Targeting the incretin system has become an important therapeutic approach for treating type 2 diabetes. Two drug classes have been developed: glucagon-like peptide (GLP)-1 receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors. Clinical data have revealed that these therapies improve glycemic control while reducing body weight (GLP-1 receptor agonists, specifically) and systolic blood pressure (SBP) in patients with type 2 diabetes. Furthermore, incidence of hypoglycemia is relatively low with these treatments (except when used in combination with a sulfonylurea) because of their glucose-dependent mechanism of action. There are currently two GLP-1 receptor agonists available (exenatide and liraglutide), with several more currently being developed. This review considers the efficacy and safety of both the short- and long-acting GLP-1 receptor agonists. Head-to-head clinical trial data suggest that long-acting GLP-1 receptor agonists produce superior glycemic control when compared with their short-acting counterparts. Furthermore, these long-acting GLP-1 receptor agonists were generally well tolerated, with transient nausea being the most frequently reported adverse effect.
Careful consideration should be given to the selection of therapies for managing type 2 diabetes. In particular, antidiabetic agents that offer improved glycemic control without increasing cardiovascular risk factors or rates of hypoglycemia are warranted. At present, many available treatments for type 2 diabetes fail to maintain glycemic control in the longer term because of gradual disease progression as β-cell function declines. Where sulfonylureas or thiazolidinediones (common oral antidiabetic drugs) are used, the risk of hypoglycemia and weight gain can increase (1,2). The development of new therapies for the treatment of type 2 diabetes that, in addition to maintaining glycemic control, could reduce body weight and hypoglycemia risk (3,4), may help with patient management. Indeed, guidelines have been developed that support the consensus that blood pressure, weight reduction, and avoidance of hypoglycemic events should be targeted in type 2 diabetes management alongside glycemic targets. For example, the American Diabetes Association (ADA) defines multiple goals of therapy that include A1C <7.0% and SBP <130 mmHg and no weight gain (or, in the case of obese subjects, weight loss) (5). In particular, incretin-based therapies (GLP-1 receptor agonists, specifically) can help meet these new targets by offering weight reduction, blood pressure reduction, and reduced hypoglycemia in addition to glycemic control.
WHAT IS GLP-1?
The incretin effect, responsible for 50–70% of total insulin secretion after oral glucose administration, is defined as the difference in insulin secretory response from an oral glucose load compared with intravenous glucose administration (6) (Supplementary Fig. 1).
There are two naturally occurring incretin hormones that play a role in the maintenance of glycemic control: glucose-dependent insulinotropic polypeptide and GLP-1, both of which have a short half-life because of their rapid inactivation by DPP-4 (7). In patients with type 2 diabetes, the incretin effect is reduced or, in some cases, absent (8). In particular, the insulinoptropic action of glucose-dependent insulinotropic polypeptide is lost in patients with type 2 diabetes. However, it has been shown that, after administration of pharmacological levels of GLP-1, the insulin secretory function can be restored in this population (9), and thus GLP-1 has become an important target for research into new therapies for type 2 diabetes.
GLP-1 has multiple physiological effects that make it an attractive candidate for type 2 diabetes therapy. It increases insulin secretion while inhibiting glucagon release, but only when glucose levels are elevated (6,10), thus offering the potential to lower plasma glucose while reducing the likelihood of hypoglycemia. Furthermore, gastric emptying is delayed (10) and food intake is decreased after GLP-1 administration. Indeed, in a 6-week study investigating continuous GLP-1 infusion, patients with type 2 diabetes achieved a significant weight loss of 1.9 kg and a reduction in appetite from baseline compared with patients receiving placebo, where there was no significant change in weight or appetite (11). Preclinical studies reveal other potential benefits of GLP-1 receptor agonist treatment in individuals with type 2 diabetes, which include the promotion of β-cell proliferation (12) and reduced β-cell apoptosis (13). These preclinical results indicate that GLP-1 could be beneficial in treating patients with type 2 diabetes. However, because native GLP-1 is rapidly inactivated and degraded by the enzyme DPP-4 and has a very short half-life of 1.5 min (14), to achieve the clinical potential for native GLP-1, patients would require 24-h administration of native GLP-1 (15). Because this is impractical as a therapeutic option for type 2 diabetes, it was necessary to develop longer-acting derivatives of GLP-1.
DEVELOPMENT OF DPP-4–RESISTANT GLP-1 RECEPTOR AGONISTS
Two classes of incretin-based therapy have been developed to overcome the clinical limitations of native GLP-1: GLP-1 receptor agonists (e.g., liraglutide and exenatide), which exhibit increased resistance to DPP-4 degradation and thus provide pharmacological levels of GLP-1, and DPP-4 inhibitors (e.g., sitagliptin, vildagliptin, saxagliptin), which reduce endogenous GLP-1 degradation, thereby providing physiological levels of GLP-1. In this review, we focus on the GLP-1 receptor agonist class of incretin-based therapies. The efficacy and tolerability of the DPP-4 inhibitors have been reviewed elsewhere (16). Two GLP-1 receptor agonists are licensed at present in Europe, the U.S., and Japan: exenatide (Byetta, Eli Lilly) (17) and liraglutide (Victoza, Novo Nordisk) (18). For the purposes of this review, we refer to “short-acting” GLP-1 receptor agonists as those agents having duration of action of <24 h and “long-acting” as those agents with duration of action >24 h (Table 1).
Table 1.
Short- and long-acting GLP-1 receptor agonists
| Short-acting <24 h | Long-acting ≥24 h | |
|---|---|---|
| Twice daily | Once daily | Once weekly |
| Exenatide (launched) | Liraglutide (launched) | Exenatide LAR (phase 3) |
| Taspoglutide (phase 3) | ||
| Albiglutide (phase 3) | ||
| LY2189265 (phase 2) | ||
OVERVIEW OF LICENSED GLP-1 RECEPTOR AGONISTS
Exenatide
Exenatide, an exendin-4 mimetic with 53% sequence identity to native GLP-1, is currently approved for the treatment of type 2 diabetes as monotherapy (in the U.S.) (19) and in combination with metformin ± sulfonylurea (17). Because of its half-life of 2.4 h, exenatide is recommended for twice-daily dosing.
Clinical trial results have demonstrated that exenatide, when used in combination with selected oral antidiabetic drugs, effectively reduces A1C by −0.4 to −1.5% in patients with type 2 diabetes inadequately controlled on metformin with or without a sulfonylurea (20–24). Across these studies, body weight was seen to decrease in a dose-dependent manner; treatment with 10 μg exenatide, as an add-on to metformin, resulted in the greatest weight loss (−2.8 kg) in patients previously treated with metformin alone (21). Exenatide was generally well tolerated, with mild-to-moderate gastrointestinal effects being the most common adverse effect (20–23). The number of patients experiencing nausea peaked during the initial weeks of treatment (0–8 weeks) but decreased thereafter. Rates of hypoglycemia were relatively low in these studies, although frequency of hypoglycemia was increased when exenatide was used in combination with a sulfonylurea (20). Indeed, the summary of product characteristics for exenatide states that when exenatide is used in combination with a sulfonylurea, consideration should be given to reducing the sulfonylurea dose to reduce the risk of hypoglycemia (17).
Liraglutide
Liraglutide is a GLP-1 analog that shares 97% sequence identity to native GLP-1 (25). The addition of a C16 fatty acid side chain enables once-daily dosing of liraglutide by prolonging its duration of action to over 24 h. This protraction is achieved through reversible binding to albumin and increased stability through heptamer formation mediated by the fatty acid side chain (26).
The safety and efficacy of liraglutide have been well detailed in the phase 3 Liraglutide Effect and Action in Diabetes (LEAD) trials (27–32). Data from the LEAD trials have demonstrated that liraglutide effectively improves glycemic control (up to a 1.5% decrease in A1C) in individuals with type 2 diabetes, when used as monotherapy or in combination with one or more selected oral antidiabetic drugs. Across the trials, body weight was seen to decrease; the largest weight loss resulted from treatment with liraglutide in combination with metformin ± sulfonylurea (−3.24 kg with 1.8 mg liraglutide). Reductions in SBP were also observed across the trials (mean decrease −2.1 to −6.7 mmHg) (27–32).
Liraglutide was generally well tolerated, with only transient nausea experienced toward the beginning of the studies. The rate of minor hypoglycemia was very low in these trials (incidence ranged from 0.03 to 0.6 events/patient/year with the different treatment groups [excluding those using liraglutide in combination with a sulfonylurea]). However, as seen in the exenatide trials, frequency of hypoglycemia increased slightly when liraglutide was used in combination with a sulfonylurea (incidence of major hypoglycemia: 0.056 events/patient/year; minor hypoglycemia: 1.2 events/patient/year with 1.8 mg liraglutide in combination with metformin and a sulfonylurea).
OVERVIEW OF GLP-1 RECEPTOR AGONISTS IN DEVELOPMENT
In addition to liraglutide and exenatide, there are several once-weekly GLP-1 receptor agonists in development: exenatide long-acting release (LAR) (Eli Lilly/Amylin), taspoglutide (Roche), albiglutide (GlaxoSmithKline), and LY2189265 (Eli Lilly) (Supplementary Table 1).
At the time of writing, Roche had suspended the development of taspoglutide, currently in phase 3 trials, because of the high discontinuation rates as a result of gastrointestinal tolerability and serious hypersensitivity reactions (33).
LONG- VERSUS SHORT-ACTING GLP-1 RECEPTOR AGONISTS: EFFICACY AND TOLERABILITY
A number of phase 3 head-to-head trials have been conducted investigating the efficacy and tolerability of long- versus short-acting GLP-1 receptor agonists, results of which are briefly described here.
Once-daily liraglutide versus twice-daily exenatide
The efficacy and tolerability of once-daily liraglutide were compared with twice-daily exenatide in a phase 3 randomized head-to-head trial over 26 weeks involving 464 patients (32). Results from this trial revealed that liraglutide provided a significantly greater reduction in mean A1C compared with exenatide (−1.12 vs. −0.79%; P < 0.0001) (Supplementary Fig. 2). As a result, a greater proportion of patients with type 2 diabetes reached the ADA A1C target (≤7.0%) (3) with liraglutide compared with exenatide (54 vs. 43%; P = 0.0015) (32). In addition, fasting plasma glucose significantly decreased with liraglutide treatment (−1.61 mmol/L with liraglutide vs. −0.60 mmol/L with exenatide; P < 0.0001).
The effects on body weight were similar with both liraglutide and exenatide (−3.24 vs. −2.87 kg, respectively), with a similar proportion of patients losing weight in both treatment groups (78% with liraglutide vs. 76% with exenatide) (32). Both drugs were well tolerated, with only mild-to-moderate side effects observed. Nausea was reported as the most common adverse effect with both treatments, although it was less frequent and less persistent with liraglutide. Further benefits of liraglutide treatment included a reduced number of hypoglycemic events and higher overall treatment satisfaction.
A 14-week LEAD-6 extension study was also completed, in which patients, already randomized to liraglutide, stayed on liraglutide, and those on exenatide switched to once-daily liraglutide (34). Individuals switching from exenatide to liraglutide achieved an additional reduction in A1C of −0.3%, from 7.2% at week 26 to 6.9% at week 40 (Supplementary Fig. 2). Further reductions in fasting plasma glucose (−0.9 mmol/L), body weight (−0.9 kg), and SBP (−3.8 mmHg) were also seen after the switch to liraglutide. Patients switched from exenatide to liraglutide also experienced a reduction in rates of hypoglycemia from 2.6 episodes/patient-year at week 26 to 1.3 episodes/patient-year at week 40. After the switch from exenatide to liraglutide, 3.2% of patients experienced nausea during the extension period, compared with 1.5% of individuals who continued liraglutide treatment.
Once-weekly exenatide LAR versus twice-daily exenatide
The safety and efficacy of once-weekly exenatide LAR (2 mg) versus twice-daily exenatide (10 μg) was evaluated in a phase 2/3 randomized open-label trial over 30 weeks involving 295 patients naive to drug therapy or on one or more oral antidiabetic drugs (24). Results from this trial revealed that exenatide LAR improved glycemic control significantly more than twice-daily exenatide. Reduction in A1C was significantly greater with exenatide LAR versus twice-daily exenatide (−1.9 vs. −1.5%, respectively; P = 0.0023), and a significantly greater proportion of subjects reached the A1C target of ≤7.0% with exenatide LAR versus twice-daily exenatide (77 vs. 61%, respectively; P = 0.0039) (Supplementary Fig. 3). In addition, a significantly greater reduction in fasting plasma glucose was observed with exenatide LAR versus twice-daily exenatide (−2.3 vs. −1.4 mmol/L for exenatide LAR and twice-daily exenatide, respectively; P < 0.0001).
A progressive reduction in body weight was observed throughout the study, with both treatment groups experiencing similar reductions in weight from baseline (−3.7 kg with exenatide LAR vs. −3.6 kg with twice-daily exenatide; P = 0.89). The most common adverse effects seen with exenatide LAR versus twice-daily exenatide were nausea (26.4 vs. 34.5%, respectively) and injection site pruritus (17.6 vs. 1.4%, respectively). The proportion of patients reporting minor hypoglycemic events was low in both treatment arms (0 vs. 1.1% of the study population for exenatide LAR and twice-daily exenatide, respectively); reports of minor hypoglycemia were increased in patients taking a sulfonylurea concomitantly (14.5 vs. 15.4% of the study population for exenatide LAR and twice-daily exenatide, respectively).
The A1C and fasting plasma glucose reductions seen in the first 30 weeks were maintained throughout an extension study (22 weeks), where patients either switched from twice-daily exenatide to once-weekly exenatide LAR or continued exenatide LAR treatment (35). Individuals switching from twice-daily exenatide to exenatide LAR displayed further improvements in glycemic control, achieving the same reduction in A1C from baseline (−2.0% at week 52) as subjects who had been treated only by exenatide LAR. Decreases in body weight were similar for both treatment groups. As seen in the original DURATION (Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide Once Weekly) -1 study, incidence of hypoglycemia was low and limited to patients who received exenatide in combination with a sulfonylurea.
LONG-ACTING GLP-1 RECEPTOR AGONISTS: OVERVIEW OF CLINICAL EFFICACY
Currently, there are no data directly comparing the clinical efficacy of the long-acting GLP-1 receptor agonists (liraglutide, exenatide LAR, albiglutide, taspoglutide, LY2189265). This section provides an indirect comparison of the clinical trial results achieved with long-acting GLP-1 receptor agonists to date.
A1C
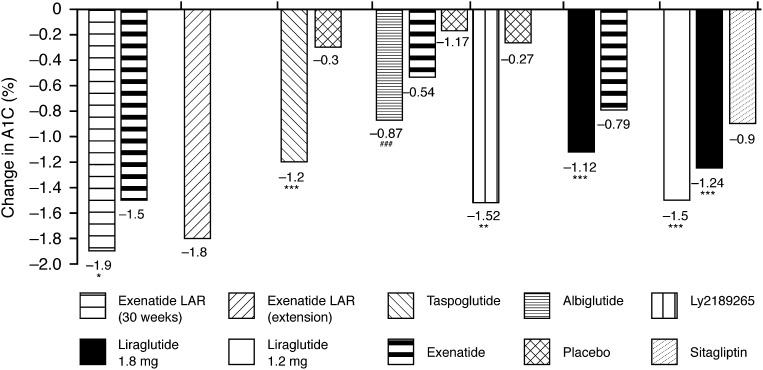
Data from published clinical trials using long-acting GLP-1 receptor agonists (liraglutide, exenatide LAR, albiglutide, taspoglutide, LY2189265) reveal that reductions in A1C from baseline range from −0.87 to −1.9% (31,33,35–39) (Fig. 1). Results with exenatide LAR demonstrated that these improvements in A1C could be maintained after 2 years (mean A1C decrease at 2 years: −1.8%) (36). Greater reductions in A1C were seen with liraglutide compared with the DPP-4 inhibitor sitagliptin (mean A1C decrease: −1.50 and −1.24% with 1.8 and 1.2 mg liraglutide, respectively, vs. −0.90% with sitagliptin; P < 0.0001) (37).
Figure 1.
Change in A1C with long-acting GLP-1 receptor agonists across the clinical trials (24,32,36–39,41). *P < 0.01 vs. comparator; **P < 0.001; ***P < 0.0001; ###P < 0.0001 vs. placebo.
Overall, at least 50% of patients reached an A1C target of <7.0% with the long-acting GLP-1 receptor agonists (31,33,36,37,39,40); results varied from 52% after 16 weeks of treatment with albiglutide (38) to 81% after 8 weeks of taspoglutide treatment (39).
Weight loss
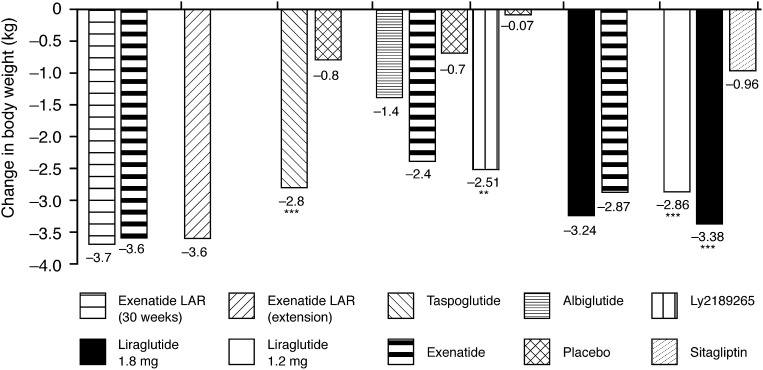
Body weight has been shown to significantly decrease in a dose-dependent manner with all of the long-acting GLP-1 receptor agonists; results varied from −1.4 kg after 16 weeks of treatment with 30 mg albiglutide (38) to −3.87 kg after 15 weeks of treatment with exenatide LAR (2.0 mg) (40) (Fig. 2; Table 2).
Figure 2.
Change in body weight with long-acting GLP-1 receptor agonists across the clinical trials (24,32,36–39,41). **P < 0.001; ***P < 0.0001.
Table 2.
Summary of efficacy and tolerability with long-acting GLP-1 receptor agonists
| Liraglutide | Exenatide LAR | Taspoglutide | Albiglutide | LY2189265 | |
|---|---|---|---|---|---|
| Change in A1C (%) | −1.1 to −1.6 | −1.9 | −1.2 | −0.9 | −1.5 |
| Change in body weight (kg) | −0.2 to −3.2 | −3.7 | −2.8 | −1.4 | −2.5 |
| Change in SBP (mmHg) | −2.3 to −6.7 | −4.7 | Not reported | −5.8 | −5.1 |
| Nausea (%) | 7–29 | 26.4 | 52 | 25.8 | 13 |
| Vomiting (%) | 4.4–17 | 10.8 | 22 | 12.9 | Not reported |
SBP
In addition to their effects on glycemic control and body weight, the long-acting GLP-1 receptor agonists have been shown to reduce SBP in patients with type 2 diabetes, ranging from −4.7 mmHg after 15 weeks with exenatide LAR (33) to −6.7 mmHg after 26 weeks with liraglutide (30) (Table 2).
LONG-ACTING GLP-1 RECEPTOR AGONISTS: OVERVIEW OF SAFETY AND TOLERABILITY
Hypoglycemia
Minor hypoglycemic events have been observed at a relatively low rate after the commencement of treatment with long-acting GLP-1 receptor agonists, with between 0 and 14.5% of patients experiencing this side effect (24,28,38). As reported previously, the greatest proportion of patients reporting minor hypoglycemic events was when adding treatments to a sulfonylurea background (24,27,31,32). No major hypoglycemic events were reported.
Gastrointestinal side effects
Gastrointestinal effects, including nausea and vomiting, appear to be the most frequently reported adverse effect seen with the long-acting GLP-1 receptor agonists (Table 2). These side effects occur early on in the treatment, but tend to be transient and rarely result in patient withdrawal (24,32,36–39,41). After taspoglutide treatment, for example, nausea and vomiting were usually resolved within 1 day, and subsequent taspoglutide administrations were less likely to induce nausea (39). Furthermore, a smaller proportion of patients reported nausea or vomiting after liraglutide treatment compared with patients treated with exenatide (25.5% of the study population vs. 28% with twice-daily exenatide; vomiting: 6.0% of the study population vs. 9.9% with twice-daily exenatide) (32).
Antibodies
Antibody formation was very low in patients treated with once-weekly GLP-1 receptor agonists. Antibodies to albiglutide, which has 95% amino acid identity with native GLP-1, were seen in 2.5% of albiglutide-treated patients (38).
Liraglutide shares 97% sequence identity with native GLP-1 and, across the LEAD trials, 8.6% of patients developed antiliraglutide antibodies (18); however, there were no indications from the clinical trial data that the formation of these antibodies affected efficacy (27–32,42). Indeed, even after 78 weeks’ treatment with liraglutide (26 weeks in the LEAD-6 trial plus a 52-week extension), only 2.6% of patients treated with liraglutide had low-titer liraglutide antibodies, and these antibodies did not affect reductions in A1C in these patients (32).
A larger proportion of patients developed antibodies to exenatide (after 26 weeks: 113/185 patients; 61%), and this is likely to be due to the lower sequence identity of exenatide with native GLP-1. Patients with high-titer exenatide antibodies exhibited a smaller decrease in A1C (−0.5%) compared with patients with low-titer antibodies (−1.0%). Following a switch to liraglutide after 26 weeks, patients previously treated with exenatide still exhibited anti-exenatide antibodies after treatment weeks 40 (49.7%) and 78 (17.5%). However, the persistence of anti-exenatide antibodies did not affect subsequent liraglutide treatment.
SUMMARY
The results achieved with long-acting GLP-1 receptor agonists appear to be superior to those achieved with short-acting GLP-1 receptor agonists, with greater improvements in glycemic control after once-daily liraglutide treatment compared with twice-daily exenatide. Furthermore, exenatide LAR provided better glycemic control than exenatide with comparable weight loss. Trials are ongoing to evaluate the efficacy of exenatide LAR when compared with insulin glargine in patients with type 2 diabetes on a metformin background with or without prior sulfonylurea treatment (DURATION-3; NCT00641056) or used as monotherapy in drug-naive patients (DURATION-4; NCT00676338).
As a drug class, long-acting GLP-1 receptor agonists increase glycemic control in patients with type 2 diabetes with a low risk of hypoglycemia because of their glucose-dependent mechanism of action. This drug class has also been demonstrated to promote weight loss and reduce SBP, which could be of benefit to patients with type 2 diabetes, reducing their cardiovascular risk. Furthermore, although nausea is a common side effect with long-acting GLP-1 receptor agonists, it tends to be transient and, overall, long-acting GLP-1 receptor agonists are generally well tolerated. Thus, long-acting GLP-1 receptor agonists may provide an effective therapeutic option for individuals with type 2 diabetes and are well placed to meet the standard of care guidelines set by the ADA in treating more than just blood glucose.
Acknowledgments
Writing assistance was provided by Watermeadow Medical, funded by Novo Nordisk A/S. A.J.G. received research support from Novo Nordisk and GlaxoSmithKline; received advisory board/consultant/speaker honoraria from Novo Nordisk, GlaxoSmithKline, and Roche; served on the speakers’ bureaus of Merck, Novo Nordisk, and GlaxoSmithKline; served on advisory boards for GlaxoSmithKline, Roche, Novo Nordisk, and Merck; served as a consultant for GlaxoSmithKline, Roche, Novo Nordisk, and Sankyo; and attended speakers' bureaus for GlaxoSmithKline, Novo Nordisk, Merck, and Sankyo. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-s231/-/DC1.
This publication is based on the presentations at the 3rd World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, F. Hoffmann-La Roche, Janssen-Cilag, Johnson & Johnson, Novo Nordisk, Medtronic, and Pfizer.
References
- 1.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 3.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 4.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Standards of medical care in diabetes—2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 8.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986;29:46–52 [DOI] [PubMed] [Google Scholar]
- 9.Højberg PV, Vilsbøll T, Rabøl R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009;52:199–207 [DOI] [PubMed] [Google Scholar]
- 10.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:741–744 [DOI] [PubMed] [Google Scholar]
- 11.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824–830 [DOI] [PubMed] [Google Scholar]
- 12.Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 2003;52:124–132 [DOI] [PubMed] [Google Scholar]
- 13.Farilla L, Hui H, Bertolotto C, et al. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 2002;143:4397–4408 [DOI] [PubMed] [Google Scholar]
- 14.Vilsbøll T, Agersø H, Krarup T, Holst JJ. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 2003;88:220–224 [DOI] [PubMed] [Google Scholar]
- 15.Larsen J, Hylleberg B, Ng K, Damsbo P. Glucagon-like peptide-1 infusion must be maintained for 24 h/day to obtain acceptable glycemia in type 2 diabetic patients who are poorly controlled on sulphonylurea treatment. Diabetes Care 2001;24:1416–1421 [DOI] [PubMed] [Google Scholar]
- 16.Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008:CD006739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amylin/Eli Lilly. Byetta summary of product characteristics: Europe [Internet]. Available from http://www.ema.europa.eu/humandocs/PDFs/EPAR/byetta/emea-combined-h698en.pdf Accessed 17 May 2010
- 18.Novo Nordisk A/S. Victoza summary of product characteristics [Internet]. Available from http://www.ema.europa.eu/humandocs/PDFs/EPAR/victoza/H-1026-PI-en.pdf Accessed 17 May 2010
- 19.Amylin/Eli Lilly. Byetta summary of product characteristics: US [Internet]. Available from http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf Accessed 21 May 2010
- 20.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083–1091 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 22.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD;; Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 23.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; GWAA Study Group Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559–569 [DOI] [PubMed] [Google Scholar]
- 24.Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 25.Russell-Jones D. Molecular, pharmacological and clinical aspects of liraglutide, a once-daily human GLP-1 analogue. Mol Cell Endocrinol 2009;297:137–140 [DOI] [PubMed] [Google Scholar]
- 26.Steensgaard DB, Thomsen JK, Olsen HB, Knudsen LB. The molecular basis for the delayed absorption of the once-daily human GLP-1 analogue, liraglutide. Diabetes 2008;57(Suppl. 1):A164
- 27.Marre M, Shaw J, Brändle M, et al. Liraglutide,1a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009;26:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373:473–481 [DOI] [PubMed] [Google Scholar]
- 30.Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009;32:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009;52:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 33.Fiercebiotech. Roche faces long delay for diabetes drug taspoglutide [Internet]. Available from http://www.fiercebiotech.com/story/roche-faces-long-delay-diabetes-drug-taspoglutide/2010-06-18 Accessed 26 November 2010
- 34.Buse JB, Sesti G, Schmidt WE, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care 2010;33:1300–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buse JB, Drucker DJ, Taylor KL, et al. DURATION-1: exenatide once weekly provides sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010;33:1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trautmann M, Wilhelm K, Taylor K, Kim T, Zhuang D, Porter L. Exenatide once-weekly treatment elicits sustained glycaemic control and weight loss over 2 years. Diabetologia 2009;52(Suppl. 1):S286 [Google Scholar]
- 37.Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–1456 [DOI] [PubMed] [Google Scholar]
- 38.Rosenstock J, Reusch J, Bush M, Yang F, Stewart M; Albiglutide Study Group Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care 2009;32:1880–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nauck MA, Ratner RE, Kapitza C, Berria R, Boldrin M, Balena R. Treatment with the human once-weekly glucagon-like peptide-1 analog taspoglutide in combination with metformin improves glycemic control and lowers body weight in patients with type 2 diabetes inadequately controlled with metformin alone: a double-blind placebo-controlled study. Diabetes Care 2009;32:1237–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007;30:1487–1493 [DOI] [PubMed] [Google Scholar]
- 41.Umpierrez G, Blevins T, Rosenstock J, Cheng C, Bastyr E, Anderson J. The effect of LY2189265 (GLP-1 analogue) once weekly on HbA1c and beta cell function in uncontrolled type 2 diabetes mellitus: the EGO study analysis. Diabetologia 2009;52(Suppl. 1):S59 [Google Scholar]
- 42.Garber A, Marre M, Nauck M, et al. The efficacy of liraglutide is not impacted in subjects positive for anti-liraglutide antibodies: a pooled analysis [article online]. Available from http://www.comtecmed.com/codhy/2010/orals.aspx#o8 Accessed 26 May 2010
- 43.Bergenstal R, Wysham C, Yan P, Macconell L, Malloy J, Porter L. DURATION-2: exenatide once weekly demonstrated superior glycaemic control and weight reduction compared to sitagliptin or pioglitazone after 26 weeks of treatment. Abstract 6-LB presented at the American Diabetes Association 2009 Scientific Sessions, 5–9 June 2009, New Orleans, Louisiana