Abstract
The importance of mechanical signals during embryogenesis and development, through both intercellular and extracellular signals, are coming into focus. It is widely hypothesized that physical forces help to guide the shape, cellular differentiation and the patterning of tissues. To test these ideas many classical engineering principles and imaging technologies are being adapted. Recent advances in microscopy, mechanical testing and genetic and pharmacological techniques, alongside computational models are helping to dissect the activity of mechanical signals in development at the cellular and molecular level. These inroads are permitting the study of mechanical changes in tissue structure and stiffness, and will provide deeper insights into the role of mechanics in both developmental biology and disease.
Keywords: deformation, cell shape change, tissue and cellular mechanics, stress, traction, modulus, elasticity, viscoelasticity
Introduction
Quantitative descriptions of motion and deformation are the foundation for any biomechanical analysis of morphogenesis. These descriptions of structures and their deformations provide a framework to understand the physics of biological structures as they react to force and mechanical stress. Optical microscopy and other imaging techniques are the core elements of devices that probe mechanical properties of materials, providing researchers with the ability to apply defined forces or deformations to biological samples and to investigate the transmission of mechanical strain and stress throughout a tissue. Imaging can provide precise descriptions of variations in tissue structure but can also serve as a tool to both interrogate gene expression and manipulate protein function. The use of light-based tools to manipulate signaling pathways and the use of fluorescence-based biosensors are revolutionizing the field of biomechanics providing for the first time the ability to manipulate mechanics and stimulate molecular signaling pathways while simultaneously measuring the response of embryonic tissues.
Whereas simple physical models can be used to describe forces and deformation acting on simple materials and structures (see Box), it is more challenging to formulate and test predictive models that include the heterogeneous and dynamic environments of cells, tissues and developing embryos. The mechanics of these complex biological structures can include both viscous and elastic properties [2]. Multidisciplinary efforts from a number of groups have developed new techniques to study these complex mechanical structures. These efforts have driven improvements in understanding regulation and consequences of single cell mechanics [3, 4], the role of tissue-level mechanics in disease [5, 6], the role of mechanics during morphogenesis [7-9] and the influence of cell and tissue mechanics on embryonic patterning [10-13]. A combination of modeling and experimental measurements of bulk properties, along with high-resolution analyses using confocal imaging and mechanical micromanipulation are helping to elucidate the role of mechanics during development. Ultimately, efforts to understand the evolution of embryonic shape from the elementary principles of mechanics will need to consider dynamic developmental programs that include heterogeneous mechanical properties of cells and tissues and how those developmental programs are influenced by differentiation and feedback signals.
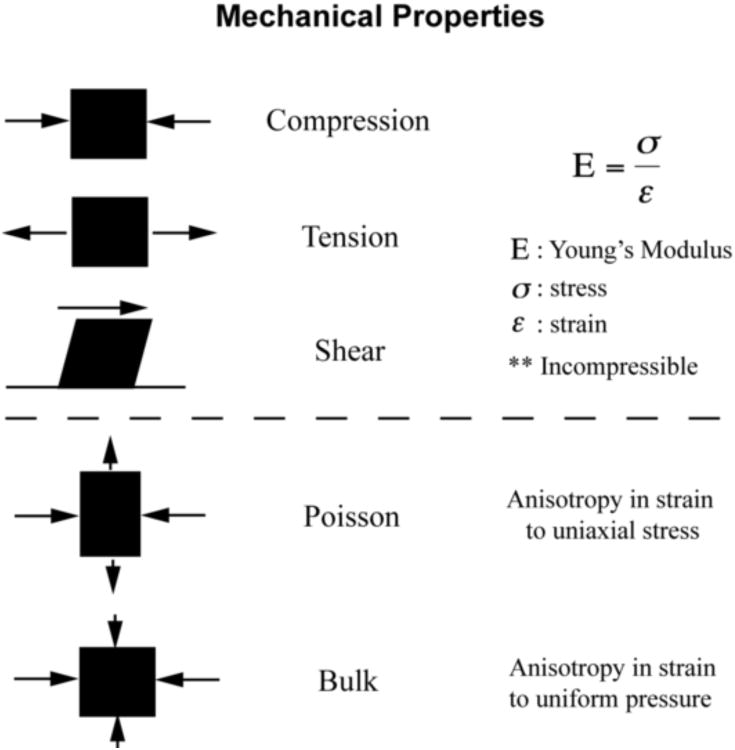
Box.
Mechanical properties are described by deformation in response to forces applied over a surface (stress (σ)). The descriptive moduli of compression, tension and shear are determined from the strains (ε) observed in response to applied stress. The poisson ratio and bulk modulus indicate the compressibility of materials under stress.
In this review we present the role of optical techniques in biomechanics, survey less familiar imaging modalities and highlight recent advances in manipulating mechanics and controlling signaling pathways relevant to studies of morphogenesis and development.
Mechanical measurements of tissue and cellular components
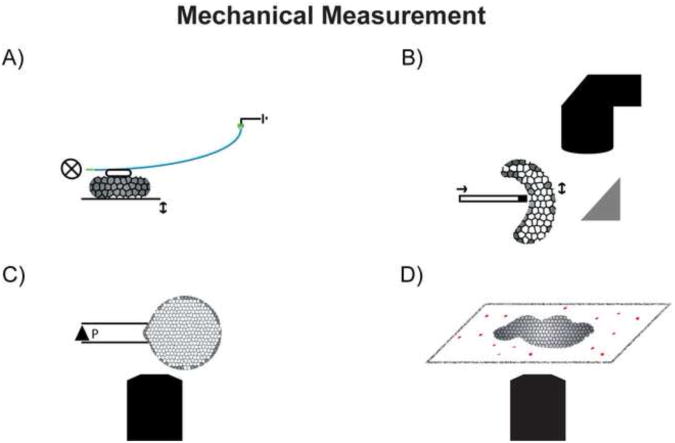
Optical techniques are key elements of biomechanical measurements and are critically important to interpreting experimental results. One of the first direct mechanical tests to be scaled and modified to tissue studies uses a simple deflecting beam to compress isolated embryonic tissues [14]. The deflecting beam consists of an optical fiber whose lateral displacement, measured by a sensitive quadrant detector, acts as the spring to apply a defined force to the face of regularly shaped tissue explants (figure 1a). This biomechanical test, an example of uniaxial compression, is one of the simplest to interpret. Spring-and-dashpot physical models permit the description of mechanical and viscoelastic attributes of embryonic tissues in terms of a time-dependent Young's modulus. Viscoelastic properties of tissue can be correlated with their tissue-scale anatomy and cellular microstructure to provide clues on how the genome controls the mechanics of morphogenesis.
Figure 1.
Mechanical testing of biological samples requires sensitive measurement tools that are adaptable to physiological environments. A) An optical fiber can act as a spring in a simple compression test, with applied force recorded by a quadtrant detector as the fiber deflects. B) Another variant on a compression test relies on image tracking of local tissue deformation in response to an indenter. Measurements across the surface of the tissue are used to map spatial heterogeneity in mechanical properties. C) Surface tension or membrane stiffness can be measured by aspirating a portion of the embryo into a capillary and measuring the distance the tissue moves into the channel. D) Traction forces can be measured by tracking displacement of a compliant substrate, typically ecm coated polyacrylamide gels with imbedded microspheres.
Another technique uses tissue indentation to map spatial heterogeneity in developing tissues. For this technique, the position of a blunt capillary tip is moved linearly with a piezoelectric crystal. Movement of the tip occurs when a voltage is placed across the crystal. As the tip pushes into the tissue with a constant force the depth of the indentation can be resolved by tracking the capillary to provide a local measurement of the compression modulus (Figure 1b). Spatial or temporal heterogeneity within the tissue can be mapped by recording the modulus at multiple positions or times. One example of indentation demonstrated a change in relative stiffness as the primitive chick heart tube is shaped [15]. These indentation-based techniques provide many advantages for the non-destructive measurement and mapping of mechanical properties over complex tissue topologies.
Using indentation on a much finer scale, atomic force microscopy (AFM) can be used to map topology and stiffness-like properties [16] and can also probe chemical composition with functionalized indenting tips. Functionally derivatized tips are capable of spatially probing the surface mechanics, molecular organization and chemical activity of multicellular tissues. These tips have been useful in studying the binding energy of specific proteins [17] and cellular adhesion forces [18]. Such tips can also mechanically stimulate cells on a very fine scale[19].
Biological measurements of tensile and shear stress are possible using uniaxial tension or micro-aspiration techniques (Figure 1c). Simple tensile tests have been conducted whereby a tissue fragment is stretched between two parallel wires [20]. Alternative biophysical descriptions for the regulation of surface tension by cell-cell adhesions have been theorized for many years. The differential adhesion hypothesis has found support in microscopically observed phenomena such as cell sorting, aggregation and engulfment studies. Such interfacial phenomena have been observed in the forming Drosophila eye [21] but can also be recreated by a number of biophysical and biomechanical processes such as differential contractility [18, 22, 23] and compound mechanical structures [24, 25]. Novel biomechanical approaches will be needed to visualize cells movements as shear stresses are controllably applied to resolve the roles of adhesion and contraction in shaping embryonic tissues.
Microscopy and imaging
Central to any biomechanical analysis of morphogenesis is the description of embryonic anatomy including the size and position of various tissues and cells and their composition. Microscopy techniques of varied resolving powers, contrast mechanisms and stress-inducing methods have been essential to studies of embryo mechanics. Stereoscopes offer simplicity in optics and a resolving power that is sufficient to many studies of gross tissue deformation [26] and mechanical anisotropy [27]. The compound microscope increases resolving power to offer sub cellular resolutions, but lacks broad field of view and depth of focus, although technological efforts are underway to address these shortcomings. Both stereo and compound designs are compatible with two of the most versatile contrast methods in biological research: white light and fluorescence. White light is robust and powerful at resolving changes in pigmentation and refractive index. The contrast that these tools provide can be complemented with fluorescent probes that allow the localization of genetic markers, cellular structures and individual proteins, and can provide physiological information about the chemical microenvironment.
Fluorescent probes can provide considerably more information than basic localization studies. Förster resonance energy transfer [28, 29] and polarization anisotropy [30] permit direct observation of changes in protein structure or higher order complex formation under mechanical perturbation. The emission characteristics of endogenous fluorophores [31] can be used to reveal metabolic states [32]. Structural proteins such as tubulin, collagen and myosin may exhibit scattering effects and elicit second harmonic generation [33-35]. Combined with genetic and pharmacologic studies, optical techniques are now being directed at mapping stress-strain fields and protein dynamics involved in tissue-level and sub cellular phenomena.
Localized tissue and cellular mechanics
Dynamic cell- and sub-cellular measurements of stress and force production are being realized through high-resolution microscopy approaches. Traction forces directed onto deformable substrates are obtained from tracking the strain fields of fluorescent beads imbedded within a thin gel of known stiffness (Figure 1d) [36]. Traction forces analyzed from explanted Xenopus tissues reveal higher contractility related to the depolymerization of microtubules and activation of a Rho-GEF, which also translates to a stiffening of the bulk tissue properties [37]. Traction forces adding to the assembly of a fibronectin matrix have been noted to increase in the presence of increased tension [38]. Measurements from force generation at the edges of bulk tissue have extrapolated the theory of traction force to tissue growth in three dimensions [39]. In addition to the traction forces, intrinsic or exogenous contrast may be analyzed together to map internal molecular, physiological, or metabolic heterogeneities from cellular to molecular scales.
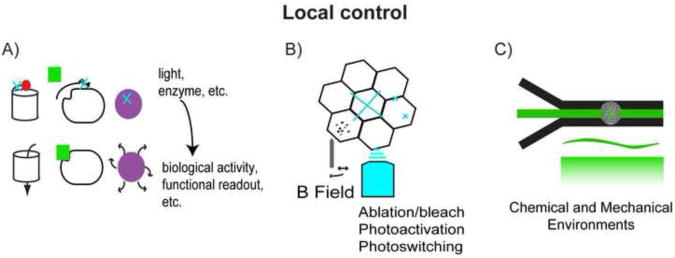
The status of signaling pathways and the composition of large multi-protein complexes such as focal adhesions [40] are critical to understanding the cellular and molecular response to mechanical stimulation and the capacity of multi-cellular tissues to transmit force. Biosensors have been used for many years to directly study metabolism, pH and calcium dynamics within developing embryos. Novel biosensors developed for cultured cell studies may be used to probe the activity of signal transduction pathways [41], intramolecular strain [42, 43] and protein complex formation [44] in vivo. In addition to the development of tools for laser ablation or “drilling” (discussed below), a variety of optically-triggered reagents have been developed to control or perturb intracellular signaling pathways, including: optically activated proteins [45-47], photoconvertable fluorophores [48] and ablation [49, 50](Figure 2a). New options to control molecular signaling pathways and interrogate their status in live cells will allow studies into the coupling between physiological and biomechanical processes.
Figure 2.
A variety of techniques can control the local physiological environment at the tissue, cellular and molecular scales. A) Biosensors amenable to optical manipulation have been designed to open membrane channels, activate signaling pathways or release apoptotic signals. B) Localized laser ablation can induce changes in biosensors and can be used to cause local injury. Additionally, magnetic tweezers can be used to locally distort the tissue. C) The chemical and mechanical environment can be manipulated in a microfluidic culture system.
Ablation studies allow further control over the mechanical microenvironment through disruption of multicellular arrays, down to the cell membrane and underlying cytoskeleton, while permitting researchers to monitor the effects on fluorescently tagged proteins and structures. Laser induced injuries have recently been used to study the recruitment of proteins and dynamic remodeling of the cytoskeleton in single cell wounds [51] and to study the tissue-mechanical responses to whole cell or cell-boundary ablation [52](Figure 2b). In a multicellular embryo, stresses transmitted along cell-cell junctions have been studied by ablation of a membrane region and monitoring morphology of the surrounding response [53-57]. In mammalian cells, single actin stress fibers have been ablated to study the unstressed fiber relaxation and subsequent mechanical response at the substrate [58]. Likewise, magnetic tweezers have shown promise as a local modulator of the tissue environment [59] (Figure 2b). The cross correlation of signals from fluorescence channels with local manipulation provides a rich set of inputs to test and refine mechanical models of epithelial morphogenesis.
Mechanics, computation and modeling
Computational approaches lend a unique set of tools for biomechanical analysis, as they provide the means to understand experimental observations and to direct new mechanical studies. For example, the coordinated movements of mesodermal and ectodermal cells during Drosophila gastrulation could only be observed through large scale cell tracking and computational analysis [60]. Given the challenge of direct biomechanical analyses, computational studies are attempting to infer forces from image data representing estimates of mechanical strains alone. Large-scale cell tracking has been use to discern between shape changes and intercalation, demonstrating tissue specific differences in the modes of convergence and extension [61]. In a related approach, tissue deformation patterns during invagination of the ventral furrow in Drosophila have been investigated in the context of local cell strain maps determined from cell boundaries collected using time-lapse confocal microscopy. This technique, referred to as video force microscopy, reveals that spatiotemporally complex forces are needed to drive invagination; high forces must act within the apical surface of the mesoderm and lower forces must be present within the basal ectoderm. However, the assumption of homogeneous and static mechanical properties are essential elements of the core model fit by the algorithm [62]. Such hybrid analyses combining computer-simulated mechanical models with image data are helping to decipher specific protein activities and the feedback nature of select signaling pathways [63-67].
Is there a role for mechanotransduction in vivo?
Cellular studies suggest a role for mechanotransduction during progression of diseases such as cancer [68] and heart disease [69] and during development. These studies suggest that morphogenesis may integrate a wide repertoire of responses to mechanical stimulation. However, clean experimental tests of mechanotransduction in the embryo and tissue isolates have been difficult. Such tests require both precisely controlled mechanical stimulation and simultaneous observation of signaling and cell mechanical responses. Several recent studies have applied sophisticated image based segmentation and quantitative analysis and have begun to dissect the relative contribution of signaling and mechanics. For instance, contractile pulses of myosin activity and adherens junction remodeling coordinate furrow formation during Drosophila gastrulation [70, 71]. Another study in Xenopus found that extracellular fibronectin matrix assembly is governed by tension via a complex pathway involving both cadherin and Wnt mediated signals [38]. It is believed that interplay between fibronectin, cadherins, and cytoskeleton elements help to establish long distance force transduction and sensing [37, 72], but the relationships and associated forces have been difficult to accurately decipher.
Testing putative mechanotransduction pathways may be possible by harnessing the properties of laminar flow to control both the chemical and mechanical microenvironment of developing embryos. Microfluidic streams have been used to control temperature [73], study translocation of sub cellular components [74] and deliver signaling factors [75, 76](Figure 2c). Careful design of microfluidic culture systems may allow paracrine and autocrine signaling loops and gradients of exogenous factors to be differentially studied [77, 78]. Since microfluidic chambers can be mounted on conventional compound microscopes, a combination of precise fluidic control and light-based manipulations can simultaneously modulate the tissue environment. Combined molecular manipulation, micromechanical stimulation and image-based interrogation of signaling pathways now provide the essential tools for future biomechanical studies.
Conclusion
Imaging tools and techniques to study the biomechanics of morphogenesis and tissue assembly are poised to advance our understanding of the inside-out and outside-in signaling pathways active during development and disease. Advanced microscopy has been and will continue to be critically important for new discoveries. To dissect the coupling between biology and mechanics requires tools to measure and control mechanical attributes and sub cellular protein activities. As our understanding of the coupling between biology and mechanics advances, mechanical and computational models will become more descriptive and predictive allowing a clearer vision of the role of mechanical processes in biological signaling and development.
Acknowledgments
We would like to apologize in advance to colleagues whose work we did not have the room to cite due to space constraints. This work was supported by grants from the NIH (R01-HD044750 and R21-ES019259) and the NSF (IOS-0845775).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vogel S. Comparative biomechanics: life's physical world. 1st. Vol. 550 Princeton: Princeton University Press; 2003. [Google Scholar]
- 2.Findley WN, Lai JS, Onaran K. Creep and relaxation of nonlinear viscoelastic materials: With an introduction to linear viscoelasticity. Amsterdam: North-Holland Publishing Company; 1976. [Google Scholar]
- 3.Wilson CA, Tsuchida MA, Allen GM, Barnhart EL, Applegate KT, Yam PT, Ji L, Keren K, Danuser G, Theriot JA. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465(7296):373–7. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Lockwood GA, Martin LJ, Knight JA, Byng JW. Mammographic densities and breast cancer risk. Breast disease. 1998;10(3):113–126. doi: 10.3233/bd-1998-103-412. [DOI] [PubMed] [Google Scholar]
- 6.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson DAW. On Growth and Form. 1st. Cambridge University Press; 1917. [Google Scholar]
- 8.Trinkaus JP. Cells into Organs: the forces that shape the embryo. Second. Englewood Cliffs: Prentice-Hall Inc; 1984. [Google Scholar]
- 9.Koehl MAR. Biomechanical approaches to morphogenesis. Seminars in Developmental Biology. 1990;1:367–78. [Google Scholar]
- 10.Keller R, Danilchik M. Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development. 1988;103(1):193–209. doi: 10.1242/dev.103.1.193. [DOI] [PubMed] [Google Scholar]
- 11.Taber LA, Keller BB, Clark EB. Cardiac mechanics in the stage-16 chick embryo. J Biomech Eng. 1992;114(4):427–34. doi: 10.1115/1.2894091. [DOI] [PubMed] [Google Scholar]
- 12**.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10(1):34–43. doi: 10.1038/nrm2592. Excellent review surveying mechanics from cells to development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson LA, von Dassow M. Multi-scale mechanics from molecules to morphogenesis. International Journal of Biochemistry and Cell Biology. 2009;41:2147–2162. doi: 10.1016/j.biocel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore SW. A fiber optic system for measuring dynamic mechanical properties of embryonic tissues. IEEE Trans Biomed Eng. 1994;41(1):45–50. doi: 10.1109/10.277270. [DOI] [PubMed] [Google Scholar]
- 15.Zamir EA, Srinivasan V, Perucchio R, Taber LA. Mechanical asymmetry in the embryonic chick heart during looping. Ann Biomed Eng. 2003;31(11):1327–36. doi: 10.1114/1.1623487. [DOI] [PubMed] [Google Scholar]
- 16.Wu HW, Kuhn T, Moy VT. Mechanical properties of L929 cells measured by atomic force microscopy: effects of anticytoskeletal drugs and membrane crosslinking. Scanning. 1998;20(5):389–97. doi: 10.1002/sca.1998.4950200504. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner W, Hinterdorfer P, Ness W, Raab A, Vestweber D, Schindler H, Drenckhahn D. Cadherin interaction probed by atomic force microscopy. Proc Natl Acad Sci U S A. 2000;97(8):4005–10. doi: 10.1073/pnas.070052697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puech PH, Taubenberger A, Ulrich F, Krieg M, Muller DJ, Heisenberg CP. Measuring cell adhesion forces of primary gastrulating cells from zebrafish using atomic force microscopy. J Cell Sci. 2005;118(Pt 18):4199–206. doi: 10.1242/jcs.02547. [DOI] [PubMed] [Google Scholar]
- 19.Upadhye KV, Candiello JE, Davidson LA, Lin H. Whole-cell electrical activity under direct mechanical stimulus by AFM cantilever using planar patch clamp chip approach. Cellular and Molecular Bioengineering. 2011;4(2):270–280. doi: 10.1007/s12195-011-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiebe C, Brodland GW. Tensile properties of embryonic epithelia measured using a novel instrument. J Biomech. 2005;38(10):2087–94. doi: 10.1016/j.jbiomech.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431(7009):647–52. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- 22.Harris AK. Is Cell sorting caused by differences in the work of intercellular adhesion? A critique of the Steinberg hypothesis. J Theor Biol. 1976;61(2):267–85. doi: 10.1016/0022-5193(76)90019-9. [DOI] [PubMed] [Google Scholar]
- 23.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10(4):429–36. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 24.Luu O, David R, Ninomiya H, Winklbauer R. Large-scale mechanical properties of Xenopus embryonic epithelium. Proc Natl Acad Sci U S A. 2011;108(10):4000–5. doi: 10.1073/pnas.1010331108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Dassow M, Strother JA, Davidson LA. Surprisingly simple mechanical behavior of a complex embryonic tissue. PLoS One. 2010;5(15):e15359. doi: 10.1371/journal.pone.0015359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling P, Taber LA, Humphrey JD. Approach to quantify the mechanical behavior of the intact embryonic chick heart. Ann Biomed Eng. 2002;30(5):636–45. doi: 10.1114/1.1483080. [DOI] [PubMed] [Google Scholar]
- 27.Sorzano CO, Thevenaz P, Unser M. Elastic registration of biological images using vector-spline regularization. IEEE Trans Biomed Eng. 2005;52(4):652–63. doi: 10.1109/TBME.2005.844030. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Puhl HL, 3rd, Koushik SV, Vogel SS, Ikeda SR. Measurement of FRET efficiency and ratio of donor to acceptor concentration in living cells. Biophys J. 2006;91(5):L39–41. doi: 10.1529/biophysj.106.088773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–46. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- 30.Thaler C, Koushik SV, Puhl HL, 3rd, Blank PS, Vogel SS. Structural rearrangement of CaMKIIalpha catalytic domains encodes activation. Proc Natl Acad Sci U S A. 2009;106(15):6369–74. doi: 10.1073/pnas.0901913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A. 2003;100(12):7075–80. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skala MC, Squirrell JM, Vrotsos KM, Eickhoff JC, Gendron-Fitzpatrick A, Eliceiri KW, Ramanujam N. Multiphoton microscopy of endogenous fluorescence differentiates normal, precancerous, and cancerous squamous epithelial tissues. Cancer research. 2005;65(4):1180. doi: 10.1158/0008-5472.CAN-04-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth S, Freund I. Second harmonic generation in collagen. The Journal of chemical physics. 1979;70(Journal Article):1637. [Google Scholar]
- 34.Campagnola PJ, Loew LM. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nature biotechnology. 2003;21(11):1356–1360. doi: 10.1038/nbt894. [DOI] [PubMed] [Google Scholar]
- 35.Plotnikov SV, Millard AC, Campagnola PJ, Mohler WA. Characterization of the myosin-based source for second-harmonic generation from muscle sarcomeres. Biophysical journal. 2006;90(2):693–703. doi: 10.1529/biophysj.105.071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelham RJ, Jr, Wang Y. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol Biol Cell. 1999;10(4):935–45. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Zhou J, Kim HY, Wang JH, Davidson LA. Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development. 2010;137(16):2785–94. doi: 10.1242/dev.045997. A molecular analysis from traction force to explant viscoelasticity. A variety of image modalities are used to investigate the role of microtubules and Rho GTPases in the mechanics of morphogenisis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev Cell. 2009;16(3):421–32. doi: 10.1016/j.devcel.2009.01.008. Analysis of the mechanical aspects of cadherin wnt pathways by traction force microscopy, as well as an elegant approach to create mechanical stress for studying downstream fibronectin deposition and morphology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon VD, Valentine MT, Gardel ML, Andor-Ardo D, Dennison S, Bogdanov AA, Weitz DA, Deisboeck TS. Measuring the mechanical stress induced by an expanding multicellular tumor system: a case study. Exp Cell Res. 2003;289(1):58–66. doi: 10.1016/s0014-4827(03)00256-8. [DOI] [PubMed] [Google Scholar]
- 40.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468(7323):580–4. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461(7260):99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci U S A. 2002;99(8):5139–43. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466(7303):263–6. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerppola TK. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat Protoc. 2006;1(3):1278–86. doi: 10.1038/nprot.2006.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446(7136):633–9. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 46**.Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 2010;12(6):591–7. doi: 10.1038/ncb2061. Light mediated activation of motility in development demonstrating migration barriers to the guidance of cells during migration. Provides an in vivo demonstration for a novel class of photoactivatable reagents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461(7260):104–8. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci U S A. 2002;99(20):12651–6. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24(1):95–9. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 50*.Monier B, Pelissier-Monier A, Brand AH, Sanson B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol. 2010;12(1):60–1. doi: 10.1038/ncb2005. sup pp 1-9. CALI study that uses GFP-MRLC as the photosensitizer. Localized laser ablation of this biosensor causes inhibition of myosoin activity and demonstrates that acto-myosin is responsible for segregating cells into compartments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168(3):429–39. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joshi SD, von Dassow M, Davidson LA. Experimental control of excitatble embryonic tissues: three stimuli induce rapid epithelial contraction. Experimental Cell Research. 2010;316(1):103–114. doi: 10.1016/j.yexcr.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rauzi M, Lenne P, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468(December):1110–1114. doi: 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- 54.Hutson MS, Tokutake Y, Chang MS, Bloor JW, Venakides S, Kiehart DP, Edwards GS. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300(5616):145–9. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- 55.Ma X, Lynch HE, Scully PC, Hutson MS. Probing embryonic tissue mechanics with laser hole drilling. Phys Biol. 2009;6(3):036004. doi: 10.1088/1478-3975/6/3/036004. [DOI] [PubMed] [Google Scholar]
- 56.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11(4):459–70. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17(5):736–43. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J. 2006;90(10):3762–73. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15(3):470–7. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 60.McMahon A, Supatto W, Fraser SE, Stathopoulos A. Dynamic analyses of Drosophila gastrulation provide insights into collective cell migration. Science. 2008;322(5907):1546–50. doi: 10.1126/science.1167094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blanchard GB, Kabla AJ, Schultz NL, Butler LC, Sanson B, Gorfinkiel N, Mahadevan L, Adams RJ. Tissue tectonics: morphogenetic strain rates, cell shape change and intercalation. Nat Methods. 2009;6(6):458–64. doi: 10.1038/nmeth.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62*.Brodland GW, Conte V, Cranston PG, Veldhuis J, Narasimhan S, Hutson MS, Jacinto A, Ulrich F, Baum B, Miodownik M. Video force microscopy reveals the mechanics of ventral furrow invagination in Drosophila. Proc Natl Acad Sci U S A. 2010;107(51):22111–6. doi: 10.1073/pnas.1006591107. Computational model for predicting stresses held at membranes from videomicroscopy data sets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137(7):1331–42. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 64**.Sherrard K, Robin F, Lemaire P, Munro E. Sequential activation of apical and basolateral contractility drives ascidian endoderm invagination. Curr Biol. 2010;20(17) doi: 10.1016/j.cub.2010.06.075. Through a combination of theoretical predictions and experimental tests, the process of invagination, as occurring during gastrulation, is found to occur from a balance in contracting endoderm and resisting mesoderm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65*.Gibson WT, Veldhuis JH, Rubinstein B, Cartwright HN, Perrimon N, Brodland GW, Nagpal R, Gibson MC. Control of the mitotic cleavage plane by local epithelial topology. Cell. 2011;144(3):427–38. doi: 10.1016/j.cell.2010.12.035. Integrated imaging and mechanical analysis of cell division patterns in fly wing epidermis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butler LC, Blanchard GB, Kabla AJ, Lawrence NJ, Welchman DP, Mahadevan L, Adams RJ, Sanson B. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat Cell Biol. 2009;11(7):859–64. doi: 10.1038/ncb1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farhadifar R, Roper JC, Aigouy B, Eaton S, Julicher F. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr Biol. 2007;17(24):2095–104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 68.Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol. 2011;3(1):a003228. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89–144. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- 70**.Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. J Cell Biol. 2010;188(5):735–49. doi: 10.1083/jcb.200910099. An imaging study of the actomyosin dynamics involved in apical constriction. Cross correlations and spatial analysis gives insight into activities of molecular players and their multicellular coordination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457(7228):495–9. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136(4):677–88. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lucchetta EM, Lee JH, Fu LA, Patel NH, Ismagilov RF. Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature. 2005;434(7037):1134–8. doi: 10.1038/nature03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takayama S, Ostuni E, LeDuc P, Naruse K, Ingber DE, Whitesides GM. Selective chemical treatment of cellular microdomains using multiple laminar streams. Chem Biol. 2003;10(2):123–30. doi: 10.1016/s1074-5521(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 75.Kim Y, Joshi SD, Messner WC, LeDuc PR, Davidson LA. Detection of dynamic spatiotemporal response to periodic chemical stimulation in a Xenopus embryonic tissue. PLoS One. 2011;6(1):e14624. doi: 10.1371/journal.pone.0014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim Y, Joshi SD, Davidson LA, Leduc PR, Messner WC. Dynamic control of 3D chemical profiles with a single 2D microfluidic platform. Lab Chip. 2011 doi: 10.1039/c1lc20077a. [DOI] [PubMed] [Google Scholar]
- 77.Yu H, Alexander CM, Beebe DJ. Understanding microchannel culture: parameters involved in soluble factor signaling. Lab Chip. 2007;7(6):726–30. doi: 10.1039/b618793e. [DOI] [PubMed] [Google Scholar]
- 78.Abhyankar VV, Lokuta MA, Huttenlocher A, Beebe DJ. Characterization of a membrane-based gradient generator for use in cell-signaling studies. Lab Chip. 2006;6(3):389–93. doi: 10.1039/b514133h. [DOI] [PubMed] [Google Scholar]