The lack of adequate insulin secretion characterizes all hyperglycemic states. When insulin action is normal, as in type 1 diabetes, there is a near total loss of insulin secretory function. In type 2 diabetes, the abnormalities in insulin secretion are multiple. One of the initial defects is a loss of the early phase of meal-stimulated insulin secretion. This is followed by an inability of the β-cell to increase insulin secretion sufficient to overcome hepatic and peripheral insulin resistance. Type 2 diabetes is characterized by a progressive decrease in both β-cell mass and secretory function so that, in most individuals, absolute insulin deficiency occurs in the late stages of the disease.
It would seem logical that the ideal treatment for type 2 diabetes should be early and continuing insulin therapy. Unfortunately, there are several characteristics of insulin treatment and insulin action in type 2 diabetes that limit the usefulness of insulin treatment and that suggest that chronic insulin therapy is best used in the later stages of diabetes when there is an absolute deficiency of insulin.
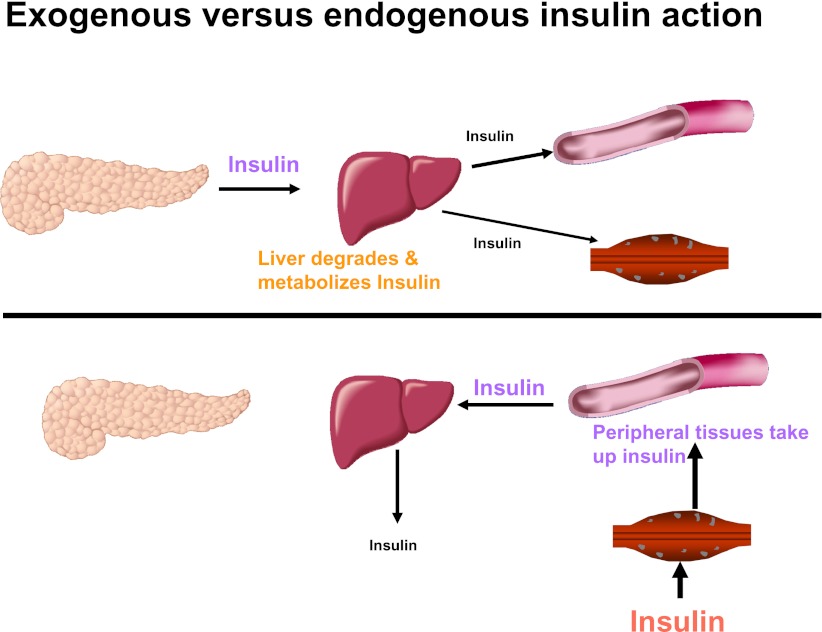
In normal physiology, β-cell insulin secretion is coupled immediately with changes in the plasma glucose level (1). The secretory response is rapid (within a minute or two), and because the half-life of insulin is ~5 min, there is little lag time in the glucose regulatory system. Endogenously secreted insulin goes via the portal vein to the liver, where 30–80% of it is either metabolized or used (2). The portal vein-to-peripheral arterial insulin ratio is ~2:1. The administration of insulin exogenously eliminates the rapid regulation of plasma glucose, since the insulin must be taken up slowly and without regulation from the subcutaneous injection site. The kinetics are determined by the nature of the injected insulin formulation. Additionally, as illustrated in Fig. 1, it is necessary to create hyperinsulinemia in the periphery to achieve adequate insulin in the liver (portal-to-peripheral insulin levels ~1:2) to appropriately regulate hepatic glucose production and/or glucose uptake.
Figure 1.
Administration of exogenous insulin provides a different insulin gradient than that occurring after endogenous insulin secretion. Endogenous insulin secretion acts initially on the liver where a major portion of it is taken up and <50% reaches the peripheral tissues. Exogenously administered insulin must circulate through the peripheral tissues before it can reach the liver; therefore, peripheral hyperinsulinemia is necessary to attain adequate insulin to regulate the liver.
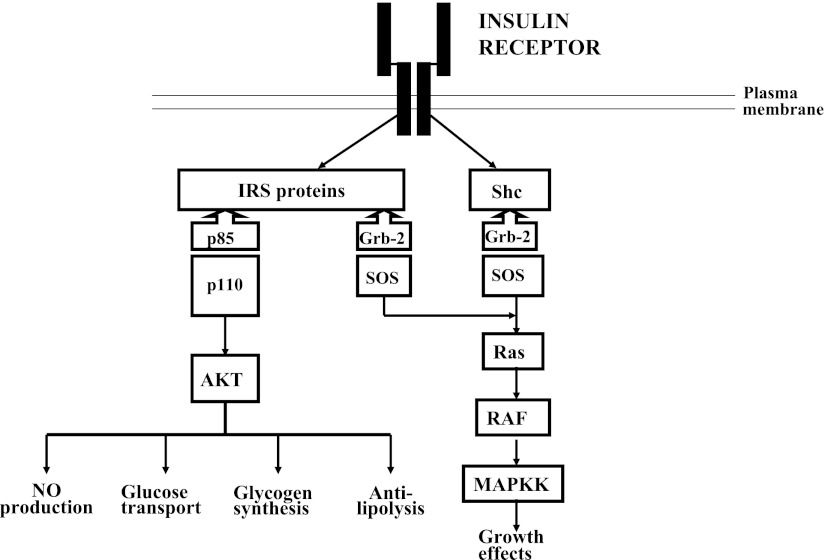
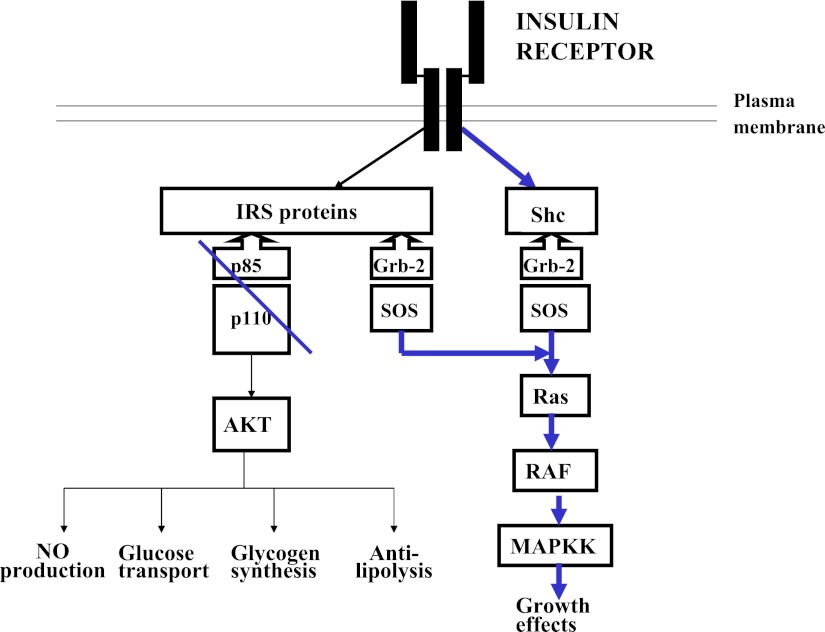
Insulin resistance as occurs in type 2 diabetes adds additional complexities to insulin administration, since it modifies the relative mitogenic insulin activity to the metabolic insulin activity. Figure 2 depicts the two intracellular pathways of insulin action: the phosphatidylinositol 3-kinase (PI 3-kinase) pathway mediates the metabolic effects of insulin, and the adapter protein Grb-2-SOS pathway mediates the mitogenic effects of insulin (3). Insulin resistance in type 2 diabetes occurs at the level of the insulin receptor substrate 1–PI 3-kinase molecular interaction and decreases only the metabolic effects of insulin (4). Replacement with insulin to overcome the deficiencies of the metabolic deficiency will result in exaggerated mitogenic activity (Fig. 3). The evidence that inhibition of PI 3-kinase activity in humans can lead to exaggerated insulin mitogenic activity comes from studies in patients with pseudoacromegaly. Pseudoacromegaly is a disorder in which acromegalic features develop in individuals who are markedly insulin resistant with significantly elevated plasma insulin levels and normal plasma growth hormone and IGF-1 levels (5–7). Investigations of insulin action on their fibroblasts in culture have shown that insulin activation of PI 3-kinase is materially reduced (32% of normal), resulting in deficient glucose transport, whereas insulin activation of mitogen-activated protein (MAP) kinase phosphorylation and thymidine incorporation into DNA is normal (8) Thus, impairment of PI 3-kinase activity increases the mitogenic activity of insulin relative to its metabolic activity, and the administration of insulin to restore metabolic activity would be expected to cause an exaggeration in mitogenic activity. The potential long-term clinical consequences of this exaggerated mitogenic activity may be related in part to the side effects associated with chronic intensive insulin therapy in patients with type 2 diabetes. This effect would not be seen in patients with type 1 diabetes, since there is no alteration in the relationship between the mitogenic and metabolic pathways.
Figure 2.
Insulin has two signaling pathways within the insulin-sensitive cell: one that uses the PI-3 pathway regulates metabolic activity, and the other that uses the MAP kinase regulates mitogenic activity. In the normal cell, these activities are balanced. IRS, insulin receptor substrate. MAPKK, mitogen-activated protein kinase kinase.
Figure 3.
Insulin resistance in obesity and type 2 diabetes occurs because of a block in the transmission of the insulin signal through PI-3 kinase. The MAP kinase pathway is not affected. Increasing insulin availability to overcome the blockade of the metabolic pathway leads to an exaggerated activation of the mitogenic pathway.
ARE THERE UNIQUE BENEFITS TO STARTING CHRONIC INSULIN TREATMENT EARLY?
Starting chronic insulin treatment early in the course of type 2 diabetes would be advantageous if insulin treatment had a unique benefit in decreasing the rate of β-cell apoptosis and had a more durable effect in maintaining glycemic control than other antihyperglycemic agents. The available data do not support such an effect. In the UK Prospective Diabetes Study (UKPDS) insulin treatment arm, HbA1c rose progressively during the 10 years of follow-up from ~6.3 to 8.0% (9). The percentage of insulin-treated patients who maintained an HbA1c <7% was 47% at 3 years, 37% at 6 years, and 28% at 9 years and was not different from individuals treated with sulfonylureas (10). More recent studies such as the 3-year Treating To Target in Type 2 Diabetes (4-T) study, which added insulin therapy to patients inadequately controlled on oral therapies, were able to achieve and maintain HbA1c at ~7% by combining multiple different insulin strategies and progressively increasing the daily doses of insulin administered (11). The consequences, as noted in the section on complications of therapy, were a progressive increase in weight and a considerable incidence of hypoglycemia.
If chronic insulin therapy does not lead to a greater durability of glycemic control, another potential advantage would be if it caused a greater benefit in clinical outcomes than other antihyperglycemic therapies. Two long-term clinical trials have evaluated the benefit of intensive insulin therapy compared with other therapies in reducing clinical events. The University Group Diabetes Program (UGDP) was a randomized controlled trial of patients with early adult-onset diabetes treated with placebo (diet), tolbutamide, or insulin (12,13). After 8.5 years, the trial was stopped because the tolbutamide-treated group had an increase in cardiovascular mortality compared with the placebo-treated group. The insulin-treated group showed no difference in cardiovascular mortality compared with the placebo-treated group. The UKPDS randomized type 2 diabetic patients to diet, glibenclamide, chlorpropamide, or insulin treatment. At the end of 11 years of treatment, the reduction in vascular complications with insulin was no greater than that with sulfonylureas (9). Thus, there is not a long-term randomized controlled trial that shows improved outcomes in insulin-treated type 2 diabetic patients compared with other treatments.
SIDE EFFECTS ASSOCIATED WITH LONG-TERM INTENSIVE INSULIN TREATMENT OF PATIENTS WITH TYPE 2 DIABETES
Hypoglycemia and increased mortality
Until recently, it was thought that the prevalence of severe hypoglycemia associated with insulin therapy in patients with type 2 diabetes was low. However, recent long-term clinical trials in which patients with type 2 diabetes have been treated to a target HbA1c of ≤7% with intensive insulin therapy added on to oral antihyperglycemic agents show an increasing incidence of severe hypoglycemia as the HbA1c is lowered to ≤7.0% (11,14–17). Table 1 summarizes the incidence of severe hypoglycemia in two recent trials: Action to Control Cardiovascular Risk in Diabetes (ACCORD) (14) and Veterans Affairs Diabetes Trial (VADT) (15,16). In both trials, intensive glycemic control greatly increased the incidence of severe hypoglycemia. The occurrence of one or more severe hypoglycemic episode in either intensively or moderately controlled patients increased the hazard ratio (HR) for mortality during the studies. A similar phenomenon was noted in the Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study, in which intensive insulin therapy to achieve normal blood glucose levels in intensive care patients resulted in a 90-day mortality that was increased by 14% compared with individuals treated to blood glucose levels in the moderate range (~140 mg/dL) (18). The recent 4-T study of the efficacy of different insulin regimens added to oral agents in patients with type 2 diabetes demonstrated the high incidence of clinically relevant hypoglycemia in patients assigned to prandial insulin therapy (median number of events per year 5.5) and mixed insulin therapy twice a day (3.0 events per year) (11,17). Cardiovascular mortality was greater in the prandial insulin–treated group (nine events vs. four in the mixed insulin–treated group and one in the basal insulin–treated group, P = 0.002) (11).
Table 1.
Relationship of severe hypoglycemia to mortality outcome in recent clinical trials assessing intensive glycemic control
| Clinical trial | Incidence of severe hypoglycemia (%) | Mortality during study (% per year) | HR |
|---|---|---|---|
| ACCORD (10) | |||
| Standard glycemic control | 5.0 | ||
| No severe hypoglycemia | 1.0 | ||
| Severe hypoglycemia (≥1 episode) | 4.9 | ||
| Intensive glycemic control | 15.9 | ||
| No severe hypoglycemia | 1.3 | ||
| Severe hypoglycemia (≥1 episode) | 2.8 | ||
| VADT (11,12) | |||
| Moderate glycemic control | 9.7 | ||
| Intensive glycemic control | 21.1 | ||
| HR if severe hypoglycemia | |||
| All-cause mortality | 6.37 | ||
| Cardiovascular mortality | 3.73 | ||
| NICE-SUGAR study (14) | |||
| Intensive glycemic control | 6.8 | ||
| Ordinary glycemic control | 0.5 | ||
| HR for mortality in intensive control as compared with ordinary glycemic control | 1.14 |
In all of these studies, it is unclear how the episodes of severe hypoglycemia are related to the increased mortality (14–16). A recent retrospective analysis of the U.K. General Practice Research Database identified two cohorts of patients with type 2 diabetes: one with 27,965 patients whose treatment had been intensified from oral monotherapy to combination oral antihyperglycemic therapy and the other with 20,005 patients whose monotherapy regimen had been changed to regimens that included insulin (19). The insulin-treated cohort showed a progressive increase in mortality as the treatment median HbA1c decreased from 7.5 to 6.4% (adjusted HR 1.52 [95% CI 1.32–1.76]). There was also an increase in cardiovascular events in the lower deciles of glycemic control compared with those in the 7.12–7.68 HbA1c deciles.
Weight gain
Weight gain accompanies insulin treatment. The magnitude of the weight gain is influenced by the level of the initial glycemic control, the treatment glycemic control achieved, the duration of insulin therapy, the insulin regimen used, and which combination of oral agents are concomitantly used (20–24). For example, in a study normalizing the HbA1c during 6 months of intensive multiple-dose insulin therapy, the mean weight gain was 8.7 kg (25). The 3-year data from the 4-T trial of insulin treatment regimens added to oral agents showed a 1.4% decrease in HbA1c and 6.4-kg weight gain in the patients assigned to prandial insulin treatment and a 1.3% decrease in HbA1c and 5.7-kg weight gain in the patients assigned to mixed insulin twice a day (11). In the ACCORD study, 28% of the intensively treated cohort and 14% of the ordinarily treated cohort gained >10 kg during a mean treatment period of ~3.5 years (26).
The mechanism responsible for the weight gain in insulin-treated patients is complex and can result in part from the decrease in glycosuria; however, direct effects of insulin are clearly operative, since some other forms of treatment for hyperglycemia are either weight neutral (dipeptidyl peptidase-4 inhibitors) or actually cause weight loss (metformin, glucagon-like peptide 1 [GLP-1] receptor agonists).
The effect of the weight gain associated with insulin therapy on diabetes management and complications has not been determined. The increase in weight is associated with a striking increase in waist circumference that presumably is associated with an increase in visceral adiposity (11). In patients with type 1 diabetes, intensive insulin therapy is associated with a significant increase in body weight in many patients, and the consequences are the development of the metabolic syndrome in as many as 30–40% of the patients and a long-term increase in cardiovascular risk (27,28).
Risk of cancer
Recently, there has been a growing concern about the association of an increase in the incidence of specific cancers with the type of therapy of type 2 diabetes. It has been well established that pancreatic, hepatobiliary, colon, and breast cancer occur in a higher incidence in patients with type 2 diabetes than in control populations (29). The reasons for these associations may be multiple and include obesity, hyperglycemia, insulin resistance, and type of antihyperglycemic therapy. There are little data coming from prospective randomized controlled clinical trials. The vast bulk of available data come from retrospective analyses of various databases. The underlying pathophysiology of insulin resistance and the peripheral hyperinsulinemia accompanying exogenous insulin administration raise the specter that chronic intense insulin treatment might facilitate neoplastic growth.
A retrospective analysis comparing 465 patients with hepatocellular carcinoma to 618 patients with liver cirrhosis and 490 control subjects (30) found an odds ratio (OR) for hepatocellular carcinoma in patients treated with sulfonylureas or insulin to be 2.99 (95% CI 1.34–6.65, P = 0.007), whereas that for patients treated with metformin was 0.33 (0.1–0.7, P = 0.006). The prevalence of diabetes in the patients with hepatocellular carcinoma was 31.2%, and in the control subjects, the prevalence was 12.7%. Onset of diabetes preceded the diagnosis of hepatocellular carcinoma in 84.9% of the patients and by a mean of 141 months.
An analysis of antidiabetic therapy and the risk of pancreatic cancer in a retrospective case-controlled study (31) showed that after adjusting for age, sex, race, smoking, alcohol consumption, BMI, family history of cancer, duration of diabetes, and the use of insulin, the OR of developing pancreatic cancer was 4.99 (95% CI 2.59–9.61, P < 0.001) in patients having had insulin therapy compared with patients who had never had insulin therapy. A similar result was noted in patients who had had diabetes for >2 years before their diagnosis of pancreatic carcinoma.
An analysis of a Saskatchewan Health database (32) of 10,309 new users of sulfonylurea or metformin with an average follow-up of 5.4 years found that sulfonylurea use was associated with an OR of cancer-related mortality (after adjusting for the chronic disease score) of 1.3 (95% CI 1.1–1.6) and insulin use of 1.9 (1.5–2.4). The recent nonrandomized prospective Zwolle Outpatient Diabetes Project Integrating Available Care (ZODIAC) study (33) adds to the observations that patients with diabetes have a higher standardized cancer mortality rate (1.47) and that metformin therapy decreases the HR to 0.43 (0.23–0.80). The Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) study (34) found that rosiglitazone decreased the development of pancreatic carcinoma over a 5.5-year period in patients with type 2 diabetes from 13 in the metformin/sulfonylurea-treated group to 2 (P = 0.0074).
Not all studies implicate insulin treatment as the potential cause of the increase in cancers. A recent publication reported that in Chinese patients with type 2 diabetes, hyperglycemia predicts cancer incidence and insulin treatment was associated with better glycemic control and a reduced cancer risk (35).
Insulin-sensitizing therapies and cardiovascular benefits in specific populations of type 2 diabetic patients
As noted in the introduction, there are relatively little data indicating that clinical outcomes are better with any one specific antihyperglycemic treatment compared with any other. Because of their effects in improving surrogate markers, it had been speculated that agents that reduce insulin resistance might have better outcomes than agents that provide insulin. Clinical trials such as Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) and RECORD failed to demonstrate such a benefit. Two studies (one a retrospective analysis of patients with type 2 diabetes after an acute myocardial infarction [36] and one a subanalysis of the Sibrafiban versus aspirin to Yield Maximum Protection from ischemic Heart events post-acute cOronary sYndrome [SYMPHONY] study [37]) suggested that survival and clinical outcomes after acute coronary artery disease is better after a treatment strategy emphasizing insulin-sensitizing therapies than insulin-providing therapies. As shown in Table 2, clinical outcomes in the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI-2D) trial were shown to be better in patients with type 2 diabetes and coronary heart disease undergoing coronary bypass surgery and treated with an insulin-sensitizing strategy than in patients treated with an insulin-providing strategy (38). These data are somewhat tenuous but still suggest that insulin-providing strategies may not be as good in some patients with type 2 diabetes as insulin-sensitizing strategies.
Table 2.
Bari-2D study: Kaplan-Meier estimates for major cardiovascular events at 5 years
| Major cardiovascular events (% with events) | P | ||
|---|---|---|---|
| Revascularization | Medical therapy | ||
| PCI stratum | |||
| Insulin sensitization | 21.1 | 20.4 | 0.36 |
| Insulin providing | 24.9 | 21.7 | 0.28 |
| P | 0.30 | 0.51 | 0.84 |
| CABG stratum | |||
| Insulin sensitization | 18.7 | 32.0 | 0.002 |
| Insulin providing | 26.0 | 29.9 | 0.58 |
| P | 0.066 | 0.51 | 0.07 |
CABG, coronary artery bypass graft. PCI, percutaneous coronary intervention.
CONCLUSIONS
Starting insulin therapy early in the course of chronic treatment of patients with type 2 diabetes would imply that there are unique benefits to insulin treatment. As addressed above, there is little evidence to support such a view. Insulin treatment is neither durable in maintaining glycemic control nor is unique in preserving β-cells. Better clinical outcomes than those that occur with other antihyperglycemic regimens have not been shown. The downside of insulin therapy is the need to increase the dose and the regimen complexity with time, the increase in severe hypoglycemia, and the potential increase in mortality as well as the potential increased risk for specific cancers.
What then should be the role for insulin therapy in patients with type 2 diabetes?
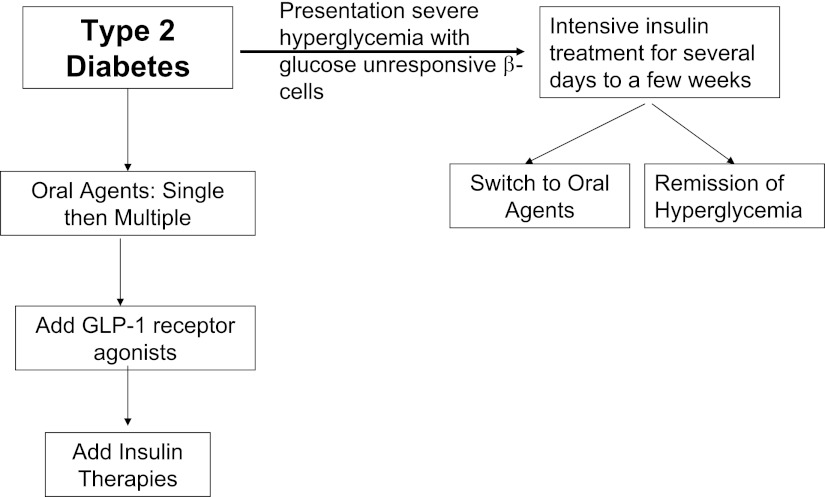
Figure 4 presents two strategies that can take advantage of insulin therapies characteristics and minimize its downsides. There is a subset of patients whose initial presentation with type 2 diabetes is severe hyperglycemia with or without ketosis (39,40). In these individuals, the β-cell has become unresponsive to glucose, but can regain its sensitivity after several days to several weeks of euglycemia. Such individuals benefit from acute intensive insulin therapy applied for several weeks since it is quite effective in maintaining short-term euglycemia (40–42). About 30 to 40% of these patients will have a remission of their diabetes that may last for several years. The remainder can usually be switched to oral therapies quite effectively. For the vast majority of patients with type 2 diabetes, insulin therapy is best reserved until other therapies can no longer maintain the target glycemic goals. With the availability of GLP-1 receptor agonists that provide for glucose-regulated insulin secretion delivered through the portal vein and facilitate modest weight loss (43,44), it would seem appropriate to add insulin therapy in patients failing oral agents plus a GLP-1 receptor agonist. This therapy has shown promising results in several recent clinical studies (45), however, this combination is currently not approved for clinical use.
Figure 4.
Scheme for insulin use in patients with type 2 diabetes. Type 2 diabetic patients presenting initially with severe hyperglycemia should be treated with a short course of insulin to achieve normoglycemia and restore β-cell responsiveness to glucose. A total of 30–40% of such patients will develop a remission of their hyperglycemia, and the rest can be controlled on oral agents. The majority of type 2 diabetic patients presenting with modest and slowly progressively rising glucose levels can be managed with a scheme that includes oral agents, to which GLP-1 receptor agonists can be added. Insulin therapy would be reserved for patients with developed marked insulin deficiency and cannot be controlled with the other antihyperglycemic strategies.
Acknowledgments
H.E.L. is on the advisory boards for Amylin, Biocon, and Merck; is a consultant for AstraZeneca and Intarcia; is a speaker for GlaxoSmithKline and sanofi-aventis; and is a shareholder of Merck and MetaCure. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This publication is based on the presentations at the 3rd World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, F. Hoffmann-La Roche, Janssen-Cilag, Johnson & Johnson, Novo Nordisk, Medtronic, and Pfizer.
References
- 1.Marchetti P, Dotta F, Lauro D, Purrello F. An overview of pancreatic beta-cell defects in human type 2 diabetes: implications for treatment. Regul Pept 2008;146:4–11 10.1016/j.regpep.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 2.Ferrannini E, Cobelli C. The kinetics of insulin in man. II. Role of the liver. Diabetes Metab Rev 1987;3:365–397 10.1002/dmr.5610030202 [DOI] [PubMed] [Google Scholar]
- 3.Kido Y, Nakae J, Accili D. Clinical review 125: The insulin receptor and its cellular targets. J Clin Endocrinol Metab 2001;86:972–979 10.1210/jc.86.3.972 [DOI] [PubMed] [Google Scholar]
- 4.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520 10.1152/physrev.00024.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flier JS, Moller DE, Moses AC, et al. Insulin-mediated pseudoacromegaly: clinical and biochemical characterization of a syndrome of selective insulin resistance. J Clin Endocrinol Metab 1993;76:1533–1541 10.1210/jc.76.6.1533 [DOI] [PubMed] [Google Scholar]
- 6.Kausch C, Bergemann C, Hamann A, Matthaei S. Insulin-mediated pseudoacromegaly in a patient with severe insulin resistance: association of defective insulin-stimulated glucose transport with impaired phosphatidylinositol 3-kinase activity in fibroblasts. Exp Clin Endocrinol Diabetes 1999;107:148–154 10.1055/s-0029-1212090 [DOI] [PubMed] [Google Scholar]
- 7.Yaqub A, Yaqub N. Insulin-mediated pseudoacromegaly: a case report and review of the literature. W V Med J 2008;104:12–15 [PubMed] [Google Scholar]
- 8.Dib K, Whitehead JP, Humphreys PJ, et al. Impaired activation of phosphoinositide 3-kinase by insulin in fibroblasts from patients with severe insulin resistance and pseudoacromegaly: a disorder characterized by selective postreceptor insulin resistance. J Clin Invest 1998;101:1111–1120 10.1172/JCI119884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 10.Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 10.1001/jama.281.21.2005 [DOI] [PubMed] [Google Scholar]
- 11.Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 10.1056/NEJMoa0905479 [DOI] [PubMed] [Google Scholar]
- 12.University Group Diabetes Program A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. Diabetes 1970;19(Suppl. 2):747–830 [PubMed] [Google Scholar]
- 13.Knatterud GL, Klimt CR, Levin ME, Jacobson ME, Goldner MG; UGDP Group Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA 1978;240:37–42 10.1001/jama.240.1.37 [DOI] [PubMed] [Google Scholar]
- 14.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909 10.1136/bmj.b4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 10.2337/dc08-9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. New Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 17.Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 10.1056/NEJMoa075392 [DOI] [PubMed] [Google Scholar]
- 18.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 10.1056/NEJMoa0810625 [DOI] [PubMed] [Google Scholar]
- 19.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort. Lancet 2010;375:481–489 [DOI] [PubMed]
- 20.Yki-Jarvinen H, Kauppila M, Kujansuu E, et al. Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1992;327:1426–1433 10.1056/NEJM199211123272005 [DOI] [PubMed] [Google Scholar]
- 21.Yki-Jarvinen H, Ryysy L, Nikkila K, Tulokas T, Vanamo R, Heikkila M. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus: a randomized, controlled trial. Ann Intern Med 1999;30:389–396 [DOI] [PubMed] [Google Scholar]
- 22.Yki-Järvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care 2001;24:758–767 10.2337/diacare.24.4.758 [DOI] [PubMed] [Google Scholar]
- 23.Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 10.2337/diacare.26.11.3080 [DOI] [PubMed] [Google Scholar]
- 24.Riddle MC, Schneider J; Glimepiride Combination Group Beginning insulin treatment of obese patients with evening 70/30 insulin plus glimepiride versus insulin alone. Diabetes Care 1998;21:1052–1057 10.2337/diacare.21.7.1052 [DOI] [PubMed] [Google Scholar]
- 25.Henry RR, Gumbiner B, Ditzler T, Wallace P, Lyon R, Glauber HS. Intensive conventional insulin therapy for type II diabetes: metabolic effects during a 6-mo outpatient trial. Diabetes Care 1993;16:21–31 10.2337/diacare.16.1.21 [DOI] [PubMed] [Google Scholar]
- 26.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. JAMA 1998;280:140–146 10.1001/jama.280.2.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorn LM, Forsblom C, Fagerudd J, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 2005;28:2019–2024 10.2337/diacare.28.8.2019 [DOI] [PubMed] [Google Scholar]
- 29.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009;16:1103–1123 10.1677/ERC-09-0087 [DOI] [PubMed] [Google Scholar]
- 30.Donadon V, Balbi M, Ghersetti M, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol 2009;15:2506–2511 10.3748/wjg.15.2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009;137:482–488 10.1053/j.gastro.2009.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006;29:254–258 10.2337/diacare.29.02.06.dc05-1558 [DOI] [PubMed] [Google Scholar]
- 33.Landman GWD, Kleefstra N, van Hateren KJJ, Groenier KH, Gans ROB, Bilo HJG. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 2010;33:322–326 10.2337/dc09-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125–2135 10.1016/S0140-6736(09)60953-3 [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Ko GTC, So WY, et al. Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes 2010;59:1254–1260 10.2337/db09-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inzucchi SE, Masoudi FA, Wang Y, et al. Insulin-sensitizing antihyperglycemic drugs and mortality after acute myocardial infarction: insights from the National Heart Care Project. Diabetes Care 2005;28:1680–1689 10.2337/diacare.28.7.1680 [DOI] [PubMed] [Google Scholar]
- 37.McGuire DK, Newby LK, Bhapkar MV, et al. Association of diabetes mellitus and glycemic control strategies with clinical outcomes after acute coronary syndromes. Am Heart J 2004;147:246–252 10.1016/j.ahj.2003.07.024 [DOI] [PubMed] [Google Scholar]
- 38.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–2515 10.1056/NEJMoa0805796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banerji MA, Chaiken RL, Lebovitz HE. Long-term normoglycemic remission in black newly diagnosed NIDDM subjects. Diabetes 1996;45:337–341 10.2337/diabetes.45.3.337 [DOI] [PubMed] [Google Scholar]
- 40.McFarlane SI, Chaiken RL, Hirsch S, Harrington P, Lebovitz HE, Banerji MA. Near-normoglycaemic remission in African-Americans with type 2 diabetes mellitus is associated with recovery of beta cell function. Diabet Med 2001;18:10–16 10.1046/j.1464-5491.2001.00395.x [DOI] [PubMed] [Google Scholar]
- 41.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 10.1016/S0140-6736(08)60762-X [DOI] [PubMed] [Google Scholar]
- 42.Chen H-S, Wu T-E, Jap T-S, Hsiao L-C, Lee S-H, Lin H-D. Beneficial effects of insulin on glycemic control and β-cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy. Diabetes Care 2008;31:1927–1932 10.2337/dc08-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007;50:259–267 10.1007/s00125-006-0510-2 [DOI] [PubMed] [Google Scholar]
- 44.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; GWAA Study Group Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559–569 [DOI] [PubMed] [Google Scholar]
- 45.Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther 2009;31:1511–1523 10.1016/j.clinthera.2009.07.021 [DOI] [PubMed] [Google Scholar]