EPIDEMIOLOGY
The importance of diabetes as a cause of mortality and morbidity is well known. The number of patients increases alongside aging of the population, raising the prevalence of obesity and a sedentary lifestyle. As a disease that is strongly associated with both micro- and macrovascular complications, diabetes results in organ and tissue damage. Cardiovascular disease (CVD) causes up to 70% of all deaths in people with diabetes. The epidemic of diabetes will thus be followed by an epidemic of diabetes-related vascular diseases. Almost two of three patients who present with symptomatic chronic heart disease have abnormal glucose homeostasis. Although the mortality from coronary artery disease (CAD) in patients without diabetes has declined over the past 20 years, the mortality in men with type 2 diabetes has not changed significantly. Moreover, diabetes is an independent risk factor for heart failure, and there are a substantial number of patients with diabetes and heart failure. The main macrovascular complications, for which diabetes has been a well-established risk factor throughout the cardiovascular system, are CAD, peripheral vascular disease, increased intima-media thickness, and stroke. Ischemic heart disease and stroke account for the highest proportion of comorbid diseases associated with diabetes.
The joint guidelines of the European Society of Cardiology and the European Association of Diabetes take into account the close reciprocal relationship between diagnostics and therapeutics in cardiology and diabetology.
Patients with diabetes and CVD have an unfavorable prognosis (1). Mortality rates due to heart disease are two to four times higher among people with diabetes compared with those without diabetes after correction for traditional risk factors for CVD such as age, obesity, smoking, dyslipidemia, and hypertension. It appears, however, that the presence of even one of these risk factors leads to poorer outcomes among people with diabetes compared with those without diabetes. People with diabetes have an up to fivefold-higher risk for a first myocardial infarction (MI) and a twofold-greater risk for a recurrent MI than people who previously had an MI but do not suffer from diabetes. Patients with diabetes with prior MI have the worst prognosis (2). Furthermore, people with diabetes have a poorer long-term prognosis after MI, including an increased risk for congestive heart failure and death. People with diabetes are two to four times more likely to develop stroke than people without diabetes.
Diabetes accounted for a significant percentage of patients with a diagnosis of heart failure in numerous epidemiologic studies such as The Framingham Study, UK Prospective Diabetes Study (UKPDS), Cardiovascular Health Study, and Euro Heart Failure Surveys. Data from UKPDS regarding the adjusted rate of heart failure demonstrate a rise from 2.3 events per 100 person-years in people with HbA1c levels <6% to 11.9 events per 100 person-years in those presenting with HbA1c levels >10% (3). An increase in HbA1c of 1% correlates to an increment of 8% in heart failure (3,4). Diabetes is a powerful predictor of cardiovascular morbidity and mortality and is an independent risk factor for death in patients with established heart failure. In addition, the prevalence of heart failure in elderly diabetic patients was up to 30% (5). Diabetic women are more likely to develop heart failure than men if compared with age-matched control subjects (5.1-fold vs. 2.1-fold increase) (6). The reason for this difference is not yet fully understood, but may be in part due to a worse comorbid risk factor profile, and the permissive effect upon outcome, particularly in diabetic women (7).
The combination of hyperglycemia, insulin resistance, dyslipidemia, hypertension, and chronic inflammation injures the vascular endothelium, resulting in microvascular damage (alterations in capillary density and vascular permeability), macrovasculopathy, and CVD. Most importantly, more than 70% of people with diabetes have high blood pressure or are being treated with medications for hypertension. Because prediabetic subjects often present with multiple CVD risk factors such as insulin resistance, obesity, central obesity, elevated blood pressure, elevated total triglycerides, and low HDL cholesterol, the onset of cardiovascular damage is not closely related to hyperglycemia alone, but has to be seen in the concert of metabolic derangement (8). The cardiac risk in diabetic patients is not only with respect to type 1 or type 2 diabetic patients, but also to several pathophysiological mechanisms and features such as CAD, heart failure, and autonomic neuropathy.
HISTORY AND DIAGNOSIS
Despite decades of basic and clinical investigations, diabetic cardiomyopathy as a clinical entity remains elusive. A diagnostic method for the identification of diabetic cardiomyopathy is still not available. Since the first report in 1972 by Rubler et al. (9) who analyzed autopsy data from four patients with diabetic renal microangiopathy and dilated left ventricles in the absence of other common causes, evidence and acceptance of diabetic cardiomyopathy as a clinical entity has been rising. Looking decades back, it was in 1881 that Leyden (10) commented that heart failure was a “frequent and noteworthy complication of diabetes” and Mayer (11) stated that “heart disease in diabetes can be traced to an abnormality in metabolism.”
Diabetic cardiomyopathy describes diabetes-associated changes in the structure and function of the myocardium that are not directly linked to other confounding factors such as CAD or hypertension. As a multifactorial disease entity, it is clinically characterized by an initial increase in left ventricular stiffness and subclinical diastolic dysfunction. However, this may advance to compromised left ventricular systolic function with loss of contractile function and progress into an overt congestive heart failure. Diabetic cardiomyopathy is known to be associated with changes in cardiac structure such as myocardial hypertrophy, fibrosis, and fat deposition. In many patients, particularly those with type 2 diabetes, the diabetes-associated clinical findings are amplified by the existence of these comorbidities, augmenting the development of left ventricular hypertrophy, increasing the susceptibility of the heart to ischemic injury, and increasing the overall likelihood of developing heart failure.
The use of appropriate diagnostic strategies, which may help correctly identify the disease at early asymptomatic stages and lead to the implementation of suitable corrective therapies, is imperative. Diabetes-associated vascular alterations include anatomic, structural, and functional changes leading to organ dysfunction (12). Early changes in cardiac function are typically manifested as abnormal diastolic function that consecutively progresses to loss of contractile function. In its progression, diabetic cardiomyopathy may advance to compromised left ventricular systolic function that with time leads to loss of contractile function and results in an overt congestive heart failure (13). Echocardiography-based methods currently stand as the preferred diagnostic approach for diabetic cardiomyopathy. Newer echocardiographic methods such as tissue-Doppler imaging have been developed for early and very discrete detection of cardiac dysfunction before manifest symptoms are present (14). Diastolic dysfunction precedes the development of systolic dysfunction. The use of flow and tissue-Doppler techniques suggests a prevalence of diastolic dysfunction as high as 40–75% in individuals with type 1 and type 2 diabetes without overt CAD (15).
In addition to conventional techniques, magnetic resonance imaging and spectroscopy along with contrast agents are now leading new approaches in the diagnosis of myocardial fibrosis and cardiac and hepatic metabolic changes. The measurements of metabolic flux via positron emission tomography are useful to demonstrate local metabolic maladaption of the myocardium. By assessing the myocardial function through the evaluation of myocardial substrate metabolism in asymptomatic men with well-controlled uncomplicated type 2 diabetes and verified absence of cardiac ischemia, Rijzewijk et al. (16) were able to demonstrate that the left ventricular diastolic function was impaired and that the myocardial substrate metabolism was altered in patients compared with age-matched healthy control subjects.
CARDIAC METABOLISM IN GENERAL
Metabolic and morphologic defects concern myocardial energy metabolism, reduced flow reserve, formation of advanced glycation end products (AGEs) and structural alterations that impair cardiac function (17). The flexibility in myocardial substrate metabolism for energy production is fundamental to cardiac health. The loss in variability leads to fixation on special substrates. Predominance in fatty acid metabolism is characteristic of diabetic heart disease and is associated with pressure-overload left ventricular hypertrophy. Myocardial metabolic remodeling is central to the pathogenesis of a variety of cardiac disease processes, such as left ventricular hypertrophy. It is the process in which the heart loses its ability to use different substrates, becoming dependent primarily on the metabolism of a single substrate for energy production (18). The most important result of cardiac metabolism in diabetes is the switch from carbohydrates and fatty acids as a source of energy to an excessive use of fatty acids. In animal models of diabetes, cardiac dysfunction coexists with increased myocardial nonesterified fatty acid use, triglyceride accumulation, and subsequent increased production of toxic intermediates, which, in the presence of hyperglycemia, contribute to increased formation of reactive oxygen species (ROS), mitochondrial uncoupling, decreased adenosine triphosphate (ATP) synthesis, mitochondrial dysfunction, and finally apoptosis. These deleterious processes are commonly referred to as lipotoxicity (19).
ROLE OF HYPERGLYCEMIA
Chronic hyperglycemia plays a major role in the initiation of diabetic vascular complications through many metabolic and structural derangements. Four main hypotheses and pathomechanisms have been proposed to explain how hyperglycemia and the inhibition of glyceraldehyde-3-phosphate dehydrogenase by superoxides can cause all of the diabetes complications (20).
Increased polyol pathway flux
Increased hexosamine pathway flux and modification of proteins
Increased formation of AGEs
Increased protein kinase C (PKC) isoform expression
Increased levels of the upstream glycolytic metabolite glyceraldehyde-3-phosphate activate the AGE pathway by forming the major intracellular AGE precursor methylglyoxal from glyceraldehyde-3-phosphate. The classic PKC pathway is activated, too, since the activator of PKC, diacylglycerol, is formed from glyceraldehyde-3-phosphate. Further upstream in glycolysis, levels of the glycolytic metabolite fructose-6-phosphate increase, leading to a pronounced flux through the hexosamine pathway, resulting in the conversion of fructose-6-phosphate to uridine diphosphate N-acetylglucosamine by the enzyme glutamine:fructose-6-phosphate amidotransferase. Finally, inhibition of glyceraldehyde-3-phosphate dehydrogenase increases intracellular levels of glucose, which is converted to fructose during the polyol pathway. According to this hypothesis, all these pathogenic mechanisms are linked by a single, unifying, hyperglycemia-induced process: the overproduction of superoxide by the mitochondrial electron transport chain resulting in oxidative stress, which is regarded as the pathomechanism underlying insulin resistance, CVD, diabetes, and diabetes complications (20).
Besides the direct effects of glucose on the cells, oxidative stress and nonenzymatic glycation are of major concern. AGEs are elevated in serum and tissues of diabetic patients and affect the structural components of the extracellular matrix such as collagen (21). Hyperglycemia and the associated increase in AGEs results in structural changes by the increase of arterial stiffness and increased amounts of ROS, which contribute to cellular dysfunction and apoptosis (22). In addition to the intracardiac deposition of triglycerides and glycogen granules, which indicate the metabolic derangement in diabetic heart failure, irregularly distributed capillaries and perivascular plaques of collagen are frequent. Myocytolysis and deterioration as well as myofilament fragmentation are common structural findings. Areas of focal necrosis and contraction can be found regularly. All these observations indicate severe morphological and structural alterations occurring in the diabetic heart (19,23).
In addition, mitochondria are severely damaged and increased in diameter, and the number of apoptotic cells was clearly increased in the hearts of type 2 diabetic patients (cardiomyocytes, fibroblasts, endothelial cells) (24). As none of these lesions seem to be specific for diabetes, concomitant hypertension may play a central role. This fact underlines the favorable effect of antihypertensive therapy in people with diabetes.
Mechanisms leading to increased diastolic stiffness of the diabetic heart are different in heart failure with reduced and normal left ventricular ejection fraction (LVEF). In cases of reduced LVEF, fibrosis occurs and AGEs are detectable, whereas in hearts with normal LVEF, cardiomyocyte resting tension is more relevant (23,24). Diabetic heart failure patients without symptoms of CAD showed higher diastolic left ventricular stiffness independently of LVEF. Myocardial collagen volume fraction as well as myocardial AGE deposition was increased in patients with diabetes and reduced LVEF (24).
In addition to the above described mechanisms, hyperglycemia inhibits the production of nitric oxide (NO), leads to elevated levels of free fatty acids (FFAs), lipid deposition in the form of lipid droplets, and stimulates the production of endothelin-1, which has direct vasoconstrictive effects on the endothelium as well as indirect fluid volume effects by stimulation of water and salt retention and the activation of the renin-angiotensin system (25). Impaired endothelial function characterized by the loss of vasodilative action through inhibition of NO is related to the presence of hyperglycemia. The endothelial NO synthase (eNOS) is inhibited and the production of ROS is increased, which in turn leads to further inhibition of eNOS. Upon insulin resistance, the vascular endothelium loses its ability to produce NO-activated tissue plasminogen activator (26). These circumstances rather account for the effects on the microvasculature, whereas, regarding the macrovasculature, effects on adhesion, inflammation, and blood pressure are of relevance.
The formation of AGEs inhibits NO production, too, and further impairs the vasodilatory response in diabetes. Hyperglycemia-stimulated PKC pathway effects on NO and ROS generation and diabetes-associated impaired fibrinolytic capacity may contribute to a prothrombotic state. Platelet hyperactivity is another factor involved in the development and progression of macrovascular disease in diabetes, which contributes to the risk for atherosclerosis progression and consecutive thrombus formation. The increased risk of atherosclerotic and atherothrombotic events in patients with diabetes accounts for most of the CVDs. It is obvious that atherosclerosis of the epicardial arteries and heart failure are independently linked with insulin resistance and the metabolism, suggesting a causal link between these comorbidities.
OTHER FACTORS
Impaired insulin action (insulin resistance) is characterized by compensatory hyperinsulinemia, which is the major metabolic dysfunction associated with the early stages of type 2 diabetes. Elevated plasma insulin levels can lead to numerous metabolic and pathological derangements in various tissues, including the heart (27).
Inflammation represents another diabetes-related mechanism for macrovascular disease. Inflammatory cells (e.g., monocytes and T cells) enter damaged endothelial cells and migrate into the intima media, ingesting oxidized LDL and—as a consequence—forming foam cells. Foam cells are main components of atherosclerotic fatty streaks and represent an early marker of macrovascular disease. In people with diabetes, the levels of adhesion molecules are elevated, facilitating the process of foam cell formation. Furthermore, diabetes is associated with smooth muscle cell dysfunction, which may be associated with similar mechanisms for endothelial cell dysfunction, including activation of the PKC pathway, deposition of AGEs, as well as activation of their receptor RAGE and overproduction of growth factors. In the development of atherosclerosis, activated smooth muscle cells in the medial layer of arteries migrate to the atherosclerotic fatty streaks in the intimal layer and produce an extensive extracellular matrix. This leads to a solidifying of the streaks and reciprocally reduces the protective strengthening function in the medial layer, resulting in an unstable atherosclerotic plaque being prone to rupture. These hyperglycemia-stimulated events act in conjunction over time to produce atherosclerosis and thrombosis (28).
ENERGETIC ASPECTS OF THE FAILING HEART
The daily turnover rate of ATP of more than 6 kg exacerbates the available energy pool of the heart. The energy yield of the healthy heart is around 25% of the energy from substrates, mainly fatty acids and— to a lesser extent—glucose and lactate. Thus, the metabolic disarrangement comprising disturbances in cellular uptake of energy components as well as generation of metabolites found in insulin resistance mainly accounts for the cardiac energy starvation (29). Cardiac energy metabolism comprises three components. Besides the use of substrates derived from glucose and FFAs via the Krebs cycle, the oxidative phosphorylation for the generation of ATP and finally the use of ATP are main parts of cardiac energy cycle. Myocardial energy and lipid metabolism are essential for heart structure and function. Energy surplus as well as energy starvation may lead to disarrangement in myocardial tissue. Seventy percent of cardiac ATP are derived from fatty acid oxidation; glucose and lactate account for 30% of the energy (30). Glucose is the preferred substrate under hypoxic conditions like ischemia and increased workload, because the glycolytic ATP production through conversion of glucose to lactate is independent from oxygen. The healthy heart is able to switch rapidly between different energy sources to accommodate to different physiological and pathological conditions involving altered extracellular hormones, substrate availability, and energy demand (31). The concept of energy starvation as a main reason for myocardial failing was discussed long ago and detected by reduced amounts of creatine phosphate content (rev. in 29). Creatine is produced by the liver and kidneys and transported to the heart where it is taken up by a specific plasma-membrane creatine transporter. Creatine kinase catalyzes the phosphorylation of about two-thirds of the total creatine pool in the heart to phosphocreatine, whereas the other one-third remains as free creatine (32). The creatine kinase system acts as an energy buffer, when high energy demands exceed the energy supply. In this case the phosphocreatine level decreases, which keeps ATP at a normal level but increases the free ADP level (33). The augmented level of free ADP inhibits the function of many intracellular enzymes, causing failure of the muscle contraction mechanism that relay on intracellular signaling. Thus, a metabolic derangement in the cardiac myocyte can occur when phosphocreatine levels fall and free ADP levels rise, even if ATP levels remain unchanged. Obviously, the level of phosphocreatine correlate to the stage of the disease (16).
In cases where ATP demand exceeds ATP synthesis, phosphocreatine levels decline first and ATP decreases only when phosphocreatine is substantially depleted because the creatine kinase reaction equilibrium favors ATP synthesis over phosphocreatine. In chronic heart failure, the total creatine level falls leading to a further reduction of the phosphocreatine-to-ATP ratio (34). Myocardial phosphocreatine-to-ATP ratios are reduced in heart failure, and they are in correlation with New York Heart Association classes and with systolic and diastolic function (rev. in 29). Thus, the phosphocreatine-to-ATP ratio may be a strong predictor of both total mortality and mortality in CVD (35).
SUBSTRATE METABOLISM: A LOOK AT THE DETAILS
Cardiac glucose uptake depends on the transmembrane glucose gradient and the content of sarcolemmal glucose transporter GLUT1 and GLUT4. GLUT1 is primarily located in the membrane and GLUT4 is localized predominantly intracellularly and is translocated to the membrane by insulin stimulation (36).
Upon binding of insulin to the cell surface receptor, the intracellular tyrosine kinase domain is activated. Besides the insulin receptor, insulin receptor substrates are phoshorylated (37). As a consequence, lipid kinase phosphatidylinositol 3‘-kinase (PI3K) is activated. After binding to PI3K, Akt is phosphorylated and activated. The activation of the PI3K/Akt pathway is of importance in the regulation of fatty acid metabolism and glucose metabolism, as well as gene expression and cell survival (37). In addition to the effect on GLUT4 stimulation, glucose uptake and promotion of glycolysis through activation of 6-phosphofructo-2-kinase, insulin increases the myocardial blood flow through the Akt arranged phosphorylation of eNOS (38). Besides the effects on glucose uptake, insulin promotes the formation of glycogen by inhibiting the glycogen synthase kinase 3β (39). In aerobic conditions, <10% of total ATP generated is derived by glycolysis. Following glycolysis, the generated pyruvate can be further metabolized by three pathways: carboxylation to oxalacetate or malate, reduction to lactate, or decarboxylation to acetyl-CoA.
The diabetic heart relies on fatty acid oxidation and is less able to switch to the use of glucose as a process with lower oxygen consumption; substrate fixation is a hallmark of diabetic cardiomyopathy. The accumulation of FFAs leads to the phenomenon of lipotoxicity, which in turn results in impaired β-oxidation, thereby generating even more FFAs (40).
The heart relies on a continuous and well organized supply of energy compounds. Triglyceride-rich lipoproteins like VLDLs and chylomikrons are the main source for fatty acids and are hydrolyzed by lipoprotein lipase (LPL) (41). LPL therefore plays a central role in the regulation of fatty acid delivery, on the surface of cardiomyocytes LPL increases lipid uptake and produces cardiomyopathy (42). Our own results indicate that LPL levels are upregulated in heart failure, supporting the hypothesis that in heart failure excessive uptake of fatty acids leads to the deposition of excessive fatty acids as lipid droplets and to the production of ceramides, acyl-CoA, and carnitines, which account for ROS production and apoptosis as well as derangements of the oxidative phosphorylation. The oxidation of long-chain fatty acids (LCFAs) is the major source of energy for the healthy heart. The uptake of LCFAs is dependent on the energy demand and is regulated by transporting systems such as fatty acid translocase (CD36), plasmalemmal fatty acid binding protein, and fatty acid transport proteins (FATPs), mainly FATP1 and FATP6 (43). Overexpression of CD36 or FATP has been found to dramatically increase fatty acid metabolism (44,45), suggesting a central role of these transporters in fatty acid uptake. Following uptake, LCFAs are bound to soluble fatty acid binding proteins, which transport LCFAs to the outer mitochondrial membrane where they are converted to acyl-CoA to enter the process of β-oxidation.
Peroxisome proliferator–activated receptors (PPARs) are transcription factors activated by fatty acids. Their target genes participate in lipid metabolism, and therefore PPARs are involved in cardiac lipid metabolism. PPARα is a key regulator of fatty acid metabolism. This ligand-activated transcription factor is highly expressed in tissues that derive most of their energy from fatty acid oxidation, including liver, heart, kidney, and skeletal muscle. Target genes of PPARα participate in lipid metabolism, for example the genes of heart type fatty acid binding protein, LPL, CD36, carnitine-palmitoyltransferase 1, and uncoupling protein-3. Knock-out of PPARα abolishes fasting-induced overexpression of fatty acid metabolic genes and switches substrate selection from fatty acids to glucose whereas fatty acid uptake and oxidation are increased by the overexpression of cardiac PPARα (46). As FFAs activate PPARα, the expression of genes involved in fatty acid oxidation and uptake accumulation of fatty acid in the cardiomyocyte is promoted.
Increased uptake and metabolism of fatty acids not only leads to accumulation of fatty acids and triglycerides but also increases oxygen consumption and generation of ROS. Augmented fatty acid uptake through overexpression of LPL or fatty acid transporters, or by stimulating PPARα expression or long-chain CoA synthase results in a cardiac phenotype resembling diabetic cardiomyopathy (42,44).
Diabetic alterations of myocardial metabolism result mainly from malfunctions of acetyl-CoA carboxylase, carnitine-palmitoyl transferase 1, which imports the acyl-CoA into the mitochondrium, and pyruvate dehydrogenase. This induces an overflow of fatty acid oxidation and inhibits glucose oxidation.
The regulation of cardiac energy substrate handling occurs at the level of substrate uptake at the sarcolemmal membrane as well as at the level of mitochondrial oxidation (Randle cycle) (47). The accumulation of fatty acids impairs insulin-mediated uptake of glucose through inhibition of insulin receptor substrate and protein kinase B (Akt) (48). The amounts of intracellular fatty acids derivatives like fatty acyl-CoA, diacylglycerol, and ceramide increase with augmenting intracellular fatty acid content. Although there is an overflow of substrates, the heart resembles an engine running out of fuel; mainly disturbances in the key signal pathways account for the disbalance between energy demand and cardiac efficiency.
INSULIN RESISTANCE AND DIABETES
The concept of myocardial insulin resistance is based on the fact that even in the absence of CAD a decreased in vivo stimulation of myocardial glucose uptake is detectable. Insulin-mediated glucose uptake rates were positively correlated with peripheral muscle insulin sensitivity. ROS may trigger the development of insulin resistance (49). A sustained presence of CD36 in the sarcolemmal membrane has been detected in the insulin-resistant heart, which is associated with higher rates of fatty acid uptake (50). LCFAs are no longer delivered to the heart by fuel demand but constitutively. Contractile dysfunction is detected in these hearts, which can in part be reversed by antisteatotic therapy (51). At least the induction of apoptosis by FFAs as a mechanism leading to cardiac dysfunction is discussed (52).
The change in myocardial energy preferences might be a result of adaption/maladaption to elevated fatty acid concentrations (53,54). Obese diabetic women demonstrate increased fatty acid use, increased oxygen consumption, and decreased cardiac efficiency. In patients without ischemic heart disease, elevated levels of cardiac triglycerides and increased expression of PPARα target genes have been observed. In addition to the stimulatory effects of PPARα, direct or indirect inhibitory effects on genes involved in glucose uptake, glycolysis, and glucose oxidation are observed (55).
The normal adaptive response of a failing heart is the shift from fatty acid oxidation to a more efficient and less oxygen-consuming glucose metabolism mainly by the downregulation of pyruvate dehydrogenase kinase (56). With decreased expression of the PPARα/retinoid X receptor complex and enzymes critical to FFA metabolism, namely carnitine-palmitoyl transferase 1 and medium-chain acyl-CoA dehydrogenase, FFA metabolism is decreased (57,58). To further maximize efficiency, uncoupling proteins that generate heat rather than energy are downregulated in the failing heart (59).
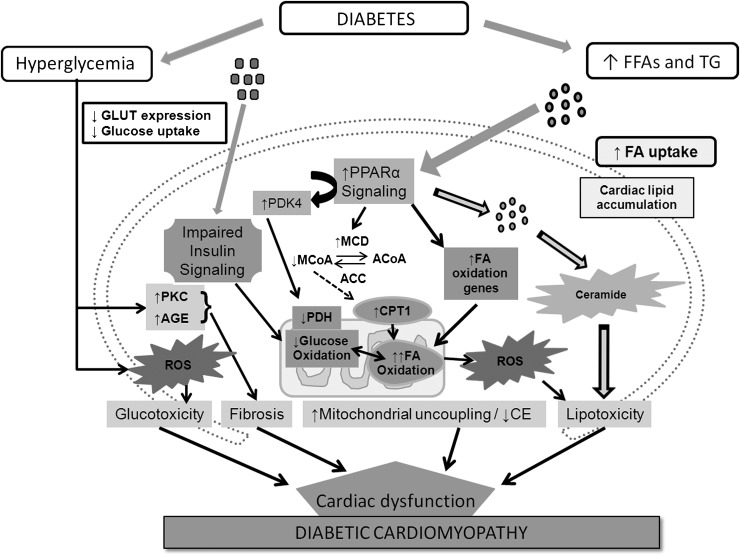
In the case of insulin resistance, FFA metabolism is upregulated, resulting in an increased demand of oxygen, decreased cardiac efficiency, and lipotoxicity (60). This dysregulation is already obvious in patients with obesity and insulin resistance lacking symptoms of heart failure. Figure 1 summarizes the effects on metabolism and its derangement on the development of diabetic cardiomyopathy.
Figure 1.
Orchestra of contributing factors to the development of diabetic cardiomyopathy concerning fatty acid and glucose metabolism, (adapted from Boudina and Abel [60]). ACC, acetyl-CoA carboxylase; ACoA, acetyl-CoA; CE, cardiac efficiency; CPT1, carnitine-palmitoyltransferase 1; FA, fatty acid; MCD, malonyl-CoA decarboxylase; MCoA, malonyl-CoA; PDH, pyruvate dehydrogenase; PDK4, pyruvate dehydrogenase kinase 4; TG, triglycerides.
CONCLUSIONS
The prevalence of diabetes has increased and will double until 2030. This dramatic increase has serious implications from a cardiovascular perspective, and thus the aggressive management of blood pressure, dyslipidemia, and blood glucose in diabetes is of great importance. Although the increase in cardiovascular mortality and heart failure is due in part to accelerated atherosclerosis, epidemiological and clinical data indicate that diabetes increases the risk for cardiac dysfunction and heart failure independently of other risk factors such as CAD and hypertension.
The failing diabetic heart faces complex structural macrovascular derangements such as hypertrophy and loss of function due to glycation. The metabolic switch in heart failure is complex and involves signaling mechanisms, altered substrate preference and finally structural alterations. The uptake of fatty acids is enhanced by debiting glucose use. Lipotoxicity accounts for fat accumulation in the heart muscle as well as the development of ROS. AGEs, as a result of high blood glucose levels, contribute to structural impairment and reduced cardiac power by increased arterial stiffness and collagen modification. Metabolic disorders like insulin resistance or diabetes accelerate these derangements in heart failure by several insulin-mediated mechanisms that are of relevance for microvascular events as well.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
This publication is based on the presentations at the 3rd World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, F. Hoffmann-La Roche, Janssen-Cilag, Johnson & Johnson, Novo Nordisk, Medtronic, and Pfizer.
References
- 1.Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004;292:2495–2499 [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 3.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation 2001;103:2668–2673 [DOI] [PubMed] [Google Scholar]
- 5.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 2003;26:2433–2441 [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 7.Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 2004;27:2898–2904 [DOI] [PubMed] [Google Scholar]
- 8.Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Cardiovascular risk factors clustering with endogenous hyperinsulinaemia predict death from coronary heart disease in patients with type II diabetes. Diabetologia 2000;43:148–155 [DOI] [PubMed] [Google Scholar]
- 9.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 1972;30:595–602 [DOI] [PubMed] [Google Scholar]
- 10.Leyden E. Asthma und Diabetes. Zeitschr. Klin. Med. 1881;3:358–364 [in German] [Google Scholar]
- 11.Mayer J. Über den Zusammenhang des Diabetes mit Erkrankungen des Herzens. Zeitschr. Klin. Med. 1888;14:212–239 [in German] [Google Scholar]
- 12.de Simone G, Devereux RB, Chinali M, et al. Metabolic syndrome and left ventricular hypertrophy in the prediction of cardiovascular events: the Strong Heart Study. Nutr Metab Cardiovasc Dis 2009;19:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation 2007;116:434–448 [DOI] [PubMed] [Google Scholar]
- 14.von Bibra H, St John Sutton M. Diastolic dysfunction in diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia 2010;53:1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res 2007;101:335–347 [DOI] [PubMed] [Google Scholar]
- 16.Rijzewijk LJ, van der Meer RW, Lamb HJ, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol 2009;54:1524–1532 [DOI] [PubMed] [Google Scholar]
- 17.Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 2004;107:539–557 [DOI] [PubMed] [Google Scholar]
- 18.Haffner SM, Mykkänen L, Festa A, Burke JP, Stern MP. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation 2000;101:975–980 [DOI] [PubMed] [Google Scholar]
- 19.Borisov AB, Ushakov AV, Zagorulko AK, et al. Intracardiac lipid accumulation, lipoatrophy of muscle cells and expansion of myocardial infarction in type 2 diabetic patients. Micron 2008;39:944–951 [DOI] [PubMed] [Google Scholar]
- 20.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 21.Kalousová M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes. Physiol Res 2002;51:597–604 [PubMed] [Google Scholar]
- 22.Rösen P, Du X, Tschöpe D. Role of oxygen derived radicals for vascular dysfunction in the diabetic heart: prevention by alpha-tocopherol? Mol Cell Biochem 1998;188:103–111 [PubMed] [Google Scholar]
- 23.Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res 2000;87:1123–1132 [DOI] [PubMed] [Google Scholar]
- 24.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008;117:43–51 [DOI] [PubMed] [Google Scholar]
- 25.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 2006;113:1888–1904 [DOI] [PubMed] [Google Scholar]
- 26.Bian K, Doursout MF, Murad F. Vascular system: role of nitric oxide in cardiovascular diseases. J Clin Hypertens (Greenwich) 2008;10:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: a meta-analysis. Circulation 1998;97:996–1001 [DOI] [PubMed] [Google Scholar]
- 28.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation 2003;108:1917–1923 [DOI] [PubMed] [Google Scholar]
- 29.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007;356:1140–1151 [DOI] [PubMed] [Google Scholar]
- 30.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest 1988;82:2017–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King KL, Okere IC, Sharma N, et al. Regulation of cardiac malonyl-CoA content and fatty acid oxidation during increased cardiac power. Am J Physiol Heart Circ Physiol 2005;289:H1033–H1037 [DOI] [PubMed] [Google Scholar]
- 32.Wyss M, Wallimann T. Creatine metabolism and the consequences of creatine depletion in muscle. Mol Cell Biochem 1994;133–134:51–66 [DOI] [PubMed] [Google Scholar]
- 33.Ingwall JS. Is creatine kinase a target for AMP-activated protein kinase in the heart? J Mol Cell Cardiol 2002;34:1111–1120 [DOI] [PubMed] [Google Scholar]
- 34.Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, Radda GK. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 1991;338:973–976 [DOI] [PubMed] [Google Scholar]
- 35.Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997;96:2190–2196 [DOI] [PubMed] [Google Scholar]
- 36.Luiken JJ, Coort SL, Koonen DP, et al. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch 2004;448:1–15 [DOI] [PubMed] [Google Scholar]
- 37.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 2006;7:85–96 [DOI] [PubMed] [Google Scholar]
- 38.Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep 2003;3:279–288 [DOI] [PubMed] [Google Scholar]
- 39.Cohen P. The Croonian Lecture 1998. Identification of a protein kinase cascade of major importance in insulin signal transduction. Philos Trans R Soc Lond B Biol Sci 1999;354:485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. Lancet 2004;364:1786–1788 [DOI] [PubMed] [Google Scholar]
- 41.Teusink B, Voshol PJ, Dahlmans VE, et al. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes 2003;52:614–620 [DOI] [PubMed] [Google Scholar]
- 42.Yagyu H, Chen G, Yokoyama M, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest 2003;111:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luiken JJ, Coort SL, Koonen DP, Bonen A, Glatz JF. Signalling components involved in contraction-inducible substrate uptake into cardiac myocytes. Proc Nutr Soc 2004;63:251–258 [DOI] [PubMed] [Google Scholar]
- 44.Chiu HC, Kovacs A, Blanton RM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 2005;96:225–233 [DOI] [PubMed] [Google Scholar]
- 45.Ibrahimi A, Bonen A, Blinn WD, et al. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem 1999;274:26761–26766 [DOI] [PubMed] [Google Scholar]
- 46.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA 1999;96:7473–7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res 1997;34:25–33 [DOI] [PubMed] [Google Scholar]
- 48.Paz K, Hemi R, LeRoith D, et al. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem 1997;272:29911–29918 [DOI] [PubMed] [Google Scholar]
- 49.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944–948 [DOI] [PubMed] [Google Scholar]
- 50.Coort SL, Hasselbaink DM, Koonen DP, et al. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese Zucker rats. Diabetes 2004;53:1655–1663 [DOI] [PubMed] [Google Scholar]
- 51.Golfman LS, Wilson CR, Sharma S, et al. Activation of PPARgamma enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab 2005;289:E328–E336 [DOI] [PubMed] [Google Scholar]
- 52.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 2000;97:1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation 2002;105:1861–1870 [DOI] [PubMed] [Google Scholar]
- 54.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation 2002;105:1727–1733 [DOI] [PubMed] [Google Scholar]
- 55.Finck BN, Han X, Courtois M, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci USA 2003;100:1226–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dávila-Román VG, Vedala G, Herrero P, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2002;40:271–277 [DOI] [PubMed] [Google Scholar]
- 57.Taegtmeyer H, Passmore JM. Defective energy metabolism of the heart in diabetes. Lancet 1985;1:139–141 [DOI] [PubMed] [Google Scholar]
- 58.Osorio JC, Stanley WC, Linke A, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 2002;106:606–612 [DOI] [PubMed] [Google Scholar]
- 59.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation 2001;104:2923–2931 [DOI] [PubMed] [Google Scholar]
- 60.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223 [DOI] [PubMed] [Google Scholar]