Abstract
Background and Purpose
Inflammatory and fibrogenic processes play a crucial role in the radiation-induced injury in the lung. The aim of the present study was to examine whether additive LPS exposure in the lung (to simulate respiratory infection) would affect pneumonitis or fibrosis associated with lung irradiation.
Material and Methods
Wildtype C57Bl/6J (WT-C57) and TNFα, TNFR1 and TNFR2 knockout (−/−) mice, in C57Bl/6J background, were given whole thorax irradiation (10Gy) with or without post-irradiation intratracheal administration of LPS (50μg/mice). Functional deficit was examined by measuring breathing rate at various times after treatment. Real-time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and immunohistochemistry were used to analyse the protein expression and m-RNA of Interleukin-1 alpha (IL-1α), Interleukin-1 beta (IL-1β), Interleukin-6 (IL-6), Tumour Necrosis Factor alpha (TNFα) and Transforming Growth Factor beta (TGFβ) in the lung at various times after treatment. Inflammatory cells were detected by Mac-3 (macrophages) and Toluidine Blue (mast cells) staining. Collagen content was estimated by hydroxyproline (total collagen) and Sircol assay (soluble collagen). Levels of oxidative damage were assessed by 8-hydroxy-2-deoxyguanosine (8-OHdG) staining.
Results
LPS exposure significantly attenuated the breathing rate increases following irradiation of WT-C57, TNFR1−/− and TNFR2−/− mice and to a lesser extent in TNFα−/− mice. Collagen content was significantly reduced after LPS treatment in WT-C57, TNFR1−/− and TNFα−/− mice and there was a trend in TNFR2−/− mice. Similarly there were lower levels of inflammatory cells and cytokines in the LPS treated mice.
Conclusions
This study reveals a mitigating effect of early exposure to LPS on injury caused by irradiation on lungs of C57Bl mice. The results suggest that immediate infection post irradiation may not impact lung response negatively in radiation-accident victims, however, further studies are required in different animal models, and with specific infectious agents, to confirm and extend our findings.
Keywords: TNF alpha −/−, TNFR1−/− and TNFR2−/− mice, lung, radiation, lipopolysaccharide (LPS), pneumonitis, fibrosis
Introduction
Lung is one of the more sensitive organs to irradiation and recent concerns for the accidental or deliberate exposure of the general population to irradiation due to terrorism have resulted in studies of agents to mitigate or treat the symptoms of pneumonitis or fibrosis [1–3]. Radiation-induced pneumonitis and fibrosis are distinguished by their time of expression after irradiation and characteristic histologic changes [4]. Although the exact mechanisms involved in tissue response after lung exposure to irradiation remain uncertain, there is evidence for increased expression of inflammatory cytokines in lung taking place within hours to days to weeks after irradiation, consistent with a prolonged inflammatory response [5–10].
The aim of the present study was to examine whether additive LPS exposure in the lung (to simulate respiratory infection) would affect pneumonitis or fibrosis associated with lung irradiation. LPS, a glycolipid, is the only lipid present in the outer membrane of gram-negative bacteria. On release of LPS into the circulation, a series of tissue responses are activated that may trigger severe reactions resulting in septic shock and death. Major events that lead to LPS-induced pathogenesis include inflammatory responses via NF-kB activation and TNF signaling. The effects of TNFα are mediated through two distinct cell surface receptors, the 55-kDa type 1 TNFR1 and the 75-kDa type 2 TNFR2. TNFR-1 mediates most pro-inflammatory and cytotoxic effects of TNFα, including shock and tissue injury induced by endotoxins such as LPS [11–15]. The role of TNFR2 is indirect. It aids in the recruitment of TNF to the cell membrane and passes the signal to TNFR1 or regulates the amount of TNF which is accessible to TNFR1 [16, 17]. Expression of TNFR1 has been reported to be increased in various models of inflammatory lung injury and disease, suggesting that TNFα may be a major mediator of the pathogenic response to toxicants [18–21]. This is supported by findings that mice lacking TNFR1 are protected from lung injury induced by pulmonary irritants such as ozone, silica, bleomycin and radiation [22–24]. Furthermore, TNFR1 knockout mice fail to develop fibroproliferative lesions in lung after asbestos exposure [25]..
In this study we explored the effects of LPS on pneumonitis and fibrosis and investigated the pro- and anti-inflammatory cytokine and fibrotic response produced by radiation-induced lung injury in C57Bl/6J wild type and TNFα knockout (−/−), TNFR1−/− and TNFR2−/− mice. Our previous studies had found that TNFα knockout (−/−) mice had reduced sensitivity to lung irradiation [10].
Methods
Mice
Eight-week old female C57-WT, TNFα−/−, TNFR1−/− and TNFR2−/−, mice were housed at the Ontario Cancer Institute/Princess Margaret Hospital small animal facility accredited by the Canadian Council on Animal Care and were treated in accordance with approved protocols. The W/T mice were purchased from JAX Laboratories. The knockout mice were bred in house from stock obtained from the laboratories of Drs Khokha and Mak. They had been backcrossed at least 10 generations into the C57BL/6 background. Mice from each strain were divided into three experimental groups: control, radiation, radiation plus LPS. Mice were sacrificed at 12, 20 and 24 weeks post irradiation for subsequent analysis.
Irradiation
All mice were irradiated in an image guided small animal irradiator (X-Rad 225Cx, Precision X-ray, North Branford, CT, USA). The x-ray tube in this unit is mounted on a rotating C arm with a flat panel detector opposite for image-guided set-up. The imaging characteristics of the unit have been described previously [26]. The x-ray tube was calibrated at 100kVp, 30mA following the AAPM TG-61 protocol [27] for radiation treatments. Specifically for this study, a 2.2 cm diameter surface collimator was used for targeting the whole lung. Further dosimetry on this collimator was done by using EBT Gafchromic films in solid water at the depth of 0.5cm. The dose rate at 100kVp, 30mA (HVL: 2.95mm Al, added filtration: 2mm Al) was estimated as 3.13 Gy/min. Each animal was anesthetized by isofluorane inhalation and immobilized supine in a Lucite jig. Two circular lead surface collimators (OD: 4.9cm and ID: 2.2cm) were inserted on the surfaces of the jig to further facilitate targeting of the lung volume with minimized diaphragm in the radiation field. Animals were imaged and adjusted inside the jig for targeting the whole lung volume inside the lead surface collimators. Anatomical features were used for targeting this volume. The integrated targeting software was used to locate the mid plane of the animal, at a depth of 0.5cm, on the iso-center of the unit. All animals were irradiated with two beams anterior-posterior (a-p) and p-a to a total dose of 10Gy. We have found previously that this dose leads to some lethality in C57Bl mice at about 26 weeks after irradiation (data not shown). The imaging dose was estimated to be less than 1cGy.
LPS treatment
LPS, 50 μg/mice (Escherichia coli, 026-B6, Sigma-Aldrich) was administered intratracheally approximately one hour after the 10 Gy irradiation. Injections were performed in mice anesthetised by halothane inhalation. After an incision was made in the soft tissue overlying the trachea, 100 μl of LPS was injected via a 27gauge needle into the visualized trachea. We found that this dose of LPS caused an early increase in cytokine levels in the normal lung of C57Bl W/T mice (data not shown).
Breathing rate
The breathing rate of mice was measured weekly starting at Day 0 up to week 24 post-irradiation using a whole body plethysmograph (Columbus Instruments, Columbus, Ohio, USA). Mice were allowed to acclimatize for one minute before each measurement. The reading for each mouse was taken for one minute and the data of at least 3 readings were selected manually from regions free of noise. Due to movement it was not always possible to obtain data at each time point for every animal. A minimum of 15 animals contributed to each data point for the C57-WT mice (out of a total of 30/treatment gp) and a minimum of 5 for the knockout mice (out of a total of 10/treatment gp). Data are represented as the mean ± SE.
Lung extraction
For lung extraction, mice were lethally anaesthetized and the lungs removed. The left lung was inflated with 1–2 ml of 10% formalin. The right lobes were frozen and used for the other assays involving digestion and analysis of the lung tissue. The left lobes were placed in 10% formalin and afterwards embedded in paraffin, and sectioned at an average thickness of 5 μm for subsequent histological and immunohistochemical analysis. There were a minimum of 6 mice per treatment group at each time point.
PCR analysis
RNA extraction
Total RNA preparations were performed following manufacturers’ instructions with minimal modifications, using the RNeasy® Mini Kit (Qiagen, Mississauga, Ontario, Canada). Briefly, 60mg of tissue was removed from RNAlater solution and homogenized using a rotor-stator homogenizer (IKA, Wilmington, NC, USA). The resulting RNA sample was stored at −80°C until needed. RNA concentration was determined by spectrophotometer absorption at 260 nm and the integrity of the RNA was assessed by running mini agarose gel electrophoresis.
First-strand complementary DNA (cDNA) synthesis
Reverse Transcription was carried out using an Omniscript Reverse Transcription Kit (Qiagen, Mississauga, Canada) according to the manufacturer’s protocol. Oligo-dT primers were used for generating first-strand cDNA in a final reaction mix of 20 μl. Samples were stored at −80°C for no longer than 1 week.
Real-Time RT-PCR
A master mix using the QuantiTectTM SYBR1 Green PCR kit (Qiagen, Mississauga, Ontario, Canada) was prepared according to the manufacturer’s protocol. GAPDH was used as a control since we have previously shown that expression of this gene in lung is not affected by irradiation [9]. The expression of IL-1α, IL-1β, IL-6, and TNFα and TGFβ was quantified by RT-PCR (ABI Prism77001 Sequence Detection System, Applied Biosystems, and Foster City, CA, USA).
Immunohistochemistry
Tissue sections (5um thick) were stained with Haematoxylin and Eosin (H & E), Toluidine Blue and antibodies to detect 8′-hydroxy-2-deoxyguanosine (JaICA Cat # MOG-100P), MAC3 (BD Pharmingen Cat #550292), IL-1α (Santa Cruz #sc-9983, Santa Cruz Biotechnology), IL-1β (Santa Cruz #sc-7884), IL-6 (Santa Cruz #sc-1265), TNFα (SantaCruz #sc-1348) and TGFβ (Santa Cruz #sc-146). Immunohistochemistry was performed in the Pathology core facility of the Toronto General Hospital. Following staining, the slides were scanned using the ScanScope XT (Aperio Technologies, Vista, CA, USA). This is a brightfield scanner that digitizes the whole microscope slide at 20x and 40x magnifications and provides high resolution images. The images can then be viewed with ImageScope (Aperio Technologies) for quantitative analysis. Using the Positive Pixel Algorithm, the whole slide was analyzed and the number of positive pixels/number of positive and negative pixels x 100% was recorded (% positivity). Air spaces were excluded.
Hydroxyproline Assay
Hydroxyproline content was measured using a kit following the manufacturer’s instructions (Sigma-Aldrich Canada, Oakville, ON, Canada). Lung tissue (100 mg) was digested at 60°C for 48 hours and then subjected to acid hydrolysis for 18 hours at 110°C. Free hydroxyproline was released from protein and peptides into the solution that was then neutralized. The hydroxyproline was oxidized into a pyrrole with chloramine T. This intermediate turns pink in color with the addition of Ehrlich’s Reagent (4-dimethylaminobenzaldehyde). The absorbance was measured at 560 nm.
Sircol assay
The Sircol assay (Biocolor Ltd., Belfast, United Kingdom) was performed following the manufacturer’s instructions. Briefly, 65 mg of lung tissue was prepared as an homogenate and Sirius red reagent was added to each lung homogenate and mixed for 30 minutes. The collagen–dye complex was precipitated and separated by centrifugation at 12,000 g for 10 minutes, and dissolved in alkali reagent. Finally, the samples were loaded into a microplate reader and the absorbance determined at 540 nm.
Statistical analysis
For comparison between the control and the various time points, an Analysis of Variance (ANOVA) was performed. Multiple linear regressions and Tukey’s method for the adjustment of least square means in multiple comparisons were used for analysis of the data sets. Mixed modeling was used to examine time trends in the breathing rate data. SAS (enterprise guide-4) software was used for the analysis.
Results
Breathing Rate
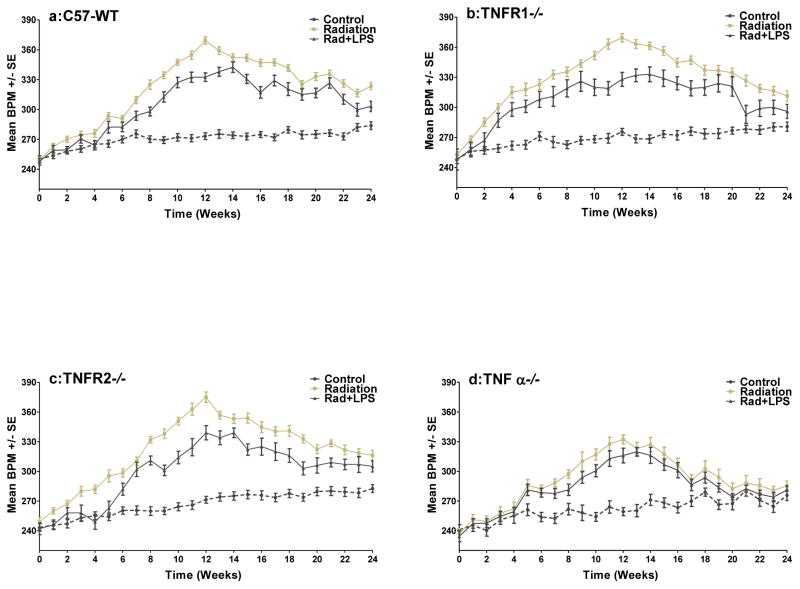
Increases in breathing rate are widely used in rodent models as an indicator of pneumonitis caused by radiation. As seen in Figure 1 (a–d) the irradiated C57-WT mice and the three different knockout mice all exhibited significantly higher (p<0.001) breathing rates (with a peak at 12 weeks) compared with non-irradiated control groups. The increase in the breathing rate showed an earlier response in the TNFR1−/− mice (p< 0.05) and the extent of the increase was reduced in the TNFα−/− mice (p< 0.01). The TNFR2−/− mice showed a response that was similar to that of the C57-WT mice. Exposure to LPS within 1 hr of irradiation resulted in a reduced breathing rate increase (p<0.01) in TNFR1−/− and TNFR2−/− mice (Figs 1b & c). This is also seen in C57-WT mice (Fig 1a) as we have reported previously [10]. A decrease in the TNFα−/− mice was also observed (Fig 1d) despite the reduced effect of irradiation but the reduction was less than for the other groups of mice. Nevertheless the breathing rate curve for these mice was significantly below that for the other groups of mice particularly at the later times (p<0.05). The earlier increase in the breathing rate for the TNFR1−/− mice is still seen in the LPS exposed mice. At 20–24 weeks after radiation the breathing rate increase in the TNFα−/− mice had returned to control levels but for the other three groups of mice there was still a small elevation (Figs 1a–d).
Figure 1.
The mean breathing rate (±SEM) for groups of mice (a) C57WT, (b) TNFR1 −/−, (c) TNFR2−/−, (d) TNFα−/−, as a function of time after being given either 0 or 10 Gy (+/− LPS) to the whole lung at time zero. Dashed black line indicates the control mice, gray line indicates radiation treated mice and black line indicates radiation + LPS treated mice. A preliminary version of some of these results has been published previously (10).
Cytokine expression
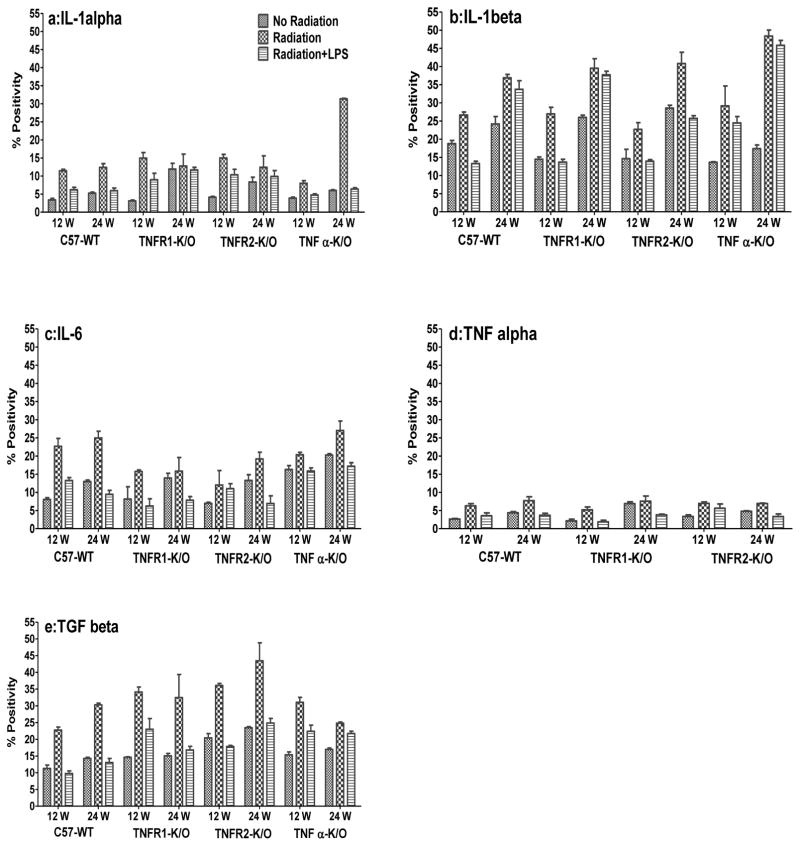
To examine possible reasons for the effect of the LPS exposure and the reduced breathing rate increase for the TNFα−/− mice, we determined the expression of a variety of inflammatory cytokines. Quantitative results for positive protein staining for IL-1α, IL-1β, IL-6, TNFα and TGFβ in the lung at two different time-points (12 and 24 wks) after irradiation are shown in Figure 2 (a–e) and IHC images of the staining are shown in Supplementary Figure 1 (A-C). Results for mRNA expression in the lung tissue are shown in Supplementary Figure 2. Results obtained at 20 wks were similar to those at 24 weeks (data not shown). For IL-1α and IL-1β there was not a clear relation between the changes in the mRNA and protein expression but for the other three cytokines there was reasonable agreement. For both IL-1α and IL-1β the mRNA expression (Supplementary Fig 2a, b) was lower in the irradiated lungs than in age-matched controls but protein expression (Fig 2a, b) was higher, particularly in the TNFα−/− mice. Similarly, addition of LPS exposure showed a trend for an increase at the mRNA level but a decrease at the protein level. The extent of these decreases in IL-1α protein staining varied between the two time points and the different mouse groups but it was significant for the C57-WT and TNFα−/− mice (p <0.01).
Figure 2.
Quantitative analysis of IHC staining for cytokines IL-1α (a), IL-1β (b), IL-6 (c), TNFα (d) and TGFβ (e), at 12 and 24 weeks following lung irradiation (10Gy) with/without post-irradiation (within 1h) intratracheal administration of LPS. Percent positivity is the ratio of positive pixels/total number of positive and negative pixels in the tissue section (air spaces excluded). Each bar represents the mean (±SEM for 6 mice).
For IL-6 there were higher levels of mRNA expression (Supplementary Fig 2c) in the controls in the C57-WT mice relative to the other three groups of mice and there was a significantly (p <0.001) greater increase in mRNA expression at 12 and 24 wks after irradiation in the C57-WT mice. This difference was not so clear in the IHC (protein) staining (Fig 2c) but the increases remained significant for the C57-WT (p<0.01) mice. The TNFα−/− mice showed higher IL-6 protein levels in the controls and a smaller relative increase following irradiation. Exposure to LPS caused a decrease in protein expression in all groups of mice at both times (p<0.05) except for TNFR1−/− mice. Decreases following LPS were less apparent in the mRNA expression levels
TNFα mRNA expression in the C57-WT, TNFR1−/− /and TNFR2−/− mice increased following irradiation (Supplementary Fig 2d). This increase was significant (p<0.001) in the C57-WT mice when compared to the controls but was only a trend in the TNFR1−/− and TNFR2−/− mice. TNFα protein staining in the lung tissue exhibited low levels (Fig 2d) but there was a significant (p<0.05) increase in all the irradiated groups of mice at 12 and 24 wks when compared to the controls. Exposure to LPS caused a decrease of the TNFα staining level in comparison to the irradiated groups at both time points. The decrease was significant in C57-WT and TNFR2−/− mice (p<0.01) at 24 wks and TNFR1−/− (p<0.01) at 12 wks. There was a similar but less pronounced trend for the mRNA levels.
TGFβ mRNA expression (Supplementary Fig 2e) and protein staining (Fig 2e) in the lung tissue showed an increase following irradiation at all the time-points for all the groups of mice (p<0.01). Additional exposure to LPS decreased the TGFβ mRNA expression (trend) and protein staining levels (p<0.05) when compared to irradiation alone in all groups of mice at both time points.
MAC-3
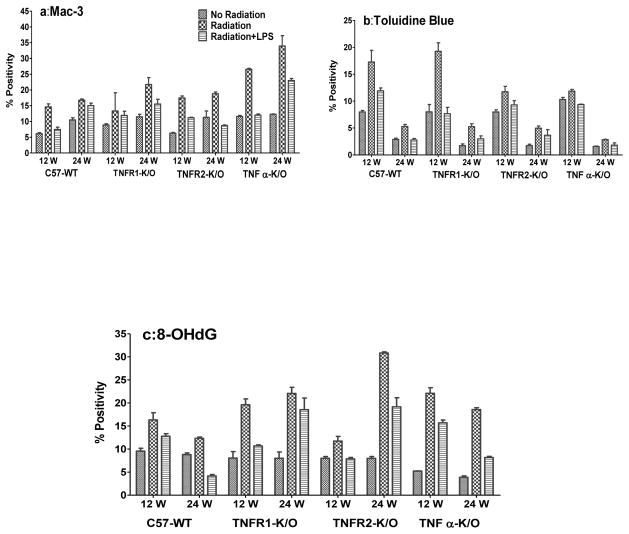
Analysis of macrophage activation as demonstrated by MAC-3 staining is shown in Figure 3a. The MAC-3 staining showed a significant increase in expression in all the irradiated mouse groups at both times (p<0.05) except in TNFR1−/− mice at 12 wks. Additional exposure to LPS post irradiation significantly decreased the macrophage activation levels as compared to irradiation alone (p<0.05) in all the mice at both times with the exception of the TNFR1−/− mice at 12 wks and in the C57-WT mice at 24 wks.
Figure 3.
(a) Mac-3 staining for activated macrophages in the mouse lungs at 12 and 24 weeks following lung irradiation (10Gy) with/without post-irradiation (within 1h) intratracheal administration of LPS. (b) Toluidine Blue staining for mast cells in the mouse lungs at 12 and 24 weeks following lung irradiation (10Gy) with/without post-irradiation (within 1h) intratracheal administration of LPS. (c) 8-OHdG staining (oxidative stress/DNA damage) in the mouse lungs at 12 and 24 weeks following lung irradiation (10Gy) with/without post-irradiation (within 1h) intratracheal administration of LPS. Percent positivity is the ratio of positive pixels/total number of positive and negative pixels in the tissue section (air spaces excluded). Each bar represents the mean (±SEM for 6 mice).
Toluidine Blue Staining
Analysis of Toluidine Blue staining (for mast cells) is demonstrated in Figure 3b. As expected because the mast cell influx is primarily associated with pneumonitis [28] the increase in Toluidine Blue staining was greater at 12 wks than 24 wks. Irradiation increased the levels significantly (p<0.05) in all the mice at both time points compared to control groups with a greater effect in the C57-WT and TNFR1−/− mice. LPS exposure after irradiation significantly decreased the staining in comparison to irradiation alone in C57-WT, TNFα−/− and TNFR1−/− mice (p<0.05) at 12 and 24 wks suggesting a decrease in mast cell recruitment. There was a trend for a decrease in TNFR2−/− mice after LPS exposure at 12 and 24 wks.
Oxidative damage (8-OHdG)
Radiation is known to cause significant oxidative stress in tissue, consequently we analysed 8-OHdG staining, a biomarker for oxidative damage to DNA. The results presented in Figure 3c show that the percent of positive 8-OHdG staining increased significantly at 12 wks (p<0.05) and 24 wks (p<0.01) after irradiation compared to non-irradiated mice in all the groups, although the increases were greater in the knockout groups of mice. Additional exposure to LPS significantly decreased the 8-OHdG expression in all the mice at 12 wks (p<0.05) and at 24 wks (p<0.001) except for the TNFR1−/− mice which only showed a downward trend.
Collagen Assays
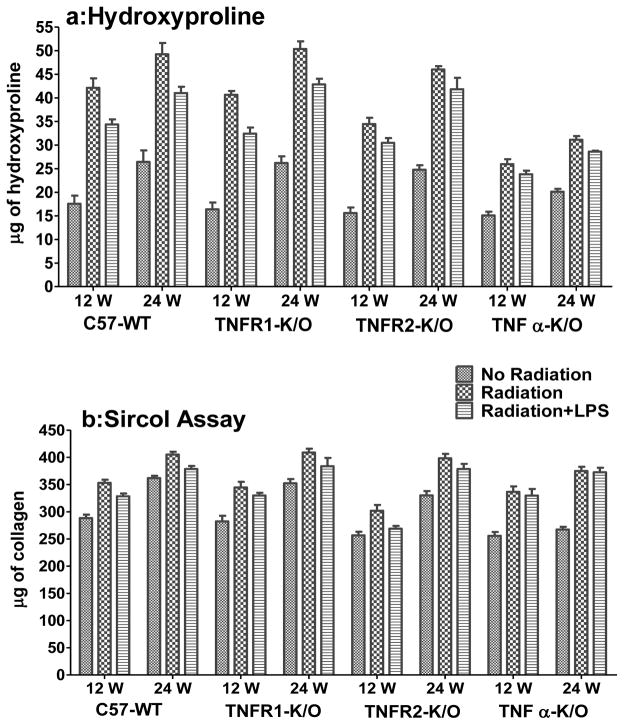
We measured deposition of collagen in the lungs following irradiation as a marker of the development of fibrosis. Hydroxyproline content is a quantitative measure of total collagen level in the lung, whilst the Sircol assay measures recently synthesized (RS) soluble collagen. Figure 4a shows the hydroxyproline content of lung tissue at 12 and 24 weeks. A significant increase in collagen content after radiation was observed in all groups of mice (p<0.001) at both times, although the extent of the increase was smaller in the TNFα−/− mice. Results for the Sircol assay (Fig 4b) also show a significant increase (p<0.01) in recently-synthesized (RS) collagen after irradiation in all groups of mice at both times. Additional exposure to LPS caused a significant decrease in hydroxyproline content (p<0.05) in C57-WT and TNFR1−/− mice at both time points and TNFα−/− mice at 24 wks relative to the mice treated only with radiation. The TNFR2−/− mice show a downward trend at both time points. The RS collagen levels were not significant decreased in the LPS exposed mice except in C57-WT mice (p<0.05). An issue for the measurement of these changes is that they could be influenced by differential accumulation of edema in the lungs of the treated mice, since the analysis was based on wet weight of tissue. If the irradiated mice had accumulated edema relative to the control the actual increase in collagen content could have been underestimated.
Figure 4.
(a) Hydroxyproline content (μg of hydroxyproline/100 mg of wet lung tissue) at 12 and 24 weeks following lung irradiation (10Gy) with/without post-irradiation (within 1h) intratracheal administration of LPS. (b) Sircol assay (mg collagen/65mg wet lung tissue) showed a small increase in recently-synthesized (RS) collagen after irradiation in the mouse lungs at 12 and 24 weeks following lung irradiation (10Gy) with/without post-irradiation (within 1h) intratracheal administration of LPS. Each bar represents the mean (±SEM for 6 mice).
Discussion
Recent concerns for the accidental or deliberate exposure of the general population to irradiation due to terrorism have resulted in studies of agents to mitigate or treat the symptoms of radiation exposure. In a previous study we examined the effects of a combination of low dose whole body irradiation and lung irradiation as a likely scenario in accidental exposures [29]. In the present study we investigated radiation-induced lung damage in the context of early post radiation exposure to LPS (to simulate respiratory infection). The results presented build on our previous report [10] which showed; 1) that C57-WT mice show reduced pneumonitis when exposed to LPS shortly after lung irradiation and 2) that mice knocked out for TNFα show reduced pneumonitis relative to C57-WT and TNFR1−/− and TNFR2−/− mice. Here we investigated possible mechanisms for these effects by examining the expression of the inflammatory cytokines IL-1α,IL-1β, IL-6, TNFα and TGFβ, macrophage activation, influx of mast cells and levels of oxidative damage. We also examined possible effects on radiation-induced fibrosis.
We observed that LPS treatment shortly after irradiation also mitigated the radiation effects in the three groups of knockout mice, although the effect was reduced in the TNFα−/− mice. Combined with our similar finding in C57-WT mice, these results raised the possibility that early exposure to LPS may modify the expression of inflammatory cytokines following irradiation and alter the secondary waves of chronic inflammation that occur. Our analysis of cytokine levels at different times after lung irradiation and exposure to LPS demonstrated that the combination treatment does indeed result in reduced cytokine expression and reduced expression of activated macrophages, for most of the animals and time points studied, relative to irradiation alone, consistent with the reduced pneumonitis observed. Interestingly a recent study in T-cell deficient mice has implicated T-cell infiltration in irradiated lung tissue as a mechanism that may reduce lung fibrosis [30]. Thus there is a possibility that LPS may have accelerated this process but this remains speculative since we did not directly investigate this issue.
Consistent with our findings, mast cells have also been reported to be increased in radiation pneumonitis in rats and mice [31–35]. In particular Haston et. al., [34] reported that both C3H/HeJ and C57/Bl6 mice showed increased mast cell numbers in lung tissue during the pneumonitis response to radiation exposure but they found that C3H/HeJ mice showed much higher levels than C57/Bl6 mice. Our results showed significant increases in mast cell levels in C57/Bl6 mice at 12 weeks following irradiation and we observed a reduction in the lungs of mice treated with LPS, consistent with the reduced level of pneumonitis as assessed by the reduced breathing rate increase. Measures of the bronchoalveolar lavage of patients after radiotherapy have revealed increased numbers of mast cells and neutrophils [36] to be present in this fluid.
That an inflammatory agent actually mitigated the radiation effect might reflect the fact that LPS-induced inflammation is normally short-lived [37] and that the early induction of inflammation following irradiation in mice is reported to play little role in the later development of pneumonitis [38]. However, studies in patients with FDG, which is taken up in areas of inflammation, has suggested that increased uptake at 1–2 weeks during lung radiotherapy may reflect the later development of pneumonitis [39–40]. That LPS and certain inflammatory cytokines have a radioprotective effect against lethal doses of ionizing radiation in mice when given before irradiation has been known for a long time [41–45]. Also a mitigating effect of exposure to LPS following irradiation has been reported for early damage to the intestine in mice [46], however, prior irradiation has been reported to sensitize lung to increased inflammation following late (6–15 months post irradiation) exposure to LPS [47, 48].
Our results also showed that, despite significant effects of irradiation on the cytokines and inflammatory cells at all three time points examined (12, 20 and 24 wks post irradiation) none of the measurements tracked with the reduced pneumonitis (breathing rate increase) observed in the TNFα−/− mice. The only difference observed in the TNFα−/− mice relative to the other groups was a slightly greater increase in macrophage activation following irradiation. This might represent a compensation strategy to help protect vasculature, since it has been reported that blockade of TNFα signalling reduces the production of VEGF by macrophages following irradiation [49].
We found that exposure to LPS also caused a significant decrease in hydroxyproline content relative to the mice treated with radiation alone in the C57-WT, TNFR1−/− and TNFα−/− mice. There was a trend for a decrease in TNFR2−/− mice. Consistent with these findings there was also a decrease in the TGFβ1 staining in all the groups of mice. TGFβ1 is a multifunctional cytokine that induces extracellular matrix production [50] and is a key cytokine in the fibrotic process and radiation-induced lung fibrosis[51–54] We further observed that the TNFα −/− mice showed a reduced increase in hydroxyproline levels at both 12 and 24 wks after irradiation relative to the other three groups of mice. Thus the development of fibrosis tracked with reduced pneumonitis in these mice. Interestingly there was no difference in the increased levels of soluble collagen seen in the lungs of the different groups of mice as detected by the Sircol assay. This may suggest that the lack of expression of TNFα results in modified enzyme levels causing reduced cross-linking of the collagen or increased breakdown.
Overall our results suggest strongly that TNFα plays a significant role in the induction of radiation-induced lung damage. TNFα is a pleiotropic cytokine with a key role in inflammatory and immunological responses, and is the first cytokine that is synthesized or released by activated monocytes/macrophages during inflammation [55]. TNFα upregulates other cytokines, such as IL-1, GM-CSF, IL-6, chemokines, prostaglandin E (PGE), and proteinases [56]. TNFα and IL-1 are known to stimulate NF-κB, a transcription factor that is also stimulated by oxidative stress. Nuclear factor-κB (NF-κB) is considered to be one of the central mediators of inflammation as a result of the activation of a wide range of pro-inflammatory genes including IL1α, IL-1β and IL-6 [13, 57–62]. Our results are consistent with the findings of Rube et al. [8, 63], who examined the potential role of TNFα, IL-1α and IL-6 as determinants of toxicity in lung tissue of C57Bl/6J mice treated with thoracic irradiation. They observed an acute and a delayed response resulting in the release of these three cytokines in the lungs of mice and concluded that the increased expression of these cytokines and the induction of a cytokine-triggered inflammatory response may be responsible for the lung toxicity. Our own recent studies with Sprague-Dawley rats have confirmed the chronic and cyclic nature of the increased expression of the inflammatory cytokines studied here [3, 9, 10]. Furthermore treatment of the rats with genistein resulted in substantial suppression of the increased expression of TNFα and a reduction in both pneumonitis and fibrosis consistent with the observations in the current study. Also according to Saito-Fujita et al. [64] knockout of IL-6 reduces fibrosis induced by carbon ion irradiation in C57BL/6J mice.
In conclusion, in this study we examined the role of LPS in reducing the effects of radiation-induced injury in the lungs of C57-WT, TNFα−/−, and TNFR1−/− and TNFR2−/− mice. The findings from our study provide evidence that LPS can act as a mitigator of radiation-induced injury and they extend our previous study to indicate that knockout of TNFα reduces both radiation-induced pneumonitis and fibrosis. There were reductions in the expression of pro- and anti-inflammatory cytokines following LPS exposure which are consistent with the findings of reduced radiation-induced pneumonitis and fibrosis in the lungs. Further studies are warranted to determine the effects of the LPS on irradiation-induced lung response using other strains of mice, such as C3H that are particularly prone to the induction of pneumonitis following irradiation.
Supplementary Material
Supplementary Figure 1: Immunohistochemical staining for the various cytokines at 12 and 24 wks after irradiation. (a) Control (non-treated), (b) Radiation, (c) Radiation + LPS. Panel (A) IL-1 alpha and IL-1 beta; Panel (B) IL-6 and TNF alpha: Panel (C) TGF beta and Mac-3. Original magnification 200X
Supplementary Figure. 2: Expression of IL-1α (a), IL-1β (b), IL-6 (c), TNF α (d) and TGFβ (e) mRNA relative to GAPDH in the right lung of mice (6 mice per group) 12 and 24 weeks following lung irradiation (10Gy) with/without post-irradiation (within 1h) intratracheal administration of LPS. Each bar represents the mean (±SEM for 6 mice).
Acknowledgments
This work was supported by funds from an NIAID/NIH U19 program (U19 AI-067734) and from the Canadian Institutes of Health Research. Partial support was also provided by the Princess Margaret Hospital Foundation and the Ontario Ministry of Health and Long Term Care (OMHLTC). The views expressed do not necessarily reflect those of OMHLTC.
Footnotes
Conflicts: The authors declare no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS) Int J Radiat Biol. 2011;87(8):851–68. doi: 10.3109/09553002.2011.560996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim K, Damoiseaux R, Norris AJ, et al. High throughput screening of small molecule libraries for modifiers of radiation responses. Int J Radiat Biol. 2011;87(8):839–45. doi: 10.3109/09553002.2011.560994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol. 2011;87(8):889–901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks LB, Yu X, Vujaskovic Z, Small W, Jr, Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13(3):333–45. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 5.Johnston CJ, Piedboeuf B, Baggs R, et al. Differences in correlation of mRNA gene expression in mice sensitive and resistant to radiation-induced pulmonary fibrosis. Radiat Res. 1995;142(2):197–203. [PubMed] [Google Scholar]
- 6.Johnston CJ, Piedboeuf B, Rubin P, et al. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145(6):762–7. [PubMed] [Google Scholar]
- 7.Rubin P, Johnston CJ, Williams JP, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33(1):99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 8.Rube CE, Uthe D, Wilfert F, et al. The bronchiolar epithelium as a prominent source of pro-inflammatory cytokines after lung irradiation. Int J Radiat Oncol Biol Phys. 2005;61(5):1482–92. doi: 10.1016/j.ijrobp.2004.12.072. [DOI] [PubMed] [Google Scholar]
- 9.Calveley VL, Khan MA, Yeung IW, et al. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005;81(12):887–99. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- 10.Hill RP, Zaidi A, Mahmood J, Jelveh S, et al. Investigations into the role of inflammation in normal tissue response to irradiation. Radiother Oncol. 2011;101(1):73–9. doi: 10.1016/j.radonc.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darnay BG, Aggarwal BB. Early events in TNF signaling: a story of associations and dissociations. J Leukoc Biol. 1997;61(5):559–66. doi: 10.1002/jlb.61.5.559. [DOI] [PubMed] [Google Scholar]
- 12.Erickson SL, de Sauvage FJ, Kikly K, et al. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372(6506):560–3. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- 13.Peschon JJ, Torrance DS, Stocking KL, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160(2):943–52. [PubMed] [Google Scholar]
- 14.Pfeffer K, Matsuyama T, Kundig TM, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73(3):457–67. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 15.Rothe J, Lesslauer W, Lotscher H, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 16.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334(26):1717–25. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 17.Zhou T, Mountz JD, Kimberly RP. Immunobiology of tumor necrosis factor receptor superfamily. Immunol Res. 2002;26(1–3):323–36. doi: 10.1385/IR:26:1-3:323. [DOI] [PubMed] [Google Scholar]
- 18.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214(2):149–60. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 19.Chen B, Tong Z, Ye Q, et al. Expression of tumour necrosis factor receptors by bronchoalveolar cells in hypersensitivity pneumonitis. Eur Respir J. 2005;25(6):1039–43. doi: 10.1183/09031936.05.00084704. [DOI] [PubMed] [Google Scholar]
- 20.Dai H, Guzman J, Chen B, Costabel U. Production of soluble tumor necrosis factor receptors and tumor necrosis factor-alpha by alveolar macrophages in sarcoidosis and extrinsic allergic alveolitis. Chest. 2005;127(1):251–6. doi: 10.1378/chest.127.1.251. [DOI] [PubMed] [Google Scholar]
- 21.Ermert M, Pantazis C, Duncker HR, Grimminger F, et al. In situ localization of TNFalpha/beta, TACE and TNF receptors TNF-R1 and TNF-R2 in control and LPS-treated lung tissue. Cytokine. 2003;22(3–4):89–100. doi: 10.1016/s1043-4666(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 22.Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-alpha receptors. Am J Physiol Lung Cell Mol Physiol. 2001;280(3):L537–46. doi: 10.1152/ajplung.2001.280.3.L537. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz LA, Lasky J, Lungarella G, Cavarra E, et al. Upregulation of the p75 but not the p55 TNF-alpha receptor mRNA after silica and bleomycin exposure and protection from lung injury in double receptor knockout mice. Am J Respir Cell Mol Bio. 1999;20(4):825–33. doi: 10.1165/ajrcmb.20.4.3193. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Qian J, Xing X, Kong FM, et al. Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung. Clin Cancer Res. 2008;14(6):1868–76. doi: 10.1158/1078-0432.CCR-07-1894. [DOI] [PubMed] [Google Scholar]
- 25.Brass DM, Hoyle GW, Poovey HG, et al. Reduced tumor necrosis factor-alpha and transforming growth factor-beta1 expression in the lungs of inbred mice that fail to develop fibroproliferative lesions consequent to asbestos exposure. Am J Pathol. 1999;154(3):853–62. doi: 10.1016/s0002-9440(10)65332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarkson R, Lindsay PE, Ansell S, Wilson G, et al. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med Phys. 2011;38(2):845–56. doi: 10.1118/1.3533947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma CM, Coffey CW, DeWerd LA, Liu C, et al. AAPM protocol for 40–300 kV x-ray beam dosimetry in radiotherapy and radiobiology. Med Phys. 2001;28(6):868–93. doi: 10.1118/1.1374247. [DOI] [PubMed] [Google Scholar]
- 28.Thomas DM, Fox J, Haston CK. Imatinib therapy reduces radiation-induced pulmonary mast cell influx and delays lung disease in the mouse. Int J Radiat Biol. 2010;86(6):436–44. doi: 10.3109/09553001003674863. [DOI] [PubMed] [Google Scholar]
- 29.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of lung injury after accidental exposure to radiation. Radiat Res. 2011;176(6):770–80. doi: 10.1667/rr2562.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cappuccini F, Eldh T, Bruder D, et al. New insights into the molecular pathology of radiation-induced pneumopathy. Radiother Oncol. 2011 Oct;101(1):86–92. doi: 10.1016/j.radonc.2011.05.064. [DOI] [PubMed] [Google Scholar]
- 31.Vergara JA, Raymond U, Thet LA. Changes in lung morphology and cell number in radiation pneumonitis and fibrosis: a quantitative ultrastructural study. Int J Radiat Oncol Biol Phys. 1987;13(5):723–32. doi: 10.1016/0360-3016(87)90291-4. [DOI] [PubMed] [Google Scholar]
- 32.Ward HE, Kemsley L, Davies L, Holecek M, Berend N. The pulmonary response to sublethal thoracic irradiation in the rat. Radiat Res. 1993;136(1):15–21. [PubMed] [Google Scholar]
- 33.Ward WF, Molteni A, Ts’ao CH, Hinz JM. Captopril reduces collagen and mast cell accumulation in irradiated rat lung. Int J Radiat Oncol Biol Phys. 1990;19(6):1405–9. doi: 10.1016/0360-3016(90)90351-j. [DOI] [PubMed] [Google Scholar]
- 34.Aldenborg F, Nilsson K, Jarlshammar B, Bjermer L, Enerback L. Mast cells and biogenic amines in radiation-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1993;8(1):112–7. doi: 10.1165/ajrcmb/8.1.112. [DOI] [PubMed] [Google Scholar]
- 35.Haston CK, Begin M, Dorion G, Cory SM. Distinct loci influence radiation-induced alveolitis from fibrosing alveolitis in the mouse. Cancer Res. 2007;67(22):10796–803. doi: 10.1158/0008-5472.CAN-07-2733. [DOI] [PubMed] [Google Scholar]
- 36.Majori M, Poletti V, Curti A, et al. Bronchoalveolar lavage in bronchiolitis obliterans organizing pneumonia primed by radiation therapy to the breast. J Allergy Clin Immunol. 2000;105(2 Pt 1):239–44. doi: 10.1016/s0091-6749(00)90071-x. [DOI] [PubMed] [Google Scholar]
- 37.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288(2):L333–41. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 38.Hong JH, Chiang CS, Tsao CY, et al. Can short-term administration of dexamethasone abrogate radiation-induced acute cytokine gene response in lung and modify subsequent molecular responses? Int J Radiat Oncol Biol Phys. 2001;51(2):296–303. doi: 10.1016/s0360-3016(01)01702-3. [DOI] [PubMed] [Google Scholar]
- 39.De Ruysscher D, Houben A, Aerts HJ, et al. Increased (18) F-deoxyglucose uptake in the lung during the first weeks of radiotherapy is correlated with subsequent Radiation-Induced Lung Toxicity (RILT): a prospective pilot study. Radiother Oncol. 2009;91(3):415–20. doi: 10.1016/j.radonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 40.McCurdy MR, Castillo R, Martinez J, et al. [(18) F]-FDG uptake dose-response correlates with radiation pneumonitis in lung cancer patients. Radiother Oncol. 2012 May 9; doi: 10.1016/j.radonc.2012.04.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith WW, Alderman IM, Gillespie RE. Increased survival in irradiated animals treated with bacterial endotoxins. Am J Physiol. 1957;191(1):124–30. doi: 10.1152/ajplegacy.1957.191.1.124. [DOI] [PubMed] [Google Scholar]
- 42.Neta R, Douches S, Oppenheim JJ. Interleukin 1 is a radioprotector. J Immunol. 1986;136(7):2483–5. [PubMed] [Google Scholar]
- 43.Neta R, Oppenheim JJ, Douches S. Interdependence of the radioprotective effects of human recombinant interleukin 1 alpha, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and murine recombinant granulocyte-macrophage colony-stimulating factor. J Immunol. 1988;140(1):108–11. [PubMed] [Google Scholar]
- 44.Redlich CA, Gao X, Rockwell S, Kelley M, Elias JA. IL-11 enhances survival and decreases TNF production after radiation-induced thoracic injury. J Immunol. 1996;157(4):1705–10. [PubMed] [Google Scholar]
- 45.Zsebo KM, Smith KA, Hartley CA, et al. Radioprotection of mice by recombinant rat stem cell factor. Proc Natl Acad Sci USA. 1992;89(20):9464–8. doi: 10.1073/pnas.89.20.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riehl TE, Newberry RD, Lorenz RG, Stenson WF. TNFR1 mediates the radioprotective effects of lipopolysaccharide in the mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G166–73. doi: 10.1152/ajpgi.00537.2002. [DOI] [PubMed] [Google Scholar]
- 47.Johnston CJ, Williams JP, Elder A, Hernady E, Finkelstein JN. Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res. 2004;30(5):369–82. doi: 10.1080/01902140490438915. [DOI] [PubMed] [Google Scholar]
- 48.Johnston CJ, Manning C, Hernady E, et al. Effect of total body irradiation on late lung effects: hidden dangers. Int J Radiat Biol. 2011;87(8):902–13. doi: 10.3109/09553002.2011.573439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng Y, Beckett MA, Liang H, et al. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70(4):1534–43. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 51.Burger A, Loffler H, Bamberg M, Rodemann HP. Molecular and cellular basis of radiation fibrosis. Int J Radiat Biol. 1998;73(4):401–8. doi: 10.1080/095530098142239. [DOI] [PubMed] [Google Scholar]
- 52.Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, et al. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med. 1997;186(11):1831–41. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong JH, Jung SM, Tsao TC, Wu CJ, et al. Bronchoalveolar lavage and interstitial cells have different roles in radiation-induced lung injury. Int J Radiat Biol. 2003;79(3):159–67. doi: 10.1080/0955300031000076894. [DOI] [PubMed] [Google Scholar]
- 54.Yi ES, Bedoya A, Lee H, Chin E, et al. Radiation-induced lung injury in vivo: expression of transforming growth factor-beta precedes fibrosis. Inflammation. 1996;20(4):339–52. doi: 10.1007/BF01486737. [DOI] [PubMed] [Google Scholar]
- 55.Fujita M, Ikegame S, Harada E, et al. TNF receptor 1 and 2 contribute in different ways to resistance to Legionella pneumophila-induced mortality in mice. Cytokine. 2008;44(2):298–303. doi: 10.1016/j.cyto.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 56.Marasco WA, Ward PA. Chemotactic factors of bacterial origin. Methods Enzymol. 1988;162:198–214. doi: 10.1016/0076-6879(88)62077-5. [DOI] [PubMed] [Google Scholar]
- 57.Christman JW, Lancaster LH, Blackwell TS. Nuclear factor kappa B: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Med. 1998;24(11):1131–8. doi: 10.1007/s001340050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McFarlane SM, Pashmi G, Connell MC, et al. Differential activation of nuclear factor-kappaB by tumour necrosis factor receptor subtypes. TNFR1 predominates whereas TNFR2 activates transcription poorly. FEBS Lett. 2002;515(1–3):119–26. doi: 10.1016/s0014-5793(02)02450-x. [DOI] [PubMed] [Google Scholar]
- 59.Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today. 1999;5(3):123–32. doi: 10.1016/s1357-4310(98)01430-0. [DOI] [PubMed] [Google Scholar]
- 60.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 61.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 62.Liou HC, Baltimor D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993;5(3):477–87. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 63.Rube CE, Wilfert F, Uthe D, et al. Increased expression of pro-inflammatory cytokines as a cause of lung toxicity after combined treatment with gemcitabine and thoracic irradiation. Radiother Oncol. 2004;72(2):231–41. doi: 10.1016/j.radonc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Saito-Fujita T, Iwakawa M, Nakamura E, et al. Attenuated lung fibrosis in interleukin 6 knock-out mice after C-ion irradiation to lung. J Radiat Res (Tokyo) 2011;52(3):270–7. doi: 10.1269/jrr.10094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Immunohistochemical staining for the various cytokines at 12 and 24 wks after irradiation. (a) Control (non-treated), (b) Radiation, (c) Radiation + LPS. Panel (A) IL-1 alpha and IL-1 beta; Panel (B) IL-6 and TNF alpha: Panel (C) TGF beta and Mac-3. Original magnification 200X
Supplementary Figure. 2: Expression of IL-1α (a), IL-1β (b), IL-6 (c), TNF α (d) and TGFβ (e) mRNA relative to GAPDH in the right lung of mice (6 mice per group) 12 and 24 weeks following lung irradiation (10Gy) with/without post-irradiation (within 1h) intratracheal administration of LPS. Each bar represents the mean (±SEM for 6 mice).