Abstract
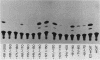
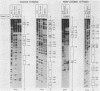
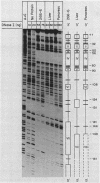
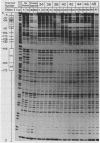
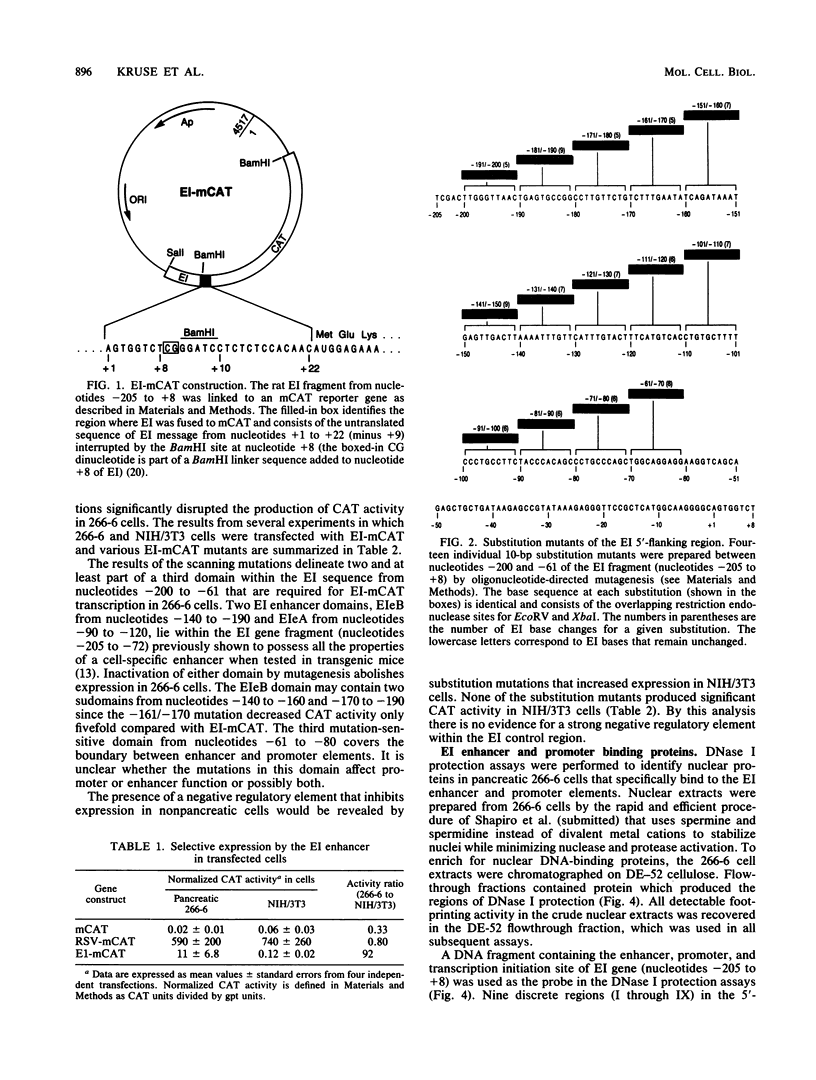
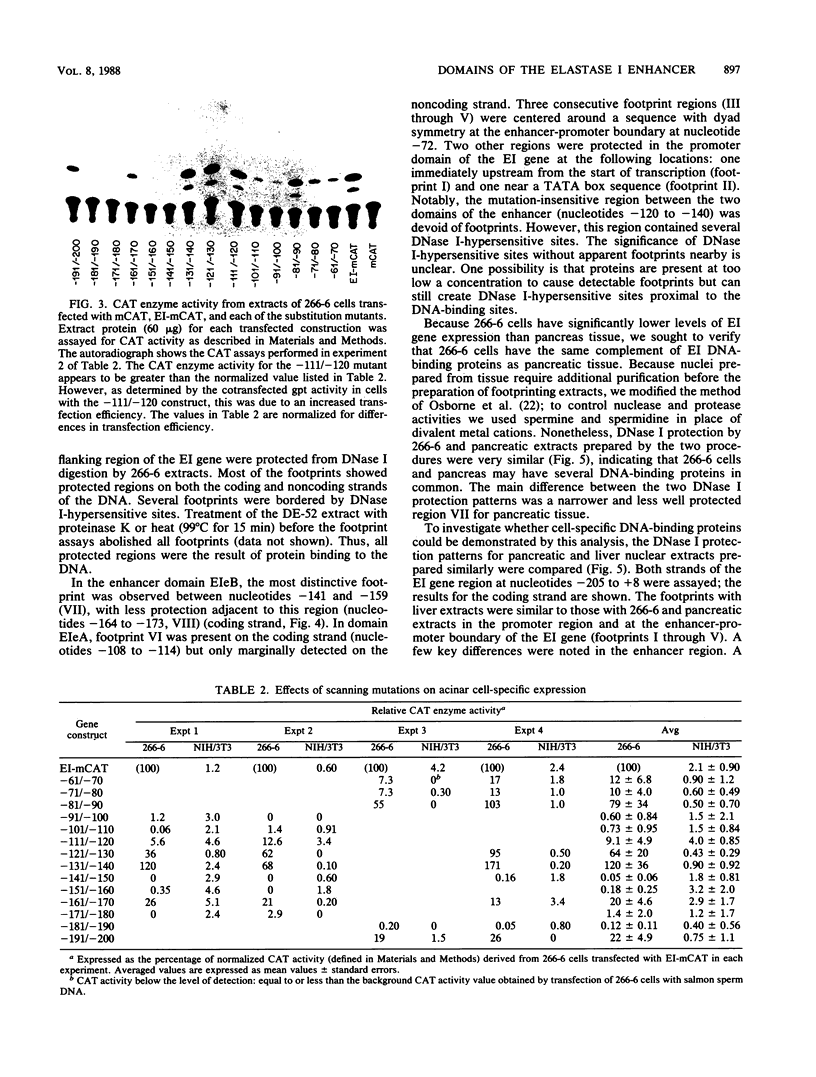
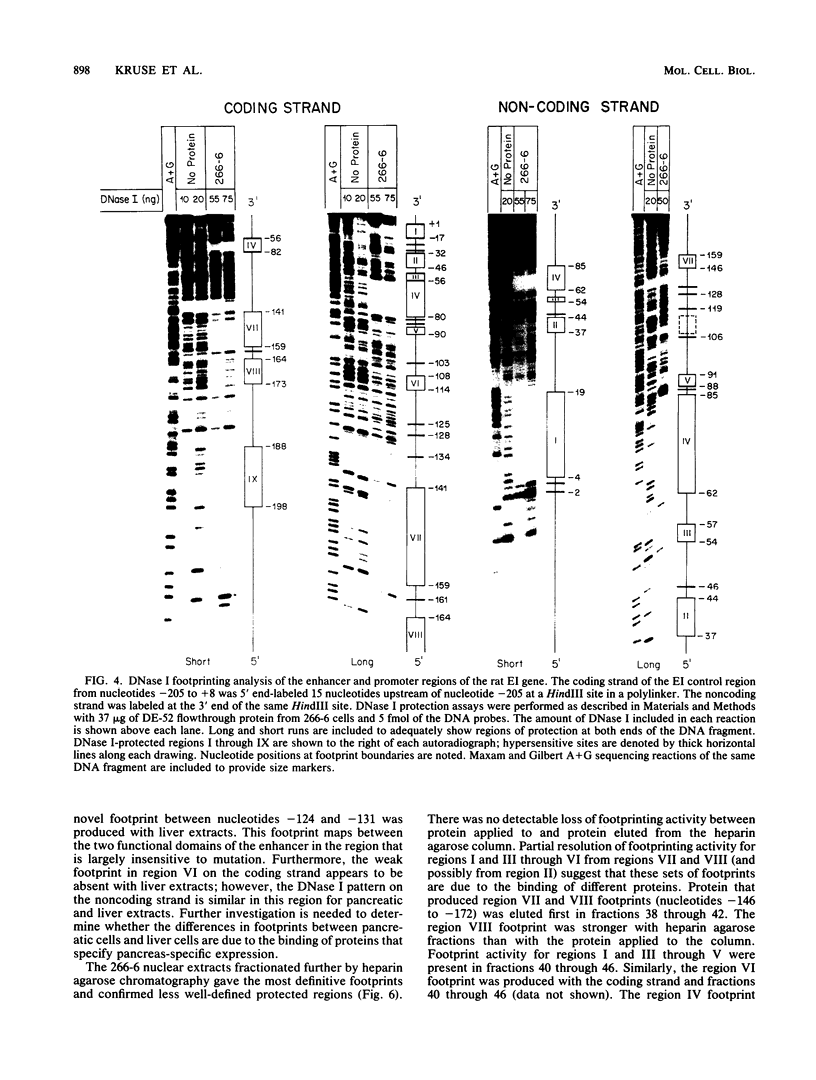
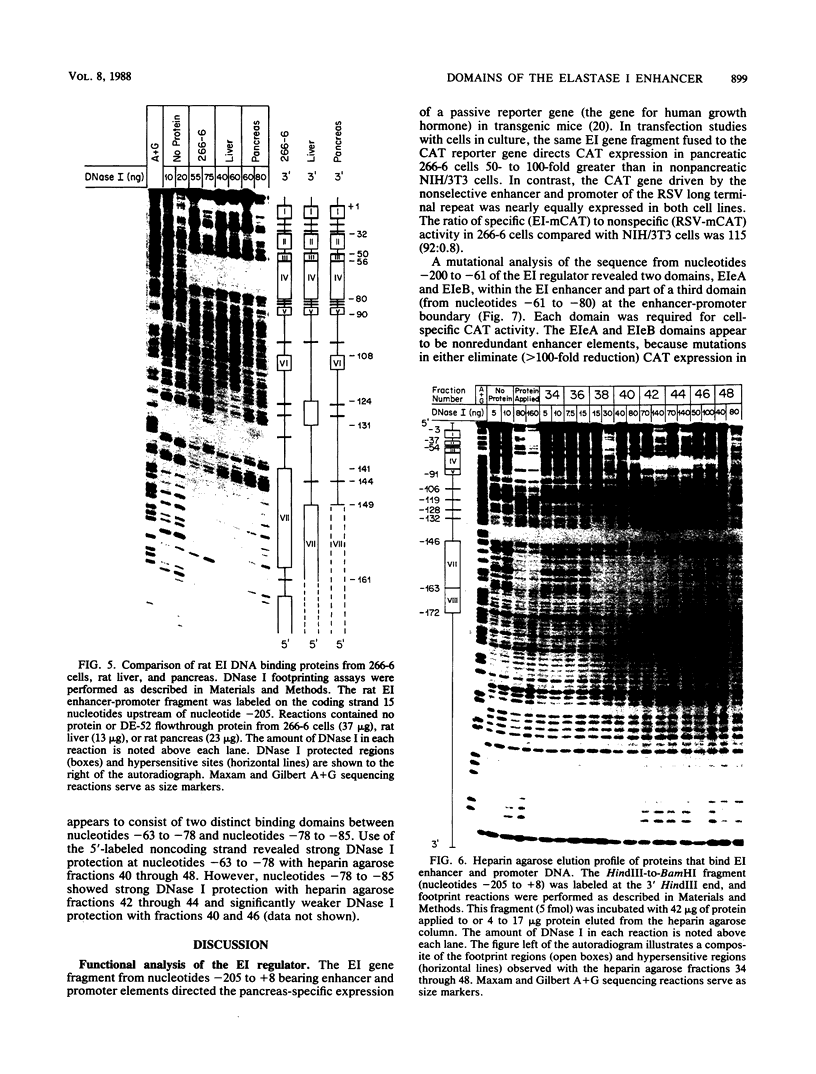
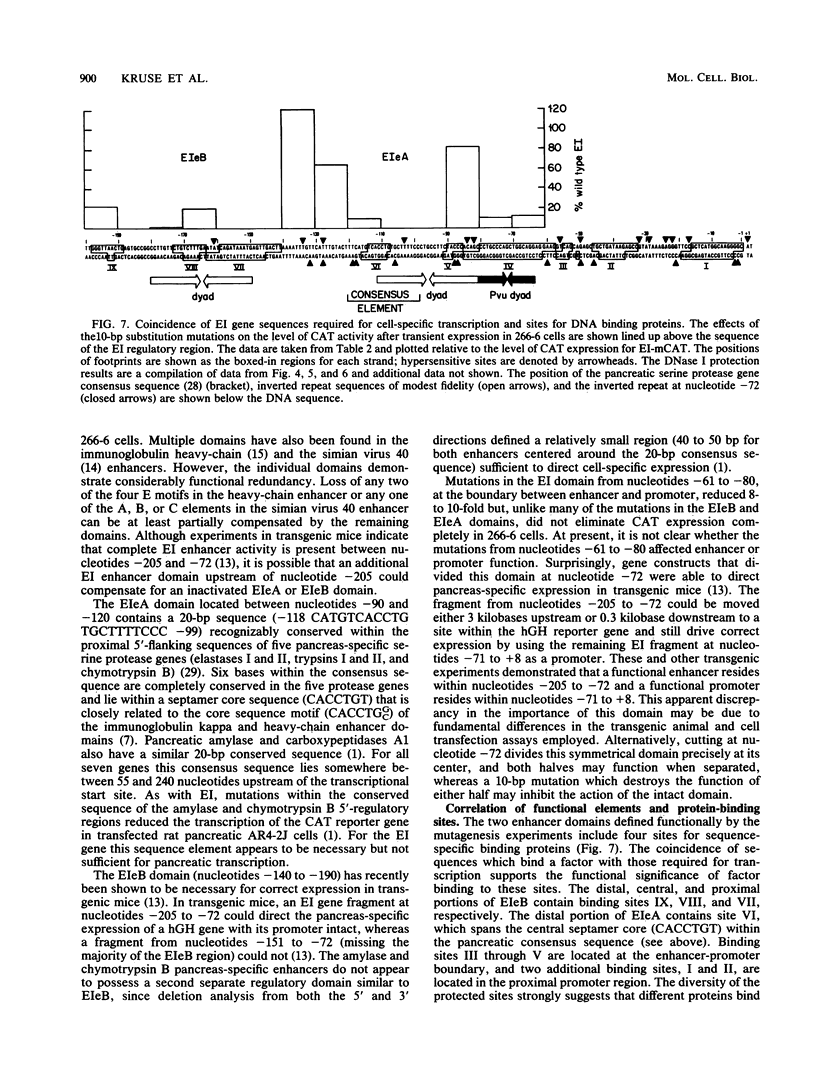
Two separate domains within the 134-base-pair rat elastase I enhancer and a third domain at the enhancer-promoter boundary are required for selective expression in pancreatic acinar cells. The domains were detected by a series of 10-base-pair substitution mutations across the elastase I gene regulatory region from positions -200 to -61. The effect of each mutant on the pancreas-specific expression of a linked chloramphenicol acetyltransferase gene was assayed by transfection into pancreatic 266-6 acinar cells and control NIH/3T3 cells. The two enhancer domains are nonredundant, because mutations in either eliminated (greater than 100-fold reduction) expression in 266-6 cells. DNase I protection studies of the elastase I enhancer-promoter region with partially purified nuclear extracts from pancreatic tissue and 266-6 cells revealed nine discrete protected regions (footprints) on both DNA strands. One of three footprints that lie within the two functional domains of the enhancer contained a sequence, conserved among several pancreas-specific genes, which when mutated decreased linked chloramphenicol acetyltransferase expression up to 170-fold in 266-6 cells. This footprint may represent a binding site for one or more pancreas-specific regulatory proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulet A. M., Erwin C. R., Rutter W. J. Cell-specific enhancers in the rat exocrine pancreas. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3599–3603. doi: 10.1073/pnas.83.11.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chu G., Berg P. Rapid assay for detection of Escherichia coli xanthine-guanine phosphoribosyltransferase activity in transduced cells. Nucleic Acids Res. 1985 Apr 25;13(8):2921–2930. doi: 10.1093/nar/13.8.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Swift G. H., Ornitz D. M., Quaife C. J., Palmiter R. D., Brinster R. L., MacDonald R. J. The rat elastase I regulatory element is an enhancer that directs correct cell specificity and developmental onset of expression in transgenic mice. Mol Cell Biol. 1987 Aug;7(8):2956–2967. doi: 10.1128/mcb.7.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Clarke J. The SV40 enhancer is composed of multiple functional elements that can compensate for one another. Cell. 1986 May 9;45(3):461–470. doi: 10.1016/0092-8674(86)90332-6. [DOI] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J. Expression of the pancreatic elastase I gene in transgenic mice. Hepatology. 1987 Jan-Feb;7(1 Suppl):42S–51S. doi: 10.1002/hep.1840070708. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Hammer R. E., Swift G. H., Davis B. P., Brinster R. L. Transgenic progeny inherit tissue-specific expression of rat elastase I genes. DNA. 1986 Oct;5(5):393–401. doi: 10.1089/dna.1986.5.393. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Palmiter R. D., Hammer R. E., Brinster R. L., Swift G. H., MacDonald R. J. Specific expression of an elastase-human growth hormone fusion gene in pancreatic acinar cells of transgenic mice. Nature. 1985 Feb 14;313(6003):600–602. doi: 10.1038/313600a0. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Palmiter R. D., Messing A., Hammer R. E., Pinkert C. A., Brinster R. L. Elastase I promoter directs expression of human growth hormone and SV40 T antigen genes to pancreatic acinar cells in transgenic mice. Cold Spring Harb Symp Quant Biol. 1985;50:399–409. doi: 10.1101/sqb.1985.050.01.050. [DOI] [PubMed] [Google Scholar]
- Osborne T. F., Gil G., Brown M. S., Kowal R. C., Goldstein J. L. Identification of promoter elements required for in vitro transcription of hamster 3-hydroxy-3-methylglutaryl coenzyme A reductase gene. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3614–3618. doi: 10.1073/pnas.84.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Krovatin W., Jeffrey A., Sauer R. T. The N-terminal arms of lambda repressor wrap around the operator DNA. Nature. 1982 Jul 29;298(5873):441–443. doi: 10.1038/298441a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Westphal H. M., Carlson C., Bosshard H., Beato M. Molecular model of the interaction between the glucocorticoid receptor and the regulatory elements of inducible genes. DNA. 1986 Oct;5(5):383–391. doi: 10.1089/dna.1986.5.383. [DOI] [PubMed] [Google Scholar]
- Schick J., Kern H., Scheele G. Hormonal stimulation in the exocrine pancreas results in coordinate and anticoordinate regulation of protein synthesis. J Cell Biol. 1984 Nov;99(5):1569–1574. doi: 10.1083/jcb.99.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. B. Interaction of the cAMP receptor protein with the lac promoter. Nucleic Acids Res. 1980 Feb 25;8(4):759–766. [PMC free article] [PubMed] [Google Scholar]
- Swift G. H., Craik C. S., Stary S. J., Quinto C., Lahaie R. G., Rutter W. J., MacDonald R. J. Structure of the two related elastase genes expressed in the rat pancreas. J Biol Chem. 1984 Nov 25;259(22):14271–14278. [PubMed] [Google Scholar]
- Swift G. H., Hammer R. E., MacDonald R. J., Brinster R. L. Tissue-specific expression of the rat pancreatic elastase I gene in transgenic mice. Cell. 1984 Oct;38(3):639–646. doi: 10.1016/0092-8674(84)90258-7. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Shuey D. J., Kibbe W. A., Parker C. S. The Saccharomyces and Drosophila heat shock transcription factors are identical in size and DNA binding properties. Cell. 1987 Feb 13;48(3):507–515. doi: 10.1016/0092-8674(87)90201-7. [DOI] [PubMed] [Google Scholar]
- Zassenhaus H. P., Butow R. A., Hannon Y. P. Rapid electroelution of nucleic acids from agarose and acrylamide gels. Anal Biochem. 1982 Sep 1;125(1):125–130. doi: 10.1016/0003-2697(82)90392-x. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]
- de Vries E., van Driel W., van den Heuvel S. J., van der Vliet P. C. Contactpoint analysis of the HeLa nuclear factor I recognition site reveals symmetrical binding at one side of the DNA helix. EMBO J. 1987 Jan;6(1):161–168. doi: 10.1002/j.1460-2075.1987.tb04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]