Abstract
BACKGROUND
The disease risks from cigarette smoking increased in the United States over most of the 20th century, first among male smokers and later among female smokers. Whether these risks have continued to increase during the past 20 years is unclear.
METHODS
We measured temporal trends in mortality across three time periods (1959–1965, 1982–1988, and 2000–2010), comparing absolute and relative risks according to sex and self-reported smoking status in two historical cohort studies and in five pooled contemporary cohort studies, among participants who became 55 years of age or older during follow-up.
RESULTS
For women who were current smokers, as compared with women who had never smoked, the relative risks of death from lung cancer were 2.73, 12.65, and 25.66 in the 1960s, 1980s, and contemporary cohorts, respectively; corresponding relative risks for male current smokers, as compared with men who had never smoked, were 12.22, 23.81, and 24.97. In the contemporary cohorts, male and female current smokers also had similar relative risks for death from chronic obstructive pulmonary disease (COPD) (25.61 for men and 22.35 for women), ischemic heart disease (2.50 for men and 2.86 for women), any type of stroke (1.92 for men and 2.10 for women), and all causes combined (2.80 for men and 2.76 for women). Mortality from COPD among male smokers continued to increase in the contemporary cohorts in nearly all the age groups represented in the study and within each stratum of duration and intensity of smoking. Among men 55 to 74 years of age and women 60 to 74 years of age, all-cause mortality was at least three times as high among current smokers as among those who had never smoked. Smoking cessation at any age dramatically reduced death rates.
CONCLUSIONS
The risk of death from cigarette smoking continues to increase among women and the increased risks are now nearly identical for men and women, as compared with persons who have never smoked. Among men, the risks associated with smoking have plateaued at the high levels seen in the 1980s, except for a continuing, unexplained increase in mortality from COPD.
The disease risks from cigarette smoking increased over most of the 20th century in the United States as successive generations of first male and then female smokers began smoking at progressively earlier ages. American men began smoking manufactured cigarettes early in the 20th century; by the 1930s, the average age at initiation fell below 18 years.1,2 Relatively few women smoked regularly before World War II; their average age at initiation continued to decrease through the 1960s. Women were not included in the earliest prospective epidemiologic studies in the 1950s,3–5 since mortality from lung cancer among women was not yet increasing in the general population.6 The landmark 1964 U.S. Surgeon General’s Report concluded only that “cigarette smoking is causally related to lung cancer in men.”7 Neither sex had yet experienced the full effects of smoking from adolescence throughout adulthood.
The first large, prospective study of smoking and mortality involving both women and men was Cancer Prevention Study I (CPS I), initiated by the American Cancer Society (ACS) in 1959.8,9 The relative risk of death from lung cancer during the first 6 years of follow-up among current smokers, as compared with persons who had never smoked, was 2.69 (95% confidence interval [CI], 2.14 to 3.37) for women and 11.35 (95% CI, 9.10 to 14.15) for men.10 In 1982, the ACS initiated the second Cancer Prevention Study (CPS II), which included nearly 1.2 million men and women nationwide.11 During the intervening 20 to 25 years, the relative risk of death from lung cancer had increased to 11.94 (95% CI, 9.99 to 14.26) for female smokers and to 22.36 (95% CI, 17.77 to 28.13) for male smokers.10,12
Several factors may have altered the health risks incurred by smokers. Smoking patterns have changed. Women who began smoking in the 1950s or thereafter have smoked more like men than they did in previous generations (i.e., starting at an earlier age and smoking more heavily).1 Daily cigarette consumption peaked during the 1970s among male smokers and during the 1980s among female smokers13; smoking prevalence in the two groups has since decreased in parallel. Contemporary smokers have spent much of their lives smoking filtered cigarettes made of blended tobacco.2,14 Women have more difficulty quitting than men; thus, for both current and former female smokers, the number of years of smoking has increased. Male and female smokers today are less educated and less affluent than smokers were 20 to 40 years ago.15 Since the 1950s, there has been a more rapid proportional decrease in the background risk of death from cardiovascular conditions among persons who have never smoked than among smokers.16,17
We calculated death rates and the relative risks associated with active cigarette smoking and smoking cessation during three time periods — 1959–1965, 1982–1988, and 2000–2010 — using data from the two historical ACS cohorts (CPS I and CPS II) and pooled data from five contemporary cohort studies in the United States.18–23 A central question is whether the hazards for women are now approaching those for men as their lifetime smoking behaviors have become increasingly similar.
METHODS
STUDY POPULATIONS
Descriptions of the study populations are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org. Because of the age distribution in the contemporary cohorts, analyses were restricted to participants who attained an age of 55 years or older during follow-up. CPS I analyses were based on 183,060 men and 335,922 women, enrolled in 1959 and followed through September 31, 1965. CPS II analyses are based on 293,592 men and 452,893 women enrolled in 1982 and followed through December 31, 1988. The five contemporary cohort studies representing the most recent period (2000– 2010) included the National Institutes of Health– American Association of Retired Persons Diet and Health Study (NIH–AARP),18 the ACS CPS II Nutrition Cohort19 (a subset of the original CPS II mortality study), the Women’s Health Initiative (WHI),20,21 the Nurses’ Health Study (NHS),22 and the Health Professionals Follow-up Study (HPFS) (Table S1 in the Supplementary Appendix).23 These are among the largest U.S. cohort studies that collected updated smoking information at least once during the period from 2000 through 2010.
ASSESSMENT OF SMOKING STATUS
The criteria used to define current smokers, former smokers, and those who had never smoked cigarettes in the various cohorts are described in the Supplementary Appendix. In CPS I and CPS II, we used only the information about smoking obtained at the time of enrollment, whereas in the contemporary cohort studies, we used updated information when available in time-dependent analyses. The frequency of updating varied across cohorts. Analyses of former smokers were restricted to those who had quit 2 or more years before the start of follow-up.
FOLLOW-UP OF VITAL STATUS
We restricted the follow-up time for participants in the CPS I and CPS II to 6 years to minimize the effects of smoking cessation on mortality. Follow-up of the contemporary cohorts began on January 1, 2000, and ended on or before December 31, 2010. More detailed information about follow-up is provided in the Supplementary Appendix.
STATISTICAL ANALYSIS
We tabulated age-specific deaths, person-years at risk, and death rates according to smoking status in each of the contributing cohorts, pooling the data for the five contemporary cohorts. Death rates were standardized according to the U.S. age distribution in 2000. Cox proportional-hazards regression was used to calculate age-adjusted and multivariable-adjusted relative-risk estimates according to smoking status (former smokers and current smokers vs. those who never smoked), according to the intensity and duration of smoking among current smokers, and according to the age at the time of quitting among former smokers. Multivariable-adjusted analyses were stratified according to cohort and age at baseline (in 1959, 1982, or 2000) and were further adjusted according to race and educational level.
We performed sensitivity analyses to assess whether educational level modified the relationship of current or former smoking to each end point, using both stratified analyses and interaction terms. To assess whether the lack of fully updated smoking information in some cohorts or differences in the follow-up periods might have biased the observed associations, we compared the results from the CPS II Nutrition cohort according to the time when smoking status was recorded: at baseline, 2 years before the end of follow-up, or at the most recent update through 2005.
RESULTS
STUDY POPULATIONS
Most of the participants were white; the majority were married and had a higher level of education than the general population24,25 (Table 1). In the contemporary cohorts, approximately half the participants had at least a college or nursing-school education. At least 20% of participants in all the cohorts had no education beyond high school, a proportion that allowed us to perform analyses stratified according to or adjusted for educational level. In the contemporary cohorts, the prevalence of current smoking decreased over time to 9.3% among men and 9.7% among women, findings that are consistent with trends in the educated general population.26 More than half the current smokers in the contemporary cohorts reported smoking fewer than 20 cigarettes per day in 2000; about 25% had smoked for 50 or more years. Additional baseline characteristics are provided in Table S1 in the Supplementary Appendix.
Table 1.
Baseline Characteristics and Number of Deaths According to Study Cohort.*
| Characteristic | CPS I Cohort (1959–1965)† | CPS II Cohort (1982–1988)† | Contemporary Cohort (2000–2010)‡ | |||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Participants included in analysis (no.) | 183,060 | 335,922 | 293,592 | 452,893 | 421,702 | 535,054 |
|
| ||||||
| Deaths (no.) | ||||||
|
| ||||||
| All causes | 22,363 | 25,787 | 32,335 | 30,342 | 73,800 | 62,956 |
|
| ||||||
| Lung cancer | 1,027 | 266 | 3,158 | 1,799 | 6,635 | 4,785 |
|
| ||||||
| COPD | 571 | 211 | 1,296 | 832 | 3,478 | 3,034 |
|
| ||||||
| Ischemic heart disease | 9,706 | 8,103 | 9,580 | 6,728 | 14,753 | 7,869 |
|
| ||||||
| Other heart disease | 1,494 | 2,658 | 3,043 | 3,054 | 5,261 | 4,641 |
|
| ||||||
| Any type of stroke | 2,213 | 3,677 | 1,720 | 2,317 | 3,431 | 4,105 |
|
| ||||||
| Mean age§ | 59.0±7.6 | 60.2±8.3 | 60.9±7.8 | 61.4±8.3 | 66.7±6.2 | 66.5±6.5 |
|
| ||||||
| Educational level (%)¶ | ||||||
|
| ||||||
| High school or less | 65.2 | 64.5 | 39.0 | 34.4 | 20.9 | 20.7 |
|
| ||||||
| Some college | 16.3 | 20.7 | 27.0 | 29.9 | 27.7 | 28.6 |
|
| ||||||
| College or nursing school or more | 17.1 | 13.5 | 32.6 | 34.1 | 49.4 | 49.1 |
|
| ||||||
| Race (%)|| | ||||||
|
| ||||||
| White | 97.1 | 96.4 | 94.6 | 93.8 | 93.8 | 90.2 |
|
| ||||||
| Black | 1.9 | 2.1 | 3.3 | 4.2 | 2.3 | 5.2 |
|
| ||||||
| Other or missing data | 1.0 | 1.5 | 2.4 | 2.0 | 3.9 | 4.6 |
|
| ||||||
| Ethnic group (%)** | ||||||
|
| ||||||
| Non-Hispanic | 99.3 | 99.3 | 97.6 | 97.4 | ||
|
| ||||||
| Hispanic | 0.8 | 0.7 | 1.6 | 2.0 | ||
|
| ||||||
| Married at time of enrollment (%) | 94.8 | 68.2 | 94.8 | 71.7 | 87.7 | 64.1 |
|
| ||||||
| Smoking status (%)†† | ||||||
|
| ||||||
| Never smoked | 43.5 | 81.0 | 33.6 | 61.2 | 36.2 | 49.3 |
|
| ||||||
| Current smoker | 39.5 | 15.2 | 23.5 | 18.0 | 9.3 | 9.7 |
|
| ||||||
| Former smoker | 17.0 | 3.8 | 42.9 | 20.8 | 54.5 | 41.0 |
|
| ||||||
| Characteristics of current smokers | ||||||
|
| ||||||
| No. of cigarettes smoked per day (%)‡‡ | ||||||
|
| ||||||
| <10 | 14.7 | 34.4 | 11.2 | 16.3 | 24.0 | 42.9 |
|
| ||||||
| 10–19 | 23.5 | 31.2 | 17.3 | 24.5 | 36.0 | 35.9 |
|
| ||||||
| 20–39 | 51.8 | 32.2 | 50.4 | 43.5 | 34.3 | 18.4 |
|
| ||||||
| ≥40 | 8.7 | 2.2 | 17.9 | 8.0 | 4.4 | 1.2 |
|
| ||||||
| Mean duration of smoking by age group (yr)§§¶¶ | ||||||
|
| ||||||
| 50–59 yr | 34.9±6.0 | 26.3±8.8 | 36.8±5.2 | 34.0±6.6 | 35.2±7.9 | 34.6±8.9 |
|
| ||||||
| 60–69 yr | 43.6±7.3 | 29.2±9.6 | 45.7±5.8 | 41.9±8.1 | 44.0±8.9 | 41.7±9.8 |
|
| ||||||
| 70–79 yr | 50.9±9.8 | 33.5±10.0 | 54.5±6.6 | 47.8±9.9 | 51.2±10.4 | 48.2±10.7 |
|
| ||||||
| ≥80 yr | 58.6±12.5 | 39.9±10.8 | 63.6±9.1 | 50.3±13.3 | 56.3±15.5 | 48.9±13.7 |
|
| ||||||
| Age at initiation of smoking (%)‡‡§§ | ||||||
|
| ||||||
| <15 yr | 13.1 | 0.9 | 16.5 | 4.2 | 13.7 | 6.5 |
|
| ||||||
| 15–19 yr | 44.5 | 10.0 | 52.0 | 40.1 | 49.9 | 51.9 |
|
| ||||||
| 20–29 yr | 27.4 | 27.4 | 24.4 | 35.6 | 31.6 | 35.4 |
|
| ||||||
| ≥30 yr | 9.0 | 56.8 | 3.1 | 16.2 | 3.7 | 6.0 |
Plus–minus signs are means ±SD. COPD denotes chronic obstructive pulmonary disease.
Participants were enrolled in Cancer Prevention Study I (CPS I) in 1959 and in Cancer Prevention Study II (CPS II) in 1982. Analyses of both studies excluded participants younger than 50 years of age.
The contemporary cohort consisted of cohorts from five studies. Participants were enrolled in the Nurses’ Health Study (NHS) in 1976, the Health Professionals Follow-up Study (HPFS) in 1986, the CPS II Nutrition Cohort in 1992, the National Institutes of Health–American Association of Retired Persons (NIH–AARP) Diet and Health Study in 1995–1996, and the Women’s Health Initiative (WHI) in 1993–1998.
Age at the start of follow-up is shown.
For women, the spouse’s educational level was used if it was more advanced.
Race was self-reported.
Ethnic group was self-reported. The HPFS was not included in this analysis.
Smoking status was recorded as of 1959 for CPS I, 1982 for CPS II, 1999 for the CPS II Nutrition Cohort, 2000 for the HPFS and the NHS, 1996 for the NIH–AARP cohort, and 1995–2001 for the WHI.
These data were age-adjusted to the U.S. population standard for the year 2000.
Current smokers in the NIH–AARP cohort were excluded from this analysis because the age at smoking initiation was available only for the subgroup that completed the 2004 survey.
Categories refer to age at the start of follow-up.
MORTALITY
All Causes
Among the participants who had never smoked, the age-standardized rates of death from any cause were approximately 50% lower in the contemporary period than in the 1959–1965 period for both sexes (Tables 2 and 3). In contrast, there was no temporal decrease in the all-cause death rate among women who were current smokers (Table 2) and there was a 23.6% decrease among men who were current smokers (Table 3). Thus, the age-standardized relative risk for death from all causes among current smokers, as compared with those who had never smoked, increased across all three time periods, with a relative risk of 2.80 (95% CI, 2.72 to 2.88) for male smokers and 2.76 (95% CI, 2.69 to 2.84) for female smokers in the contemporary cohorts. The age-specific relative-risk estimates exceeded 3.00 for male current smokers who were 55 to 74 years of age and equaled or exceeded 3.00 for female current smokers who were 60 to 74 years of age (Table S2 in the Supplementary Appendix).
Table 2.
Age-Adjusted and Multivariable-Adjusted Relative Risks of Death from Smoking-Related Diseases among Women 55 Years of Age or Older in the Three Study Cohorts, According to Smoking Status.*
| Variable | Never Smoked | Current Smoker | Former Smoker† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CPS I Cohort (1959–1965) | CPS II Cohort (1982–1988) | Contemporary Cohort (2000–2010) | CPS I Cohort (1959–1965) | CPS II Cohort (1982–1988) | Contemporary Cohort (2000–2010) | CPS I Cohort (1959–1965) | CPS II Cohort (1982–1988) | Contemporary Cohort (2000–2010) | |
| Death from all causes | |||||||||
|
| |||||||||
| No. of deaths | 21,825 | 17,585 | 26,182 | 3214 | 7188 | 7240 | 748 | 5569 | 29,534 |
|
| |||||||||
| Rate per 100,000 | 2,884.23 | 1741.18‡ | 1247.93‡ | 3225.49 | 2953.70 | 3015.68 | 3553.76 | 2104.71‡ | 1676.23‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 1.33 | 2.08 | 2.80 | 1.29 | 1.28 | 1.44 |
|
| |||||||||
| 95% CI | 1.28–1.38 | 2.03–2.14 | 2.73–2.88 | 1.20–1.39 | 1.24–1.32 | 1.41–1.46 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 1.35 | 2.08 | 2.76 | 1.33 | 1.33 | 1.45 |
|
| |||||||||
| 95% CI | 1.30–1.40 | 2.02–2.14 | 2.69–2.84 | 1.23–1.43 | 1.29–1.37 | 1.43–1.48 | |||
|
| |||||||||
| Death from lung cancer | |||||||||
|
| |||||||||
| No. of deaths | 179 | 334 | 513 | 79 | 1084 | 1485 | 8 | 381 | 2787 |
|
| |||||||||
| Rate per 100,000 | 17.70 | 27.74‡ | 21.77‡ | 30.19 | 291.86‡ | 505.79‡ | 46.50 | 97.77 | 128.84‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 2.74 | 12.62 | 26.18 | 1.30 | 3.77 | 6.66 |
|
| |||||||||
| 95% CI | 2.07–3.62 | 11.13–14.31 | 23.65–28.98 | 0.64–2.65 | 3.25–4.38 | 6.06–7.31 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 2.73 | 12.65 | 25.66 | 1.30 | 3.85 | 6.70 |
|
| |||||||||
| 95% CI | 2.07–3.61 | 1.15–14.34 | 23.17–28.40 | 0.64–2.64 | 3.32–4.47 | 6.09–7.36 | |||
|
| |||||||||
| Death from COPD | |||||||||
|
| |||||||||
| No. of deaths | 136 | 192 | 320 | 66 | 367 | 720 | 9 | 273 | 1994 |
|
| |||||||||
| Rate per 100,000 | 15.70 | 18.71 | 16.09 | 45.74 | 199.17‡ | 312.92‡ | 43.32 | 104.14‡ | 103.40 |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 3.93 | 10.31 | 23.03 | 2.32 | 5.84 | 7.88 |
|
| |||||||||
| 95% CI | 2.88–5.38 | 8.60–12.36 | 20.15–26.31 | 1.18–4.56 | 4.83–7.05 | 7.00–8.87 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 3.95 | 10.35 | 22.35 | 2.31 | 6.10 | 8.09 |
|
| |||||||||
| 95% CI | 2.89–5.41 | 8.63–12.41 | 19.55–25.55 | 1.17–4.56 | 5.05–7.37 | 7.19–9.10 | |||
|
| |||||||||
| Death from ischemic heart disease¶ | |||||||||
|
| |||||||||
| No. of deaths | 6881 | 4266 | 3349 | 998 | 1357 | 907 | 224 | 1105 | 3613 |
|
| |||||||||
| Rate per 100,000 | 952.02 | 464.05‡ | 168.69‡ | 1148.82 | 676.13‡ | 368.00‡ | 1107.60 | 553.82‡ | 212.64‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 1.53 | 2.00 | 2.93 | 1.35 | 1.20 | 1.40 |
|
| |||||||||
| 95% CI | 1.43–1.64 | 1.87–2.13 | 2.72–3.16 | 1.19–1.55 | 1.12–1.28 | 1.34–1.47 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 1.56 | 2.00 | 2.86 | 1.39 | 1.27 | 1.44 |
|
| |||||||||
| 95% CI | 1.46–1.67 | 1.88–2.13 | 2.65–3.08 | 1.22–1.59 | 1.19–1.36 | 1.38–1.51 | |||
|
| |||||||||
| Death from other heart disease¶ | |||||||||
|
| |||||||||
| No. of deaths | 2332 | 1972 | 2212 | 252 | 597 | 374 | 74 | 485 | 2055 |
|
| |||||||||
| Rate per 100,000 | 345.59 | 220.60‡ | 115.75‡ | 348.01 | 310.07 | 188.29‡ | 383.00 | 231.04‡ | 126.83‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 1.16 | 1.89 | 1.89 | 1.35 | 1.15 | 1.21 |
|
| |||||||||
| 95% CI | 1.01–1.33 | 1.72–2.08 | 1.69–2.11 | 1.07–1.70 | 1.04–1.28 | 1.14–1.29 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 1.20 | 1.88 | 1.84 | 1.40 | 1.22 | 1.24 |
|
| |||||||||
| 95% CI | 1.04–1.37 | 1.71–2.07 | 1.65–2.06 | 1.11–1.77 | 1.10–1.35 | 1.17–1.32 | |||
|
| |||||||||
| Death from any stroke¶ | |||||||||
|
| |||||||||
| No. of deaths | 3188 | 1507 | 2015 | 390 | 474 | 358 | 99 | 336 | 1732 |
|
| |||||||||
| Rate per 100,000 | 497.58 | 177.11‡ | 100.01‡ | 445.61 | 223.16‡ | 206.39‡ | 664.09 | 169.63‡ | 114.81‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 1.48 | 2.20 | 2.12 | 1.42 | 1.12 | 1.14 |
|
| |||||||||
| 95% CI | 1.33–1.65 | 1.97–2.45 | 1.89–2.38 | 1.16–1.73 | 0.99–1.27 | 1.07–1.21 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 1.51 | 2.19 | 2.10 | 1.46 | 1.16 | 1.15 |
|
| |||||||||
| 95% CI | 1.35–1.69 | 1.96–2.44 | 1.87–2.36 | 1.19–1.78 | 1.03–1.31 | 1.07–1.22 | |||
Mortality was adjusted to the U.S. population standard for the year 2000, and relative risks were calculated with the use of age-adjusted and multivariable-adjusted Cox proportional-hazards models of the five most common diseases related to smoking. CI denotes confidence interval, and RR relative risk.
This group consists of former smokers who quit more than 2 years before the survey date.
The age-standardized rate differed significantly from that for the immediately preceding time period.
Multivariable models were adjusted for exact age, race, and educational level.
The combined rates per 100,000 participants for the three cardiovascular disease categories (ischemic heart disease, other heart disease, and any type of stroke) in those who never smoked were 1795.19 in the CPS I cohort, 861.76 in the CPS II cohort, and 384.45 in the contemporary cohort — a reduction of 79% from the 1959–1965 period to the contemporary period.
Table 3.
Age-Adjusted and Multivariable-Adjusted Relative Risks of Death from Smoking-Related Diseases among Men 55 to 85 Years of Age or Older in the Three Study Cohorts, According to Smoking Status.*
| Variable | Never Smoked | Current Smoker | Former Smoker† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CPS I Cohort (1959–1965) | CPS II Cohort (1982–1988) | Contemporary Cohort (2000–2010) | CPS I Cohort (1959–1965) | CPS II Cohort (1982–1988) | Contemporary Cohort (2000–2010) | CPS I Cohort (1959–1965) | CPS II Cohort (1982–1988) | Contemporary Cohort (2000–2010) | |
| Death from all causes | |||||||||
|
| |||||||||
| No. of deaths | 8244 | 8206 | 17,462 | 10,843 | 10,608 | 6920 | 3276 | 13,521 | 49,418 |
|
| |||||||||
| Rate per 100,000 | 4,142.19 | 2,676.85‡ | 1,917.95‡ | 6,126.42 | 5,202.46‡ | 4,679.65‡ | 4,707.95 | 3,527.17‡ | 2,716.45‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 1.78 | 2.43 | 2.98 | 1.28 | 1.43 | 1.53 |
|
| |||||||||
| 95% CI | 1.73–1.84 | 2.36–2.50 | 2.90–3.07 | 1.23–1.33 | 1.40–1.47 | 1.50–1.55 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 1.76 | 2.33 | 2.80 | 1.28 | 1.42 | 1.47 |
|
| |||||||||
| 95% CI | 1.71–1.81 | 2.26–2.40 | 2.72–2.88 | 1.23–1.34 | 1.38–1.45 | 1.45–1.50 | |||
|
| |||||||||
| Death from lung cancer | |||||||||
|
| |||||||||
| No. of deaths | 73 | 125 | 357 | 859 | 1887 | 1455 | 95 | 1146 | 4823 |
|
| |||||||||
| Rate per 100,000 | 27.35 | 34.87 | 34.07 | 305.40 | 707.14‡ | 798.81 | 101.51 | 251.74‡ | 208.05‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 12.49 | 25.30 | 27.32 | 3.50 | 7.60 | 7.13 |
|
| |||||||||
| 95% CI | 9.80–15.92 | 21.10–30.34 | 24.30–30.70 | 2.58–4.76 | 6.32–9.15 | 6.40–7.94 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 12.22 | 23.81 | 24.97 | 3.48 | 7.41 | 6.75 |
|
| |||||||||
| 95% CI | 9.59–15.58 | 19.85–28.57 | 22.20–28.09 | 2.56–4.74 | 6.16–8.91 | 6.06–7.52 | |||
|
| |||||||||
| Death from COPD | |||||||||
|
| |||||||||
| No. of deaths | 82 | 94 | 183 | 338 | 462 | 656 | 151 | 740 | 2639 |
|
| |||||||||
| Rate per 100,000 | 39.42 | 33.72 | 18.56‡ | 227.64 | 318.24‡ | 497.62‡ | 168.23 | 209.27 | 138.57‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 5.61 | 10.50 | 28.97 | 5.78 | 6.96 | 7.62 |
|
| |||||||||
| 95% CI | 4.38–7.20 | 8.39–13.13 | 24.55–34.19 | 4.40–7.59 | 5.61–8.62 | 6.56–8.85 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 5.52 | 9.98 | 25.61 | 5.75 | 6.77 | 7.05 |
|
| |||||||||
| 95% CI | 4.30–7.08 | 7.97–12.49 | 21.68–30.25 | 4.37–7.55 | 5.46–8.40 | 6.07–8.19 | |||
|
| |||||||||
| Death from ischemic heart disease¶ | |||||||||
|
| |||||||||
| No. of deaths | 3529 | 2731 | 3582 | 4711 | 2743 | 1286 | 1466 | 4106 | 9885 |
|
| |||||||||
| Rate per 100,000 | 1,678.67 | 852.71‡ | 411.17‡ | 2,403.70 | 1,289.73‡ | 891.64‡ | 1,977.97 | 1,026.76‡ | 558.93‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 1.71 | 1.86 | 2.69 | 1.29 | 1.30 | 1.49 |
|
| |||||||||
| 95% CI | 1.63–1.79 | 1.76–1.96 | 2.53–2.88 | 1.21–1.37 | 1.24–1.36 | 1.44–1.55 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 1.69 | 1.78 | 2.50 | 1.28 | 1.27 | 1.43 |
|
| |||||||||
| 95% CI | 1.61–1.77 | 1.69–1.88 | 2.34–2.66 | 1.21–1.36 | 1.21–1.33 | 1.37–1.48 | |||
|
| |||||||||
| Death from other heart disease¶ | |||||||||
|
| |||||||||
| No. of deaths | 635 | 911 | 1400 | 630 | 907 | 403 | 229 | 1225 | 3458 |
|
| |||||||||
| Rate per 100,000 | 349.30 | 320.80 | 168.46‡ | 498.03 | 502.64 | 292.70‡ | 428.68 | 364.10 | 210.74‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 1.55 | 1.99 | 2.35 | 1.28 | 1.20 | 1.34 |
|
| |||||||||
| 95% CI | 1.37–1.74 | 1.81–2.18 | 2.10–2.63 | 1.10–1.50 | 1.10–1.31 | 1.26–1.43 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 1.51 | 1.88 | 2.15 | 1.29 | 1.19 | 1.27 |
|
| |||||||||
| 95% CI | 1.34–1.70 | 1.71–2.07 | 1.92–2.41 | 1.11–1.51 | 1.09–1.29 | 1.20–1.36 | |||
|
| |||||||||
| Death from any stroke¶ | |||||||||
|
| |||||||||
| No. of deaths | 1083 | 558 | 1001 | 862 | 511 | 236 | 268 | 651 | 2194 |
|
| |||||||||
| Rate per 100,000 | 654.06 | 220.84‡ | 124.58‡ | 719.66 | 320.97‡ | 202.52‡ | 515.76 | 216.91‡ | 135.89‡ |
|
| |||||||||
| Age-adjusted RR | 1.00 | 1.00 | 1.00 | 1.41 | 2.08 | 2.00 | 0.94 | 1.07 | 1.18 |
|
| |||||||||
| 95% CI | 1.28–1.55 | 1.84–2.35 | 1.73–2.31 | 0.82–1.08 | 0.96–1.20 | 1.10–1.28 | |||
|
| |||||||||
| Multivariable-adjusted RR§ | 1.00 | 1.00 | 1.00 | 1.38 | 1.97 | 1.92 | 0.95 | 1.07 | 1.16 |
|
| |||||||||
| 95% CI | 1.26–1.52 | 1.74–2.23 | 1.66–2.21 | 0.83–1.09 | 0.95–1.20 | 1.07–1.25 | |||
Mortality was adjusted to the U.S. population standard for the year 2000, and relative risks were calculated with the use of age-adjusted and multivariable-adjusted Cox proportional-hazards models of the five most common diseases related to smoking.
This group consists of former smokers who quit more than 2 years before the survey date.
The age-standardized rate differed significantly from that for the immediately preceding time period.
Multivariable models were adjusted for exact age, race, and educational level.
The combined rates per 100,000 participants for the three cardiovascular disease categories (ischemic heart disease, other heart disease, and any type of stroke) in those who never smoked were 2682.03 in the CPS I cohort, 1394.35 in the CPS II cohort, and 704.21 in the contemporary cohort — a reduction of 74% from the 1959–1965 period to the contemporary period.
Lung Cancer
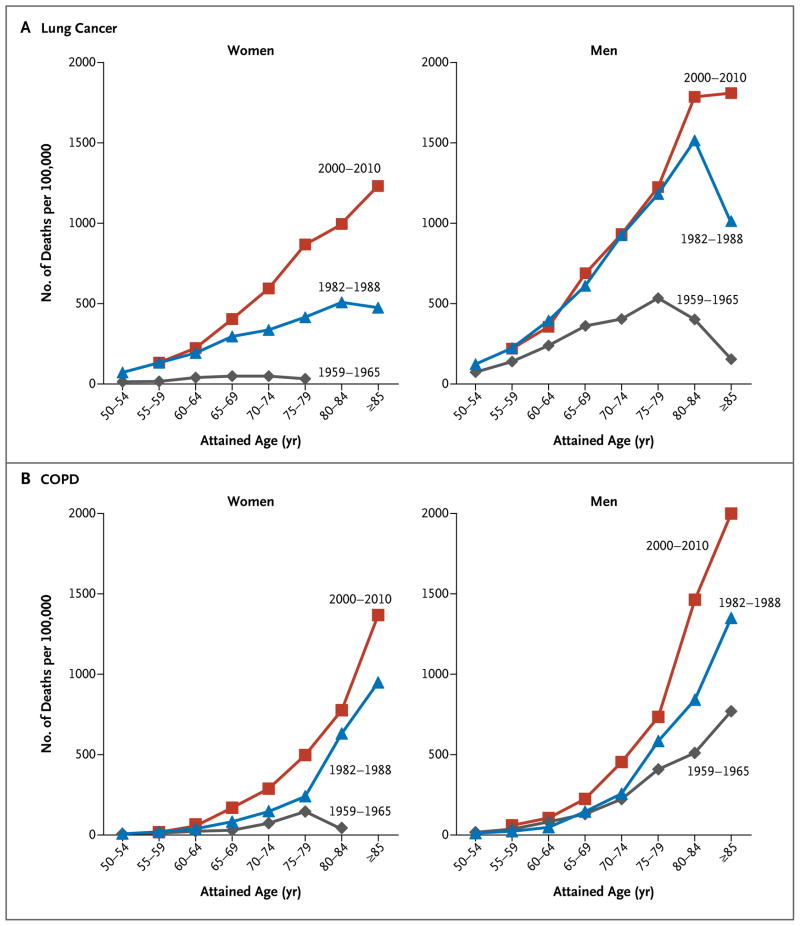
Among the participants who had never smoked, the age-standardized rate of death from lung cancer remained constant for men (Table 3) but increased slightly for women (Table 2) from the 1959–1965 period (CPS I) to the 1982–1988 period (CPS II), before decreasing in the contemporary period. Among female smokers, there was a large increase (by a factor of 16.8) in deaths from lung cancer over the entire 50-year period, about half of which occurred during the past 20 years (Table 2). In the CPS I cohort, lung-cancer mortality among male smokers was about 12 times as high as that among men who had never smoked; the mortality approximately doubled from the 1959–1965 period to the 1982–1988 period (Table 3) before stabilizing in the period after the 1980s. Only the two oldest age groups of male smokers had any increase in the rate of death from lung cancer from the 1982–1988 period to the contemporary period (Fig. 1); these age groups represent birth cohorts from 1900 to 1929 (Fig. S1 in the Supplementary Appendix). Absolute lung-cancer mortality was higher for men than for women in all three periods, irrespective of smoking status. However, in the contemporary period, the point estimates for the relative risk of death from lung cancer among current smokers, as compared with those who had never smoked, were virtually identical for men and women: 24.97 (95% CI, 22.20 to 28.09) and 25.66 (95% CI, 23.17 to 28.40), respectively (Tables 2 and 3).
Figure 1. Changes in Rates of Death from Lung Cancer and Chronic Obstructive Pulmonary Disease (COPD) over Time among Current Female and Male Smokers in the Three Time Periods.
Data were obtained from the first Cancer Prevention Study (CPS I) for the period from 1959 to 1965, from the second Cancer Prevention Study (CPS II) for the period from 1982 to 1988, and from five contemporary cohort studies for the period from 2000 to 2010.
Chronic Obstructive Pulmonary Disease
Among the participants who had never smoked, the age-standardized rate of death from chronic obstructive pulmonary disease (COPD) remained relatively constant for women (Table 2) but decreased by approximately 45% for men from the 1982–1988 period to the contemporary period (Table 3). In contrast, mortality increased for both male and female smokers across all three periods (Tables 2 and 3 and Fig. 1). The largest absolute increase in COPD mortality occurred among male smokers after the 1980s, affecting all smokers who were 55 years of age or older (Fig. 1) and all birth cohorts from 1900 through at least 1954 (Fig. S1 in the Supplementary Appendix). The multivariable adjusted relative risk of death from COPD among male smokers more than doubled from the 1982– 1988 period (9.98 [95% CI, 7.97 to 12.49]) to the contemporary period (25.61 [95% CI, 21.68 to 30.25]) (Table 3). Approximately half this increase reflected the lower background death rate among men in the contemporary period who had never smoked, as compared with those in the 1982– 1988 period. The relative risk for female smokers also more than doubled over this period, from 10.35 (95% CI, 8.63 to 12.41) to 22.35 (95% CI, 19.55 to 25.55) (Table 2).
Cardiovascular Diseases
Among participants who had never smoked, the combined rates of death from ischemic heart disease, other types of heart disease, and any type of stroke decreased from the 1959–1965 period to the contemporary period by 79% among women (Table 2) and by 74% among men (Table 3). These decreases were proportionately larger than those seen in the current smokers; consequently, the relative-risk estimates associated with current smoking increased for all three cardiovascular end points. In the contemporary cohorts, the relative risk for death from ischemic heart disease for current smokers, as compared with those who never smoked, was 2.86 (95% CI, 2.65 to 3.08) for women (Table 2) and 2.50 (95% CI, 2.34 to 2.66) for men (Table 3). The relative risk of death from ischemic heart disease exceeded 3.00 among male and female current smokers who were 55 to 74 years of age (Table S2 in the Supplementary Appendix). Hence, two thirds of the deaths due to ischemic heart disease among smokers in the contemporary cohorts were attributable to their smoking.
MORTALITY ACCORDING TO INTENSITY AND DURATION OF CURRENT SMOKING
The relative risks of death from lung cancer, death from COPD, and death from any cause among current smokers, as compared with those who had never smoked, increased according to the number of cigarettes smoked per day and the number of years of smoking during all three periods, although the relationships were less consistent for the cardiovascular end points (Tables S3, S4, and S5 in the Supplementary Appendix). Differences in these variables reported at the start of follow-up did not explain the increases from the 1980s (CPS II) to the contemporary period in rates of death from lung cancer and COPD among female smokers and the rate of death from COPD among male smokers. Even within each stratum of smoking intensity and duration, the relative-risk estimates increased over time.
MORTALITY AMONG FORMER SMOKERS
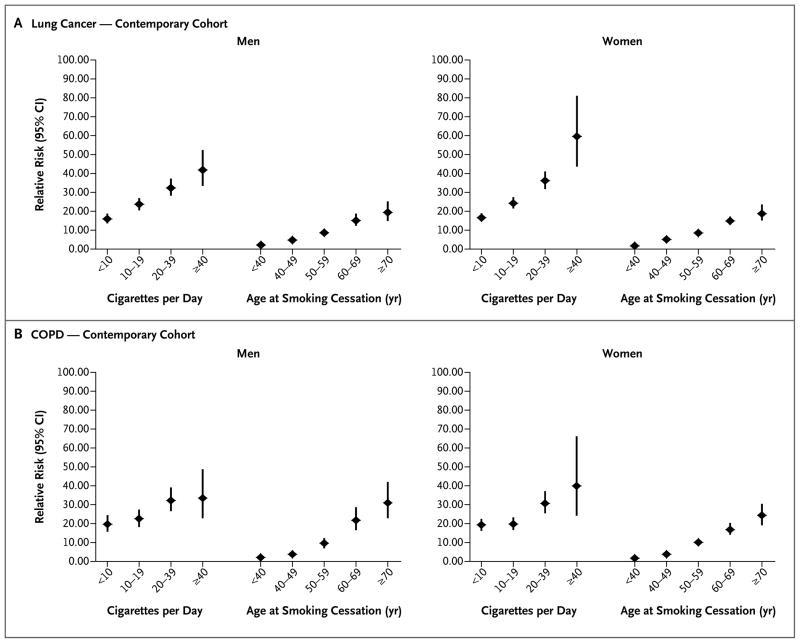
Former smokers of both sexes in the CPS II and contemporary cohorts had lower age-standardized rates of death and relative risks of death than did current smokers, for all the end points studied (Tables 2 and 3). The rates of death from cardiovascular conditions among men and women who were former smokers decreased significantly from the 1960s to the contemporary period, but the rates of death from lung cancer and COPD increased among women. Former smokers who had stopped smoking at earlier ages had progressively lower relative risks of death from lung cancer and COPD, as compared with current smokers in the contemporary cohorts (Fig. 2, and Table S6 in the Supplementary Appendix). Those who quit smoking by 40 years of age avoided nearly all the excess smoking-related deaths from these conditions; even those who quit smoking before 60 years of age had a lower relative risk than those who did not quit but smoked fewer than 10 cigarettes per day. Strong inverse relationships were also observed between years since quitting and deaths from these end points (Tables S6 through S13 in the Supplementary Appendix).
Figure 2. Relative Risks of Lung Cancer and COPD among Current Smokers, According to Number of Cigarettes Smoked per Day, and among Former Smokers, According to Age at the Time of Quitting, in the Contemporary Cohorts.
Pooled Cox proportional-hazards, multivariable models were used to determine relative risks for current or former smokers who participated in the Cancer Prevention Study II Nutrition cohort, the Health Professionals Follow-up Study, the Nurses’ Health Study, the National Institutes of Health–American Association of Retired Persons Diet and Health Study (NIH–AARP), and the Women’s Health Initiative study, 2000–2010. All models were controlled for education level, race, and cohort and were stratified according to the participant’s age in 2000. Data were not available for age at the time of quitting for former smokers in the NIH–AARP study. Former smokers who had quit more than 2 years before the survey date were included. P<0.001 for the test for trend. Vertical lines indicate 95% confidence intervals.
SENSITIVITY ANALYSES
Educational level significantly modified the association of current and former smoking with some of the mortality end points in the contemporary cohorts (P = 0.05) (Table S14 in the Supplementary Appendix). In general, the estimated relative risks for current and former smokers with only a high-school education or less were similar to or larger than the estimates for current and former smokers who were college graduates. This was consistently true with respect to the relative risks of death from COPD and ischemic and other heart diseases for women and former smokers but not for male current smokers. The timing of information on smoking status also affected the association between current smoking and certain end points, but the changes were small; in most cases, analyses based on fully updated smoking information underestimated the associations when smoking status was documented at baseline or 2 years before death or the end of follow-up for women and men (Tables S15 and S16, respectively, in the Supplementary Appendix).
DISCUSSION
Our study of cohorts from three time periods provides a 50-year perspective on the evolution of smoking-related risks in the United States. We highlight five important findings.
First, the relative and absolute risks of death from smoking continue to increase among female smokers; the relative risks of death from lung cancer, COPD, ischemic heart disease, any type of stroke, and all causes are now nearly identical for female and male smokers. This finding is new and confirms the prediction that, in relative terms, “women who smoke like men die like men.”27 Convergence of the relative risks for men and women results from the convergence of smoking patterns among men and women since the 1960s28,29 and the aging of birth cohorts with the heaviest lifetime history of smoking. The risk of death from lung cancer among male smokers appears to have stabilized since the 1980s, whereas it continues to increase among female smokers.
Second, we found that for men 55 to 74 years of age and for women 60 to 74 years of age, the rate of death from all causes combined is now at least three times as high among current smokers as among those who have never smoked. This finding parallels and extends the findings in the British Doctors’ Study,30 the Million Women Study,31 and the U.S. National Health Interview Survey.32 These studies show that more than two thirds of all deaths among current smokers in these age groups are associated with smoking.
Third, the rate of death from COPD continues to increase among both male and female smokers in contrast to a significant decrease in risk among men who never smoked. This increase is not simply a function of aging, since it affects male smokers 55 years of age or older and female smokers 60 years of age or older. Nor can it be explained by differences in the average duration of smoking or the number of cigarettes smoked per day, since daily consumption was actually lower in the contemporary cohorts than in the CPS II cohort, and the average duration of smoking did not change significantly at any age. The ability to diagnose COPD has improved over time,33 but this would probably affect the number of prevalent cases more than the number of deaths for which COPD is considered to be the underlying cause of death. A plausible explanation for the continuing increase in deaths from COPD among male smokers is that cigarettes marketed since the late 1950s have undergone design changes that promote deeper inhalation of smoke.34 For example, the introduction of blended tobacco and genetic selection of tobacco plants lowered the pH of smoke; as a result, inhalation was easier and deeper inhalation was needed for the absorption of protonated nicotine. 35 Other design changes, such as the use of more porous wrapping paper and perforated filters, also diluted the smoke. Deeper inhalation of more dilute smoke increases exposure of the lung parenchyma. These and other design changes in cigarettes may also have contributed to the shift, beginning in the 1970s, in the histologic and topographic features of lung cancers in male smokers,36 with an increase in the incidence of peripheral adenocarcinomas that largely offset the decrease in squamous-cell and small-cell cancers of the central airways. The likely net effect of deeper inhalation on COPD could be wholly detrimental, since COPD results from injury to the lung parenchyma.
Fourth, our analyses of data from former smokers confirm that quitting smoking at any age dramatically lowers mortality from all major smoking-related diseases. As reported previously, nearly all the excess risk can be avoided if a person quits smoking before 40 years of age.17,31,32 Quitting smoking is much more effective than reducing the number of cigarettes smoked.
Finally, our analyses according to educational level show that the relative-risk estimates associated with current and former smoking among smokers with only a high-school education are generally similar to or larger than those among smokers who are college graduates. Only among male current smokers were the relative-risk estimates for ischemic and other heart disease significantly lower in the least-educated group. Hence, the relative-risk estimates presented here will in general correspond to those in a less-educated population. Similarly, differences in the time when information on smoking was obtained in the contemporary cohorts will not appreciably affect the results.
The strengths of our study include its size, prospective design, national scope, and 50-year time span. Our results provide estimates of temporal changes in cause-specific mortality and the contemporary risks from smoking in the United States. Its limitations are that it principally represents whites, 50 years of age or older, who were born between 1870 and 1954. We could not assess risks among younger contemporary smokers. Most current smokers in the contemporary cohorts had smoked for at least 30 years, limiting the range over which we could examine the influence of the duration of smoking.
In conclusion, there have been large, persistent increases in the risks of smoking-related deaths among female cigarette smokers over the past half century; in relative terms, the risks for women now equal those for men. The risks among male smokers have plateaued at the high levels of the 1980s, except for a continuing, unexplained increase in deaths from COPD.
Supplementary Material
Acknowledgments
Support for the National Institutes of Health (NIH)–AARP Diet and Health Study was provided by the Intramural Research Program of the National Cancer Institute (NCI), NIH. Support for all the American Cancer Society (ACS) cohort studies was provided by the Intramural Research Programs of the ACS. Support for the Nurses’ Health Study and the Health Professionals Follow-up Study was provided by grants (P01 CA87969 and P01 CA055075, respectively) from the NCI. Support for the Women’s Health Initiative program is provided by contracts (N01WH22110, N01WH24152, N01WH32100–32102, N01WH32105, N01WH32106, N01WH32108, N01WH32109, N01WH32111, N01WH32112, N01WH32113, N01WH32115, N01WH32118, N01WH32119, N01WH32122, N01WH42107–N01WH42126, N01WH42129– N01WH42132, and N01WH44221) from the National Heart, Lung, and Blood Institute.
We thank the investigators and staff of all the study cohorts for their dedication; and the study participants for making the program possible.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Burns D, Lee L, Shen L, et al. Smoking and tobacco control monograph no. 8. Bethesda, MD: National Cancer Institute; 1997. Cigarette smoking behavior in the United States: changes in cigarette-related disease risks and their implication for prevention and control; pp. 13–112. (NIH publication no. 97-4213.) [Google Scholar]
- 2.Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW., Jr Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89:1580–6. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 3.Doll R, Hill A. The mortality of doctors in relation to their smoking habits: a preliminary report. BMJ. 1954;1:1451–5. doi: 10.1136/bmj.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond EC, Horn D. Smoking and death rates: report on forty-four months of follow-up of 187,783 men. II. Death rates by cause. JAMA. 1958;166:1294–308. doi: 10.1001/jama.1958.02990110030007. [DOI] [PubMed] [Google Scholar]
- 5.Dorn H. The mortality of smokers and nonsmokers. Proc Soc Stat Sect Am Stat Assn. 1958:34–71. [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 7.Smoking and health: report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: Department of Health, Education, and Welfare; 1964. p. 387. (PHS publication no. 1103.) [Google Scholar]
- 8.Hammond EC, Horn D. The relationship between human smoking habits and death rates: a follow-up study of 187,766 men. JAMA. 1954;155:1316–28. doi: 10.1001/jama.1954.03690330020006. [DOI] [PubMed] [Google Scholar]
- 9.Hammond E. Smoking in relation to the death rates of one million men and women. Natl Cancer Inst Monogr. 1966;19:127–204. [PubMed] [Google Scholar]
- 10.Department of Health and Human Services. Reducing the health consequences of smoking: 25 years of progress: a report of the Surgeon General. Washington, DC: Government Printing Office; 1989. (DHHS publication no. [CDC] 89–8411.) [Google Scholar]
- 11.Garfinkel L. Selection, follow-up and analysis in the American Cancer Society prospective studies. Natl Cancer Inst Monogr. 1985;67:49–52. [PubMed] [Google Scholar]
- 12.Thun MJ, Day-Lally CA, Calle EE, Flanders WD, Heath CW., Jr Excess mortality among cigarette smokers: changes in a 20-year interval. Am J Public Health. 1995;85:1223–30. doi: 10.2105/ajph.85.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns D, Major J, Shanks T. Those who continue to smoke: is achieving abstinence harder and do we need to change our interventions? Smoking and tobacco control monograph no. 15. Bethesda, MD: National Cancer Institute; 2003. Changes in number of cigarettes smoked per day: cross-sectional and birth cohort analyses using NHIS; pp. 83–99. (NIH publication no. 03–5370.) [Google Scholar]
- 14.Burns D, Major J, Shanks T, Thun M, Samet J. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Smoking and tobacco control monograph no. 13. Bethesda, MD: National Cancer Institute; 2001. Smoking lower yield cigarettes and disease risks; pp. 65–158. (NIH publication no. 02-5074.) [Google Scholar]
- 15.Chahine T, Subramanian SV, Levy JI. Sociodemographic and geographic variability in smoking in the U.S: a multilevel analysis of the 2006–2007 Current Population Survey. Tobacco Use Supplement. Soc Sci Med. 2011;73:752–8. doi: 10.1016/j.socscimed.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Thun M, Day-Lally C, Meyers D, et al. Cigarette smoking behavior in the United States: changes in cigarette-related disease risks and their implication for prevention and control. Smoking and tobacco control monograph no. 8. Bethesda, MD: National Cancer Institute; 1997. Trends in tobacco smoking and mortality from cigarette use in cancer prevention studies I (1959 through 1965) and II (1982 through 1988) pp. 305–82. (NIH publication no. 97-4213.) [Google Scholar]
- 17.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 19.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 20.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(Suppl):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(Suppl):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 22.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 23.Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310:555–9. doi: 10.1136/bmj.310.6979.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Current Population Survey annual social and economic supplement, 2010 to current. Washington, DC: Census Bureau; ( http://www.census.gov/hhes/www/poverty/publications/pubs-cps.html) [Google Scholar]
- 25.America’s families and living arrangements: 2010 — Table 1A: marital status of people 15 years and over by age, sex, personal earnings, race and Hispanic origin. Washington, DC: Census Bureau; ( http://www.census.gov/population/www/socdemo/hh-fam/cps2010.html) [Google Scholar]
- 26.Health, United States. Atlanta: Centers for Disease Control and Prevention; 2011. ( http://www.cdc.gov/nchs/hus/contents2011.htm#061) [Google Scholar]
- 27.Peto and Doll win King Olav V prize for outstanding cancer research. Science Blog. 2002 Jul; ( http://scienceblog.com/community/older/2002/E/20023717.html)
- 28.Department of Health and Human Services. Women and smoking: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention; 2001. [PubMed] [Google Scholar]
- 29.Anderson C, Burns D. Changing adolescent smoking prevalence. Smoking and tobacco control monograph no. 14. Bethesda, MD: National Cancer Institute; 2001. Pattern of adolescent initiation rates over time: national and California data. (NIH publication no. 02–5086.) [Google Scholar]
- 30.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309:901–11. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirie K, Peto R, Reeves GK, Green J, Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2012 Oct 26; doi: 10.1016/S0140-6736(12)61720-6. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-Century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:351–60. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 33.Chronic obstructive pulmonary disease surveillance — United States, 1971– 2000. MMWR Surveill Summ. 2002;51(6):1–16. [PubMed] [Google Scholar]
- 34.Burns D, Benowitz N. Risks associated with smoking cigarettes with low machine-measured tar and nicotine. Smoking and tobacco control monograph no. 15. Bethesda, MD: National Cancer Institute; 2001. Public health Implications of changes in cigarette design and marketing; pp. 1–12. (NIH publication no. 02-5074.) [Google Scholar]
- 35.Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention; 2010. [PubMed] [Google Scholar]
- 36.Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene. 2002;21:7307–25. doi: 10.1038/sj.onc.1205807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.