition may predispose individuals to depression and account for lifelong REM disturbances in them [60].
This might be particularly relevant to the increase in symptoms of anxiety and depression reported in young adolescents who had chronic, severe iron deficiency in infancy [61]. Our findings of shorter latency and prolonged duration of the first REM episode, with absence of progressive lengthening of episodes duration with advancing sleep period, are reminiscent of REM sleep patterns often observed in depressive patients [62]. In fact, REM sleep changes are a major component of all animal models of depression. Increased REM pressure at the beginning of the night (shortened sleep latency) is also found in a majority of patients with acute and remitted state depression. Several studies of people at high risk for depression, including healthy relatives of patients with depression, have demonstrated that REM sleep, particularly REM density changes, are present before the disorder’s onset and could even predict its development [63]. Finally, since altered sleep patterns have long been recognized as symptomatic of many forms of neurological dysfunction (including Parkinson disease, dementia, epilepsy, stroke, demyelinating diseases, and schizophrenia) [63], changes in REM sleep may make an iron-deficient animal model relevant to some such neurological disorders.
To the best of our knowledge, there is just one other human or non-human primate study that has reported lasting effects of early IDA on a single sleep feature [64]. Former IDA children were asked to complete a 5-point rating scale for six dimensions, one of which was sleep quality. Compared to controls, they reported “more sleep disturbance (mainly insomnia).”
3. EXPERIMENTAL ANIMAL DATA
Regarding data from animal models, we would like to emphasize the following points: (i) recent research on spontaneous motor activity has been scarce, (ii) earlier studies have not assessed sleep-wake patterns or long-term effects on sleep-related issues, and (iii) animals generally became severely anemic, which could have had significant confounding effects on their health and, therefore, upon their motor activity patterns [5,6,10, 11].
A recent study reported changes in sleep-wake patterns in a mouse model of dietary iron deprivation [65]. Following weaning, mice were assigned to iron-deficient or iron-adequate dietary treatment. Their sleep-wake patterns were continuously recorded for a 48-h period when the rats were young adults. The diet was not severe enough to negatively affect growth but effective in decreasing hematocrit without inducing severe levels of anemia. The iron-deficient group showed a marked increase in the time spent awake in the 12-h dark (active) period with a reduction in NREM sleep and a suggestive tendency for less REM sleep. Differences were most marked in the last 4 hours of the dark period, the circadian time point that corresponds to the period during which RLS would maximally disturb sleep onset and progression in humans [66].
3.1. Contributions from experimental models
Rodent models provide convincing evidence of altered metabolism and neurotransmission in different brain structures, disrupted myelination processes [5,52], and altered gene and protein profiles [67,68]. Strong evidence also exists for the impact of iron deficiency on cell metabolism and morphology. For instance, in the hippocampal formation there is a decrease in neuronal metabolism, dendritic growth and arborization, and synapse formation, which are not corrected by iron repletion [5,69].
Although there is no a clear explanation why sleep alterations associated with early IDA are long-lasting nor can we rule out that some unidentified neurobiological factor(s) may account for the association, we suggest that these sleep alterations may relate to persisting modifications of the brain processes in which iron is keenly involved. Long-lasting effects of iron deficiency on the developing dopamine (DA) system [5,6,52] are a promising example of such brain processes. Neuromodulation by the DA system plays an important role in sleep regulation, including the modulation of REM sleep quality, quantity, and timing [70-72]. In turn, recent evidence suggests that REM sleep is also characterized by an increase in mesolimbic dopamine release [73]. IDA alters DA neurotransmission in specific areas of the brain, among which are those critically involved in sleep regulation [74,75]. For instance, the basal ganglia become high in iron concentration and are more highly interconnected with REM-regulatory structures in the mesopontine tegmentum than with any other brain region [76,77]. Some changes induced by early iron deficiency in the basal ganglia are not corrected with iron supplementation [5,6,52].
The dynamic balance between neurotransmitter systems is another important consideration. Dramatic changes in neurotransmitter levels are known to occur as the brain progresses through the sleep-wake cycle. The ultradian alternance of NREM sleep/REM sleep appears to be controlled by a permanent interacting balance between brainstem aminergic and cholinergic neuronal discharges [74-75]. Relevant to this issue are findings in recent iron deficiency studies in rodent models showing alterations not only in the DA system but also central serotonin and noradrenergic transporters and levels [52,78,79]. Since only some of the changes were reversible by iron treatment at weaning [5,6], the resulting IDA-induced neurotransmission imbalance could affect the fine-tuned neural mechanisms involved in the regulation of sleep states patterning. In addition, a recently described model of REM cycling involves reciprocal inhibitory interactions between brainstem gamma-aminobutyric acid (GABA)-ergic REM-off and REM-on populations as main components of the REM switch [80]. Since iron deficiency may also affect GABA-ergic transmission systems [52,79], the ongoing balance between the GABA-ergic populations may be altered as well, contributing to the altered transitions into and out of REM sleep throughout the nighttime sleep observed in former IDA children.
Another consideration is iron’s role in normal myelination. Disruptions in iron processing, storage, or availability affect myelin quantity, quality, composition and compaction, with alterations that persist even if the iron content of the myelin achieves normal levels after iron supplementation [81]. In former IDA children, slower transmission in both auditory and visual sensory systems and the slower reaction time in neurocognitive assessments are consistent with iron’s role in myelination [12]. The effects of iron deficiency on myelination might decrease the efficiency of neural signalling not only in sensory systems but also in those involved in the circuits of sleep-wake patterns regulation. It has been postulated that the neural network organization during sleep differs from that of wakefulness and can be modulated by sensory signals [82]. In turn, the sensory input to the central nervous system may be influenced by whether the organism is asleep or awake. Indeed, the continuous sensory information input to the brain during sleep may serve to modulate the brain by activity-dependent mechanisms of neural development, as has been postulated for wakefulness. Since sleep spindles appear to modulate the transmission of auditory inputs during sleep [83], the reduced spindles density observed in IDA infants could represent another potential way through which early IDA might alter the interaction between sleep and sensory pathways (at least, the auditory one). Furthermore, studies conducted in animal models have shown altered sleep architecture and modified patterns of REM sleep phasic events in demyelinating diseases, suggesting that such sleep alterations may be useful biological markers of these kinds of diseases [84]. In this respect, it could be argued that the altered pattern of REM sleep organization in former IDA children might relate to an altered myelin status.
As a final comment, we would like to point out that early IDA may not only have a direct effect on sleep itself. In the long run, early IDA may also potentially affect sleep regulatory mechanisms indirectly through altered response to other stressors and challenges. This possibility could be particularly relevant in the context of mental and behavioral disorders during childhood and adolescence. As the WHO 2001 Mental Health Report pointed out [85], such disorders are common and “very costly to society in both human and financial terms… many of these disorders can be precursors to much more disabling disorders during later life.”
CONCLUSION
Our results show that, despite iron therapy, early IDA is associated with altered short- and long-term sleep patterns. We suggest that altered sleep features may represent an underlying mechanism that interferes with optimal functioning during sleep and wakefulness in former IDA children.
ACKNOWLEDGEMENTS
We would like to express our gratitude to the children and parents whose ongoing participation made this follow-up possible. We also thank all technicians who contributed to day and/or nighttime recordings during the course of this study and drivers for providing careful transportation services to children and parents.
Statement of financial support: Grants from National Institutes of Health, Bethesda, MD, U.S.A. (R01 HD33487) and the Chilean Agency for Funding in Science and Technology (CONICYT, Fondecyt 1070668).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: risk factors and racial/ethnic disparities. Pediatrics. 2007;120(3):568–75. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- [2].Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: Global and regional burden of disease attribution to selected major risk factors. Vol 1. World Health Organization; Geneva: 2004. pp. 163–209. [Google Scholar]
- [3].Stoltzfus RJ. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. J Nutr. 2001;131(2 Suppl 2):697–700. doi: 10.1093/jn/131.2.697S. [DOI] [PubMed] [Google Scholar]
- [4].Brotanek JM, Halterman J, Auinger P, Flores G, Weitzman M. Iron deficiency, prolonged bottle-feeding, and racial/ethnic disparities in young children. Arch Pediatr Adolesc Med. 2005;159(11):1038–42. doi: 10.1001/archpedi.159.11.1038. [DOI] [PubMed] [Google Scholar]
- [5].Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(5 Suppl 2):34–43. doi: 10.1301/nr.2006.may.S34-S43. [discussion S72-91] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28(Suppl 4):560–71. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- [7].Dahl RE. The impact of inadequate sleep on children’s daytime cognitive function. Sem Pediatr Neurol. 1996;3(1):44–50. doi: 10.1016/s1071-9091(96)80028-3. [DOI] [PubMed] [Google Scholar]
- [8].Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112(4):846–54. [PubMed] [Google Scholar]
- [9].Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in young iron deficient anemic infants: delayed maturation of auditory brainstem responses. Am J Clin Nutr. 1998;68(3):683–690. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- [10].Angulo-Kinzler RM, Peirano P, Lin E, Garrido M, Lozoff B. Spontaneous motor activity in human infants with iron-deficiency anemia. Early Hum Dev. 2002;66(2):67–79. doi: 10.1016/s0378-3782(01)00238-9. [DOI] [PubMed] [Google Scholar]
- [11].Angulo-Kinzler RM, Peirano P, Lin E, Algarín C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron-deficiency anemia. Early Hum Dev. 2002;70(1-2):85–101. doi: 10.1016/s0378-3782(02)00092-0. [DOI] [PubMed] [Google Scholar]
- [12].Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res. 2003;53(2):217–23. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- [13].Peirano P, Algarín C, Garrido M, Algarín D, Lozoff B. Iron-deficiency anemia is associated with altered characteristics of sleep spindles in NREM sleep in infancy. Neurochem Res. 2007a;32(10):1665–72. doi: 10.1007/s11064-007-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peirano P, Algarín C, Garrido M, Lozoff B. Iron deficiency anemia (IDA) in infancy is associated with altered sleep states organization in childhood. Pediatr Res. 2007b;62(6):715–9. doi: 10.1203/PDR.0b013e3181586aef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Peirano P, Algarín C, Chamorro R, Reyes S, Garrido M, Durán S, et al. Sleep and neurofunctions throughout child development: lasting effects of early iron deficiency. J Pediatr Gastroenterol Nutr. 2009;48(Suppl 1):8–15. doi: 10.1097/MPG.0b013e31819773b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].O’Brien LM, Gozal D. Neurocognitive dysfunction and sleep in children: from human to rodent. Pediatr Clin North Am. 2004;51(1):187–202. doi: 10.1016/s0031-3955(03)00184-6. [DOI] [PubMed] [Google Scholar]
- [17].Davis KF, Parker KP, Montgomery GL. Sleep in infants and young children: part two: common sleep problems. J Pediatr Health Care. 2004;18(3):130–7. doi: 10.1016/s0891-5245(03)00150-0. [DOI] [PubMed] [Google Scholar]
- [18].Cao M, Guilleminault C. Sleep difficulties and behavioral outcomes in children. Arch Pediatr Adolesc Med. 2008;162(4):385–9. doi: 10.1001/archpedi.162.4.385. [DOI] [PubMed] [Google Scholar]
- [19].Steriade M, Deschênes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54(6):1473–97. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- [20].De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7(5):423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- [21].Gibbs FA, Gibbs EL. Atlas of Electroencephalography. Vol.1. Adison Wesley; Reading, MA: 1950. [Google Scholar]
- [22].Jenni OG, Borbély AA, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol Reg Integr Comp Physiol. 2004;286(3):528–38. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- [23].Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci. 1999;22(8):337–45. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- [24].Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25(41):9398–405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Walker MP, Stickgold R. Sleep, memory and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- [26].Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10(3):385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- [27].Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Münch M, et al. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26(35):8976–82. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Maquet P, Degueldre C, Delfiore G, Aerts J, Peters JM, Luxen A, et al. Functional neuroanatomy of human slow wave sleep. J Neurosci. 1997;17(8):2807–12. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–97. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- [30].Kajimura N, Uchiyama M, Takayama Y, Uchida S, Uema T, Kato M, et al. Activity of midbrain reticular formation and neocortex during the progression of human non-rapid eye movement sleep. J Neurosci. 1999;19(22):10065–73. doi: 10.1523/JNEUROSCI.19-22-10065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, et al. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J Neurosci. 1997;17(12):4800–8. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. PNAS. 2007;104(32):13164–9. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peirano P, Algarín C, Uauy R. Sleep-wake states and their regulatory mechanisms throughout early human development. J Pediatr. 2003;143(Suppl 4):70–9. doi: 10.1067/s0022-3476(03)00404-9. [DOI] [PubMed] [Google Scholar]
- [34].Peirano P, Algarín C. Sleep in brain development. Biol Res. 2007;40(4):471–8. [PubMed] [Google Scholar]
- [35].Parmelee AH, Sigman M, Garbanati J. Neonatal electroencephalographic organization and attention in early adolescence. In: Dawson G, Fischer KW, editors. Human Behavior and the Developing Brain. Guilford; New York: 1994. pp. 537–54. [Google Scholar]
- [36].Bruni O, Ferri R, Novelli L, Terribili M, Troianiello M, Finotti E, et al. Sleep spindle activity is correlated with reading abilities in developmental dyslexia. Sleep. 2009;32(10):1333–40. doi: 10.1093/sleep/32.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schabus M, Gruber G, Parapatics S, Sauter C, Klösch G, Anderer P, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- [38].Schabus M, Hoedlmoser K, Pecherstorfer T, Anderer P, Gruber G, Parapatics S, et al. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–35. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13-15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31(2):204–11. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tamaki M, Matsuoka T, Nittono H, Hori T. Activation of fast sleep spindles at the premotor cortex and parietal areas contributes to motor learning: a study using sLORETA. Clin Neurophysiol. 2009;120(5):878–86. doi: 10.1016/j.clinph.2009.03.006. [DOI] [PubMed] [Google Scholar]
- [41].Sterman MB, Howe RC, Macdonald LR. Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science. 1970;167(921):1146–8. doi: 10.1126/science.167.3921.1146. [DOI] [PubMed] [Google Scholar]
- [42].Chase M, Morales FR. Control of motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th edition Elsevier Saunders; Philadelphia: 2005. pp. 154–68. [Google Scholar]
- [43].Engle PL, VasDias T, Howard I, Romero-Abal ME, Quan de Serrano J, Bulux J, et al. Effects of discontinuing coffee intake on iron deficient Guatemalan toddlers’ cognitive development and sleep. Early Hum Dev. 1999;53(3):251–69. doi: 10.1016/s0378-3782(98)00080-2. [DOI] [PubMed] [Google Scholar]
- [44].Kordas K, Siegel EH, Olney DK, Katz J, Tielsch JM, Chwaya HM, et al. Maternal reports of sleep in 6-18 month-old infants from Nepal and Zanzibar: Association with iron deficiency anemia and stunting. Early Hum Dev. 2008;84(6):389–98. doi: 10.1016/j.earlhumdev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- [45].Kordas K, Siegel EH, Olney DK, Katz J, Tielsch JM, Kariger PK, et al. The effects of iron and/or zinc supplementation on maternal reports of sleep in infants from Nepal and Zanziba. J Dev Behav Pediatr. 2009;30(2):131–9. doi: 10.1097/DBP.0b013e31819e6a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Scher A. Infant sleep at 10 months of age as a window to cognitive development. Early Hum Dev. 2005;81(3):289–92. doi: 10.1016/j.earlhumdev.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [47].Cortese S, Konofal E, Bernardina BD, Mouren MC, Lecendreux M. Sleep disturbances and serum ferritin levels in children with attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2009;18(7):393–9. doi: 10.1007/s00787-009-0746-8. [DOI] [PubMed] [Google Scholar]
- [48].Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56(2):263–5. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- [49].Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26(6):735–8. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]
- [50].Konofal E, Cortese S, Marchand M, Mouren MC, Arnulf I, Lecendreux M. Impact of restless legs syndrome and iron deficiency on attention-deficit/hyperactivity disorder in children. Sleep Med. 2007;8(7-8):711–5. doi: 10.1016/j.sleep.2007.04.022. [DOI] [PubMed] [Google Scholar]
- [51].Picchietti MA, Picchietti DL. Restless legs syndrome and periodic limb movement disorder in children and adolescents. Semin Pediatr Neurol. 2008;15(2):91–9. doi: 10.1016/j.spen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- [52].Beard JL, Connor JR. Iron status and neural functioning. Ann Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- [53].Connor JR, Xin-Sheng W, Allen RP, Beard JL, Wiesinger JA, Felt BT, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132(Pt 9):2403–12. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gillberg M, Akerstedt T. Sleep restriction and SWS-suppression: effects on daytime alertness and night-time recovery. J Sleep Res. 1994;3(3):144–51. doi: 10.1111/j.1365-2869.1994.tb00121.x. [DOI] [PubMed] [Google Scholar]
- [55].Ferrara M, De Gennaro L, Bertini M. Selective slow-wave sleep (SWS) deprivation and SWS rebound: do we need a fixed SWS amount per night? Sleep Res Online. 1999;2(1):15–9. [PubMed] [Google Scholar]
- [56].Karacan I, Williams RL, Finley WW, Hursch CJ. The effects of naps on nocturnal sleep: influence on the need for stage-1 REM and stage 4 sleep. Biol Psychiat. 1970;2(11):391–9. [PubMed] [Google Scholar]
- [57].Feinberg I, March JD, Floyd TC, Jimison R, Bossom-Demitrack L, Katz PH. Homeostatic changes during post-nap sleep maintain baseline levels of delta EEG. Electroencephalogr clin Neurophysiol. 1985;61(2):134–7. doi: 10.1016/0013-4694(85)91051-x. [DOI] [PubMed] [Google Scholar]
- [58].Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31(5):673–90. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69(1-2):1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- [60].Vogel GW, Feng P, Kinney GG. Ontogeny of REM sleep in rats: possible implications for endogenous depression. Physiol Behav. 2000;68(4):453–61. doi: 10.1016/s0031-9384(99)00207-3. [DOI] [PubMed] [Google Scholar]
- [61].Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105(4):e51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- [62].Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–68. doi: 10.1001/archpsyc.1992.01820080059010. [discussion 669-70] [DOI] [PubMed] [Google Scholar]
- [63].Modell S, Lauer CJ. Rapid eye movement (REM) sleep: an endophenotype for depression. Cur Psychiat Rep. 2007;9(6):480–5. doi: 10.1007/s11920-007-0065-z. [DOI] [PubMed] [Google Scholar]
- [64].Yehuda S, Yehuda M. Long lasting effects of infancy iron deficiency – Preliminary results. J Neural Transm. 2006;71(Suppl):197–200. doi: 10.1007/978-3-211-33328-0_20. [DOI] [PubMed] [Google Scholar]
- [65].Dean T, Jr, Allen RP, O’Donnell P, Earley CJ. The effects of dietary iron deprivation on murine circadian sleep architecture. Sleep Med. 2006;7(8):634–40. doi: 10.1016/j.sleep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [66].Hening WA, Walters AS, Wagner M, Rosen R, Chen V, Kim S, et al. Circadian rhythm of motor restlessness and sensory symptoms in the idiopathic restless legs syndrome. Sleep. 1999;22(7):901–12. doi: 10.1093/sleep/22.7.901. [DOI] [PubMed] [Google Scholar]
- [67].Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl. 2006;71:173–96. doi: 10.1007/978-3-211-33328-0_19. [DOI] [PubMed] [Google Scholar]
- [68].Wang X, Wiesinger J, Beard J, Felt B, Menzies S, Earley C, et al. Thy1 expression in the brain is affected by iron and is decreased in restless legs syndrome. J Neurol Sci. 2004;220(1-2):59–66. doi: 10.1016/j.jns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- [69].Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133(10):3215–21. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- [70].Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, et al. Dopaminergic control of sleep-wake status. J Neuroscience. 2006;26(41):10577–89. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11(2):113–33. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- [72].Crochet S, Sakai K. Dopaminergic modulation of behavioural states in mesopontine tegmentum: a reverse microdialysis study in freely moving cats. Sleep. 2003;26(7):801–6. doi: 10.1093/sleep/26.7.801. [DOI] [PubMed] [Google Scholar]
- [73].Lena I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81(6):891–9. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- [74].Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21(6):482–93. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- [75].McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8(4):302–30. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- [76].Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20(9):757–88. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- [77].Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27(10):585–8. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- [78].Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171(2):261–70. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, et al. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci. 2005;8(1):31–8. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- [80].Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- [81].Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57(5):467–78. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- [82].Velluti RA. Interactions between sleep and sensory physiology. J Sleep Res. 1997;6(2):61–77. doi: 10.1046/j.1365-2869.1997.00031.x. [DOI] [PubMed] [Google Scholar]
- [83].Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron. 2000;28(3):991–9. doi: 10.1016/s0896-6273(00)00169-0. [DOI] [PubMed] [Google Scholar]
- [84].Anch AM, Laposky AD. Rat sleep and eye movement density as biological markers of demyelinating disease. Physiol Behav. 2000;71(3-4):269–75. doi: 10.1016/s0031-9384(00)00328-0. [DOI] [PubMed] [Google Scholar]
- [85].Saraceno B. The WHO World Health Report 2001 on mental health. Epidemiol Psychiatr Soc. 2002;11(2):83–7. doi: 10.1017/s1121189x00005546. [DOI] [PubMed] [Google Scholar]