Abstract
Background and Purpose
Post-stroke hyperglycemia is associated with resistance to tissue plasminogen activator (tPA) reperfusion, higher risk of intracerebral hemorrhage (ICH) and worse neurological outcomes. In this study we asked whether minocycline combined with intravenous tPA may ameliorate inflammation and brain injury after focal embolic stroke in type 1 diabetic rats.
Methods
Type I diabetic rats were subjected to a focal embolic stroke. Three treatment groups: (1) saline at 1.5 hours after stroke; (2) tPA alone at 1.5 hours after stroke; (3) combined minocycline (IV) at 1 hour plus tPA at 1.5 hours, and second treatment of minocycline (IP) at 12 hours after stroke. Acute brain tissue damages were assessed at 24 hours after stroke. Inflammatory biomarkers interleukin 1β (IL-1β) and matrix metalloproteinase 2 and 9 (MMP-2/-9) were examined in plasma. Neutrophil infiltration, microglia activation, MMP activation and degradation of the tight junction protein claudin-5 were examined in the brain.
Results
Compared to saline or tPA alone treatments, minocycline plus tPA combination therapy significantly reduced brain infarction, intracerebral hemorrhage and hemispheric swelling at 24 hours after stroke. The combination also significantly suppressed the elevated plasma levels of MMP-9 and IL-1 up to 24 hours after stroke. At 16 hours after stroke, neutrophil infiltration, microglia activation, MMP-9 activity and tight junction protein claudin-5 degradation in the peri-infarct brain tissues were also significantly attenuated by the combination therapy.
Conclusions
Combination therapy with minocycline plus tPA may be beneficial in ameliorating inflammation and reducing infarction, brain swelling and hemorrhage after ischemic stroke with diabetes/hyperglycemia.
Keywords: Minocycline, tissue plasminogen activator, combination therapy, embolic stroke, diabetes, hyperglycemia
Introduction
Emerging evidence suggest that stroke comorbidities are associated with tPA-associated ICH complication, a major limitation of tPA therapy 1, 2. Clinical investigations have documented a correlation between increased ICH and worse neurological outcomes after tPA thrombolysis in stroke patients with diabetes mellitus or post-stroke hyperglycemia3. Therefore, development of therapeutic approaches for improving tPA thrombolysis in patients with diabetes or post-stroke hyperglycemia is considered a high clinical priority.
Although the pathophysiology of stroke patients with diabetic or post-hyperglycemia is complex, experimental animal studies have suggested that vascular inflammation and BBB disruption may be two major contributors to increased hemorrhagic transformation and worse neurological outcomes after stroke and tPA thrombolytic therapy 4-6. Combination of neuroprotective and anti-inflammatory agents with tPA thrombolysis would be a reasonable approach to overcome above shortcomings2. Minocycline might be a compelling candidate 7-9.
Minocycline is a semisynthetic tetracycline, which has been clinically used as an antibiotic and anti-inflammatory drug. Accumulating experimental evidence has demonstrated minocycline is neuroprotective in multiple neurological disorders including ischemic and hemorrhagic stroke8-10. Ongoing clinical trials suggest that minocycline may be safe and potentially beneficial in humans 11, 12. Although its underlying molecular mechanisms remain to be fully defined, minocycline possess a wide array of anti-inflammatory, anti-apoptotic, anti-oxidative and vascular protective properties 9, 13. These effects may be especially important in the context of stroke patients with vascular comorbidities. A previous study has demonstrated that minocycline dose not affect tPA fibrinolytic activity in vitro and dose not compromise tPA clot lysis in the stroke animal model 14. We have previously shown that minocycline plus tPA may be effective in hypertensive rats15. In this study, we now ask whether this combination therapy may also be effective in a rat model of hyperglycemia/type 1 diabetes.
Materials and Methods
Induction of type I diabetes in rats
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Eight-weeks-old male Wistar rats (Charles River Laboratories, Wilmington, MA) with an initial body weight of 200–220 g were used to induce type-I diabetes by a standard intraperitoneal injection of streptozotocin (60 mg/kg; Sigma, St. Louis, MO). Seven days after streptozotocin administration, rats with blood glucose concentrations of > 15 mmol/L were retained as the diabetic rats as we previously described16.
Focal embolic stroke model and treatment groups
14-week-old (Type 1 diabetes for 6 weeks) streptozotocin-induced diabetic (male, Wistar) rats with blood glucose concentration 18 to 31 mmol/L were subjected to focal embolic strokes as we described previously16. This focal embolic stroke rat model was originally established by Dr. Chopp’s group, its thrombolytic reperfusion time window and hemorrhagic transformation closely mimic clinical situation, which has been most commonly used for thrombolytic stroke studies 17. Totally 63 rats were used in two sets of experiments. In the first set of experiments, 45 rats were divided randomly into 3 treatment groups: (1) intravenous saline at 1.5 hour after ischemia (n=15); (2) intravenous 10 mg/kg of tPA at 1.5 hour after ischemia (n=15); (3) combination of intravenous 10mg/kg of minocycline at 1 hour plus tPA at 1.5 hours, and second minocycline treatment (IP, 45mg/kg) at 12 hours after stroke (n=15). Minocycline dose was selected according to the established dose regimen 9, 10, 18, the relative higher dose used in rodents was based on the fact that the half life of minocycline in rodents is about 2-3 hours 19, whereas in human being the half life is about in a range of 15 to 24 hours 12, 20. Regional cerebral blood flow was used to monitor reperfusion for 1.5 h after induction of ischemia, and then continuously monitored for 1 h after treatments as we previously described16. Only animals survived for 24 hours after stroke were included for outcome assessments; animals dead within 24 hours after stroke were counted for overall mortality rate to all groups. In the second set of experiments, totally 18 rats were used to investigated therapeutic effects of minocycline plus tPA combination in MMP activity, inflammatory responses, and vascular damage. Investigators were blinded to all drug treatments and experimental assessments.
Measurement of ischemic brain infarction volume, hemispheric swelling and intracerebral hemorrhage volume
To examine acute brain tissue outcomes, rats were euthanized 24 hours after ischemia. Seven coronal brain sections (2 mm thick) were stained with 2,3,5-triphenyltetrazolium chloride (TTC, Sigma) to quantify infarct volumes and hemispheric swelling using computer-assisted image analysis. TTC-stained brain sections were used for quantification of intracerebral hemorrhage volume with spectrophotometric hemoglobin assay as we previously described16, 21
Measurements of plasma MMP-2/9 activity and IL-1β levels
To determine changes of systematic inflammatory biomarkers, blood plasma samples were collected before ischemia, at 1.5 hours after stroke (before treatment), 1 hour after treatments (2.5 hours after stroke), and 24 hours after ischemia. The levels of MMP-2 and MMP-9 activity in plasma were measured by gelatin zymogram following our previously described techniques22. Data were expressed as a percentage of human MMP-9 standards in each gel. A commercial ELISA kit (DY401, R&D Systems, Minneapolis, MN) was used to measure IL-1β concentrations in the plasma samples according to the manufacturer’s instructions. 10 μl of plasma sample was applied in each well and all the samples were run in duplicates.
In situ MMP zymography and immunohistochemistry analyses
We collected ischemic brain tissues at 16 hours after stroke and assessed in situ MMP zymography and immunohistochemistry analyses. In brief, we selected brain sections at +1.3, −0.7, −2.7 and −4.7 mm from bregma (axial sections 3, 4, 5, and 6, for quantification, because these represented locations of maximal cortical infarctions). MMP enzyme activity in tissue sections was conducted by following a standard method using a commercial kit (EnzCheck Gelatinase Assay Kit; Molecular Probes, Eugene, OR) as we previously described21, 23. For semi-quantification of the MMP activity, two cortical and two subcortical fields of ischemic boundary area and four fields of contralateral homologous area were acquired using a 20 objective through the fluorescent microscope (Nikon). All readings from four sections were averaged for each rat brain. Data were presented as a percentage of the integrated optical density (IOD) of the saline. Immunohistochemistry was performed by following a standard method as we previously described23. Briefly, we used a rabbit anti-iba-1 antibody (Wako, Osaka, Japan) to examine microglia activation at a titer of 1:200, a polyclonal rabbit anti-human MPO (myeloperoxidase, DAKO, Carpinteria, CA) antibody to examine neutrophil accumulation at a titer of 1:500, and a mouse anti-rat claudin-5 antibody (Invitrogen, Grand Island, NY) to detect the tight junction degradation of BBB at a titer of 1:200. For semi-quantification of microglia activation or neutrophil infiltration, numbers of iba-1 or MPO were counted throughout four fields of view, respectively. For semi-quantification of claudin-5 antigen on two cortical and two subcortical fields of ischemic boundary area and four fields of contralateral homologous area were acquired using a 20 objective through the fluorescent microscope (Nikon), and data were presented as a percentage of the positive immunoreactivity area of the contralateral area (pixel). Totally 18 ischemic rat brains were examined. Fresh brain samples with PBS cardiac perfusion were used for MMP in situ zymography and claudin-5 immunohistochemistry analysis (n=3, per group), and PFA perfused brain samples were used for MPO and Iba-1 immunohistochemistry analysis (n=3 per group).
Statistical analysis
Data were expressed as mean+s.d. All measurements were assessed with ANOVA analysis followed by Tukey–Kramer tests. Differences with P < 0.05 were considered statistically significant.
Results
Streptozotocin-induced type 1 diabetes in rats
At 6 weeks after streptozotocin injection (14 weeks old), the range of blood glucose concentration was approximately 18 to 31 mmol/L, and glycosylated hemoglobin was significantly increased from normal baseline 3.9 ± 0.2% to 9.7±0.7%. These findings confirmed that this standard model may mimic key aspects of hyperglycemia and Type I diabetes mellitus in rats. Blood glucose concentrations were monitored at 0, 1.5, 2.5, and 24 hours after stroke, and there were no significant differences between groups at all time points (Supplement Figure 1).
Minocycline combined with tPA reduced brain tissue damage at 24 hours after stroke
The standard rat dose tPA at 10 mg/kg alone and combined with pretreatment of intravenous 10 mg/kg minocycline only slightly increased reperfusion at 10.3% and 15.6% compared to saline control in the focal embolic stroke of diabetic rats, respectively (Supplement Figure 2). At 24 hours after stroke, tPA alone reduced brain infarction (16.6% reduction) (Figure 1A, 1B), but had no effects in hemispheric swelling (Figure 1C). Instead, tPA alone significantly worsened intracerebral hemorrhage in these diabetic rats (Figure 1D). Combination treatment with minocycline plus tPA significantly reduced brain infarction (30.1% reduction) (Figure 1A, 1B), and suppressed hemispheric swelling (63.9% reduction compared to saline; 60.6% reduction compared to tPA) (Figure 1C). Furthermore, this minocycline-tPA combination also significantly decreased tPA-induced hemorrhagic transformation (20.8% reduction in ICH volume) (Figure 1D). There was no significant difference for mortality within 24 hours after stroke between the three treatment groups (3/15 for saline, 4/15 for tPA alone, and 1/15 for the combination, respectively). Although we did not detect the significant difference in 24 hours mortality, but the statistical significance of the reduced mortality by minocycline plus tPA might be reached with a larger sample size testing. In addition, physiological parameters measured before ischemia, 1.5 hours after ischemia, and 2.5 hours after ischemia remained within the normal range in all groups (data not shown).
Figure 1.
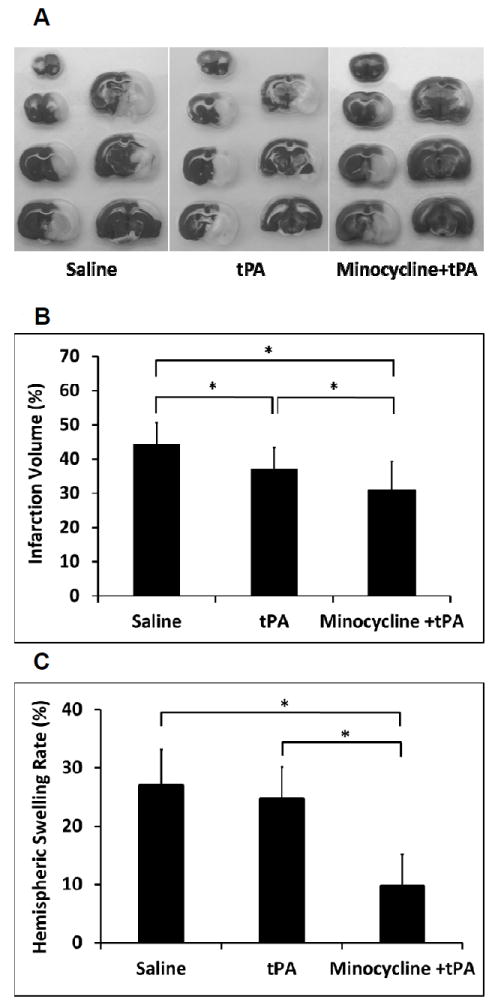
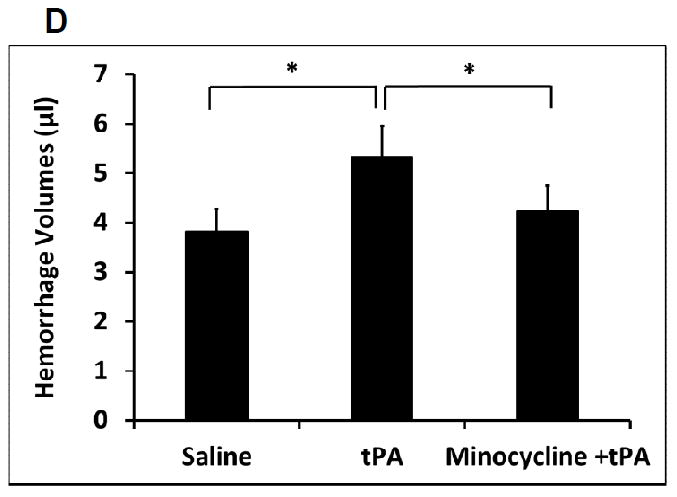
Measurements of acute brain tissue damages in a focal embolic stroke model of diabetic rats. A. Representative images for TTC stained ischemic brain infarctions. B. Ischemic infarct volumes were quantified at 24 hours after stroke. C. Hemispheric swelling were quantified on TTC-stained brain slices. D. Intracerebral hemorrhage volumes were quantified at 24 hours after stroke by hemoglobin assay. E. Blood glucose concentrations were measured at before, 1.5, 2.5, and 24 hours after stroke. Data were expressed as mean + s.d., * P<0.05, n=11-14 per group.
Minocycline combined with tPA reduced MMP-9 levels in plasma and ischemic brains
After stroke onset, total plasma MMP-9 was significantly elevated, and combination of minocycline with tPA significantly suppressed the total plasma MMP-9 compared to the tPA alone treatment group (Figure 2A, 2B). Focal stroke dramatically increased MMP activity in the ischemic brain hemisphere, and the combination of minocycline plus tPA significantly lowered MMP activity compared with tPA alone (Figure 2C, 2D).
Figure 2.
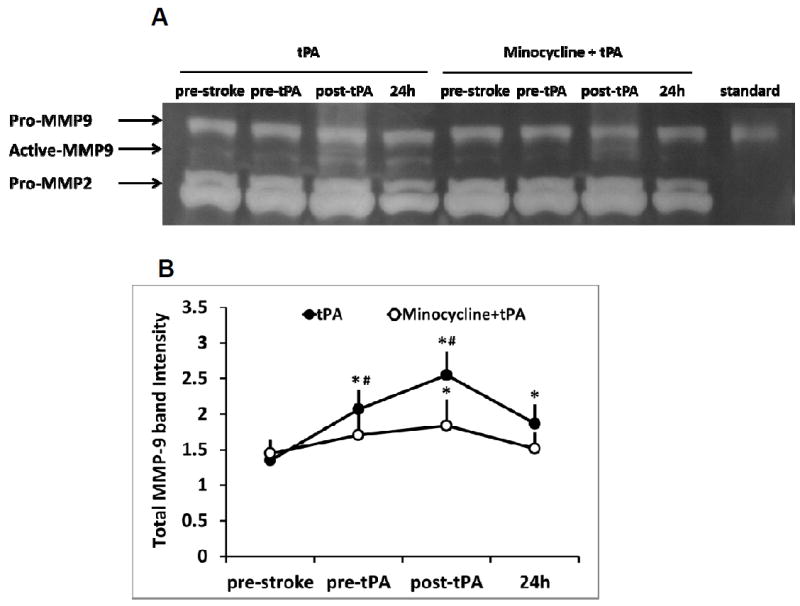
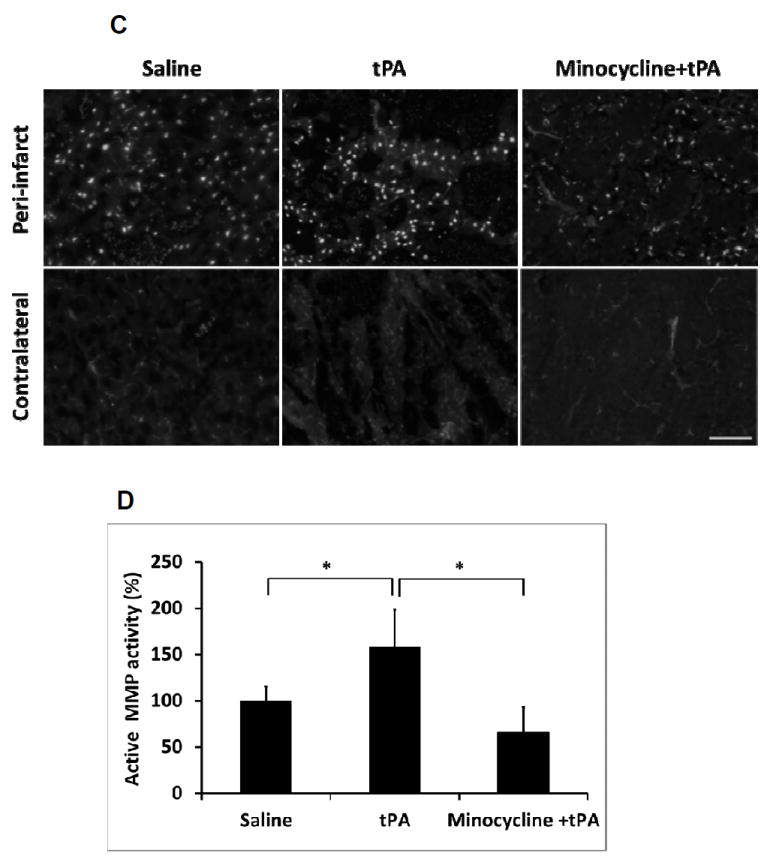
Measurements of MMP-9 activity in plasma and brain tissues of a focal embolic stroke model in diabetic rats. Plasma samples were collected before stroke (pre-stroke), 1.5 hours (pre-tPA), 2.5 hours (post-tPA) and 24 hours after stroke. A. Representative zymography comparing tPA vs minocycline plus tPA. B. Quantitative data of MMP-9 activity. Data were expressed as mean + s.d., *P<0.05 vs pre-stroke, # P<0.05 vs tPA treatment, n=6 per group. C. At 16 hours after stroke, representative MMP activity assessed by in situ zymography on rat brain sections treated with saline, tPA and minocycline combined with tPA, respectively. Bar=100 μm. D. Quantitative data analysis of MMP activity on brain sections. Data were expressed as mean + s.d., * P<0.05, n=3 per group.
Minocycline combined with tPA decreased inflammatory biomarkers after stroke
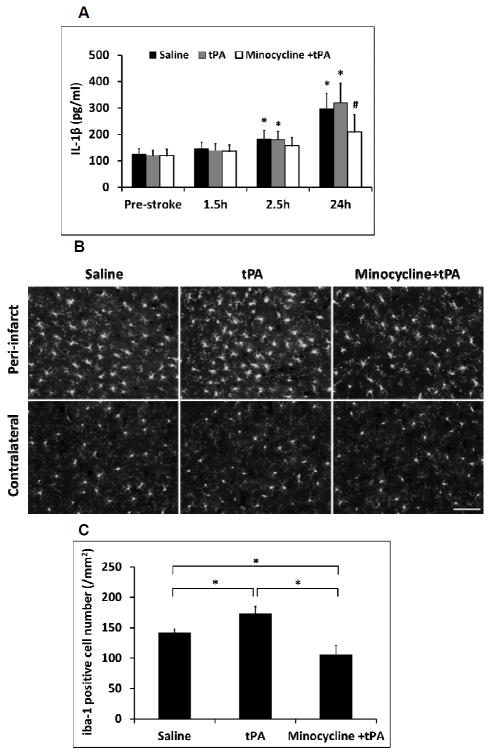
Total plasma IL-1β levels were significantly elevated after stroke. Minocycline combined with tPA significantly decreased total plasma IL-1β levels at 24 hours after stroke (Figure 3A). Activated and proliferating microglia/macrophages were observed in the peri-infarct area of ischemic brains at 16 hours after stroke. tPA significantly increased the number of activated microglia/macrophages, while it was significantly reduced by the combination of minocycline plus tPA. All the three treatments had no significant effect on the number of resting microglia in contralateral hemispheres (Figure 3B, 3C).
Figure 3.
Measurements of inflammatory IL-1β concentration in plasma and microglia activation in ischemic brains of diabetic rats. A. Plasma samples were collected before stroke (Pre-stroke), 1.5 hours, 2.5 hours and 24 hours after stroke. IL-1β concentrations in plasma were examined by ELISA analysis. Data were expressed as mean + s.d., *P<0.05 vs pre-stroke, # P<0.05 vs saline and tPA treatment at 24 hours after stroke, n=6 per group. B. At 16 hours after stroke, representative iba-1 positive cells by immunostaining on rat brain sections treated with saline, tPA and minocycline combined with tPA, respectively. Bar=100 μm. C. Quantitative data of microglia/macrophages activation (iba-1 positive cells). Data were expressed as mean + s.d., * P<0.05, n=3 per group.
Minocycline combined with tPA decreased neutrophil infiltration of ischemic brains
Numerous MPO-positive cells, presumably infiltrated neutrophils, were detected in the peri-infarct area of ischemic brains at 16 hours after stroke, which was significantly increased in the brains of tPA treated stroke rats. However, minocycline combined with tPA treatment significantly reduced neutrophil infiltration in the peri-infarct areas (Figure 4A, 4B).
Figure 4.
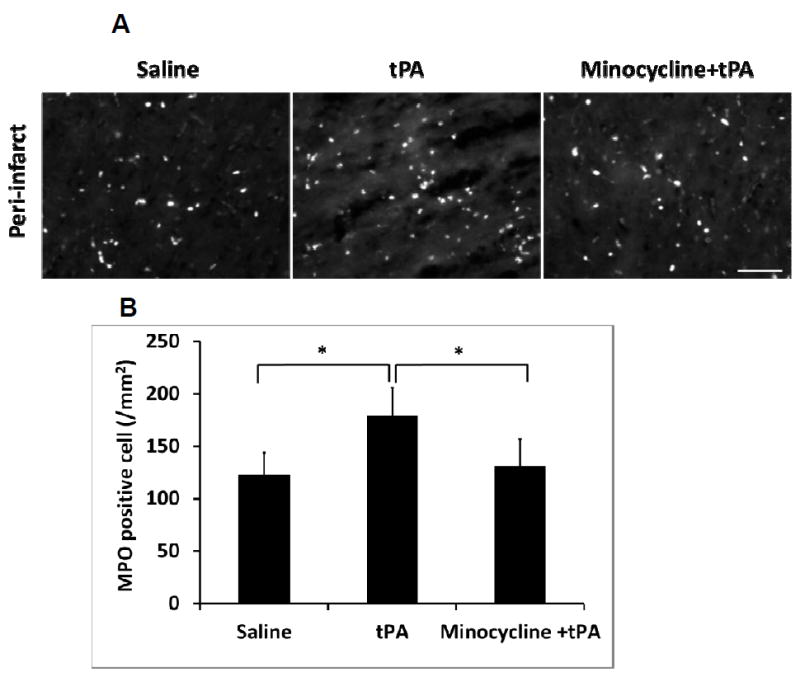
Measurements of inflammatory neutrophil infiltration in ischemic brains of diabetic rats. A. At 16 hours after stroke, representative MPO positive cells by immunostaining on rat brain sections treated with saline, tPA and minocycline combined with tPA, respectively. Bar=100 μm. B. Quantitative data of neutrophils infiltration (MPO positive cells). Data were expressed as mean + s.d., * P<0.05, n=3 per group.
Minocycline combined with tPA ameliorated tPA induced tight junction protein claudin-5 degradation
At 16 hours after stroke, there was a significant reduction in claudin-5 antigen levels in the peri-infarct area of ischemic brains treated with tPA, indicating a degradation of tight junction integrity in cerebral microvessels. The degradation of claudin-5 was significantly diminished by minocycline combined with tPA treatment compared with tPA alone (Figure 5A, 5B).
Figure 5.
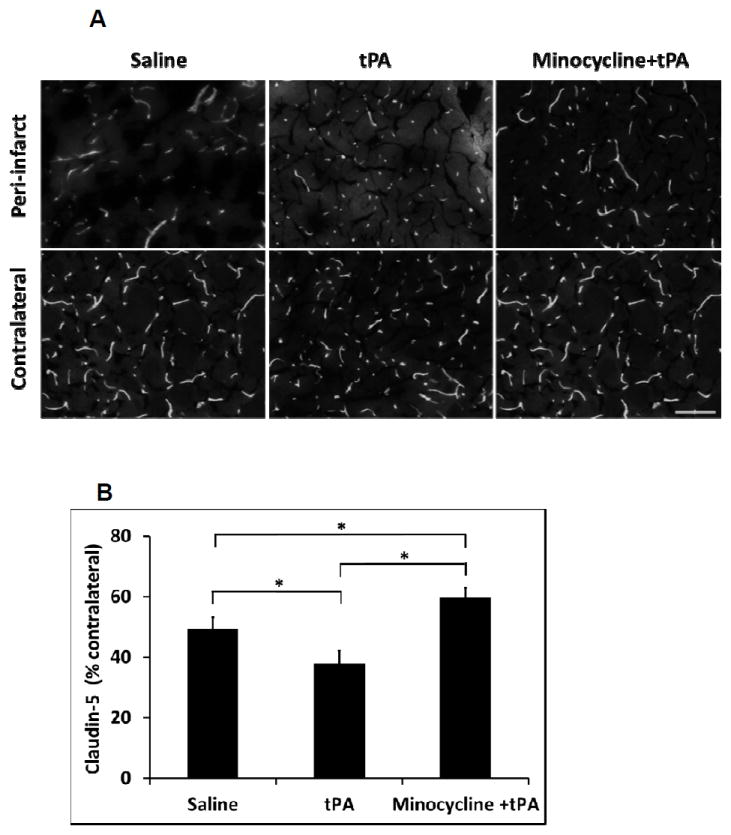
Measurement of tight junction protein claudin-5 degradation in ischemic brains of diabetic rats. A. At 16 hours after stroke, representative claudin-5 expression by immunostaining on rat brain sections treated with saline, tPA and minocycline combined with tPA, respectively. Bar=100 μm. B. Quantitative data of claudin-5 expression. Data were expressed as mean + s.d., * P<0.05, n=3 per group.
Discussion
Experimental results from this study showed that minocycline plus tPA combination therapy significantly reduced brain infarction, intracerebral hemorrhage and hemispheric swelling at 24 hours after stroke. Furthermore, our initial findings also suggest that these beneficial effects of minocycline plus tPA may be mediated by the amelioration of MMP-9, inflammatory responses, and microvascular tight junction degradation.
MMPs, especially MMP-9 activation triggers extracellular matrix proteolysis dysfunction that contributes to the ischemic brain tissue and cerebrovascular damage and tPA-mediated ICH2, 24, 25. Animal studies have also shown that MMP plays an important role in cerebrovascular damage following permanent focal stoke of diabetic rats26, 27. Importantly, it has been reported that minocycline has potent inhibitory effects in both MMP-9 gene expression and proteolytic activity 18,14, 15, 28. Previous studies have suggested that the early increased plasma MMP-9 is released from circulating neutrophils 29, 30. Results from present study showed that increased plasma MMP-9 and brain MMP activity after stroke and tPA thrombolysis were significantly inhibited by the combination of minocycline with tPA in diabetic rats. This effect may underlie the ability of the tPA-minocycline combination to reduce hemorrhagic transformation in our model.
Increased systemic and cerebrovascular inflammation is one of key pathophysiological features in diabetes and its vascular complications31, 32. Moreover, ischemic stroke and tPA thrombolysis further stimulate acute inflammation cascades that contribute to ischemic brain damage and intracerebral hemorrhage transformation2. It has been reported that minocycline has a strong anti-inflammatory effect in ischemic stroke animals33. Hence, based on this rationale, we examined three well-established inflammatory biomarkers i.e. plasma IL-1β, microglia activation and neutrophil infiltration into ischemic brain tissue 34-36. Our results showed that minocycline combined with tPA treatment significantly decreased total plasma IL-1β levels at 24 hours after stroke compared to tPA alone. In addition, at 16 hours after stroke, the combination significantly reduced the number of activated microglia/macrophages and MPO-positive neutrophils in the peri-infarct area compared to saline and tPA alone treatments. These results are consistent with the overall ability of minocycline to dampen ischemic inflammation.
Ischemic stroke and tPA thrombolysis cause vascular damage which may cause ICH and edema formation 2. Recently, experimental studies have found exacerbated vascular damage after focal ischemic stroke in both acute hyperglycemic and diabetic rats27. In this study, we examined a commonly used biomarker for cerebrovascular damage, i.e. degradation of claudin-5 protein, a key integral membrane proteins and components of tight junction strands37. We found tPA alone significantly increased degradation of claudin-5 protein at 16 hours after stroke, but this was significantly reduced by the tPA-minocycline combination.
Taken together, our experimental results suggest that minocycline combined with tPA may lessen ischemic brain damage and reduce risk of intracerebral hemorrhagic transformation by suppressing MMP activity in circulation and brain tissues, decreasing brain inflammation and protecting against cerebrovascular damage. We acknowledge that these complex pathways have many feedback loops2. Nevertheless, we believe these mechanisms are critical for minocycline’s beneficial properties, i.e. minocycline interrupts multifactorial pathogenic pathways that operate in parallel after stroke and tPA thrombolysis that result in overall synergistic protective effects7-10, 13.
There are a number of caveats and limitations in this work. First, our blood, tissue and molecular data are collected only within an acute timeframe. This may be consistent with the fact that post-stroke hyperglycemia typically affects acute infarct development3, and most tPA thrombolysis-associated ICH transformation occur within 12-36 hrs after stroke38. However, for translation and clinical utility, further studies are required to confirm that these acutely beneficial results truly correlate with improved long-term neurological outcomes. Second, we only induced hyperglycemia to mimic type I diabetes in young male rats. These mechanisms may be affected by age and gender, so further studies to test female and aged models should be useful. Third, we were unable to detect early reperfusion after tPA or in combination with tPA for up to 1 hour. In part, this may be related to our relatively brief period of LDF measurements in a restricted cortical region39. The effects of our combination therapy on the late treatment window and prolonged profile of thrombolytic reperfusion warrant further studies. Finally, minocycline has many pleiotropic properties. Besides targeting MMPs, inflammation and cerebrovascular tight junctions, other mechanisms involving anti-oxidative tissue damage and direct neuroprotection may also be taking place9, 13, 34. Further experiments to dissect and potentially optimize these multifactorial pathways would be clinical important.
In summary, this study showed that minocycline combined with tPA significantly reduced ischemic brain infarction, hemispheric swelling, and tPA-associated intracerebral hemorrhagic transformation at 24 hours after focal embolic stroke of type 1 diabetic rats. These beneficial effects of tPA-minocycline combination therapy may be mediated in part by the suppression of MMP-9 activity, decrease in brain tissue inflammation and protection against cerebrovascular damage. Further translation studies to test develop this therapeutic approach for diabetic brains may be warranted.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported in part by the National Institute of Health grants R01-NS065998 and UO1-NS072324 (to X.W.).
Footnotes
Disclosures: None
References
- 1.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Rosell A, Lo EH. Targeting extracellular matrix proteolysis for hemorrhagic complications of tpa stroke therapy. CNS Neurol Disord Drug Targets. 2008;7:235–242. doi: 10.2174/187152708784936635. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Sabin J, Molina CA, Ribo M, Arenillas JF, Montaner J, Huertas R, et al. Impact of admission hyperglycemia on stroke outcome after thrombolysis: Risk stratification in relation to time to reperfusion. Stroke. 2004;35:2493–2498. doi: 10.1161/01.STR.0000143728.45516.c6. [DOI] [PubMed] [Google Scholar]
- 4.Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by increasing superoxide production. Annals of neurology. 2011;70:583–590. doi: 10.1002/ana.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Silva DA, Ebinger M, Christensen S, Parsons MW, Levi C, Butcher K, et al. Baseline diabetic status and admission blood glucose were poor prognostic factors in the epithet trial. Cerebrovascular diseases. 2010;29:14–21. doi: 10.1159/000255969. [DOI] [PubMed] [Google Scholar]
- 6.Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;32:617–622. doi: 10.2337/dc08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess DC, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: Minocycline. Pharmacotherapy. 2010;30:55S–61S. doi: 10.1592/phco.30.pt2.55S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagan SC, Cronic LE, Hess DC. Minocycline development for acute ischemic stroke. Transl Stroke Res. 2011;2:202–208. doi: 10.1007/s12975-011-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet neurology. 2004;3:744–751. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhao F, Hua Y, He Y, Keep RF, Xi G. Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke. 2011;42:3587–3593. doi: 10.1161/STROKEAHA.111.623926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, et al. Minocycline treatment in acute stroke: An open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- 12.Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, et al. Minocycline to improve neurologic outcome in stroke (minos): A dose-finding study. Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Archives of neurology. 2010;67:1442–1448. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machado LS, Sazonova IY, Kozak A, Wiley DC, El-Remessy AB, Ergul A, et al. Minocycline and tissue-type plasminogen activator for stroke: Assessment of interaction potential. Stroke. 2009;40:3028–3033. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan X, Qiu J, Yu Z, Dai H, Singhal AB, Lo EH, et al. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke. 2012;43:567–570. doi: 10.1161/STROKEAHA.111.635250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain research. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 18.Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, et al. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25:460–467. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- 19.Colovic M, Caccia S. Liquid chromatographic determination of minocycline in brain-to-plasma distribution studies in the rat. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:337–343. doi: 10.1016/s1570-0232(03)00247-2. [DOI] [PubMed] [Google Scholar]
- 20.Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clinical pharmacokinetics. 1988;15:355–366. doi: 10.2165/00003088-198815060-00001. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Fan X, Yu Z, Liu J, Murata Y, Lu J, et al. Annexin a2 combined with low-dose tpa improves thrombolytic therapy in a rat model of focal embolic stroke. J Cereb Blood Flow Metab. 2010;30:1137–1146. doi: 10.1038/jcbfm.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park KP, Rosell A, Foerch C, Xing C, Kim WJ, Lee S, et al. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke. 2009;40:2836–2842. doi: 10.1161/STROKEAHA.109.554824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 25.Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 26.Elgebaly MM, Prakash R, Li W, Ogbi S, Johnson MH, Mezzetti EM, et al. Vascular protection in diabetic stroke: Role of matrix metalloprotease-dependent vascular remodeling. J Cereb Blood Flow Metab. 2010;30:1928–1938. doi: 10.1038/jcbfm.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elgebaly MM, Ogbi S, Li W, Mezzetti EM, Prakash R, Johnson MH, et al. Neurovascular injury in acute hyperglycemia and diabetes: A comparative analysis in experimental stroke. Transl Stroke Res. 2011;2:391–398. doi: 10.1007/s12975-011-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuadrado E, Ortega L, Hernandez-Guillamon M, Penalba A, Fernandez-Cadenas I, Rosell A, et al. Tissue plasminogen activator (t-pa) promotes neutrophil degranulation and mmp-9 release. J Leukoc Biol. 2008;84:207–214. doi: 10.1189/jlb.0907606. [DOI] [PubMed] [Google Scholar]
- 30.Gautier S, Ouk T, Petrault O, Caron J, Bordet R. Neutrophils contribute to intracerebral haemorrhages after treatment with recombinant tissue plasminogen activator following cerebral ischaemia. British Journal of Pharmacology. 2009;156:673–679. doi: 10.1111/j.1476-5381.2009.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. The Journal of clinical endocrinology and metabolism. 2009;94:3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 32.Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, et al. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun. 2011;25:1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu LS, Fang SH, Zhou Y, Yu GL, Wang ML, Zhang WP, Wei EQ. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol Sin. 2007;28:763–772. doi: 10.1111/j.1745-7254.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 34.Pabreja K, Dua K, Sharma S, Padi SS, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: Possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661:15–21. doi: 10.1016/j.ejphar.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Ritter L, Davidson L, Henry M, Davis-Gorman G, Morrison H, Frye JB, et al. Exaggerated neutrophil-mediated reperfusion injury after ischemic stroke in a rodent model of type 2 diabetes. Microcirculation. 2011;18:552–561. doi: 10.1111/j.1549-8719.2011.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Rosenberg GA. Mmp-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods in molecular biology. 2011;762:333–345. doi: 10.1007/978-1-61779-185-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Intracerebral hemorrhage after intravenous t-pa therapy for ischemic stroke. The ninds t-pa stroke study group. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 39.Henninger N, Bouley J, Bratane BT, Bastan B, Shea M, Fisher M. Laser doppler flowmetry predicts occlusion but not tpa-mediated reperfusion success after rat embolic stroke. Experimental neurology. 2009;215:290–297. doi: 10.1016/j.expneurol.2008.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.