Abstract
Although quinolones are the most commonly prescribed antibacterials, their use is threatened by an increasing prevalence of resistance. The most common causes of quinolone resistance are mutations of a specific serine or acidic residue in the A subunit of gyrase or topoisomerase IV. These amino acids are proposed to serve as a critical enzyme-quinolone interaction site by anchoring a water-metal ion bridge that coordinates drug binding. To probe the role of the proposed water-metal ion bridge, we characterized wild-type, GrlAE85K, GrlAS81F/E85K, GrlAE85A, GrlAS81F/E85A and GrlAS81F Bacillus anthracis topoisomerase IV, their sensitivity to quinolones and related drugs and their use of metal ions. Mutations increased the Mg2+ concentration required to produce maximal quinolone-induced DNA cleavage and restricted the divalent metal ions that could support quinolone activity. Individual mutation of Ser81 or Glu85 partially disrupted bridge function, whereas simultaneous mutation of both residues abrogated protein–quinolone interactions. Results provide functional evidence for the existence of the water-metal ion bridge, confirm that the serine and glutamic acid residues anchor the bridge, demonstrate that the bridge is the primary conduit for interactions between clinically relevant quinolones and topoisomerase IV and provide a likely mechanism for the most common causes of quinolone resistance.
INTRODUCTION
Quinolones are the most commonly prescribed antibacterial drugs currently in clinical use (1). They are broad-spectrum agents and are used to treat a wide variety of Gram-negative and Gram-positive bacterial infections. Several quinolones have been approved for use in the USA, including ciprofloxacin, levofloxacin, moxifloxacin and sparfloxacin (2–6).
Quinolones kill bacteria by increasing levels of DNA strand breaks generated by gyrase and topoisomerase IV (3,5,7–9). Although both type II topoisomerases are physiological targets for quinolones, their relative importance to drug efficacy appears to be species- and drug-dependent (7,10–15). Gyrase and topoisomerase IV are comprised of two protomer subunits (GyrA and GyrB in gyrase; GrlA and GrlB in Gram-positive topoisomerase IV) and have an A2B2 quaternary structure (6,8,16–21). The A subunits contain the active site tyrosine residues involved in DNA cleavage and ligation, and the B subunits bind and hydrolyse adenosine triphosphate (ATP), which is required for overall catalytic activity (16–18,20). Gyrase and topoisomerase IV alter DNA topology by generating a double-stranded break in the nucleic acid backbone and passing a separate double helix through the transient DNA gate (16–22). Both enzymes play critical roles in maintaining the bacterial genome and are required for fundamental processes such as DNA replication and chromosome segregation (16–20,22).
Quinolone usage is becoming threatened by an increasing prevalence of resistance, which currently extends to nearly every bacterial infection treated by this drug class (4,5). Quinolone resistance most often is associated with mutations in gyrase and/or topoisomerase IV (as opposed to influx/efflux pumps, drug metabolizing enzymes, etc.) (3,5–8,10,23–26). Generally, mutation of one type II enzyme confers ≤10-fold drug resistance. Selection for higher levels of resistance (∼10–100-fold) usually yields strains with mutations in both enzymes (3,5,7,8,10,24–26). The most common resistance-conferring mutations occur at a highly conserved serine residue in the A subunit of gyrase or topoisomerase IV (27). This residue originally was described as Ser83 in Escherichia coli gyrase (28,29). The second most common mutations occur at a conserved acidic residue (usually glutamic acid) that is four amino acids downstream from the serine (Glu87 in E. coli GyrA) (5,23,27,30). Although the involvement of these amino acid residues in quinolone resistance has been known for more than a decade, the mechanistic basis by which their alteration leads to resistance remains an enigma.
Four recent structural studies of DNA cleavage complexes formed with bacterial type II topoisomerases in the presence of quinolones have been reported (31–34). In all of these studies, quinolones were located in the same binding pocket, which was in the vicinity of the conserved serine and acidic residues. However, there was disagreement regarding drug orientation within the pocket, and in no case was the quinolone in close enough proximity to either amino acid to form direct contacts.
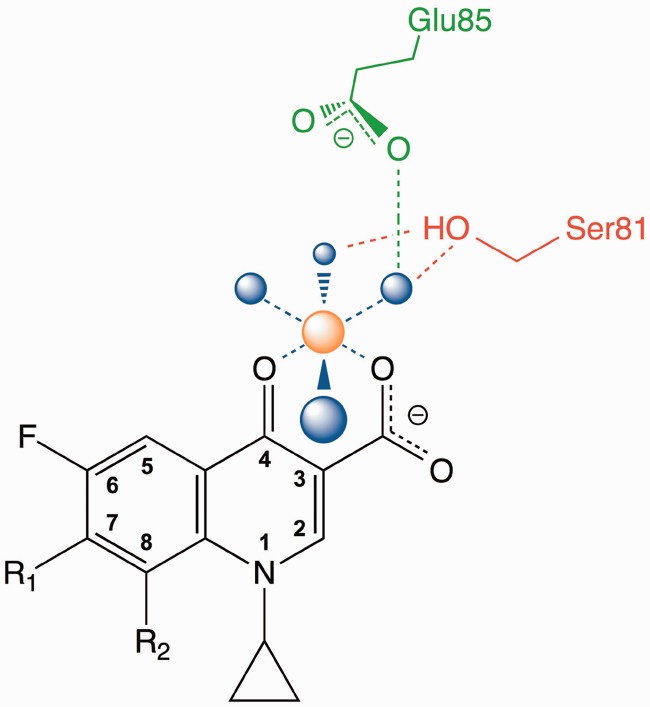
One of the structures (Acinetobacter baumannii topoisomerase IV) does, however, provide a potential mechanism by which mutations of the serine or acidic residue could lead to quinolone resistance (Supplementary Figure S1) (34). In this structure, the C3/C4 keto acid of moxifloxacin chelated a non-catalytic magnesium ion that appeared to be coordinated to four water molecules. Two of these water molecules were situated close enough to Ser84 and Glu88 (equivalent to E. coli GyrA Ser83 and Glu87) to form hydrogen bonds. Thus, the authors suggested that interactions between quinolones and bacterial type II topoisomerases were mediated by this water-metal ion coordination. A generalized diagram of the proposed water-metal ion ‘bridge’ that facilitates quinolone interactions with the conserved serine and acidic residues is shown in Figure 1.
Figure 1.
Diagram of the proposed water-metal ion bridge that mediates critical interactions between quinolones and topoisomerase IV. For simplicity, only interactions with the protein (and not DNA) are shown. Residue numbering is that of B. anthracis topoisomerase IV. A generic quinolone structure is depicted in black, water molecules are in blue, Mg2+ is in orange and the coordinating serine and glutamic acid residues are in red and green, respectively. Blue dashed lines indicate the octahedral coordination sphere of the divalent metal ion interacting with four water molecules and the C3/C4 keto acid of the quinolone. The red dashed lines represent hydrogen bonds between the serine side chain hydroxyl group and two of the water molecules. The green dashed line represents a hydrogen bond between the glutamic acid side chain carboxyl group and one of the water molecules. Adapted from Wohlkonig et al. (34).
The first functional evidence for the water-metal ion bridge came from a study on GrlAS81F in Bacillus anthracis topoisomerase IV, which displayed a high level of quinolone resistance (35). This study focused on analysing the biochemical properties of the mutant enzyme and identifying quinolone-like drugs that could overcome this resistance. As part of this analysis, however, one experiment demonstrated that GrlAS81F required a higher Mg2+ concentration (as compared with wild-type topoisomerase IV) to achieve a maximal level of quinolone-induced DNA cleavage. This finding addressed the existence of the proposed water-metal ion bridge and suggested that the underlying mechanism of quinolone resistance caused by ‘Ser83’ mutations results from the partial disruption of this bridge.
To further test the proposed water-metal ion bridge model, we analysed the activity, drug susceptibility and metal ion requirements of B. anthracis topoisomerase IV carrying the GrlAE85K mutation. This alteration, which is in the other amino acid residue predicted to anchor the bridge, has been reported in clinical and laboratory isolates of quinolone-resistant strains, including B. anthracis (23,24,26,27,30). We also characterized the metal ion requirements for drug-induced DNA cleavage with wild-type, GrlAS81F and GrlAE85K topoisomerase IV as well as the activity and the drug sensitivity of GrlAS81F/E85K, GrlAE85A and GrlAS81F/E85A topoisomerase IV. Based on the results of these studies, we propose that the water-metal ion bridge facilitates a critical interaction between clinically relevant quinolones and bacterial type II topoisomerases, that the bridge is anchored by the conserved serine and acidic residues and that quinolone resistance is caused by the loss of coordination of the bridge to the enzyme.
MATERIALS AND METHODS
Enzymes and materials
Genes encoding wild-type B. anthracis GrlA and GrlB and drug-resistant GrlAS81F, GrlAE85K, GrlAE85A, GrlAS81F/E85K and GrlAS81F/E85A were expressed and purified as described in Dong et al. (36). Wild-type genes were polymerase chain reaction-amplified from B. anthracis Sterne 34F2 chromosomal DNA and cloned into the pET15b expression vector (Novagen), which added a 6× His tag to the N-terminus of the expressed protein. Mutant genes were generated by QuikChange (Stratagene) site-directed mutagenesis, and recombinant topoisomerase IV subunits were individually expressed in E. coli strain BL21(DE3). The resulting proteins were purified by affinity chromatography, dialysed into 20 mM Tris–HCl (pH 7.5), 200 mM NaCl and 20% glycerol and stored at −80°C. In all assays, topoisomerase IV was used as a 1:1 mixture of GrlA:GrlB.
Negatively supercoiled pBR322 plasmid DNA was prepared from E. coli using a Plasmid Mega Kit (Qiagen) as described by the manufacturer. Relaxed pBR322 plasmid DNA was generated by treatment with topoisomerase I for 30 min as described previously (37), followed by phenol-chloroform-isoamyl alcohol extraction, ethanol precipitation and resuspension in 5 mM Tris–HCl (pH 8.5) and 500 µM Na2EDTA. Histone H1 was obtained from Boehringer Mannheim. [γ-32P]ATP (∼3000 Ci/mmol) was obtained from Perkin-Elmer. Plastic-backed 20 × 20 cm polyethyleneimine-impregnated cellulose F thin layer chromatography plates were obtained from EMD Chemicals Inc.
Ciprofloxacin was obtained from LKT Laboratories, stored at −20°C as a 40 mM stock solution in 0.1 N NaOH and diluted 5-fold with 10 mM Tris–HCl (pH 7.9) immediately before use. The 3-amino-7-[(3S)-3-(aminomethyl)-1-pyrrolidinyl]-1-cyclopropyl-6-fluoro-8-methyl-2,4(1H,3H)-quinazolinedione (referred to as 8-methyl-2,4-dione) was synthesized using established methods as previously reported (38) and stored at 4°C as a 20 mM stock solution in 100% dimethyl sulfoxide. All other chemicals were analytical reagent grade.
DNA relaxation
DNA relaxation assays were based on the protocol of Fortune and Osheroff (39). Reactions (20 µl) contained 18.75 nM wild-type or mutant topoisomerase IV and 5 nM negatively supercoiled pBR322 in relaxation buffer [40 mM HEPES (pH 7.6), 100 mM potassium glutamate, 10 mM Mg(OAc)2, 50 mM NaCl and 1 mM ATP] and were incubated at 37°C. DNA relaxation was stopped at times ranging from 0 to 30 min by the addition of 3 µl of 0.77% sodium dodecyl sulphate (SDS) and 77.5 mM Na2EDTA. Samples were mixed with 2 µl of agarose gel loading buffer [60% sucrose, 10 mM Tris–HCl (pH 7.9), 0.5% bromophenol blue and 0.5% xylene cyanol FF], heated at 45°C for 5 min and subjected to electrophoresis in 1% agarose gels in 100 mM Tris–borate (pH 8.3) and 2 mM ethylenediaminetetraacetic acid (EDTA). Gels were stained with 0.75 µg/ml ethidium bromide for 30 min. DNA bands were visualized with medium-range ultraviolet light and quantified using an Alpha Innotech digital imaging system. The percentage relaxed DNA was determined by the loss of supercoiled DNA substrate.
DNA catenation
Catenation assays were based on the protocol of Fortune and Osheroff (40). Reactions (20 µl) contained 50 nM wild-type or mutant GrlAE85K topoisomerase IV and 5 nM relaxed pBR322 in relaxation buffer containing 25 mM NaCl (rather than 50 mM) and supplemented with 5 µg/ml histone H1 and were incubated at 37°C. Catenation was stopped at times ranging from 0 to 30 min by the addition of 2 µl of 250 mM Na2EDTA (pH 8.0) followed by 2 µl of 1.25% SDS. Samples were mixed with 2 µl of agarose gel loading buffer, heated at 45°C for 5 min, and subjected to electrophoresis in 1% agarose gels in 100 mM Tris-borate (pH 8.3) and 2 mM EDTA containing 0.5 µg/ml of ethidium bromide. DNA bands were visualized and quantified as described earlier in the text. The percentage catenated DNA was determined by the loss of relaxed monomers or the appearance of catenated products retained in the wells (both yielded similar results).
Non-turnover catenation reactions contained 200 nM wild-type or mutant GrlAE85K topoisomerase IV and substituted 1 mM APP(NH)P for ATP. Before electrophoresis, reactions were treated with proteinase K (2 µl of a 0.8 mg/ml solution) at 45°C for 45 min to digest the enzyme.
ATP hydrolysis
ATP hydrolysis assays were carried out as described by Kingma et al. (41). Reactions (20 µl) contained 100 nM wild-type or mutant GrlAE85K topoisomerase IV and 85 nM negatively supercoiled pBR322 in relaxation buffer supplemented with 150 nM [γ-32P]ATP and were incubated at 37°C. Reactions carried out in the absence of DNA contained 500 nM wild-type or mutant GrlAE85K topoisomerase IV. Reactions were terminated at 0–14 min by spotting 2 µl on a thin layer chromatography plate. Standards were spotted at the top of each plate and consisted of 1, 2 and 3 µl of a 50-fold diluted reaction. Plates were developed in 400 mM NH4HCO3. Following air-drying, plates were covered in plastic wrap and exposed for 30 min to a K screen. The K screen was visualized and hydrolysed phosphate was quantified using a Bio-Rad Molecular Imager FX.
DNA cleavage
DNA cleavage reactions were carried out using the procedure of Fortune and Osheroff (39). Reactions contained 75 nM wild-type or mutant topoisomerase IV and 10 nM negatively supercoiled pBR322 in a total of 20 µl of cleavage buffer [40 mM Tris–HCl (pH 7.9), 10 mM MgCl2, 50 mM NaCl and 2.5% (v/v) glycerol]. In some reactions, the concentration dependence of MgCl2 was examined (final concentration of Mg2+ in those reactions is indicated in the figure) or the divalent metal ion was replaced with the indicated concentration of CaCl2, ZnCl2, CdCl2, MnCl2 or NiCl2. Reaction mixtures were incubated at 37°C for 10 min, and enzyme–DNA cleavage complexes were trapped by the addition of 2 µl of 5% SDS followed by 2 µl of 250 mM Na2EDTA (pH 8.0). Proteinase K (2 µl of a 0.8 mg/ml solution) was added, and samples were incubated at 45°C for 45 min to digest the enzyme. Samples were mixed with 2 µl of agarose gel loading buffer, heated at 45°C for 5 min and subjected to electrophoresis in 1% agarose gels in 40 mM Tris–acetate (pH 8.3) and 2 mM Na2EDTA containing 0.5 µg/ml ethidium bromide. DNA bands were visualized and quantified as described earlier in the text. DNA cleavage was monitored by the conversion of supercoiled plasmid to linear molecules.
Assays that monitored the DNA cleavage activities of wild-type and mutant topoisomerase IV in the absence of drugs substituted 10 mM CaCl2 for 10 mM MgCl2 in the cleavage buffer. Ca2+ supports higher levels of topoisomerase IV-mediated DNA cleavage in the absence of drugs, thereby greatly facilitating quantification of basal enzyme activity. Ca2+ does not significantly alter the reversibility or the stability of cleavage complexes as compared with Mg2+. Assays that assessed the DNA cleavage activities of the wild-type and mutant enzymes in the presence of drugs contained 0–100 µM compound for the wild-type enzyme and 0–500 µM compound for the mutant enzymes.
For assays that monitored competition between ciprofloxacin (0–500 µM) and 8-methyl-2,4-dione (5 µM) with GrlAS81F/E85K topoisomerase IV, the level of cleavage seen with the corresponding concentration of ciprofloxacin in the absence of the quinazolinedione was used as a baseline and was subtracted from the cleavage level seen in the presence of both compounds. Ciprofloxacin and 8-methyl-2,4-dione were added simultaneously to reaction mixtures.
DNA religation
DNA religation assays were carried out by the procedure of Robinson and Osheroff (42). Reactions (20 µl) contained 75 nM mutant GrlAE85K topoisomerase IV and 10 nM negatively supercoiled pBR322 in cleavage buffer containing 5 mM MgCl2 (rather than 10 mM). Reactions carried out in the presence of drug contained 200 µM ciprofloxacin or 20 µM 8-methyl-2,4-dione. Reactions carried out in the absence of drug substituted 1 mM CaCl2 for 5 mM MgCl2. Initial DNA cleavage/religation equilibria were established at 37°C for 10 min. Religation was initiated by rapidly shifting the reaction temperature from 37°C to 75°C. The shift to high temperature allows enzyme-mediated religation but prevents new rounds of DNA cleavage from occurring. Thus, it results in a unidirectional sealing of the cleaved DNA. Reactions were stopped at times ranging from 0 to 135 s by the addition of 2 µl of 5% SDS followed by 2 µl of 250 mM Na2EDTA (pH 8.0). Samples were digested with proteinase K and processed as described earlier in the text for plasmid cleavage assays. Levels of DNA cleavage were set to 100% at time zero, and religation was determined by the loss of linear cleavage product over time.
Persistence of topoisomerase IV-DNA cleavage complexes
The persistence of topoisomerase IV-DNA cleavage complexes established in the presence of drugs was determined using the procedure of Gentry et al. (43). Initial reactions contained 375 nM mutant GrlAE85K topoisomerase IV, 50 nM DNA and 200 µM ciprofloxacin or 20 µM 8-methyl-2,4-dione in a total of 20 µl of DNA cleavage buffer. Reactions were incubated at 37°C for 10 min and then diluted 20-fold with DNA cleavage buffer warmed to 37°C. Samples (20 µl) were removed at times ranging from 0 to 300 min, and DNA cleavage was stopped with 2 µl of 5% SDS followed by 2 µl of 250 mM Na2EDTA (pH 8.0). Samples were digested with proteinase K and processed as described earlier in the text for plasmid cleavage assays. Levels of DNA cleavage were set to 100% at time zero, and the persistence of cleavage complexes was determined by the decay of the linear reaction product over time.
RESULTS AND DISCUSSION
Characterization of GrlAE85K B. anthracis topoisomerase IV
A structural study of the A. baumannii topoisomerase IV-DNA cleavage complex (34) and an enzymological study of wild-type and mutant GrlAS81F B. anthracis topoisomerase IV (35) suggest that clinically relevant quinolones form a critical interaction with the bacterial type II enzyme through a water-metal ion bridge (Figure 1). The divalent metal ion is chelated to the quinolone by the C3/C4 keto acid, and the water molecules are proposed to anchor the bridge to the enzyme through coordination with a conserved serine and glutamic acid residue (Ser81 and Glu85 in B. anthracis GrlA). Our previous study on quinolone function and resistance focused on the role of Ser81 (35). Therefore, as the next step towards characterizing the proposed bridge model, we analysed the activity of B. anthracis GrlAE85K topoisomerase IV. The Glu->Lys mutation was chosen because it was the only alteration of Glu85 found in isolates of B. anthracis selected for ciprofloxacin resistance (24). This mutation also has been reported in quinolone-resistant topoisomerase IV from E. coli (23) and Staphylococcus aureus (44,45).
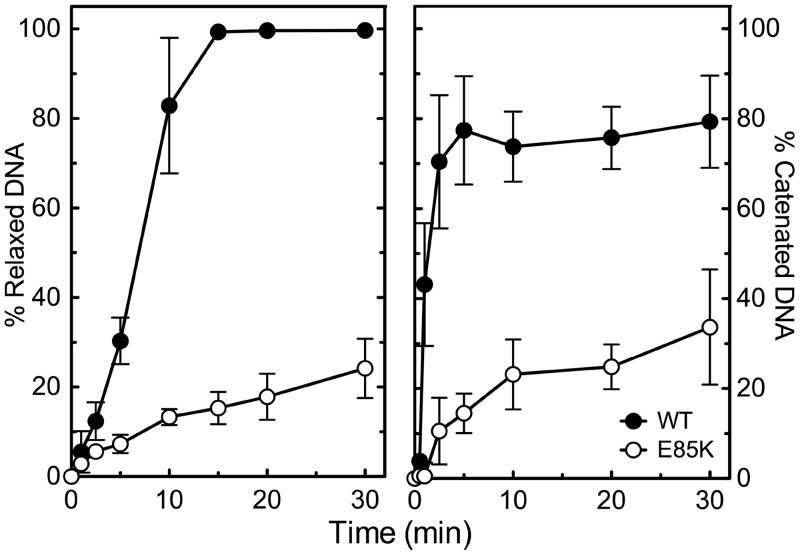
GrlAE85K topoisomerase IV displayed lower rates of DNA relaxation and catenation as compared with the wild-type enzyme (Figure 2). A similar decrease in catalytic activity has been reported for the equivalent Glu->Lys mutation in E. coli topoisomerase IV (46). These findings raise the possibility that GrlAE85K topoisomerase IV displays quinolone resistance because it has low overall activity. Therefore, several experiments were carried out to determine the underlying basis for the decreased catalytic rates.
Figure 2.
Overall catalytic activities of wild-type and GrlAE85K topoisomerase IV. The abilities of the wild-type (WT, black circles) and GrlAE85K (E85K, open circles) enzymes to relax negatively supercoiled pBR322 plasmid DNA (left) and to catenate relaxed pBR322 plasmid DNA (right) are shown. The percentage DNA relaxation was determined by the loss of supercoiled DNA substrate (FI band), and the percentage DNA catenation was determined by the loss of relaxed monomers or by the appearance of catenated products retained in the wells (both yielded similar results). Representative gels are shown in Supplementary Figure S2. Error bars represent the standard deviation of three or more independent experiments.
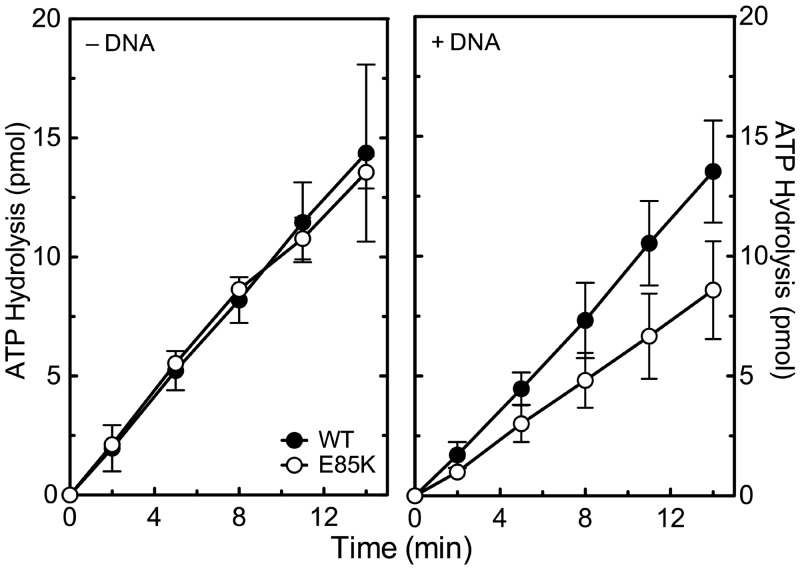
First, the ability of GrlAE85K topoisomerase IV to hydrolyse ATP was characterized. In the absence of DNA, the basal rates of ATP hydrolysis catalysed by the mutant and wild-type enzymes were indistinguishable (Figure 3, left panel). In the presence of DNA, hydrolysis rates for the mutant enzyme were slightly slower than wild-type (Figure 3, right panel), but were not sufficiently impaired to account for the large difference in the rates of catalytic DNA strand passage seen in Figure 2. Thus, the impaired overall activity of GrlAE85K does not appear to reflect an altered interaction with the ATP cofactor.
Figure 3.
ATP hydrolysis catalysed by wild-type and GrlAE85K topoisomerase IV. The abilities of the wild-type (WT, black circles) and GrlAE85K (E85K, open circles) enzymes to hydrolyse ATP in the absence (left) or presence (right) of negatively supercoiled pBR322 plasmid DNA are shown. Error bars represent the standard deviation of three or more independent experiments.
Second, the ability of GrlAE85K topoisomerase IV to carry out non-turnover DNA catenation was assessed. This reaction uses APP(NH)P, a non-hydrolyzable analogue of ATP that allows each topoisomerase IV enzyme to carry out only one DNA strand passage event. In contrast to wild-type topoisomerase IV, GrlAE85K displayed very little ability to catalyse DNA strand passage under non-turnover conditions (Figure 4). Therefore, the decreased catalytic rate of the mutant enzyme likely occurs at a step that precedes enzyme turnover.
Figure 4.
Non-turnover catenation mediated by wild-type and GrlAE85K topoisomerase IV. The ability of the wild-type (WT, black circles) and GrlAE85K (E85K, open circles) enzymes to carry out strand passage is shown. Assays used the non-hydrolyzable ATP analogue APP(NH)P and relaxed pBR322 plasmid DNA to observe a single DNA strand passage (i.e. catenation) event. The percentage DNA catenation was determined by the loss of relaxed monomers or by the appearance of catenated products retained in the wells (both yielded similar results). A representative gel is shown in Supplementary Figure S3. Error bars represent the standard deviation of three or more independent experiments.
Third, because quinolones kill bacterial cells primarily by increasing levels of DNA cleavage mediated by gyrase and topoisomerase IV, the ability of GrlAE85K B. anthracis topoisomerase IV to cleave DNA was examined. In contrast to results with DNA relaxation and catenation, the mutant enzyme cleaved plasmid DNA with an activity that was similar to (or exceeded) that of wild-type topoisomerase IV (Figure 5). This finding suggests that the steps of the catalytic cycle of GrlAE85K that precede the strand passage event [enzyme–DNA binding, DNA bending and DNA cleavage (16–21)] are relatively unaffected in the mutant enzyme and implies that the Glu85->Lys mutation decreases the overall catalytic activity of B. anthracis topoisomerase IV by impairing the DNA strand passage event. Furthermore, it provides strong evidence that quinolone resistance in GrlAE85K topoisomerase IV is not caused by a general loss of activity and likely reflects an altered interaction between the drug and the enzyme.
Figure 5.
DNA cleavage activities of wild-type and GrlAE85K topoisomerase IV. The ability of the wild-type (WT, black circles) and GrlAE85K (E85K, open circles) enzymes to cleave negatively supercoiled pBR322 plasmid DNA is shown. Assays were carried out in the presence of 10 mM CaCl2. DNA cleavage was monitored by the appearance of the linear DNA (FIII) band. A representative gel is shown in Supplementary Figure S4. Error bars represent the standard deviation of three or more independent experiments.
Effects of ciprofloxacin and 8-methyl-2,4-dione on DNA cleavage mediated by GrlAE85K topoisomerase IV
To assess the effects of the Glu85->Lys mutation on quinolone–enzyme interactions, the ability of ciprofloxacin to enhance DNA cleavage mediated by wild-type and GrlAE85K B. anthracis topoisomerase IV was compared. As seen in Figure 6 (left panel), the mutant enzyme displayed significant resistance to ciprofloxacin. The concentration of quinolone required to triple levels of enzyme-mediated DNA cleavage (CC3; ∼43 µM) increased ∼85-fold as compared with wild-type (∼0.5 µM). In contrast, at high concentrations of ciprofloxacin (∼500 µM), maximal levels of DNA cleavage mediated by GrlAE85K topoisomerase IV approached those generated by the wild-type enzyme (∼20 versus ∼30% DNA cleaved; Figure 6, inset). Therefore, ciprofloxacin resistance caused by the Glu85->Lys mutation appears to reflect primarily a decrease in quinolone potency (i.e. affinity) rather than efficacy.
Figure 6.
Effects of ciprofloxacin and 8-methyl-2,4-dione on the DNA cleavage activities of wild-type and GrlAE85K topoisomerase IV. DNA cleavage mediated by the wild-type (WT, black circles) and GrlAE85K (E85K, open circles) enzymes in the presence of a quinolone (ciprofloxacin, left) and a quinazolinedione (8-methyl-2,4-dione, right) are shown. The structures of ciprofloxacin and 8-methyl-2,4-dione are shown in their respective panels. The inset shows the maximum level of ciprofloxacin-induced cleavage generated by GrlAE85K topoisomerase IV at high drug concentrations. DNA cleavage was monitored by the appearance of the linear DNA (FIII) band. A representative gel is shown in Supplementary Figure S5. Error bars represent the standard deviation of three or more independent experiments.
Although similar in structure to quinolones, quinazolinediones lack the C3/C4 keto acid necessary to chelate the divalent metal ion. Thus, it is unlikely that they interact with the bacterial type II topoisomerase through the proposed water-metal ion bridge (35). To this point, enzymes carrying mutations at the serine residue proposed to anchor the bridge are resistant to clinically relevant quinolones but retain high sensitivity to quinazolinediones (35,47,48). Because Glu85 is the other amino acid residue proposed to anchor the water-metal ion bridge in B. anthracis topoisomerase IV, we determined the sensitivity of GrlAE85K towards a quinazolinedione (8-methyl-2,4-dione) (Figure 6, right panel). In contrast to results with ciprofloxacin, GrlAE85K topoisomerase IV displayed nearly wild-type sensitivity to 8-methyl-2,4-dione. The CC3 for the quinazolinedione increased only ∼1.5-fold compared with wild-type (∼0.2 versus ∼0.3 µM), and drug efficacy with the mutant enzyme was ∼85% of that seen with wild-type topoisomerase IV (∼22 versus ∼26% maximal DNA cleavage).
Effects of ciprofloxacin and 8-methyl-2,4-dione on DNA religation mediated by GrlAE85K topoisomerase IV and cleavage complex persistence
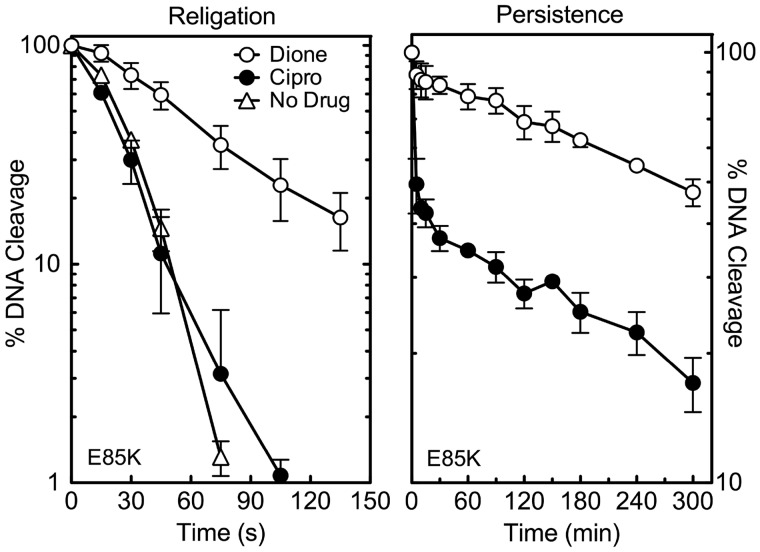
To further analyse drug interactions with GrlAE85K topoisomerase IV, the effects of ciprofloxacin and 8-methyl-2,4-dione on DNA religation and cleavage complex persistence were determined (Figure 7). The rate of religation mediated by the mutant enzyme (t1/2 ≈ 24 s) (no drug; left panel) was similar to that reported for wild-type topoisomerase IV [t1/2 ≈ 23 s (35)]. However, the two drug classes had markedly different effects on religation mediated by GrlAE85K topoisomerase IV. Although 200 µM ciprofloxacin had almost no effect on the reaction (t1/2 ≈ 20 s), 20 µM 8-methyl-2,4-dione significantly inhibited religation (t1/2 ≈ 62 s) (Figure 7, left panel).
Figure 7.
Effects of ciprofloxacin and 8-methyl-2,4-dione on the DNA religation activity of GrlAE85K topoisomerase IV and the persistence of ternary GrlAE85K topoisomerase IV-drug-DNA cleavage complexes. Results for religation assays carried out in the absence of drugs (No Drug, open triangles) or in the presence of quinolone (ciprofloxacin; Cipro, black circles) or quinazolinedione (8-methyl-2,4-dione; Dione, open circles) are shown on the left. Results for the persistence of ternary cleavage complexes formed in the presence of ciprofloxacin (Cipro, black circles) or 8-methyl-2,4-dione (Dione, open circles) are shown on the right. For persistence assays, initial DNA cleavage-religation reactions were allowed to come to equilibrium and were then diluted 20-fold with DNA cleavage buffer. In both assays, ciprofloxacin was used at 200 µM, and 8-methyl-2,4-dione was used at 20 µM. Levels of DNA cleavage at time zero were set to 100%, and results were quantified by monitoring the loss of double-stranded DNA breaks (FIII linear band) over time. Religation reactions carried out in the absence of drugs replaced Mg2+ with Ca2+ to achieve readily quantifiable levels of DNA cleavage. Rates of religation obtained in the presence of the two metal ions are similar. Representative gels are shown in Supplementary Figure S6. Error bars represent the standard deviation of three or more independent experiments.
Similar trends were seen in persistence assays with GrlAE85K topoisomerase IV [t1/2 in the absence of drug was <45 s (not shown)]. The t1/2 (∼5 min) for the decay of DNA cleavage complexes induced by 200 µM ciprofloxacin with the mutant enzyme dropped ∼18-fold as compared with wild-type [t1/2 ≈ 90 min (35)]. In contrast, the t1/2 (∼280 min) for 20 µM 8-methyl-2,4-dione decreased only ∼10% [wild-type t1/2 ≈ 310 min (35)] (Figure 7, right panel). These findings are similar to those reported for the quinolone-resistant GrlAS81F topoisomerase IV mutant (35). Therefore, mutations in either of the two amino acid residues proposed to coordinate the water molecules of the water-metal ion bridge appear to produce analogous effects on drug function.
Metal ion requirements for quinolone-induced DNA cleavage by B. anthracis topoisomerase IV
Quinolones have long been known to bind a variety of divalent metal ions (49–51), which have been hypothesized to enhance a number of drug functions (5,51–54). For example, quinolones bind DNA and alter the conformation of E. coli GyrA but only in the presence of divalent metal ions (53–55). However, because type II topoisomerases use a two-metal ion-mechanism for their DNA cleavage and religation reactions (56–59), it has been difficult to separate the requirements for the active site divalent cations from the non-catalytic metal ions that are hypothesized to participate in quinolone–enzyme interactions. Therefore, three approaches were used to elucidate the requirement for metal ions in quinolone action.
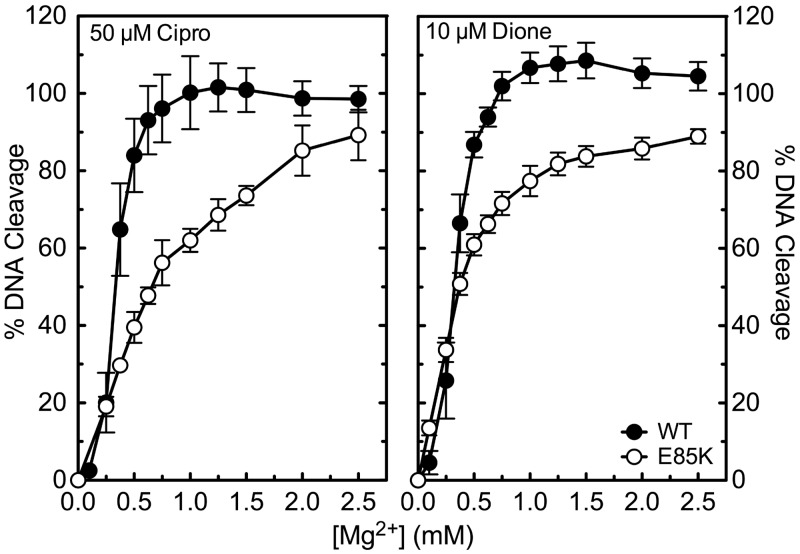
First, experiments were carried out to determine whether the Glu85->Lys mutation alters the affinity of metal ions in the proposed bridge. To this end, the Mg2+-dependence of quinolone- and quinazolinedione-induced topoisomerase IV-mediated DNA cleavage was analysed. In a previous study, quinolones (but not quinazolinediones) required increased concentrations of Mg2+ (compared with wild-type) to induce DNA scission following mutation of Ser81->Phe (35). This result was interpreted as functional evidence for the role of Ser81 in anchoring the water-metal ion bridge that purportedly stabilizes protein–quinolone interactions. To determine whether mutation of the other amino acid residue (Glu85) proposed to anchor the bridge produces a similar effect on metal ion utilization, a parallel Mg2+ titration was carried out with GrlAE85K topoisomerase IV in the presence of ciprofloxacin or 8-methyl-2,4-dione (Figure 8). The final concentrations of Mg2+ in assay mixtures are indicated in the figure. To facilitate direct comparisons between each different combination of drug and enzyme, levels of DNA cleavage generated under ‘standard conditions’ (in the presence of 10 mM Mg2+) were normalized to 100% for each drug–enzyme pair.
Figure 8.
Effects of Mg2+ on DNA cleavage mediated by wild-type and GrlAE85K topoisomerase IV in the presence of ciprofloxacin and 8-methyl-2,4-dione. Results are shown for 50 µM ciprofloxacin (Cipro, left panel) and 10 µM 8-methyl-2,4-dione (Dione, right panel) with the wild-type (WT, black circles) and GrlAE85K (E85K, open circles) enzymes. DNA cleavage was monitored by the appearance of the linear DNA (FIII) band. For each drug–enzyme pair, cleavage was normalized to 100% at 10 mM Mg2+ to facilitate direct comparisons. Error bars represent the standard deviation of three or more independent experiments.
The quinolone and quinazolinedione displayed similar requirements for Mg2+ when wild-type topoisomerase IV was used; half-maximal DNA cleavage was observed at ∼0.35 mM and ∼0.34 mM Mg2+, respectively. In contrast, the two drugs displayed markedly different Mg2+ requirements for DNA cleavage mediated by GrlAE85K. Although concentrations of Mg2+ required to generate half-maximal DNA cleavage doubled to ∼0.66 mM in the presence of the quinolone, metal ion utilization for 8-methyl-2,4-dione (∼0.36 mM) closely resembled that seen with the wild-type enzyme. The requirement for higher Mg2+ concentrations to support quinolone-induced DNA cleavage, but not quinazolinedione-induced DNA cleavage, by the mutant enzyme provides further evidence for the water-metal ion bridge that is proposed to coordinate quinolone-topoisomerase IV binding as well as the role of Glu85 in this interaction.
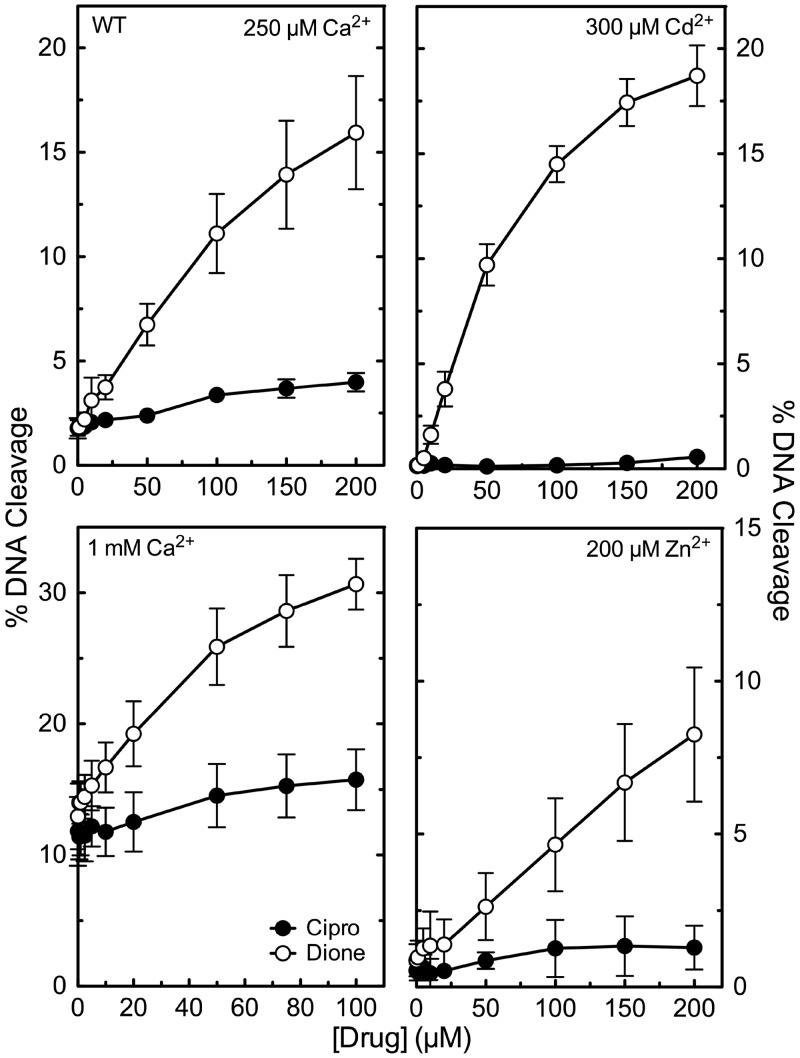
Second, experiments were carried out to establish the requirement for non-catalytic metal ions in drug function. As an initial step, it was necessary to segregate the functions of the catalytic and non-catalytic metal ions. Therefore, a variety of divalent (and trivalent) cations were screened for the ability to support DNA cleavage mediated by wild-type B. anthracis topoisomerase IV in the absence of drugs or in the presence of ciprofloxacin or 8-methyl-2,4-dione. Each metal ion was tested over a range of 50 µM to 10 mM and optimized for activity with the wild-type enzyme. Mg2+, Mn2+, Co2+, Sr2+, Ni2+, Pb2+, Al3+, Tb3+ and Eu3+ supported topoisomerase IV-mediated DNA cleavage under all three conditions (not shown). However, four divalent metal ions, Ca2+, Cd2+, Zn2+ and Ba2+, supported DNA cleavage differentially. Although they supported basal enzyme activity and high levels of DNA cleavage in the presence of 8-methyl-2,4-dione, each showed little or no ability to support DNA cleavage enhancement by ciprofloxacin (Figure 9; data with Ba2+ are not shown). These results strongly suggest that quinolones, but not quinazolinediones, require a non-catalytic divalent metal ion to induce DNA cleavage.
Figure 9.
Effects of alternative metal ions on drug-induced DNA cleavage mediated by wild-type topoisomerase IV. Assays were carried out with ciprofloxacin (Cipro, black circles) or 8-methyl-2,4-dione (Dione, open circles). The indicated divalent metal ions were substituted for the Mg2+ used in standard assays. Cd2+ (300 µM; top right) and Zn2+ (200 µM; bottom right) were used at the concentration that gave maximal enzyme-mediated DNA cleavage activity. Ca2+ was used at 250 µM (top left) or 1 mM (bottom left). DNA cleavage was monitored by the appearance of the linear DNA (FIII) band. Error bars represent the standard deviation of three or more independent experiments.
Ca2+, Cd2+, Zn2+ and Ba2+ all have been shown to complex with quinolones (50,51,60–63). Therefore, we propose that when chelated by ciprofloxacin, Ca2+, Cd2+, Zn2+ and Ba2+ cannot support the proper coordination geometry to form a functional water-metal ion bridge. However, an alternative interpretation is plausible. Sub-millimolar concentrations of metal ions were used in experiments because higher concentrations of Cd2+ and Zn2+ impaired enzyme activity and higher concentrations of Ca2+ and Ba2+ significantly increased basal levels of DNA cleavage. Therefore, it is possible that they did not support ciprofloxacin-induced DNA cleavage because the drug sequesters the divalent cations and prevents their use in the active site of the enzyme. Two controls indicate that this is not the case (Figure 9). (i) No enhancement of DNA cleavage was observed at the lowest concentrations of ciprofloxacin (<50 µM) at which concentrations of metal ions would be more than sufficient to support the DNA cleavage reaction of topoisomerase IV. In contrast, significant levels of DNA cleavage were seen in the same concentration range of 8-methyl-2,4-dione. (ii) Experiments were repeated at 1 mM Ca2+. Even though drug effects were tempered by the high levels of basal (i.e. no drug) topoisomerase IV-mediated DNA cleavage, it is obvious that the quinazolinedione, but not the quinolone, further increases DNA scission.
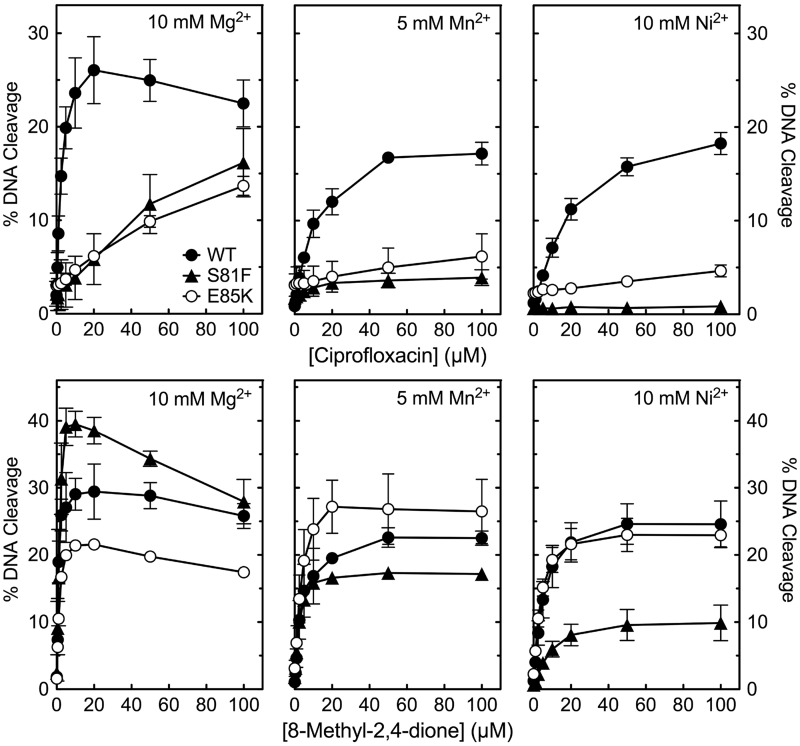
Third, experiments were carried out to further define the roles of Ser81 and Glu85 in anchoring the proposed water-metal ion bridge. Therefore, we searched for metal ions that could distinguish between wild-type and mutant B. anthracis topoisomerase IV. Metal ions that displayed optimal activity in the millimolar range were used for these studies to ensure that both the catalytic and non-catalytic sites were saturated over the drug range tested. Although Mg2+ (as well as Co2+; data not shown) supported quinolone-induced DNA cleavage mediated by wild-type, GrlAE85K or GrlAS81F topoisomerase IV, Mn2+ and Ni2+ could only do so with the wild-type enzyme (Figure 10, top). In contrast, all four of these metal ions supported quinazolinedione-induced DNA cleavage with wild-type and mutant topoisomerase IV (Figure 10, bottom; data with Co2+ are not shown). These findings suggest that mutations at either of the two amino acids that are proposed to anchor the bridge (and hence the quinolone) to the enzyme restrict the divalent cations that can be used to form a functional water-metal ion bridge.
Figure 10.
Effects of alternative metal ions on drug-induced DNA cleavage mediated by wild-type, GrlAS81F and GrlAE85K topoisomerase IV. Results are shown for cleavage mediated by the wild-type (WT, black circles), GrlAS81F (S81F, black triangles) and GrlAE85K (E85K, open circles) enzymes in the presence of ciprofloxacin (top) or 8-methyl-2,4-dione (bottom) and Mg2+ (left), Mn2+ (middle) or Ni2+ (right). Metal ions were used at the concentration that yielded maximal enzyme activity (10 mM for Mg2+ and Ni2+, 5 mM for Mn2+). DNA cleavage was monitored by the appearance of the linear DNA (FIII) band. Error bars represent the standard deviation of three or more independent experiments.
Taken together, these results support the conclusion that Ser81 and Glu85 play important roles in mediating quinolone-enzyme interactions by anchoring the water-metal ion bridge.
Simultaneous mutation of amino acid residues that are proposed to anchor the water-metal ion bridge in B. anthracis topoisomerase IV
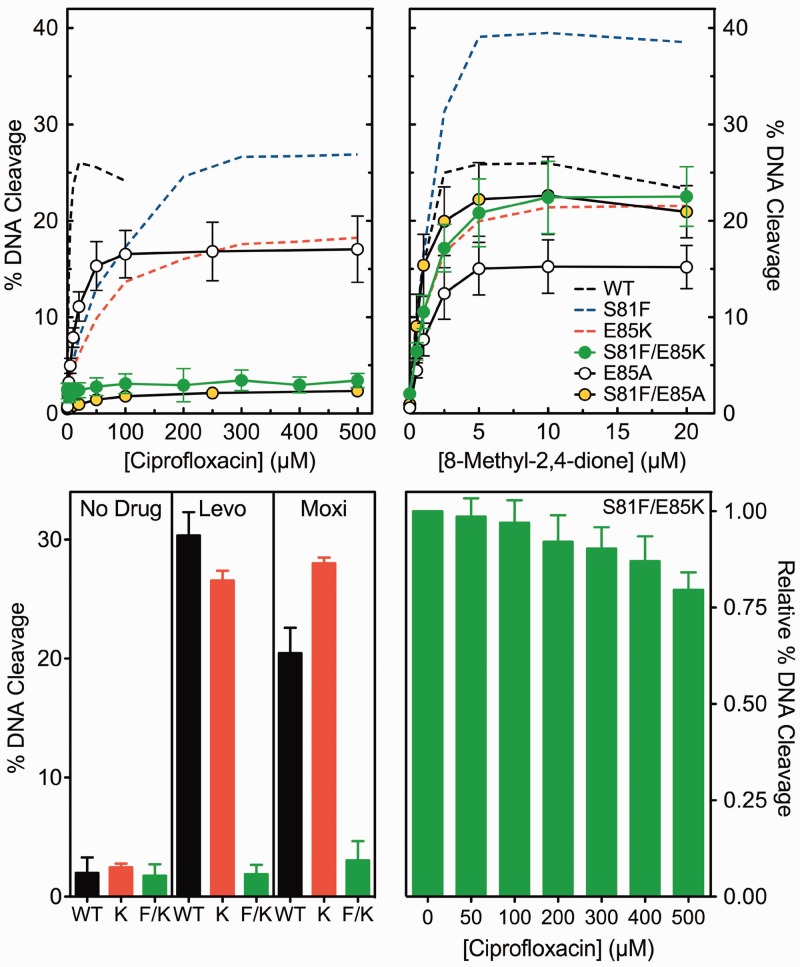
Two lines of evidence strongly suggest that mutation of either Ser81 or Glu85 results in an altered, but partially functional, water-metal ion bridge (or an altered topoisomerase IV-bridge interaction). In the presence of either individual mutation, ciprofloxacin and other clinically relevant quinolones can induce near wild-type levels of DNA cleavage at high drug concentrations [Figure 6 and Aldred et al. (35); also see Figure 12, bottom left]. In addition, ciprofloxacin requires increased Mg2+ concentrations to attain maximal levels of DNA cleavage [Figure 8 and Aldred et al. (35)].
Figure 12.
Effects of drugs on the DNA cleavage activities of GrlAS81F/E85K, GrlAE85A and GrlAS81F/E85A topoisomerase IV. DNA cleavage mediated by the GrlAS81F/E85K (S81F/E85K, green circles), GrlAE85A (E85A, open circles) or GrlAS81F/E85A (S81F/E85A, yellow circles) enzymes in the presence of ciprofloxacin (top left) or 8-methyl-2,4-dione (top right) are shown. Activity levels of the wild-type (WT, black dashed line), GrlAE85K (E85K, red dashed line) and GrlAS81F (S81F, blue dashed line) are shown for reference [data from Figure 6 and Aldred et al. (35), respectively]. The bar graph at the bottom left shows the maximum level of cleavage generated by the wild-type (WT, black bars), GrlAE85K (K, red bars) and GrlAS81F/E85K (F/K, green bars) enzymes in the absence of drug (No Drug, left) or in the presence of the clinically relevant quinolones levofloxacin (Levo, middle) or moxifloxacin (Moxi, right). Quinolone concentrations were 500 µM for the mutant enzymes and 20 µM for the wild-type enzyme. The bar graph at the bottom right shows the ability of ciprofloxacin (0–500 µM) to compete out DNA cleavage induced by 8-methyl-2,4-dione (5 µM) with GrlAS81F/E85K topoisomerase IV. The quinolone and quinazolinedione were added to reactions simultaneously. In all cases, DNA cleavage was monitored by the appearance of the linear DNA (FIII) band. Error bars represent the standard deviation of three or more independent experiments.
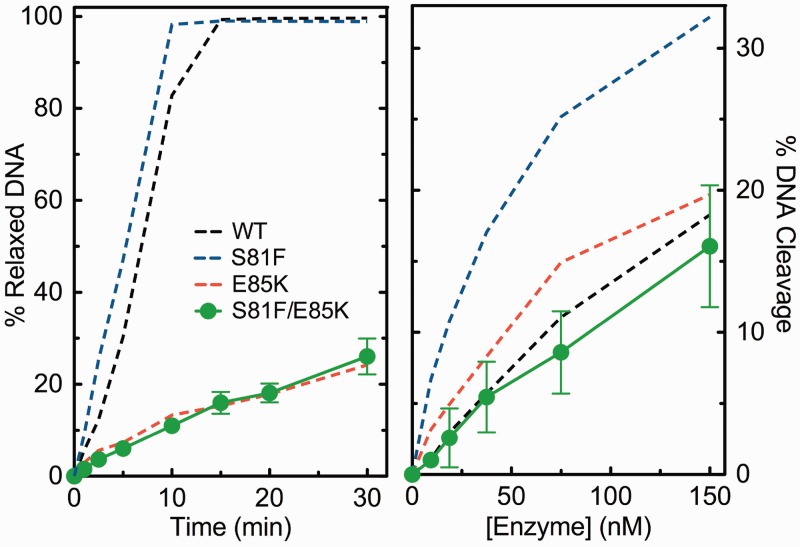
Because individual mutation of Ser81 or Glu85 did not completely disrupt bridge function, we generated and characterized GrlAS81F/E85K topoisomerase IV. The double mutant displayed an overall catalytic activity (Figure 11, left) and a DNA cleavage activity (Figure 11, right) that were similar to those of GrlAE85K topoisomerase IV. However, unlike results with the individual GrlAS81F and GrlAE85K mutants, ciprofloxacin and other clinically relevant quinolones displayed virtually no ability to enhance DNA cleavage mediated by GrlAS81F/E85K topoisomerase IV, even at high drug concentrations (Figure 12, top and bottom left). In contrast, 8-methyl-2,4-dione induced near wild-type levels of DNA cleavage with the double mutant (Figure 12, top right).
Figure 11.
DNA relaxation and cleavage activities of GrlAS81F/E85K topoisomerase IV. The abilities of the GrlAS81F/E85K (S81F/E85K, green circles) enzyme to relax (left) and cleave (right) negatively supercoiled pBR322 plasmid DNA are shown. Activities of the wild-type (WT, black dashed line), GrlAE85K (E85K, red dashed line) and GrlAS81F (S81F, blue dashed line) enzymes are shown for reference [data from Figures 2 and 5 and Aldred et al. (35), respectively]. As described earlier, DNA cleavage assays were carried out in the presence of 10 mM CaCl2 to facilitate quantification. DNA relaxation and cleavage were monitored by the disappearance of supercoiled DNA (FI band) and the appearance of the linear DNA (FIII) band, respectively. Error bars represent the standard deviation of three or more independent experiments.
The decreased potency of quinolones with the individual GrlAS81F and GrlAE85K mutants [Aldred et al. (35) and Figure 6] strongly suggests that impaired bridge function decreases the ability of quinolones to bind in the cleavage complex. Because quinolones induced virtually no DNA cleavage enhancement with GrlAS81F/E85K topoisomerase IV, it was impossible to determine quinolone potency with the double mutation. Therefore, a competition experiment was used to assess the effects of the double mutation on quinolone affinity (Figure 12, bottom right). Because structural studies indicate that the interaction domains of quinolones and quinazolinediones in the cleavage complex overlap almost completely (31–34), the ability of 0–500 µM ciprofloxacin to compete out DNA cleavage mediated by GrlAS81F/E85K topoisomerase IV in the presence of 5 µM 8-methyl-2,4-dione was determined. Even at a 100-fold molar excess of quinolone over quinazolinedione, little competition was observed (DNA cleavage dropped by ∼20%). This result indicates that in the absence of the water-metal ion bridge, the relative affinity of ciprofloxacin (compared with 8-methyl-2,4-dione) for the topoisomerase IV-DNA complex decreases >100-fold. (The relative potencies for these two compounds with wild-type topoisomerase IV differ by <3-fold.)
The aforementioned results strongly suggest that the proposed water-metal ion bridge represents the major conduit by which ciprofloxacin and other clinically relevant quinolones interact with topoisomerase IV in the DNA cleavage complex. However, it is possible that the insertion of the positively charged lysine residue in place of Glu85 disrupts enzyme–quinolone interactions by a mechanism that reflects more than the simple loss of the glutamic acid anchor. For example, the positive charge on the lysine could alter quinolone binding by repelling the divalent metal ion that is chelated by the quinolone. Therefore, we examined the ability of drugs to poison two additional mutant topoisomerase IV enzymes, GrlAE85A and GrlAS81F/E85A. In contrast to the Glu->Lys mutants, these enzymes removed the glutamic acid moiety without replacing it with a positively charged amino acid. Both of the Glu85->Ala mutant enzymes displayed DNA cleavage activities in the absence of drug that were similar to their Glu85->Lys counterparts (data not shown). Furthermore, the effects of drugs on GrlAE85A and GrlAS81F/E85A topoisomerase IV paralleled those seen with the Glu->Lys mutant enzymes (Figure 12). Ciprofloxacin was able to poison GrlAE85A topoisomerase IV at high drug concentrations but showed little ability to enhance cleavage with the double mutant at any concentration tested. Furthermore, neither the single nor the double mutation that contained Glu85->Ala had any significant effect on the ability of the quinazolinedione to enhance topoisomerase IV-mediated DNA cleavage. Finally, as seen with the Glu->Lys mutation, GrlAE85A required an increased concentration of Mg2+ to support maximal levels of DNA cleavage in the presence of ciprofloxacin, but not in the presence of 8-methyl-2,4-dione (data not shown).
Taken together, these results strongly support the water-metal ion bridge model for quinolone-topoisomerase IV interactions. Simultaneous mutation of both amino acid residues that anchor the bridge to the protein completely disrupts bridge function and abrogates ciprofloxacin-mediated DNA cleavage enhancement. However, the mutations have little effect on the activity of a quinazolinedione that interacts with the protein independently of the bridge.
SUMMARY
The clinical use of quinolones is being threatened by the growing incidence of resistance (4,5). Most commonly, quinolone resistance is caused by mutations in a specific serine or acidic residue in the A subunit of gyrase or topoisomerase IV (5,23–27,30). On the basis of structural data, these amino acids were proposed to serve as a critical site for quinolone interaction with the protein by anchoring a water-metal ion bridge that coordinates drug binding (34).
Results of the present study provide functional corroboration for the existence of the water-metal ion bridge and strongly suggest that it serves as the primary conduit for interactions between clinically relevant quinolones and topoisomerase IV in the DNA cleavage complex. Furthermore, they solidify the proposed roles of the serine and glutamic acid residues as the anchor points for the bridge and thereby provide a likely mechanism for quinolone resistance caused by mutations at these two residues. Finally, the fact that quinazolinediones lack the C3/C4 keto acid used by quinolones to chelate metal ions suggests that these compounds are unable to form an operative water-metal ion bridge. It further implies that quinazolinediones with activity against gyrase and topoisomerase IV must rely on other structural components to form novel (or stronger) anchor points to the enzyme or DNA in the cleavage complex. If correct, this postulate provides an obvious explanation as to why bacterial enzymes that carry the most common quinolone resistance mutations (which impair bridge function) retain high sensitivity to these quinazolinediones (35,38,47,48,64,65).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Figures 1–6.
FUNDING
National Institutes of Health (NIH) [AI81775 to C.L.T., AI87671 to R.J.K. and GM33944 to N.O.]; K.J.A. was a trainee under grant [T32 CA09582] from the National Institutes of Health. Funding for open access charge: NIH [GM033944].
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Adam C. Ketron, R. Hunter Lindsey and MaryJean Campbell for critical reading of the manuscript.
REFERENCES
- 1.Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am. J. Med. 2005;118:259–268. doi: 10.1016/j.amjmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Hooper DC. Clinical applications of quinolones. Biochim. Biophys. Acta. 1998;1400:45–61. doi: 10.1016/s0167-4781(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 3.Hooper DC. Mechanisms of action of antimicrobials: focus on fluoroquinolones. Clin. Infect. Dis. 2001;32(Suppl. 1):S9–S15. doi: 10.1086/319370. [DOI] [PubMed] [Google Scholar]
- 4.Andriole VT. The quinolones: past, present, and future. Clin. Infect. Dis. 2005;41(Suppl. 2):S113–S119. doi: 10.1086/428051. [DOI] [PubMed] [Google Scholar]
- 5.Drlica K, Hiasa H, Kerns R, Malik M, Mustaev A, Zhao X. Quinolones: action and resistance updated. Curr. Top. Med. Chem. 2009;9:981–998. doi: 10.2174/156802609789630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooper DC. Mode of action of fluoroquinolones. Drugs. 1999;58(Suppl. 2):6–10. doi: 10.2165/00003495-199958002-00002. [DOI] [PubMed] [Google Scholar]
- 8.Anderson VE, Osheroff N. Type II topoisomerases as targets for quinolone antibacterials: turning Dr. Jekyll into Mr. Hyde. Curr. Pharm. Des. 2001;7:337–353. doi: 10.2174/1381612013398013. [DOI] [PubMed] [Google Scholar]
- 9.Drlica K, Malik M, Kerns RJ, Zhao X. Quinolone-mediated bacterial death. Antimicrob. Agents Chemother. 2008;52:385–392. doi: 10.1128/AAC.01617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier B, Zhao X, Lu T, Drlica K, Hooper DC. Selective targeting of topoisomerase IV and DNA gyrase in Staphylococcus aureus: different patterns of quinolone-induced inhibition of DNA synthesis. Antimicrob. Agents Chemother. 2000;44:2160–2165. doi: 10.1128/aac.44.8.2160-2165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munoz R, De la Campa AG. ParC subunit of DNA topoisomerase IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperates with DNA gyrase A subunit in forming resistance phenotype. Antimicrob. Agents Chemother. 1996;40:2252–2257. doi: 10.1128/aac.40.10.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan X-S, Ambler J, Mehtar S, Fisher LM. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan X-S, Fisher LM. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob. Agents Chemother. 1997;41:471–474. doi: 10.1128/aac.41.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan X-S, Fisher LM. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1998;42:2810–2816. doi: 10.1128/aac.42.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins PG, Fluit AC, Schmitz FJ. Fluoroquinolones: structure and target sites. Curr. Drug Targets. 2003;4:181–190. doi: 10.2174/1389450033346920. [DOI] [PubMed] [Google Scholar]
- 16.Levine C, Hiasa H, Marians KJ. DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim. Biophys. Acta. 1998;1400:29–43. doi: 10.1016/s0167-4781(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 17.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 18.Velez-Cruz R, Osheroff N. Encyclopedia of Biological Chemistry. Elsevier Inc., San Diego; 2004. pp. 806–811. [Google Scholar]
- 19.Schoeffler AJ, Berger JM. DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q. Rev. Biophys. 2008;41:41–101. doi: 10.1017/S003358350800468X. [DOI] [PubMed] [Google Scholar]
- 20.Deweese JE, Osheroff MA, Osheroff N. DNA topology and topoisomerases: teaching a “knotty” subject. Biochem. Molec. Biol. Educ. 2009;37:2–10. doi: 10.1002/bmb.20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing. Nucleic Acids Res. 2009;37:738–749. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Deibler RW, Chan HS, Zechiedrich L. The why and how of DNA unlinking. Nucleic Acids Res. 2009;37:661–671. doi: 10.1093/nar/gkp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan-Linnell SK, Becnel Boyd L, Steffen D, Zechiedrich L. Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 2009;53:235–241. doi: 10.1128/AAC.00665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price LB, Vogler A, Pearson T, Busch JD, Schupp JM, Keim P. In vitro selection and characterization of Bacillus anthracis mutants with high-level resistance to ciprofloxacin. Antimicrob. Agents Chemother. 2003;47:2362–2365. doi: 10.1128/AAC.47.7.2362-2365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grohs P, Podglajen I, Gutmann L. Activities of different fluoroquinolones against Bacillus anthracis mutants selected in vitro and harboring topoisomerase mutations. Antimicrob. Agents Chemother. 2004;48:3024–3027. doi: 10.1128/AAC.48.8.3024-3027.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bast DJ, Athamna A, Duncan CL, de Azavedo JC, Low DE, Rahav G, Farrell D, Rubinstein E. Type II topoisomerase mutations in Bacillus anthracis associated with high-level fluoroquinolone resistance. J. Antimicrob. Chemother. 2004;54:90–94. doi: 10.1093/jac/dkh294. [DOI] [PubMed] [Google Scholar]
- 27.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida H, Kojima T, Yamagishi J, Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol. Gen. Genet. 1988;211:1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]
- 29.Cullen ME, Wyke AW, Kuroda R, Fisher LM. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob. Agents Chemother. 1989;33:886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Deguchi T, Yasuda M, Kawamura T, Kanematsu E, Nishino Y, Ishihara S, Kawada Y. Alteration in the GyrA subunit of DNA gyrase and the ParC subunit of DNA topoisomerase IV in quinolone-resistant clinical isolates of Staphylococcus epidermidis. Antimicrob. Agents Chemother. 1998;42:3293–3295. doi: 10.1128/aac.42.12.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laponogov I, Sohi MK, Veselkov DA, Pan XS, Sawhney R, Thompson AW, McAuley KE, Fisher LM, Sanderson MR. Structural insight into the quinolone-DNA cleavage complex of type IIA topoisomerases. Nat. Struct. Mol. Biol. 2009;16:667–669. doi: 10.1038/nsmb.1604. [DOI] [PubMed] [Google Scholar]
- 32.Laponogov I, Pan XS, Veselkov DA, McAuley KE, Fisher LM, Sanderson MR. Structural basis of gate-DNA breakage and resealing by type II topoisomerases. PLoS One. 2010;5:e11338. doi: 10.1371/journal.pone.0011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A, Hibbs M, et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature. 2010;466:935–940. doi: 10.1038/nature09197. [DOI] [PubMed] [Google Scholar]
- 34.Wohlkonig A, Chan PF, Fosberry AP, Homes P, Huang J, Kranz M, Leydon VR, Miles TJ, Pearson ND, Perera RL, et al. Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat. Struct. Mol. Biol. 2010;17:1152–1153. doi: 10.1038/nsmb.1892. [DOI] [PubMed] [Google Scholar]
- 35.Aldred KJ, McPherson SA, Wang P, Kerns RJ, Graves DE, Turnbough CL, Jr, Osheroff N. Drug interactions with Bacillus anthracis topoisomerase IV: biochemical basis for quinolone action and resistance. Biochemistry. 2012;51:370–381. doi: 10.1021/bi2013905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong S, McPherson SA, Wang Y, Li M, Wang P, Turnbough CL, Jr, Pritchard DG. Characterization of the enzymes encoded by the anthrose biosynthetic operon of Bacillus anthracis. J. Bacteriol. 2010;192:5053–5062. doi: 10.1128/JB.00568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortune JM, Velea L, Graves DE, Osheroff N. DNA topoisomerases as targets for the anticancer drug TAS-103: DNA interactions and topoisomerase catalytic inhibition. Biochemistry. 1999;38:15580–15586. doi: 10.1021/bi991792g. [DOI] [PubMed] [Google Scholar]
- 38.Malik M, Marks KR, Mustaev A, Zhao X, Chavda K, Kerns RJ, Drlica K. Fluoroquinolone and quinazolinedione activities against wild-type and gyrase mutant strains of Mycobacterium smegmatis. Antimicrob. Agents Chemother. 2011;55:2335–2343. doi: 10.1128/AAC.00033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fortune JM, Osheroff N. Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J. Biol. Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- 40.Fortune JM, Osheroff N. Topoisomerase II-catalyzed relaxation and catenation of plasmid DNA. Methods Mol. Biol. 2001;95:275–281. doi: 10.1385/1-59259-057-8:275. [DOI] [PubMed] [Google Scholar]
- 41.Kingma PS, Fortune JM, Osheroff N. Topoisomerase II-catalyzed ATP hydrolysis as monitored by thin-layer chromatography. Methods Mol. Biol. 2001;95:51–56. doi: 10.1385/1-59259-057-8:51. [DOI] [PubMed] [Google Scholar]
- 42.Robinson MJ, Osheroff N. Effects of antineoplastic drugs on the post-strand-passage DNA cleavage/religation equilibrium of topoisomerase II. Biochemistry. 1991;30:1807–1813. doi: 10.1021/bi00221a012. [DOI] [PubMed] [Google Scholar]
- 43.Gentry AC, Pitts SL, Jablonsky MJ, Bailly C, Graves DE, Osheroff N. Interactions between the etoposide derivative F14512 and human type II topoisomerases: implications for the C4 spermine moiety in promoting enzyme-mediated DNA cleavage. Biochemistry. 2011;50:3240–3249. doi: 10.1021/bi200094z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson VE, Zaniewski RP, Kaczmarek FS, Gootz TD, Osheroff N. Quinolones inhibit DNA religation mediated by Staphylococcus aureus topoisomerase IV: changes in drug mechanism across evolutionary boundaries. J. Biol. Chem. 1999;274:35927–35932. doi: 10.1074/jbc.274.50.35927. [DOI] [PubMed] [Google Scholar]
- 45.Anderson VE, Zaniewski RP, Kaczmarek FS, Gootz TD, Osheroff N. Action of quinolones against Staphylococcus aureus topoisomerase IV: basis for DNA cleavage enhancement. Biochemistry. 2000;39:2726–2732. doi: 10.1021/bi992302n. [DOI] [PubMed] [Google Scholar]
- 46.Hiasa H. The Glu-84 of the ParC subunit plays critical roles in both topoisomerase IV-quinolone and topoisomerase IV-DNA interactions. Biochemistry. 2002;41:11779–11785. doi: 10.1021/bi026352v. [DOI] [PubMed] [Google Scholar]
- 47.Pan XS, Gould KA, Fisher LM. Probing the differential interactions of quinazolinedione PD 0305970 and quinolones with gyrase and topoisomerase IV. Antimicrob. Agents Chemother. 2009;53:3822–3831. doi: 10.1128/AAC.00113-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oppegard LM, Streck KR, Rosen JD, Schwanz HA, Drlica K, Kerns RJ, Hiasa H. Comparison of in vitro activities of fluoroquinolone-like 2,4- and 1,3-diones. Antimicrob. Agents Chemother. 2010;54:3011–3014. doi: 10.1128/AAC.00190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall AJ, Piddock LJ. Interaction of divalent cations, quinolones and bacteria. J. Antimicrob. Chemother. 1994;34:465–483. doi: 10.1093/jac/34.4.465. [DOI] [PubMed] [Google Scholar]
- 50.Turel I. The interactions of metal ions with quinolone antibacterial agents. Coord. Chem. Rev. 2002;232:27–47. [Google Scholar]
- 51.Serafin A, Stańczak A. The complexes of metal ions with fluoroquinolones. Russ. J. Coord. Chem. 2009;35:81–95. [Google Scholar]
- 52.Fan JY, Sun D, Yu H, Kerwin SM, Hurley LH. Self-assembly of a quinobenzoxazine-Mg2+ complex on DNA: a new paradigm for the structure of a drug-DNA complex and implications for the structure of the quinolone bacterial gyrase-DNA complex. J. Med. Chem. 1995;38:408–424. doi: 10.1021/jm00003a003. [DOI] [PubMed] [Google Scholar]
- 53.Sissi C, Andreolli M, Cecchetti V, Fravolini A, Gatto B, Palumbo M. Mg(2+)-mediated binding of 6-substituted quinolones to DNA: relevance to biological activity. Bioorg. Med. Chem. 1998;6:1555–1561. doi: 10.1016/s0968-0896(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 54.Sissi C, Perdona E, Domenici E, Feriani A, Howells AJ, Maxwell A, Palumbo M. Ciprofloxacin affects conformational equilibria of DNA gyrase A in the presence of magnesium ions. J. Mol. Biol. 2001;311:195–203. doi: 10.1006/jmbi.2001.4838. [DOI] [PubMed] [Google Scholar]
- 55.Palu G, Valisena S, Ciarrocchi G, Gatto B, Palumbo M. Quinolone binding to DNA is mediated by magnesium ions. Proc. Natl Acad. Sci. USA. 1992;89:9671–9675. doi: 10.1073/pnas.89.20.9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deweese JE, Burgin AB, Osheroff N. Human topoisomerase IIα uses a two-metal-ion mechanism for DNA cleavage. Nucleic Acids Res. 2008;36:4883–4893. doi: 10.1093/nar/gkn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deweese JE, Burch AM, Burgin AB, Osheroff N. Use of divalent metal ions in the DNA cleavage reaction of human type II topoisomerases. Biochemistry. 2009;48:1862–1869. doi: 10.1021/bi8023256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt BH, Burgin AB, Deweese JE, Osheroff N, Berger JM. A novel and unified two-metal mechanism for DNA cleavage by type II and IA topoisomerases. Nature. 2010;465:641–644. doi: 10.1038/nature08974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pitts SL, Liou GF, Mitchenall LA, Burgin AB, Maxwell A, Neuman KC, Osheroff N. Use of divalent metal ions in the DNA cleavage reaction of topoisomerase IV. Nucleic Acids Res. 2011;39:4808–4817. doi: 10.1093/nar/gkr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sultana N, Arayne MS, Sharif S. Levofloxacin interactions with essential and trace elements. Pak. J. Pharm. Sci. 2004;17:67–76. [PubMed] [Google Scholar]
- 61.Sultana N, Arayne MS, Furqan H. In vitro availability of lomefloxacin hydrochloride in presence of essential and trace elements. Pak. J. Pharm. Sci. 2005;18:59–65. [PubMed] [Google Scholar]
- 62.Sultana N, Arayne MS, Yasmeen N. In vitro availability of ofloxacin in presence of metals essential to human body. Pak. J. Pharm. Sci. 2007;20:42–47. [PubMed] [Google Scholar]
- 63.Zhang Y, Cai X, Lang X, Qiao X, Li X, Chen J. Insights into aquatic toxicities of the antibiotics oxytetracycline and ciprofloxacin in the presence of metal: complexation versus mixture. Environ. Pollut. 2012;166:48–56. doi: 10.1016/j.envpol.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 64.Huband MD, Cohen MA, Zurack M, Hanna DL, Skerlos LA, Sulavik MC, Gibson GW, Gage JW, Ellsworth E, Stier MA, et al. In vitro and in vivo activities of PD 0305970 and PD 0326448, new bacterial gyrase/topoisomerase inhibitors with potent antibacterial activities versus multidrug-resistant Gram-positive and fastidious organism groups. Antimicrob. Agents Chemother. 2007;51:1191–1201. doi: 10.1128/AAC.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.German N, Malik M, Rosen JD, Drlica K, Kerns RJ. Use of gyrase resistance mutants to guide selection of 8-methoxy-quinazoline-2,4-diones. Antimicrob. Agents Chemother. 2008;52:3915–3921. doi: 10.1128/AAC.00330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.