Diabetes currently affects about 5% of world’s populations, and its prevalence is rapidly increasing particularly in elderly subjects. Because over 80% of all diabetic subjects have type 2 diabetes, the increase in the number of individuals with diabetes implies an epidemic of type 2 diabetes. Diabetes is associated with hyperglycemia-specific microvascular complications. Furthermore, macrovascular complications, especially coronary artery disease (CAD) but also stroke and peripheral vascular disease, are increased by two- to fourfold in type 2 diabetes. Cardiovascular disease (CVD) is the most important long-term complication and by far the greatest cause of death in people with diabetes. In fact, over 50% of all patients with type 2 diabetes die of CAD (1).
Although much of the excess risk of CAD among patients with diabetes is accounted for by the presence of diabetes-associated CVD risk factors such as LDL cholesterol, elevated blood pressure, and smoking, a substantial proportion remains unexplained. A deleterious effect of the diabetic state on vascular and endothelial function is likely to be important via its ability to increase the potential for vasoconstriction and thrombosis. In population-based studies, the relationship of hyperglycemia to CAD is evident but the association is less strong than that of LDL cholesterol and elevated blood pressure. There is also evidence that the role of hyperglycemia with respect to the risk of CVD is larger among patients with type 1 diabetes than in patients with type 2 diabetes. In our study, an increment of 1 unit (%) of glycated hemoglobin increased CVD mortality by 52.5% (95% CI 28.4–81.3) in patients with type 1 diabetes and by 7.5% (4.3–10.8) in patients with type 2 diabetes (2).
CAD also includes a microvascular component. Diabetic cardiomyopathy, a relatively rare condition, associates with the presence of microvascular complications in diabetes. Therefore, changes in small arteries and capillaries are not only responsible for microvascular long-term complications, but also for other manifestations of heart disease in diabetes.
“COMMON SOIL” HYPOTHESIS OF DIABETES COMPLICATIONS
Elegant studies by Brownlee (3) have shown that a single unifying mechanism of diabetes complications might be hyperglycemia-induced overproduction of superoxide by the mitochondrial electron transport chain, which activates four damaging pathways: polyol pathway (hyperglycemia increases flux through the polyol pathway leading to intracellular oxidative stress), hexosamine pathway (increased flux through the hexosamine pathway leads to abnormalities of glomerular cell gene expression and hyperglycemia-induced cardiomyocyte dysfunction), protein kinase C (PKC) pathway (hyperglycemia inside the cell increases the synthesis of a molecule called diacylglycerol activating PKC that results in a variety of effects on gene expression), and advanced glycation end product (AGE) formation (hyperglycemia modifies circulating proteins that bind to AGE receptors causing vascular pathology).
In addition, insulin resistance, induced by high free fatty acid (FFA) levels causes increased production of superoxide in arterial endothelial cells. The FFA-induced overproduction of superoxide activates proinflammatory signals and leads to impaired endothelial function.
Several studies have shown that microvascular complications, especially retinopathy, predict CVD and CAD death in individuals with type 2 diabetes who are free of CVD at baseline (Table 1) (4–10). Our study showed that proliferative retinopathy predicted all-cause, CVD, and CHD death in both sexes of patients with type 2 diabetes who were free of CVD at baseline. Furthermore, overall and background retinopathy predicted all these categories of mortality in women, suggesting a sex difference in the effect of nonproliferative retinopathy on mortality. The association between retinopathy and mortality was independent not only of conventional CVD risk factors but also of glycemic control, duration of diabetes, and proteinuria (10). Thus, our results agree with the concept that similar underlying processes are responsible for micro- and macrovascular complications in diabetes.
Table 1.
Studies on retinopathy predicting cardiovascular events in type 2 diabetes
| Reference | Study subjects | Follow-up; study end points | Relative risk (95% CI) | Adjusting factors |
|---|---|---|---|---|
| Miettinen et al. (4) | 1,040 Finnish type 2 diabetic subjects | 7-year follow-up of CAD events | Background 1.38 (0.95–2.00); proliferative 2.12 (1.02–4.39) | Age, area, sex, total cholesterol, HDL cholesterol, triglycerides, smoking, hypertension, urinary protein, A1C |
| Klein et al. (5) | The Wisconsin Epidemiologic Study of Diabetic Retinopathy: 1,370 subjects with age of onset of diabetes >30 years | 16-year follow-up of all-cause, CAD, and stroke mortality | All-cause mortality: mild nonproliferative 1.34 (1.29–1.71) and proliferative 1.89 (1.43–2.50); CHD mortality: mild nonproliferative 1.21 (0.95–1.53) and proliferative 1.43 (0.94–2.17); stroke mortality: mild nonproliferative 1.30 (0.92–1.85) and proliferative 1.88 (1.03–3.43) | Age, sex, duration of diabetes, A1C, systolic blood pressure, prior CVD, smoking (pack-years), diuretic use |
| Fuller et al. (6) | The World Health Organization Multinational Study of Vascular Disease in Diabetes: 1,390 type 2 diabetic subjects | 12-year follow-up of CVD mortality | 1.2 (0.8–1.8) in men and 2.7 (1.8–4.1) in women | Age, duration of diabetes, systolic blood pressure, cholesterol, smoking, proteinuria, electrocardiographic abnormalities, glucose |
| van Hecke et al. (7) | The Hoorn Study: 631 nondiabetic and diabetic subjects | 10.7-year follow-up (median) of all-cause and CVD mortality | All-cause mortality in diabetic subjects 2.05 (1.23–3.44); CVD mortality in diabetic subjects 2.20 (1.03–4.70) | Age and sex |
| Cusick et al. (8) | The Early Treatment Diabetic Retinopathy Study (ETDRS): 2,267 type 2 diabetic subjects | 5-year follow-up of all-cause mortality | Moderate nonproliferative 1.27 (0.94–1.72); severe nonproliferative 1.48 (1.03–2.15); mild proliferative 1.28 (0.80–2.06); moderate/high proliferative 2.02 (1.28–3.19) | Age, sex, BMI, A1C, total cholesterol, triglycerides, fibrinogen, cigarette smoking, daily insulin use, the use of antihypertensive medications, other baseline diabetes complications |
| Targher et al. (9) | The Valpolicella Heart Study: 248 type 2 diabetic subjects who developed CVD during follow-up and 496 type 2 diabetic control subjects | 5-year follow-up of CVD events | Nonproliferative 1.8 (1.2–2.3); proliferative 4.1 (2.0–8.9) | Age, sex, BMI, smoking history, plasma lipids, A1C, diabetes duration, diabetes treatment |
| Juutilainen et al. (10) | 824 Finnish type 2 diabetic subjects | 18-year follow-up of total, CAD and CVD mortality | CAD: Nonproliferative 1.18 (0.74–1.89) for men and 1.79 (1.13–2.85) for women; proliferative 2.54 (1.07–6.04) for men and 4.98 (2.06–12.06) for women | Age, area of residence, smoking, hypertension, total cholesterol, HDL cholesterol, duration of diabetes, urinary protein |
Support for a common soil hypothesis also comes from clinical trials. In the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, 9,795 patients aged 50–75 years with type 2 diabetes were randomly assigned to receive fenofibrate 200 mg per day (n = 4,895) or matching placebo (n = 4,900) for 5 years (11). Previous CVD and microvascular disease were more frequent in patients who had amputations during the trial than in those who had other cardiovascular events or in those who had neither event (all P < 0.001). The risks of first amputation (45 vs. 70 events; hazard ratio [HR] 0.64 [95% CI 0.44–0.94]; P = 0.02) and minor amputation events without known large-vessel disease (18 vs. 34 events; 0.53 [0.30–0.94]; P = 0.027) were lower for patients assigned to fenofibrate than for patients assigned to placebo (11). In a substudy of the FIELD trial including 1,012 patients, standardized retinal photography was done and photographs graded with Early Treatment Diabetic Retinopathy Study criteria to determine the cumulative incidence of diabetic retinopathy and its component lesions. The requirement for first laser treatment for all retinopathy was significantly lower in the fenofibrate group than in the placebo group (164 [3.4%] patients on fenofibrate vs. 238 [4.9%] on placebo; 0.69 [0.56–0.84]; P = 0.0002; absolute risk reduction 1.5% [0.7–2.3]) (12). Thus, treatment with fenofibrate in individuals with type 2 diabetes reduced the need for laser treatment for retinopathy, although the mechanism of this effect does not seem to be related to plasma concentrations of lipids. These results are in agreement with the concept that similar underlying processes are responsible for micro- and macrovascular complications in diabetes.
Also experimental studies suggest that microvascular and macrovascular complications share some pathophysiological similarities. Both processes include impaired endothelial function, inflammation, neovascularisation, apoptosis, and the hypercoagulable state. The neovascularisation of the vessel wall is a consistent feature of the development of atherosclerotic plaque, and vasa vasorum neovascularisation precedes endothelial dysfunction (13). Because proliferative retinopathy has been a more important predictor than background retinopathy in several studies, including our study (10), this may imply that neovascularisation is an especially important common pathway leading to micro- and macrovascular complications.
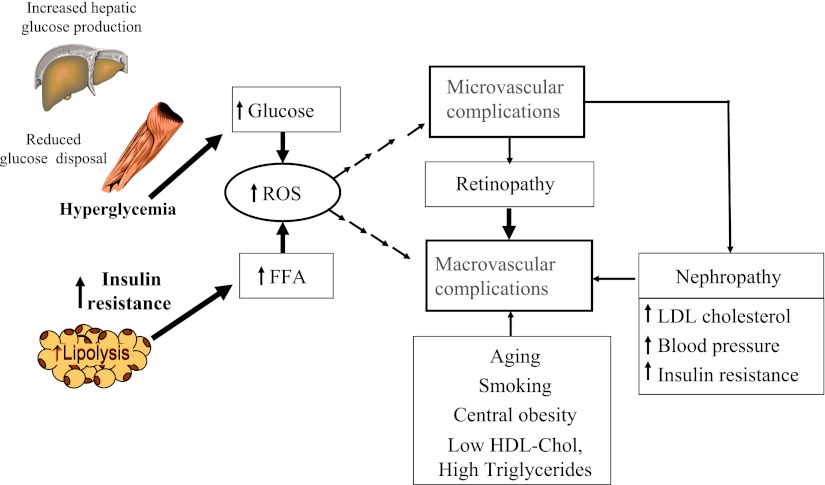
Figure 1 summarizes how micro- and macrovascular disease in type 2 diabetes could share common pathways (14). Both hyperglycemia and/or insulin resistance can lead to oxidative stress and mitochondrial overproduction of superoxide and activate damaging pathways leading to diabetic micro- and macrovascular complications. The Diabetes Control and Complications Trial showed that insulin-resistant patients with type 1 diabetes at their baseline visit were at the highest subsequent risk of developing micro- and macrovascular complications (15). Insulin-resistant patients with diabetes often have nephropathy, and a clustering of CVD risk factors that further accelerate atherothrombosis.
Figure 1.
The “common soil” hypothesis of diabetes complications (14). ROS, reactive oxygen species.
CARDIOMYOPATHY IN DIABETES
Cardiomyopathy in diabetes is characterized by the presence of myocardial dysfunction in the absence of CAD and hypertension (16). The earliest finding is diastolic dysfunction, which is characterized by impairment of relaxation and passive filling of the left ventricle. In cardiomyopathy with reduced left ventricular ejection fraction, myocardial collagen deposition and AGEs are the primary pathological processes responsible for reduced elasticity of the myocardium in patients with diabetes, whereas increased cardiomyocyte resting tension may be the predominant cause in those with preserved left ventricular ejection fraction (17).
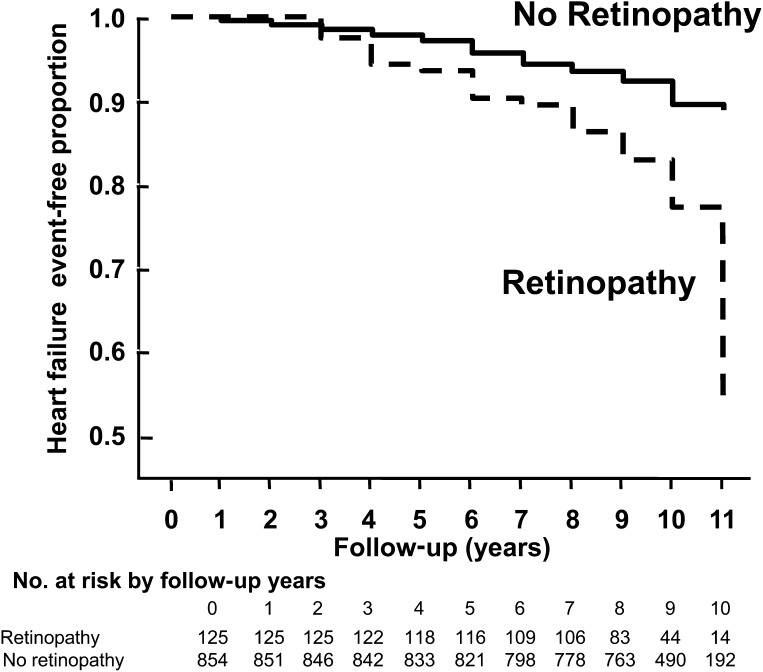
Several studies indicate that diabetes is associated with increased occurrence of heart failure even after controlling for hypertension and CAD. Cheung et al. (18) performed a population-based study included 1,021 middle-aged individuals with type 2 diabetes with normal renal function and free of clinical CAD or heart failure at baseline. A total of 106 (10.1%) participants developed incident heart failure events after 9-year follow-up. Individuals with retinopathy were more likely to develop heart failure than those without retinopathy (cumulative incidence of 21.6% and 8.5%, respectively) (Fig. 2). Participants with retinopathy had more than 2.5-fold higher risk of developing heart failure than those without retinopathy (HR 2.71; [95% CI 1.46– 5.05]) after controlling for confounding factors (age, sex, race, smoking, diabetes duration, insulin use, blood pressure, and lipid profile). This association remained significant after further adjustments for glycemic control, carotid atherosclerosis, and serum markers of endothelial dysfunction (2.20, [1.08– 4.47]). Thus, small vessel disease seen in the retina, reflected as retinopathy, may represent widespread systemic microcirculation disease, and lead to increased load to the heart and compromise cardiac performance (e.g., impair ventricular emptying and contractility) and manifest heart failure (18).
Figure 2.
Heart failure–free survival in diabetic participants with and without retinopathy in the Atherosclerosis Risk In Communities study (18).
Metabolic changes
Although cardiomyopathy in patients with diabetes is increasingly recognized, the underlying mechanisms are still incompletely understood. Most knowledge of the disease mechanisms has been gained from studies of animal models of obesity, insulin resistance, or diabetes. The relevance of these studies is not always obvious for human diabetes. However, both diabetes patients and mouse models of obesity and type 2 diabetes exhibit several similar cardiac abnormalities.
Metabolic abnormalities (hyperglycemia, hyperlipidemia, insulin resistance, and insulin deficiency) are essential for the pathogenesis of cardiomyopathy in diabetes triggering maladaptive stimuli that result in myocardial fibrosis and collagen formation (19). These processes lead to altered myocardial relaxation and manifest as diastolic dysfunction. Systolic dysfunction is a later manifestation, usually occurring after diastolic dysfunction develops. The strongest association between diabetes and cardiomyopathy is observed in patients with microvascular complications—an association that parallels the duration and severity of hyperglycemia.
Metabolic abnormalities lead to myocardial fibrosis and/or myocardial hypertrophy directly or indirectly. Several processes are responsible for these adverse changes including impaired calcium cycling, myocardial insulin resistance, increased lipid uptake, glucotoxicity, and activation of the renin-angiotensin-aldosterone system observed in mouse models of dilated cardiomyopathy (Table 2) (20). Several of these changes mirror the findings obtained from patients with type 1 and type 2 diabetes. Individuals with diabetes can develop diastolic dysfunction and cardiac hypertrophy (21,22). Furthermore, cardiac FFA uptake and use are increased in diabetes and they lead to decreased glucose oxidation and increased lipotoxicity and apoptosis (23). Subjects with diabetes also exhibit impaired cardiac mitochondrial function (24) as well as altered Ca2+ handling (25). Reactive oxygen species play a role in all stages of the development of heart failure, from cardiac hypertrophy to fibrosis, contractile dysfunction, and left ventricular failure.
Table 2.
Effects of diabetes (hyperglycemia, hyperlipidemia, insulin resistance, insulin deficiency) on myocardial function (20)
| Altered Ca2++ handling |
| Decreased sarcoendoplastic reticulum Ca2++ –ATPase 2A |
| Decreased expression of Ca2++ –ATPase 2A, Na2+/Ca2+ exchanger |
| Decreased excitation-contraction coupling |
| Cardiac insulin resistance |
| Increased mitochondrial reactive oxygen species formation |
| Increased mitochondrial uncoupling |
| Decreased mitochondrial function |
| Decreased glucose oxidation and increased fatty acid oxidation |
| Increased lipid uptake |
| Increased peroxisome proliferator–activated receptor α activation |
| Decreased glucose oxidation and increased fatty acid oxidation |
| Accumulation of ceramides |
| Increased lipotoxicity |
| Increased apoptosis |
| Glucotoxicity |
| Increased mitochondrial reactive oxygen species formation |
| Increased PKC activity |
| Increased AGE formation |
| Increased fibrosis |
| Increased renin-angiotensin-aldosterone system activation |
| Increased fibrosis |
| Increased apoptosis |
| Increased hypertrophy |
Structural changes
Two characteristic abnormalities in myocardial capillaries in human diabetes have been found in the myocardium: endothelial swelling and/or degeneration and thickening of the capillary basement membrane (26). These changes mimic microangiopathy, giving evidence that not only metabolic abnormalities but also structural abnormalities in microvessels are typical findings in heart disease related to cardiomyopathy in diabetes. Structural pathology of microvessels and cardiomyocytes is well known in animal models with cardiomyopathy, but limited information is available on humans. Increased activation of the renin-angiotensin-aldosterone pathway leads to the fibrosis formation (19).
Fischer et al. (27) performed detailed ultrastructural studies of tissue from the anterior apical segment of the heart in 145 patients undergoing coronary artery bypass grafting, and demonstrated cardiomyocyte hypertrophy and interstitial fibrosis in almost all samples. Furthermore, they found not only mitochondrial degeneration and fatty infiltration of the myofibrils but also capillary basement membrane thickening in subjects with diabetes, which could lead to complete obstruction of the lumen. This is the pathological hallmark of microangiopathy in diabetes and occurred in an uneven, patchy, segmental manner. Similar findings have also been observed in animal models of cardiomyopathy. However, these observations have not been reported in all studies.
CONCLUSIONS
Macrovascular complications of diabetes, CAD, stroke and peripheral vascular disease, are the most important causes of death among patients with diabetes. Compelling evidence also indicates that diabetes increases the risk for cardiac dysfunction and heart failure independent of CAD and its risk factors. Atherosclerosis in large arteries as well as cardiomyopathy in diabetes includes a microvascular component. Therefore, changes in small arteries and capillaries are not responsible for only microvascular long-term complications in patients with diabetes (retinopathy, nephropathy, neuropathy) but also for other manifestations of heart disease in diabetes.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
This publication is based on the presentations at the 3rd World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, F. Hoffmann-La Roche, Janssen-Cilag, Johnson & Johnson, Novo Nordisk, Medtronic, and Pfizer.
References
- 1.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 2.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care 2008;31:714–719 [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 4.Miettinen H, Haffner SM, Lehto S, Rönnemaa T, Pyörälà K, Laakso M. Retinopathy predicts coronary heart disease events in NIDDM patients. Diabetes Care 1996;19:1445–1448 [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol 1999;117:1487–1495 [DOI] [PubMed] [Google Scholar]
- 6.Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl 2):S54–S64 [DOI] [PubMed] [Google Scholar]
- 7.van Hecke MV, Dekker JM, Stehouwer CD, et al. ; EURODIAB prospective complications study Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care 2005;28:1383–1389 [DOI] [PubMed] [Google Scholar]
- 8.Cusick M, Meleth AD, Agrón E, et al. ; Early Treatment Diabetc Retinopathy Study Research Group Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: early treatment diabetic retinopathy study report no. 27. Diabetes Care 2005;28:617–625 [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Tessari R, Zenari L, Arcaro G. Retinopathy predicts future cardiovascular events among type 2 diabetic patients: The Valpolicella Heart Diabetes Study (Letter). Diabetes Care 2006;29:1178. [DOI] [PubMed] [Google Scholar]
- 10.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care 2007;30:292–299 [DOI] [PubMed] [Google Scholar]
- 11.Rajamani K, Colman PG, Li LP, et al. ; FIELD study investigators Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet 2009;373:1780–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keech AC, Mitchell P, Summanen PA, et al. ; FIELD study investigators Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007;370:1687–1697 [DOI] [PubMed] [Google Scholar]
- 13.Herrmann J, Lerman LO, Rodriguez-Porcel M, et al. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res 2001;51:762–766 [DOI] [PubMed] [Google Scholar]
- 14.Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care 2010;33:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007;30:707–712 [DOI] [PubMed] [Google Scholar]
- 16.Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 2008;121:748–757 [DOI] [PubMed] [Google Scholar]
- 17.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008;117:43–51 [DOI] [PubMed] [Google Scholar]
- 18.Cheung N, Wang JJ, Rogers SL, et al. ; ARIC (Atherosclerosis Risk In Communities) Study Investigators Diabetic retinopathy and risk of heart failure. J Am Coll Cardiol 2008;51:1573–1578 [DOI] [PubMed] [Google Scholar]
- 19.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 2010;11:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech 2009;2:454–466 [DOI] [PubMed] [Google Scholar]
- 21.Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 2001;24:5–10 [DOI] [PubMed] [Google Scholar]
- 22.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007;116:1170–1175 [DOI] [PubMed] [Google Scholar]
- 23.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004;109:2191–2196 [DOI] [PubMed] [Google Scholar]
- 24.Diamant M, Lamb HJ, Groeneveld Y, et al. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol 2003;42:328–335 [DOI] [PubMed] [Google Scholar]
- 25.Regan TJ, Lyons MM, Ahmed SS, et al. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest 1977;60:884–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asghar O, Al-Sunni A, Khavandi K, et al. Diabetic cardiomyopathy. Clin Sci (Lond) 2009;116:741–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer VW, Barner HB, Larose LS. Pathomorphologic aspects of muscular tissue in diabetes mellitus. Hum Pathol 1984;15:1127–1136 [DOI] [PubMed] [Google Scholar]