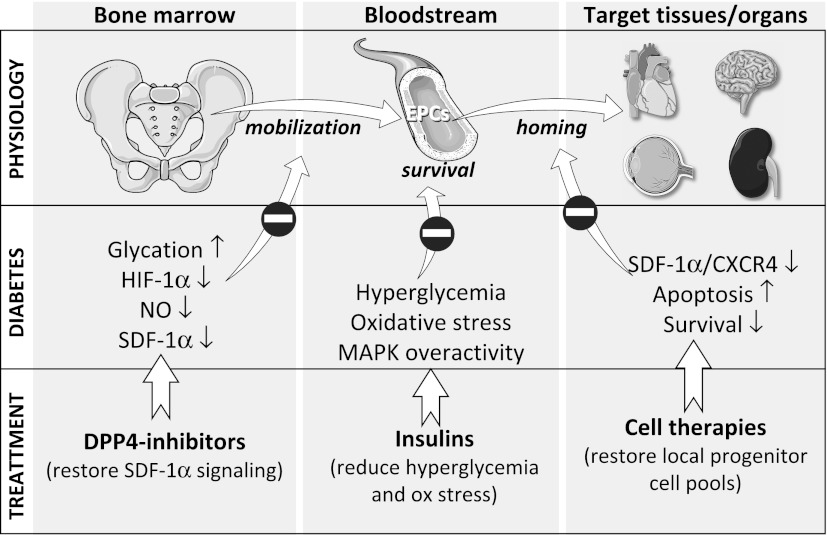
Type 2 diabetes is characterized by a two- to fourfold increased risk of cardiovascular disease. This is generally attributed to the adverse effects of hyperglycemia and oxidative stress on vascular biology. It has also been shown that patients with prediabetic conditions, such as impaired fasting glucose and impaired glucose tolerance, are at increased risk of cardiovascular disease as well (1). This result suggests that abnormalities in carbohydrate metabolism form a continuum that progressively worsens cardiovascular health; the first step of the adverse sequence of events that leads to the atherosclerotic process is thought to be endothelial dysfunction (2).
Vascular endothelial cells play a major role in maintaining cardiovascular homeostasis. In addition to providing a physical barrier between the vessel wall and lumen, the endothelium secretes a number of mediators that regulate platelet aggregation, coagulation, fibrinolysis, and vascular tone. The term “endothelial dysfunction” refers to a condition in which the endothelium loses its physiological properties: the tendency to promote vasodilation, fibrinolysis, and anti-aggregation. Endothelial cells secrete several mediators that can alternatively mediate either vasoconstriction, such as endothelin-1 and thromboxane A2, or vasodilation, such as nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor. NO is the major contributor to endothelium-dependent relaxation in conduit arteries, whereas the contribution of endothelium-derived hyperpolarizing factor predominates in smaller resistance vessels.
In patients with diabetes, endothelial dysfunction appears to be a consistent finding; indeed, there is general agreement that hyperglycemia and diabetes lead to an impairment of NO production and activity. The endothelium has a limited intrinsic capacity of self-repair, being built up by terminally differentiated cells with a low proliferative potential. That is why endothelial repair is accomplished through the contribution of circulating cells, namely endothelial progenitor cells (EPCs), in physiological and pathological conditions. In this review, we will outline the mechanisms of endothelial dysfunction in diabetes, the role of EPCs in cardiovascular homeostasis, and the implications of EPC alterations in diabetes and its complications (Fig. 1) (3).
Figure 1.
Schematic biological role of endothelial progenitor cells, their role in diabetes, and potentially available treatment aiming to restore their circulating concentration. See text for further explanations. Ox, oxidative.
BIOLOGY OF ENDOTHELIAL DYSFUNCTION AND REGENERATION IN DIABETES
It is accepted that generalized endothelial dysfunction precedes the development of atherosclerosis. When endothelium is exposed to hyperglycemia, an array of negative intracellular events promotes endothelial dysfunction. In diabetic patients, the response of coronary circulation exposed to increasing amounts of acetylcholine is a paradoxical constriction instead of vasodilation (4). Contraction instead of vasodilation induced by ACh is mediated via the M3 subtype of muscarinic receptors in coronary arteries when endothelial integrity is lost. This response suggests that endothelial cells exposed to hyperglycemia face an apoptotic process, leading to intimal denudation. The apoptotic process takes place through a complex series of events: the β1-integrin signaling pathway is an initiator/sensor for apoptosis induced by mechanical stretch: after integrin activation, mechanical stretch induces increased p38 mitogen–activated protein kinase (MAPK) and c-Jun N-terminal protein kinase (JNK) activation leading to cell apoptosis. In endothelial cells, disturbed flow stress results in endoplasmic reticulum (ER) stress response and to vascular endothelial-cadherin suppression (5). The downregulation of vascular endothelial-cadherin activates the caspase protein family, resulting in endothelial apoptosis that takes place mainly at atherosusceptible arterial sites. The consequence of the apoptotic process is detachment of endothelial cells, which are released into the bloodstream and can be recognized and measured as circulating endothelial cells. In some circumstances, endothelial cells do not detach as entire cells, but as apoptotic endothelial microparticles. McClung et al. (6) have shown that circulating endothelial cell levels are higher in type 2 diabetic patients, irrespective of glucose control, represented by HbA1c levels. Besides circulating endothelial cells, microparticles (endothelial microparticles) released from intact cells may also play a role in the normal hemostatic response, since they have a powerful procoagulant activity. Elevated endothelial cell–derived endothelial microparticle levels are predictive of the presence of coronary artery lesions, and it is a more significant independent risk factor than duration of diabetes, lipid levels, or presence of hypertension (7). The consequence of the apoptotic process is the so-called arterial denudation, which triggers important proatherosclerotic processes such as smooth muscle cell proliferation, migration, and matrix secretion. For this reason, endothelial reparatory mechanisms are fundamental in reestablishing vessel integrity.
EPCs
EPCs are immature cells capable of differentiating into mature endothelial cells. Vascular injury and tissue ischemia, through the release of growth factors and cytokines, mobilize EPCs, which, once in the peripheral circulation, specifically home in on the ischemic sites to stimulate compensatory angiogenesis. Moreover, EPCs constitute a circulating pool of cells able to form a cellular patch at sites of endothelial injury, thus contributing directly to the homeostasis and repair of the endothelial layer. EPCs have now been recognized as playing a major role in cardiovascular biology: in fact, the extent of the circulating EPC pool is now considered a mirror of cardiovascular health (8). Virtually all risk factors for atherosclerosis have been associated with decrease and/or dysfunction of circulating EPCs, whereas an expanded EPC pool is associated with a decreased cardiovascular mortality.
Definition of the antigenic phenotype of EPCs in accordance with the term “endothelial progenitors” should include at least one marker of immaturity/stemness (for instance, CD34 or CD133) plus at least one marker of the endothelial lineage. Tissue ischemia is considered the strongest stimulus for EPC mobilization, through the activation of hypoxia-sensing systems, such as hypoxia inducible factor (HIF)-1α (3). The resulting active HIF-1α binds to enhancer DNA regions and promotes the transcription of oxygen-sensible genes encoding, among others, vascular endothelial growth factor (VEGF), stromal derived factor-1α (SDF-1α), other genes involved in the angiogenic response, and erythropoietin (9). Then, growth factors allow bone marrow EPCs to undergo transendothelial migration and to pass into the peripheral blood by means of attenuating stromal cell–stem cell interactions and by rearranging extracellular matrix. EPC mobilization is characterized by both a loss in cell-cell contacts and a desensitization of chemokine signaling, notably the SDF-1α/C-X-C chemokine receptor 4 (CXCR4) axis, the fundamental signaling pathway underlying stem cell mobilization and homing during tissue homeostasis and injury (10). On the contrary, stem cell homing requires upregulation of cell adhesion molecules and activation of the SDF-1α/CXCR4 axis. The majority of cytokines that mediate stem cell migration do so via modulation either of SDF-1α or of its receptor, CXCR4. Under homeostatic conditions, most progenitors remain in the bone marrow compartment, retained by high SDF-1α expression, which is maintained by the hypoxic microenvironment. After an ischemic insult, soluble factors, such as SDF-1α, are released by injured tissue and stimulate mobilization of progenitor cells from the bone marrow. These cells are recruited to ischemic tissue by high local levels of SDF-1α, providing a permissive niche. Interestingly, SDF-1α is cleaved and inactivated by CD26/dipeptidyl peptidase 4 (DPP-4). Zaruba et al. (11) showed that the pharmacological inhibition of DPP-4 increased myocardial homing of circulating CXCR4+ stem cells, reduced cardiac remodeling, and improved heart function and survival. We recently confirmed the effect of DPP-4 inhibition on EPCs in vivo in type 2 diabetic patients. After 4 weeks, patients receiving the DPP-4 inhibitor sitagliptin, compared with control subjects, showed a significant increase of EPCs and SDF-1α and a decrease in MCP-1 (12).
To heal a damaged endothelium, EPCs themselves are required to transmigrate (extravasation) to promote new vessel growth; the rolling phase of extravasation is mediated by E- and P-selectins. Subsequent firm attachment is mediated via intercellular adhesion molecule-1/leukocyte function–associated antigen-1, vascular cell adhesion molecule-1/very late antigen (VLA)-4 ligand pairs, and junctional adhesion molecules. Integrins mediate the adhesion and transmigration of stem and progenitor cells. In particular, β2-integrins are essential for homing and improvement of neovascularization mediated by EPCs after hind limb ischemia in mice. Transendothelial migration of cells requires degradation of basement membrane, which depends on the production of matrix-degrading enzymes, especially those capable of degrading type IV collagen, such as matrix metalloproteinases (MMPs). MMP-2 and MMP-9 are known to be expressed by all cell types that migrate through the endothelial cell layer, including leukocytes, tumor cells, and hematopoietic stem cells (13).
EPC ALTERATIONS IN DIABETES
Both type 1 and type 2 diabetic patients have less circulating EPCs than matched healthy subjects (3). We have recently shown that CD34+ progenitor cell reduction marks the clinical onset of type 2 diabetes and that defective mobilization appears to be the most likely mechanism involved. We have also shown that a partial recovery of progenitor cell level occurs during the subsequent 10–20 years, but bone marrow reserve seems exhausted in the long term (14). Diabetic EPCs also display functional impairment, such as reduced proliferation, adhesion, migration, and incorporation into tubular structures (15). The mechanisms underlying EPC reduction in diabetes include weak bone marrow mobilization, decreased proliferation, and shortened survival. Recently, Sambuceti et al. (16) experimentally showed that type 2 diabetes and its related oxidative stress hamper the interaction between the vascular wall and normal EPCs by mechanisms that are, at least partially, reversed by induction of HO-1 gene expression, adiponectin, and pAMPK levels.
The expression of angiogenic factors VEGF and HIF-1α are reduced in the hearts of diabetic patients during acute coronary syndromes, and myocardial infarct size in the rat is increased because of a reduced expression of HIF-1α; therefore, poor collateral formation in diabetes may be attributed at least in part to weaker bone marrow stimulation from the ischemic tissue. We have shown that bone marrow mobilization of EPCs after ischemia-reperfusion injury is defective in diabetic rats compared with nondiabetic rats (17). The inability to mobilize EPCs was associated with a downregulation of HIF-1α and weakened release of marrow-stimulating factors, such as VEGF and SDF-1α, ultimately leading to insufficient compensatory angiogenesis after ischemia. HIF-1α deregulation in diabetes may be determined by an overproduction of reactive oxygen species (ROS): indeed, it was shown that ROS inhibition was able to normalize post-ischemic neovascularization in diabetes by positive EPC modulation (18). We found that bone marrow EPC mobilization was partially rescued in diabetic rats treated with insulin, albeit it is not known whether this favorable effect was mediated by insulin itself or by an improved glucose control. Humpert et al. (19) showed that insulin therapy in decompensated diabetic patients increased CD34+CD133+ progenitor cell count, depending on SDF-1α polymorphism. Thus, it can be hypothesized that better metabolic control, in general and specifically during acute ischemic syndromes, may induce a more efficient stimulation of EPC-mediated neovascularization in ischemic tissue, thus reducing residual ischemia. We have also shown that diabetes is characterized by altered activation of phosphatidylinositol (PI) 3-kinase/Akt pathways and by reduced NO bioavailability. Dysfunction of these subcellular pathways may be involved in the defective mobilization of EPCs from bone marrow (17).
Hyperglycemia, through the production of ROS, may directly affect EPCs. Kränkel et al. (20) demonstrated that high glucose impairs proliferation, survival, and function of cultured EPCs, with concomitant decreased NO production and MMP-9 activity. A definite demonstration is that correction of hyperglycemia by insulin therapy can indeed restore the normal EPC pool. These observations may provide a possible explanation for the delayed post-ischemic vascular healing and myocardial recovery in diabetes. Despite the evidence that high glucose can induce EPC apoptosis in culture, there are no robust data suggesting that hyperglycemia is associated with increased progenitor cell apoptosis in vivo. Indeed, we found a certain inverse correlation between peripheral blood CD34+ cell level and percentage of apoptotic CD34+ cells, but there was no increase in the apoptotic rate of CD34+ cells in diabetic patients with overt hyperglycemia compared with control subjects (14). Therefore, the mechanisms that reduce progenitor cell survival in the diabetic bloodstream need further investigation. Another possible link between diabetes and EPC alterations is the effect of insulin resistance per se. We have shown that patients with the metabolic syndrome have decreased levels of CD34+KDR+ EPCs compared with patients without the syndrome (21). Oxidative stress appears as a major determinant of long-term diabetes complications. Two studies have recently demonstrated that the natural transketolase activator, benfotiamine, which prevents the subcellular damage pathways triggered by oxidative stress, restored EPC-mediated healing of ischemic diabetic limbs in mice and prevented hyperglycemia-mediated EPC dysfunction, via modulation of the Akt pathway (22). Ceradini et al. (18) found HIF-1α function is impaired in diabetes because of ROS-induced modification by the glyoxalase-1 substrate methylglyoxal. Decreasing superoxide in diabetic mice corrects post-ischemic defects in neovascularization, oxygen delivery, and chemokine expression and normalized tissue survival. In hypoxic EPCs cultured in high glucose, overexpression of GLO1 prevented reduced expression of both the SDF-1α receptor CXCR4 and endothelial NO synthase, an enzyme essential for EPC mobilization.
EPC AND DIABETES COMPLICATIONS
The severity of macrovascular complications in diabetes has been attributed to an impaired collateralization of vascular ischemic beds, which is insufficient to overcome the loss of blood flow and leads to critical limb ischemia that often requires amputation. In animal models of diabetic vasculopathy, defective collateralization was counteracted by administration of EPCs from control animals (23). Conversely, diabetic EPCs were not able to stimulate vascularization, even becoming anti-angiogenic. We have shown that nondiabetic patients with peripheral arterial disease alone and in patients with uncomplicated diabetes had similar EPC reduction versus control subjects (24). Patients with diabetes and peripheral arterial disease had a further significant decrease in circulating EPC levels, especially in the presence of ischemic foot lesions. Remarkably, EPC levels strongly correlated with the ankle-brachial index, the most objective diagnostic and prognostic test for lower-extremity arterial disease. Diabetes also predisposes patients to heart failure. Spectroscopic and histological evidence in the human myocardium indicates a maladaptive metabolic response in diabetes, characterized by intramyocellular triglyceride accumulation. In diabetes, there is also evidence for a link between myocardial actin isoform switching, calcium homeostasis, and altered metabolism in the development of heart failure. In the diabetic heart, microvascular abnormalities are also frequently present. There are some experimental data indicating that EPC alterations are implicated in the pathogenesis of diabetic cardiomyopathy as well. Yoon et al. (25) have demonstrated that diabetic cardiomyopathy in rats is characterized by an early and progressive decline in myocardial VEGF expression that reduces capillary density, increases fibrosis, and impairs contractility. Cardiac stem cell aging and heart failure associated with diabetes can be prevented by deletion of the stress-related gene p66Shc, which we have shown to be potently upregulated in type 2 diabetic subjects (26). The discovery that alterations of circulating and/or local progenitor cells may mediate this complication could identify novel therapeutic strategies.
Diabetes is one leading cause of chronic kidney disease. Endothelial damage and microcirculatory impairment are early pathogenetic events in diabetic nephropathy and may partly depend on EPC defects (27). Moreover, it can be suggested that EPCs are pluripotent and retain the ability to transdifferentiate into disparate phenotypes (28). Therefore, a decline in EPCs may be one mechanism of defective glomerular repair and renal disease progression in diabetes. A recent study has demonstrated that type 2 diabetic patients with low CD34+ cells have a higher albumin excretion rate compared with patients with a high CD34+ cell count (29). The relationship between EPCs and microalbuminuria has also been recently confirmed in type 1 diabetic patients (30). The relationship between renal function and EPCs are more complex, because the kidney-derived hormone erythropoietin has emerged as one major regulator of EPC mobilization and differentiation (31). The oxygen-erythropoietin feedback, which depends on the hypoxia-sensing system HIF-1α, is dysregulated in diabetes: microangiopathy and progressive tubulointerstitial fibrosis increase the latency of the erythropoietin system, while production of ROS and hyperglycemia itself affect HIF-1α regulation, blunting the erythropoietin response (32). In this context, we have shown that EPC mobilization in diabetes is defective because of HIF-1α downregulation (17). For these reasons, diabetic nephropathy may be associated with a more profound EPC impairment than chronic kidney disease in general that would represent an incremental risk of cardiovascular disease and death.
Hyperglycemia induces retinal ischemia and release of angiogenic factors that stimulate the proliferation of microvessels, leading to proliferative retinopathy. Not only local endothelial cells but also bone marrow–derived EPCs may be involved in the development of proliferative retinopathy. This scenario appears paradoxical, since in diabetic patients, vascular ischemia may coexist with a condition of pathological neovascularization. With this background, to explore this so-called diabetic angiogenic paradox, we studied in vivo the levels of generic CD34+ progenitors and CD34+KDR+ EPCs and in vitro differentiation of EPCs from type 2 diabetic patients with various combinations of peripheral arterial disease and diabetic retinopathy (33). Whereas CD34+KDR+ cells and endothelial differentiation of cultured progenitors were selectively reduced in peripheral arterial disease patients, generic CD34+ progenitors were reduced in diabetic retinopathy patients, which showed instead higher clonogenic potential and enhanced endothelial differentiation in culture. Similar findings have been reported by Asnaghi et al. (34), who showed that EPCs cultured from peripheral blood of patients with type 1 diabetes and proliferative diabetic retinopathy displayed increased clonogenic potential. It was recently shown by Brunner et al. (35) that in patients with type 1 diabetes with diabetic retinopathy, EPCs undergo stage-related regulation and may play an important role in the development of human proliferative diabetic retinopathy. Interestingly, pericyte loss is an early and selective event leading to endothelial activation and proliferation in the retina, and CD34+ progenitors of perivascular cells have been demonstrated in peripheral blood (36). Thus, depletion of generic CD34+ progenitor cells may be one cause of pericyte loss, whereas increased endothelial differentiation may lead to abnormal retinal angiogenesis.
Diabetic neuropathy is caused by both imbalances in neuron metabolism and impaired nerve blood flow. Maintenance of an adequate supply of blood through vasa nervorum is essential to prevent the development of neuropathy. EPCs could be important in the homeostasis of the nutritive microvasculture, and their exhaustion or dysfunction may accelerate the course of diabetic neuropathy. Because uncommitted progenitor cells derived from adult blood can differentiate also toward the neural phenotype, it is possible that a broader derangement of immature circulating cells in diabetes predisposes to this chronic complication. Naruse et al. (37) showed that intramuscular administration of EPCs is able to reverse the impairment of sciatic nerve conduction velocity and nerve blood flow in diabetic rats. Once more, the altered EPC regulation in diabetic neuropathy may be attributed to a defective HIF-1α activation. Jeong et al. (38) investigated whether diabetic neuropathy could be reversed by local transplantation of EPCs. They found that motor and sensory nerve conduction velocities, blood flow, and capillary density were reduced in sciatic nerves of streptozotocin-induced diabetic mice but recovered to normal levels after hind-limb injection of bone marrow–derived EPCs. Injected EPCs were preferentially and durably engrafted in the sciatic nerves. Finally, they found that portions of engrafted EPCs were uniquely localized in close proximity to vasa nervorum. This study proves, for the first time, that bone marrow–derived EPCs could reverse various manifestations of diabetic neuropathy. Remarkably, it was recently shown by Busik et al. (39), using a rat model of type 2 diabetes, that the decrease in EPC release from diabetic bone marrow is caused by bone marrow neuropathy and that these changes precede the development of diabetic retinopathy. Denervation was accompanied by increased numbers of EPCs within the bone marrow but decreased numbers in circulation. Furthermore, denervation was accompanied by a loss of circadian release of EPCs and a marked reduction in clock gene expression in the retina and in EPCs themselves.
ALL THAT GLITTERS IS NOT GOLD
EPCs were proposed as a regenerative tool for treating human vascular disease. However, conflicting results have been reported in the identification, characterization, and precise role of EPCs in vascular biology. Indeed, the direct proof-of-concept of true significance of these cells in vascular repair and growth is still under scrutiny. Vascular homeostasis and specifically repair are complex multicellular processes that require several cellular phenotype-like endothelial cells, smooth muscle cells, monocytes, and bone marrow–derived cells. Hagensen et al. (40) have also recently questioned the role of EPCs in vascular repair by showing that bone marrow–derived cells rarely contribute to the endothelium of developing plaques or participate in endothelial regeneration after plaque rupture. Using orthotropic arterial transplantation, the authors convincingly demonstrate that circulating cells rarely contribute to plaque endothelium or regeneration of overlying endothelium after plaque disruption. This set of studies indirectly suggests that the local vessel is the major source of plaque endothelium and predominantly contributes to regeneration of overlying endothelium after plaque rupture. All this is further complicated by the lack of a specific marker and validated methods to unequivocally identify this circulating cell subset and by the presence of platelet microparticles in a standard assay for putative EPCs (41). A rare population of endothelial colony-forming cells has been identified in human umbilical cord blood and adult peripheral blood that embodies all of the properties of an EPC (42); however, there is no proof that the human endothelial colony-forming cells play a role in neoangiogenesis in vivo.
CONCLUSIONS
Despite some stimulating controversy regarding EPC identity and function, the literature is remarkably consistent in attributing to EPCs a central role in the development and progression of virtually all diabetes complications. Therefore, ways to reverse EPC alterations in diabetic patients should be actively pursued. Available data suggest that metabolic intervention by either lifestyle change or glucose lowering is able to improve EPC biology. In addition, many drugs commonly prescribed in diabetic patients have demonstrated significant EPC-modulating effects, and their use might be differentiated also in light of these findings. In patients with advanced complications, cell-based translational approaches may provide a novel and valid alternative in the future.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
This publication is based on the presentations at the 3rd World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, F. Hoffmann-La Roche, Janssen-Cilag, Johnson & Johnson, Novo Nordisk, Medtronic, and Pfizer.
References
- 1.Kirpichnikov D, Sowers JR. Diabetes mellitus and diabetes-associated vascular disease. Trends Endocrinol Metab 2001;12:225–230 [DOI] [PubMed] [Google Scholar]
- 2.Avogaro A, Fadini GP, Gallo A, Pagnin E, de Kreutzenberg S. Endothelial dysfunction in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 2006;16(Suppl. 1):S39–S45 [DOI] [PubMed] [Google Scholar]
- 3.Fadini GP, Sartore S, Agostini C, Avogaro A. Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care 2007;30:1305–1313 [DOI] [PubMed] [Google Scholar]
- 4.Nitenberg A, Valensi P, Sachs R, Dali M, Aptecar E, Attali JR. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes 1993;42:1017–1025 [DOI] [PubMed] [Google Scholar]
- 5.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res 2009;105:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClung JA, Naseer N, Saleem M, et al. Circulating endothelial cells are elevated in patients with type 2 diabetes mellitus independently of HbA(1)c. Diabetologia 2005;48:345–350 [DOI] [PubMed] [Google Scholar]
- 7.Nomura S. Dynamic role of microparticles in type 2 diabetes mellitus. Curr Diabetes Rev 2009;5:245–251 [DOI] [PubMed] [Google Scholar]
- 8.Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol 2006;26:257–266 [DOI] [PubMed] [Google Scholar]
- 9.Besler C, Doerries C, Giannotti G, Lüscher TF, Landmesser U. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther 2008;6:1071–1082 [DOI] [PubMed] [Google Scholar]
- 10.De Falco E, Avitabile D, Totta P, et al. Altered SDF-1-mediated differentiation of bone marrow-derived endothelial progenitor cells in diabetes mellitus. J Cell Mol Med 2009;13:3405–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaruba MM, Theiss HD, Vallaster M, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell 2009;4:313–323 [DOI] [PubMed] [Google Scholar]
- 12.Fadini GP, Boscaro E, Albiero M, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes mellitus: possible role of stromal derived factor-1α. Diabetes Care 2010;33:1607–1609 [DOI] [PMC free article] [PubMed]
- 13.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 2003;9:1370–1376 [DOI] [PubMed] [Google Scholar]
- 14.Fadini GP, Boscaro E, de Kreutzenberg S, et al. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care 2010;33:1097–1102 [DOI] [PMC free article] [PubMed]
- 15.Fadini GP, Sartore S, Albiero M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol 2006;26:2140–2146 [DOI] [PubMed] [Google Scholar]
- 16.Sambuceti G, Morbelli S, Vanella L, et al. Diabetes impairs the vascular recruitment of normal stem cells by oxidant damage, reversed by increases in pAMPK, heme oxygenase-1, and adiponectin. Stem Cells 2009;27:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadini GP, Sartore S, Schiavon M, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia 2006;49:3075–3084 [DOI] [PubMed] [Google Scholar]
- 18.Ceradini DJ, Yao D, Grogan RH, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem 2008;283:10930–10938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humpert PM, Neuwirth R, Battista MJ, et al. SDF-1 genotype influences insulin-dependent mobilization of adult progenitor cells in type 2 diabetes. Diabetes Care 2005;28:934–936 [DOI] [PubMed] [Google Scholar]
- 20.Kränkel N, Adams V, Linke A, et al. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol 2005;25:698–703 [DOI] [PubMed] [Google Scholar]
- 21.Fadini GP, de Kreutzenberg SV, Coracina A, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J 2006;27:2247–2255 [DOI] [PubMed] [Google Scholar]
- 22.Marchetti V, Menghini R, Rizza S, et al. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes 2006;55:2231–2237 [DOI] [PubMed] [Google Scholar]
- 23.Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest 2000;106:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 2005;45:1449–1457 [DOI] [PubMed] [Google Scholar]
- 25.Yoon YS, Uchida S, Masuo O, et al. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 2005;111:2073–2085 [DOI] [PubMed] [Google Scholar]
- 26.Rota M, LeCapitaine N, Hosoda T, et al. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res 2006;99:42–52 [DOI] [PubMed] [Google Scholar]
- 27.Herbrig K, Gebler K, Oelschlaegel U, et al. Kidney transplantation substantially improves endothelial progenitor cell dysfunction in patients with end-stage renal disease. Am J Transplant 2006;6:2922–2928 [DOI] [PubMed] [Google Scholar]
- 28.Fadini GP, Miotto D, Baesso I, et al. Arterio-venous gradient of endothelial progenitor cells across renal artery stenosis. Atherosclerosis 2005;182:189–191 [DOI] [PubMed] [Google Scholar]
- 29.Makino H, Okada S, Nagumo A, et al. Decreased circulating CD34+ cells are associated with progression of diabetic nephropathy. Diabet Med 2009;26:171–173 [DOI] [PubMed] [Google Scholar]
- 30.Dessapt C, Karalliedde J, Hernandez-Fuentes M, et al. Circulating vascular progenitor cells in patients with type 1 diabetes and microalbuminuria. Diabetes Care 2010;33:875–877 [DOI] [PMC free article] [PubMed]
- 31.Bahlmann FH, De Groot K, Spandau JM, et al. Erythropoietin regulates endothelial progenitor cells. Blood 2004;103:921–926 [DOI] [PubMed] [Google Scholar]
- 32.Thomas MC, Cooper ME, Rossing K, Parving HH. Anaemia in diabetes: is there a rationale to TREAT? Diabetologia 2006;49:1151–1157 [DOI] [PubMed] [Google Scholar]
- 33.Fadini GP, Sartore S, Baesso I, et al. Endothelial progenitor cells and the diabetic paradox. Diabetes Care 2006;29:714–716 [DOI] [PubMed] [Google Scholar]
- 34.Asnaghi V, Lattanzio R, Mazzolari G, et al. Increased clonogenic potential of circulating endothelial progenitor cells in patients with type 1 diabetes and proliferative retinopathy. Diabetologia 2006;49:1109–1111 [DOI] [PubMed] [Google Scholar]
- 35.Brunner S, Schernthaner GH, Satler M, et al. Correlation of different circulating endothelial progenitor cells to stages of diabetic retinopathy: first in vivo data. Invest Ophthalmol Vis Sci 2009;50:392–398 [DOI] [PubMed] [Google Scholar]
- 36.Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res 2005;37(Suppl. 1):39–43 [DOI] [PubMed] [Google Scholar]
- 37.Naruse K, Hamada Y, Nakashima E, et al. Therapeutic neovascularization using cord blood-derived endothelial progenitor cells for diabetic neuropathy. Diabetes 2005;54:1823–1828 [DOI] [PubMed] [Google Scholar]
- 38.Jeong JO, Kim MO, Kim H, et al. Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation 2009;119:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busik JV, Tikhonenko M, Bhatwadekar A, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med 2009;206:2897–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagensen MK, Shim J, Thim T, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation 2010;121:898–905 [DOI] [PubMed]
- 41.Prokopi M, Pula G, Mayr U, et al. Proteomic analysis reveals the presence of platelet microparticles in endothelial progenitor cell cultures. Blood 2009;114:723–732 [DOI] [PubMed]
- 42.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007;109:1801–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]