Abstract
Myocardial fat content refers to the storage of triglyceride droplets within cardiomyocytes. In addition, the heart and arteries are surrounded by layers of adipose tissue, exerting vasocrine and paracrine control of the subtending tissues. The rapid development of the field of noninvasive imaging has made it possible to quantify ectopic fat masses and contents with an increasing degree of accuracy. Myocardial triglyceride stores are increased in obesity, impaired glucose tolerance, and type 2 diabetes. The role of intramyocardial triglyceride accumulation in the pathogenesis of left ventricular (LV) dysfunction remains unclear. Increased triglyceride content is associated with states of fatty acid overload to the heart, saturating the oxidative capacity. It may initially serve as a fatty acid sink to circumscribe the formation of toxic lipid species and subsequently foster cardiac damage. Epicardial and perivascular fat depots may exert a protective modulation of vascular function and energy partition in a healthy situation, but their expansion turns them into an adverse lipotoxic, prothrombotic, and proinflammatory organ. They are augmented in patients with metabolic disorders and coronary artery disease (CAD). However, the progressive association between the quantity of fat and disease severity in terms of extent of plaque calcification or noncalcified areas, markers of plaque vulnerability, and number of vessels involved is less confirmed. Functional or hybrid imaging may contribute to a better definition of disease severity and unveil the direct myocardial and vascular targets of adipose tissue action.
The last decade has witnessed a renewed interest in heart adiposity, especially as the result of the rapid development in the field of noninvasive imaging, which has made it possible to quantify ectopic fat masses and contents with increasing levels of accuracy. This review addresses recent knowledge provided by imaging studies of the fatty heart in metabolic and heart disease in humans.
DEFINITIONS
Myocardial fat content refers to the storage of triglyceride droplets within cardiomyocytes, which can be measured in humans by use of proton magnetic resonance spectroscopy (1H-MRS) (1). In addition, the heart and vessels are surrounded by layers of adipose tissue, which is a complex organ composed of adypocytes, stromal cells, macrophages, and a neuronal network, all nourished by a rich microcirculation. The layers surrounding the heart include intra- and extrapericardial fat. Their thicknesses and volumes can be quantified by echocardiography and computed tomography or magnetic resonance imaging, respectively (2–4). Intrapericardial fat is in direct contact with the surface of the myocardium and coronary vessels, with no separation by a physical fascia. Thus, the diffusion of secreted molecules and the migration of cells between these adjacent structures may occur. This adherent fat layer has been defined as epicardial (between myocardium and visceral pericardium), whereas the term pericardial fat has been variably used to identify fat between myocardium and pericardium, which may include adipose tissue in the space between visceral and parietal pericardium, or just external but attached to the parietal pericardium. Perivascular fat surrounds arteries and arterioles. The epicardial fat layer originates embryologically from mesothelial cells migrating from the septum transversum and hence obtains its vascular supply from the coronary arteries. The term extrapericardial (or intrathoracic or paracardial) defines thoracic adipose tissue external to the parietal pericardium. It originates from primitive thoracic mesenchymal cells and thus derives its blood supply from noncoronary sources (5).
FAT INSIDE THE HEART
Studies with 1H-MRS show that the heart possesses an endogenous triglyceride depot of ≤1.0% organ mass in healthy lean individuals (1), which increases with age. In healthy subjects, short-term caloric restriction and starvation provoked a dose-dependent increase in myocardial triglyceride content and decrease in diastolic function (6). Instead, high-fat diets of short duration, resulting in greatly increased plasma lipid concentrations and a decline in diastolic function, had no influence on myocardial triglyceride content (6). These studies suggest that circulating free fatty acids (which are elevated in starvation) participate in the regulation of intramyocardial fat depots, and that the latter have rapid adaptive (i.e., buffering) capacities. In the physiologically aging male heart, myocardial triglyceride content increased in association with the age-related decline in diastolic function (7). Nonobese women with normal glucose tolerance have significantly less myocardial fat content, in inverse relationship with circulating adiponectin levels, compared with healthy men of similar age (8).
Obesity, diabetes, and metabolic syndrome
We and others have recently documented that cardiac steatosis is a hallmark of obesity and type 2 diabetes (1,8,9), representing a potentially remarkable endogenous source of cytosolic fatty acids. Myocardial triglyceride stores in these diseases are increased on average by two- to fourfold compared with those in control individuals (8). Cardiac adiposity is associated with greater LV mass and work, suppressed septal wall thickening, and impaired diastolic function. We observed a relationship with sex, which may play a part in lowering the risk of cardiovascular disease in nondiabetic women. Notably, chronic hyperglycemia cancelled the sex-related difference, which may to some extent explain the loss of a cardioprotective status in women developing diabetes (8).
Metabolic intervention
In patients with type 2 diabetes, the response to short-term caloric restriction, leading to an elevation in fatty acid levels, promoted an accumulation of myocardial triglycerides that was associated with a decline in LV diastolic function, as in healthy individuals. These effects were not observed when the rise in fatty acid levels was pharmacologically prevented, thus underscoring the causal role of an extramyocardial supply of fatty acids in modulating the cardiac lipid pool (10). Conversely, the prolongation of a very-low-calorie diet for 6–16 weeks reduced myocardial fat content in nondiabetic obese and in type 2 diabetic patients (11,12). This finding was accompanied by a decline in LV mass and work (12) and by an improvement in diastolic function (11). In one study, glitazones have been shown to slightly reverse cardiac steatosis in insulin-treated type 2 diabetic patients (13). In a subsequent study (14) conducted in 78 diabetic men assigned to pioglitazone or metformin or placebo for 24 weeks, neither drug changed cardiac triglyceride content, despite a decrease in cardiac work by metformin and an improvement in LV diastolic function by pioglitazone.
Does cardiac steatosis play a role in heart disease in humans?
Though diastolic dysfunction and a fatty heart are usually concomitant findings in metabolic disorders, they may (11,13) or may not (6,14) be simultaneously induced or reversed by metabolic intervention, and their changes are not always correlated (6,14). In cross-sectional evaluations (9), multivariable analysis models documented that the individual contribution of myocardial fat content in explaining the observed diastolic dysfunction in type 2 diabetic patients was modest, and its correlations with the ratio of early (E) and late (atrial, A) ventricular filling velocity (E/A) and E peak deceleration was not consistently observed (15), suggesting that other unmeasured pathologic processes, coexisting in the hearts of patients with diabetes or obesity may be more directly responsible for the dysfunction.
It is important to underline that the level of circulating fatty acids has been one main correlate of human myocardial triglyceride content. The sequence of events following fatty acid overload to the heart is summarized in Fig. 1A, showing primary hyperactivation of β-oxidation, as seen in human obesity and diabetes, leading to excess formation of reactive oxygen species (ROS) (16), and resulting in modulation of sarco(endo)plasmic reticulum Ca2+-ATPase, which is an early contributor of diastolic dysfunction in the insulin-resistant myocardium (16) and of myocardial fibrosis and hypertrophy. In mouse lines overexpressing long-chain acyl-CoA synthetase in the heart (17), LV dysfunction occurs in parallel with an over-stimulation of oxidation and ROS and ceramide formation, although cardiac steatosis and hypertrophy are concomitantly present.
Figure 1.
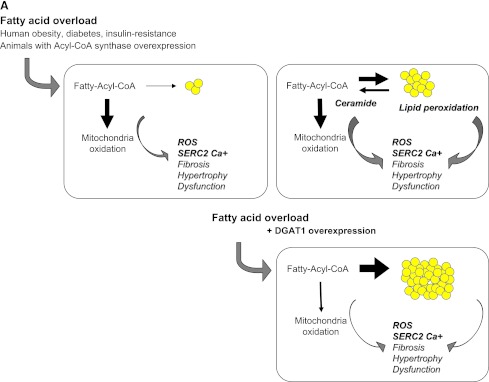
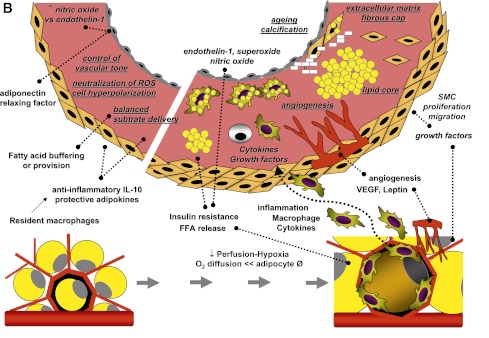
A: Fatty acids entering cardiomyocytes are conjugated with acyl-CoA and transported to the mitochondria to undergo β-oxidation for cellular energy needs. Myocardial fatty acid oxidation is, in fact, increased in human obesity and diabetes and in animal models overexpressing acyl-CoA synthase (top left). Mitochondrial respiration by the electron transport chain and NADPH oxidase are the likely predominant myocardial generators of ROS, resulting in modification of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a) as well as cardiac fibrosis and hypertrophy. As oxidation becomes saturated, triglyceride accumulation provides a buffer against the formation of fatty acid intermediate species, but progressive exhaustion of storage capacity provokes the build-up of acyl-CoA and ceramide in the cytoplasm (top right), contributing to lipotoxicity. Amplification of storage capacity by enzymatic overexpression of diacylglycerol acyltransferase 1 (DGAT1) slows the progression of cardiac damage (bottom right), suggesting a defensive role of triglyceride accumulation in fatty acid overload states. B: Adipose tissue surrounding vessels and myocardium may protectively serve as energy source and buffer and promote vascular relaxation (left panel). Its expansion is typical in metabolic and cardiovascular diseases, and leads to a cascade of events (right panel), likely triggered by adipocyte enlargement, hypoxia, consequent macrophage and T-cell recruitment, and inflammation. Changing patterns in the release of adipokines, cytokines, substrates, smooth muscle cell growth factors, and angiogenesis promoters from stromal and fat cells propagate to the subtending tissues, via simple diffusion and through newly formed adventitial vasa vasorum, and may thereby contribute to the progression of cardiac and vascular lipotoxicity, inflammation, and atherosclerosis. FFA, free fatty acid; SMC, smooth muscle cells; VEGF, vascular endothelial growth factor.
The independent role of triglyceride accumulation in the pathogenesis of disease remains elusive because the transgenic overexpression of the triglyceride-synthesizing enzyme diacylglycerol acyltransferase 1 (DGAT1) in the heart results in a physiologic hypertrophy, serving a cytoprotective function, especially in lipid overload states (18). Accordingly, in cultured cells (19) exposed to an excess of palmitic acid, this fatty acid is poorly incorporated into triglycerides and causes apoptosis, and unsaturated fatty acids rescue palmitate-induced apoptosis by channeling palmitate into triglyceride pools. In the nonischemic failing compared with normal human heart, cardiac triglyceride content was either reduced (20) or unchanged in subjects without obesity or diabetes (21), and myocardial fatty acid oxidation was activated in obese and diabetic patients with cardiac steatosis.
Taken together, these studies make it reasonable to speculate that increased triglyceride content is a consequence of fatty acid overload to the heart, progressing more rapidly after saturation of the oxidative capacity. Under these circumstances, triglyceride accumulation may be seen as a maladaptive defense response, initially serving as a fatty acid sink to circumscribe the formation of toxic lipid species, and eventually undergoing peroxidation and saturation, thereby fostering the build-up of fatty acid intermediates in the cytoplasm and functional damage to the heart. The available studies do not imply causality but suggest that accumulation of myocardial triglyceride may be at least an indirect marker of early cardiac dysfunction in selected stages of disease progression.
FAT AROUND THE HEART AND VESSELS
The heart, coronaries, and virtually all arteries are surrounded by a significant amount of adipose tissue. The thickness of epicardial fat at the level of the right ventricle free wall is normally <7 mm in healthy lean individuals (3,22). The distribution of fat thicknesses among different locations of the myocardium demonstrates a spread of values between 15 and 1 mm. Fat volumes around the heart correlate with advancing age (8,23), and they are larger in men than in women (8,23). Ethnicity is another potential confounder in epicardial fat studies, since this adipose depot is larger in Caucasians, followed by Asians, blacks, and Hispanics (23).
Fat around vessels and the heart may serve a supportive, mechanical purpose, attenuating vascular tension and torsion, participating in vessel remodeling, and being a vasocrine and paracrine source of cytokines, substrates, and adipokines (Fig. 1B). The rates of fatty acid incorporation and release are higher in the cardiac than in other adipose depots, and lipogenesis is stimulated by insulin only in this fat depot. Cardiac and perivascular adipose tissue may act as local energy supplier and/or as a buffer against toxic levels of free fatty acids in the myocardium and in the arterial circulation (24). The vasocrine action on nutritive tissue flow has been involved in modulating substrate fluxes to organs. Adiponectin and adipocyte-derived relaxing factors are released by perivascular fat to decrease contractile responses to vasoconstrictive agents, thus exerting a protective antihypertensive function via the control of endothelium-dependent (modulation of the nitric oxide-to-endothelin-1 ratio) and independent mechanisms (cell hyperpolarization, and production of ROS, hydrogen peroxide) (25,26). Moreover, resident macrophages can increase the release of the anti-inflammatory cytokine interleukin (IL)-10 (27).
Obesity, diabetes, and metabolic syndrome
A positive relationship has been established between the amount of fat surrounding the heart and vessels and several components of the metabolic syndrome. Iacobellis and Willens (3) showed associations with insulin resistance, central adiposity, and clinical parameters of cardiovascular risk, including LDL cholesterol and blood pressure, together with inverse relationships with adiponectin levels. Epicardial and pericoronary fat volumes gradually increased with the number of metabolic syndrome components (4,28), and pericardial fat showed a progressive increment from lean to obese individuals with normal glucose tolerance, to those with impaired glucose tolerance, and those with type 2 diabetes (8). Conversely, in overweight children, epicardial fat was a good indicator of visceral fat, but not an independent predictor of the metabolic syndrome (29). Epicardial fat thickness was associated with C-reactive protein (30) and with proatherogenic and proinflammatory adipokines (3). However, the Framingham Offspring study, screening for 15 biomarkers of inflammation and oxidative stress, demonstrated that intrathoracic but not pericardial fat was independently associated with C-reactive protein and urinary isoprostanes, after adjustment for other adiposity indexes (31), and that visceral adipose tissue was a stronger correlate of most metabolic risk factors (32). Instead, other authors (33) reported that the thickness rather than the volume of epicardial fat was an independent predictor of metabolic syndrome and C-reactive protein levels, also when accounting for intrathoracic and visceral fat depots.
In brief, most studies show relationships between epicardial/perivascular adiposity and metabolic and inflammatory markers, though some suggest that these correlations may be at least partly mediated by the confounding association existing between extra- and intrapericardial and visceral abdominal fat volumes.
Metabolic intervention
A 6-month very-low-calorie diet program (3) decreased epicardial fat thickness relatively more than other fat depots, and the observed changes in LV mass and diastolic function were more strongly correlated with epicardial fat changes than with those of other adiposity indices. A 12-week exercise training program in obese men (34) resulted in a greater percent reduction in epicardial fat thickness than in waist and BMI, and the change was independently related with those in systolic blood pressure and insulin sensitivity. Conversely, a 24-week study comparing the effects of pioglitazone and metformin treatment in type 2 diabetic patients (35) showed an increase in pericardial fat volume in pioglitazone-treated patients, in spite of an improvement in diastolic function, leading the authors to question the notion of a causal relationship between pericardial fat volume and LV dysfunction.
The lack of a contextual measurement of intracardiac triglycerides, which are strongly correlated with epicardial fat volume, diastolic dysfunction, and cardiac mass in patients with diabetes or obesity, may limit the mechanistic interpretation of these findings.
Does adjacent adipose tissue have a role in cardiac mechanical dysfunction?
We documented that the entire mass (intra- and extrapericardial) of fat surrounding the heart ranges on average from 100 g (in healthy lean individuals) to 400 g (in type 2 diabetic patients), extending to values of 800–900 g in some patients (8). This magnitude may pose a noticeable mechanical burden on cardiac expansion. It was associated (though not independently) with cardiac remodelling and mass, peripheral vascular resistance, and lower ejection fraction and cardiac output. Pathology and in vivo imaging studies suggest that during the hypertrophic process, pericardial fat and cardiac mass increase in parallel. Results of the Framingham study (36) showed that the association was not independent of, or stronger than, that of other proxy measures of visceral adiposity. An important exception was left atrial dimension in men, extending the previous evidence of an association between epicardial fat thickness and atria enlargement or impairment in diastolic filling in morbidly obese subjects (3).
Overall, the above studies indicate that the systemic effects of obesity on cardiac structure and function may outweigh the local pathogenic effects of pericardial fat. Conversely, the latter may directly affect LV diastolic filling and consequently induce atrial enlargement.
Does adjacent adipose tissue have a role in cardiovascular disease?
Table 1 summarizes studies conducted to explore relationships between cardiac and perivascular fat and CAD. Vascular aging and subclinical atherosclerosis, as revealed by carotid stiffness and intima-media thickness, were related to epicardial fat thickness better than waist circumference in hypertensive patients (22). In the Multi-Ethnic Study of Atherosclerosis the association with carotid stiffness, but not intima-media thickness persisted after adjusting for cardiovascular risk factors (37,38). In the Second Manifestations of ARTerial Disease (SMART) study (39), intra-abdominal fat accumulation was associated with larger infrarenal aortic diameters in patients with clinically evident arterial disease.
Table 1.
Studies on epicardial and perivascular fat versus CAD prediction or staging
| Ref. | Sampling technique(s) | Number of patients (specific features) | Evaluation of CAD | Relationship vs. epi- or pericardial or perivascular fat | Adjusted for risk factors and/or adiposity |
|---|---|---|---|---|---|
| (2) | Computerized tomography + invasive angiography | 251 | presence of CAD | YES | yes/yes |
| severity | YES | ||||
| (40) | Echocardiography + invasive angiography | 139 | presence of CAD | NO | n.a. |
| degree + no. stenoses | NO | ||||
| (42) | Echocardiography + invasive angiography | 203 | presence of CAD | YES | yes/yes |
| severity (Gensini score) | YES | ||||
| (30) | Echocardiography | 527 | presence of CAD | YES | yes/yes |
| degree of stenosis | YES | ||||
| unstable angina | YES | ||||
| (45) | Computerized tomography | 190 | presence of CAD | YES | yes/yes |
| degree + no. stenoses | NO | ||||
| coronary calcium score | NO | ||||
| calcium × cut-off | YES | ||||
| (52) | Computerized tomography | 573 (healthy women) | coronary calcium score | YES | n.d./no |
| (49) | Computerized tomography | 159 (mixed ethnicity) | calcified plaques (+/−) | YES | yes/yes |
| coronary calcium score | YES | ||||
| (50) | Computerized tomography | 150 | prevalence of CAD | YES | n.d. |
| calcium × cut-off | YES | ||||
| (47) | Computerized tomography | 128 *whole BMI range ^BMI <27 kg/m2 | no. stenoses | NO*/YES^ | n.d. in the low BMI category |
| coronary calcium score | NO*/YES^ | ||||
| (39) | Computerized tomography | 2,726 (with arterial disease) | infrarenal aortic diameter | YES | yes/n.a. |
| (32) | Computerized tomography | 1,155 (clinical CVD excluded) | coronary calcium score | YES | yes/yes |
| aortic calcium score | YES | ||||
| (43) | Computerized tomography | 264/286 (22 excluded) | presence of CAD | YES | yes/n.d. |
| no. plaques + no. segments | YES | ||||
| atherosclerosis score | YES | n.d./yes | |||
| plaque composition | NO | ||||
| (44) | Computerized tomography | 1,267 (9.7% with CVD) | presence of CVD | YES | no/yes |
| prevalent CHD | YES | n.d./yes | |||
| prevalent infarction | YES | n.d./yes | |||
| prevalent stroke | NO | n.d./no | |||
| (48) | Computerized tomography | 1,119 (147 events) (mixed ethnicity) | incident CVD vs. EAT measured at postvisit | YES | yes/yes |
| (41) | Echocardiography + invasive angiography | 150 | presence of CAD | YES | yes/n.d. |
| no. single vs. multivessel | YES | ||||
| Gensini score | YES | ||||
| (22) | Echocardiography + carotid ultrasound | 459 (hypertensive patients) | carotid wall IMT | YES | |
| carotid stiffness | YES | yes/no | |||
| (28) | Computerized tomography + invasive angiography | 71 | stenosis score | YES | |
| atheromatosis score | YES | ||||
| history of ACS | YES | ||||
| total coronary occlusion | YES | yes/yes | |||
| (55) | Echocardiography + invasive angiography | 68 (only women, chest pain, CVD excluded) | coronary flow reserve | YES | yes/n.d. |
| (51) | Computerized tomography | 1,067 (clinical CVD excluded) | coronary calcium score | YES | yes/yes |
| thoracic aorta calcium | YES | no/yes | |||
| abdominal aorta calcium | YES | yes/yes | |||
| (54) | Computerized + positron emission tomography | 161 successful of 292 (cancer patients) | FDG uptake in LAD vs. EAT | YES | yes/yes |
| vs. calcium score | YES | ||||
| (37) | Computerized tomography + carotid ultrasound | 5,770 (mixed ethnicity) | carotid stiffness | YES | yes/yes |
| IMT | YES | ||||
| coronary calcium score | YES | ||||
| (38) | Computerized tomography + carotid ultrasound | 996 | IMT common carotid artery | YES | no |
| internal carotid artery | YES | yes in men | |||
| (53) | Computerized tomography | 311 (coronary segments for plaques and fat volume) | presence of CAD | YES | yes/yes |
| plaque burden | YES | ||||
| (46) | Computerized tomography | 171 (suspected CAD) | presence of CAD | YES | yes/yes |
| stenotic plaque (+/−) | NO | ||||
| calcif. vs. mix vs. noncalcif. plaque | NO | ||||
| (23) | Computerized tomography | 214 (mixed ethnicity) | a) calcif. vs. no plaque | NO | |
| b) mixed vs. noncalcif. | NO | ||||
| a) vs. b) | YES | yes/yes | |||
| calcium score | YES | yes/yes | |||
| severity of stenoses | YES | yes/yes |
Studies include patients referred to imaging for known or suspected CAD, with few exceptions given in parentheses. The last column shows whether the relationships are maintained after adjustment for cardiovascular risk factors or (/) alternative indices of adiposity, namely BMI (in a majority of studies) or waist or amount of visceral fat. ACS, acute coronary syndrome; CHD, coronary heart disease; CVD, cardiovascular disease; calcif., calcification; EAT, epicardial adipose tissue; FDG, 18F-fluorodeoxyglucose; IMT, intima-media thickness; LAD, left anterior descending coronary artery; n.a., not applicable; n.d., indicates that the confounding variables have not been measured, or that they have been measured but not included in a multivariate regression model investigating the target relationship; noncalcif., noncalcified.
Atherosclerotic lesions are absent in segments of coronary arteries lacking pericardial fat, such as intramyocardial bridges, as compared with intraepicardial portions of the main coronary artery in both humans and animals (5). A host of noninvasive imaging studies—with one exception (40)—have shown that patients with CAD have greater depots of adipose tissue within the pericardium and around arteries compared with healthy individuals. However, the existence of a progressive association relating the amount of pericardial fat with the severity of atherosclerosis, evaluated as number of stenotic vessels and/or degree of obstruction (23,41–43), total coronary occlusion (28), stable versus unstable angina (30), or prevalent myocardial infarction (44) was reported in some but not in other studies (40,45–47) or was found only in selected subgroups (47). A recent report (48) suggested that pericardial fat is a better predictor of incident CAD than are more general measures of adiposity, but cardiac adiposity was determined at follow-up and not at baseline.
The extent of vessel wall or plaque calcification has been used as an additional index of severity of disease. In subjects free from clinical CAD, pericardial fat was independently associated with vascular calcification (32,49,50), and the relationship was consistent between sexes and groups of different ethnicity (49). Thoracic aortic fat was associated with abdominal aortic and coronary artery calcification (51). In healthy postmenopausal women (52), pericoronary fat thickness was related to calcification in respective coronaries. Instead, in patients with CAD the relationship was not progressive (45), or was found only in subjects with normal BMI (47). As suggested, the presence of mere calcification could represent an advanced but stable phase of atherosclerosis, and pericardial fat may be more strongly associated with an active process as proven by the presence of noncalcified plaques. This association was observed in one retrospective (23), but not in three subsequent studies (43,46,53).
It is important to keep in mind that the number of coronary stenoses and size of plaque/obstruction may not be optimal predictors of cardiovascular events or the best guidance for management, and that the paracrine and vasocrine nature of the interaction between fat and myocardium or arteries is most likely mediated by functional rather than anatomical outcomes, including active inflammation, vascular overtone, and tissue ischemia. The uptake of 18F-fluorodeoxyglucose in the left anterior descending coronary artery, measured with positron emission tomography as a marker of plaque inflammation and vulnerability, was correlated with CAD, calcified plaque burden, and pericardial fat volume (54). The assessment of coronary flow to identify ischemic regions in women complaining of chest pain (55) demonstrated that epicardial fat thickness was the only independent inverse predictor of coronary flow reserve, as opposed to traditional risk factors for atherosclerosis. Our findings extend this observation to patients of both sexes with and without CAD showing that only those with a severe impairment in coronary vasodilatation have a significant increase in intrapericardial fat.
Mechanisms
Epicardial and perivascular fat depots are important mechanical guides of contracting organs and vessels, and critical regulators of substrate fluxes to subtending organs because they store or release fatty acids with great flexibility to fulfill the energy needs of arterial walls and heart muscle and to avoid lipotoxicity. Their protective vasocrine function is likely mediated by adiponectin and unidentified relaxing factors. In metabolic and cardiovascular disease states, these fat tissues expand, becoming hypoxic and dysfunctional (25,56) and recruiting phagocytic cells (57). The changes in adipocyte size and increase in the infiltration of macrophages and T cells (57) reduce the production of protective in favor of detrimental adipocytokines such as leptin, resistin, IL-6, tumor necrosis factor-α, or IL-17. These molecules can reach the myocardial tissue and vessel walls by direct diffusion or by traveling in adventitial neovascularization, and it has been recently shown that epicardial adipose tissue can partially contribute to adiponectin levels in the coronary circulation (3). Thus, inflammation may propagate to the underlying arterial walls, and alter the balance between vascular nitric oxide, endothelin-1, and superoxide production, promoting vasoconstriction (26). Samples of pericardial fat from CAD patients showed increased mRNA and protein levels of chemokine and inflammatory cytokines relative to subcutaneous fat (58), and lower expression of adiponectin relative to that in patients without CAD. Perivascular adipose tissue can stimulate smooth muscle cell proliferation via release of hydrosoluble protein growth factor(s) and contribute to the progression of atherosclerosis (59). Large and inflamed adipocytes display insulin resistance and greater release of fatty acids, likely overflowing toward the myocardium, thereby increasing cardiac work and oxygen consumption and alimenting cardiac steatosis. In fact, pericardial and myocardial adiposities are strongly correlated (8). In response to the reduced vascular density and hypoxia in obesity, macrophages may express platelet-derived growth factor in adipose tissue to facilitate capillary formation (56). This process and mediators may extend to the vessel wall of arteries adjacent to the adipose depot. Plaque neoangiogenesis is associated closely with plaque progression and intraplaque hemorrhage, and is predominantly thought to arise from the adventitia vasa vasorum (60). This complex series of events is summarized in Fig. 1B.
CONCLUSIONS
Cardiac adiposity was characterized in the 19th century, including the distinction between surface and intracellular fat, its association with obesity and coronary obstruction, and its dual protective or hazardous roles. Subsequently, cardiac damage was thought to be caused by inflammation; more recently this was supplanted by the idea that coronary obstruction is the central pathogenetic mechanism of cardiovascular disease. Current advancements in technology and knowledge indicate that adiposity, inflammation, and arterial obstruction are simultaneously operative in modulating tissue ischemia and plaque vulnerability. In this interaction, adipose tissue and intracellular triglycerides may shift from being protective to being detrimental, depending on their residual substrate buffering capacity and inflammatory status. The magnitude of adiposity appears as a relative index in these complex dynamics, but its determination alone may be insufficient to predict its functional impact on the vulnerability of adjacent tissues. The complementary use of molecular/functional imaging to depict substrate oxidation, active inflammation, and organ perfusion can aid in this assessment.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
This publication is based on the presentations at the 3rd World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, F. Hoffmann-La Roche, Janssen-Cilag, Johnson & Johnson, Novo Nordisk, Medtronic, and Pfizer.
References
- 1.Szczepaniak LS, Dobbins RL, Metzger GJ, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med 2003;49:417–423 [DOI] [PubMed] [Google Scholar]
- 2.Taguchi R, Takasu J, Itani Y, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis 2001;157:203–209 [DOI] [PubMed] [Google Scholar]
- 3.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr 2009;22:1311–1319; quiz 1417–1418 [DOI] [PubMed] [Google Scholar]
- 4.Gorter PM, van Lindert AS, de Vos AM, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis 2008;197:896–903 [DOI] [PubMed] [Google Scholar]
- 5.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 2007;153:907–917 [DOI] [PubMed] [Google Scholar]
- 6.Lamb HJ, Smit JW, van der Meer RW, et al. Metabolic MRI of myocardial and hepatic triglyceride content in response to nutritional interventions. Curr Opin Clin Nutr Metab Care 2008;11:573–579 [DOI] [PubMed] [Google Scholar]
- 7.van der Meer RW, Rijzewijk LJ, Diamant M, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J 2008;29:1516–1522 [DOI] [PubMed] [Google Scholar]
- 8.Iozzo P, Lautamaki R, Borra R, et al. Contribution of glucose tolerance and sex to cardiac adiposity. J Clin Endocrinol Metab 2009;94:4472–4482 [DOI] [PubMed] [Google Scholar]
- 9.Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008;52:1793–1799 [DOI] [PubMed] [Google Scholar]
- 10.Hammer S, van der Meer RW, Lamb HJ, et al. Short-term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2008;295:E714–E718 [DOI] [PubMed] [Google Scholar]
- 11.Hammer S, Snel M, Lamb HJ, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 2008;52:1006–1012 [DOI] [PubMed] [Google Scholar]
- 12.Viljanen AP, Karmi A, Borra R, et al. Effect of caloric restriction on myocardial fatty acid uptake, left ventricular mass, and cardiac work in obese adults. Am J Cardiol 2009;103:1721–1726 [DOI] [PubMed] [Google Scholar]
- 13.Zib I, Jacob AN, Lingvay I, et al. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med 2007;55:230–236 [DOI] [PubMed] [Google Scholar]
- 14.van der Meer RW, Rijzewijk LJ, de Jong HW, et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation 2009;119:2069–2077 [DOI] [PubMed] [Google Scholar]
- 15.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007;116:1170–1175 [DOI] [PubMed] [Google Scholar]
- 16.Ritchie RH. Evidence for a causal role of oxidative stress in the myocardial complications of insulin resistance. Heart Lung Circ 2009;18:11–18 [DOI] [PubMed] [Google Scholar]
- 17.Chiu HC, Kovacs A, Ford DA, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 2001;107:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Shi X, Bharadwaj KG, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem 2009;284:36312–36323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 2003;100:3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walecki J, Michalak MJ, Michalak E, Bilinska ZT, Ruzyllo W. Usefulness of 1H MR spectroscopy in the evaluation of myocardial metabolism in patients with dilated idiopathic cardiomyopathy: pilot study. Acad Radiol 2003;10:1187–1192 [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 2004;18:1692–1700 [DOI] [PubMed] [Google Scholar]
- 22.Natale F, Tedesco MA, Mocerino R, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr 2009;10:549–555 [DOI] [PubMed] [Google Scholar]
- 23.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 2010;210:150–154 [DOI] [PubMed] [Google Scholar]
- 24.Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B 1989;94:225–232 [DOI] [PubMed]
- 25.Greenstein AS, Khavandi K, Withers SB, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009;119:1661–1670 [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Zhang C. Regulation of microvascular function by adipose tissue in obesity and type 2 diabetes: evidence of an adipose-vascular loop. Am J Biomed Sci 2009;1:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueno K, Anzai T, Jinzaki M, et al. Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J 2009;73:1927–1933 [DOI] [PubMed] [Google Scholar]
- 29.Mazur A, Ostański M, Telega G, Malecka-Tendera E. Is epicardial fat tissue a marker of metabolic syndrome in obese children? Atherosclerosis 2010;211:596–600 [DOI] [PubMed] [Google Scholar]
- 30.Ahn SG, Lim HS, Joe DY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart 2008;94:e7. [DOI] [PubMed] [Google Scholar]
- 31.Tadros TM, Massaro JM, Rosito GA, et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring) 2010;18:1039–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008;117:605–613 [DOI] [PubMed] [Google Scholar]
- 33.Wang TD, Lee WJ, Shih FY, et al. Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J Clin Endocrinol Metab 2009;94:662–669 [DOI] [PubMed] [Google Scholar]
- 34.Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol 2009;106:5–11 [DOI] [PubMed] [Google Scholar]
- 35.Jonker JT, Lamb HJ, van der Meer RW, et al. Pioglitazone compared with metformin increases pericardial fat volume in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010;95:456–460 [DOI] [PubMed] [Google Scholar]
- 36.Fox CS, Gona P, Hoffmann U, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation 2009;119:1586–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinkley TE, Hsu FC, Carr JJ, Hundley WG, Bluemke DA, Polak JF, Ding J. Pericardial fat is associated with carotid stiffness in the Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 11 February 2010. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 38.Soliman EZ, Ding J, Hsu FC, Carr JJ, Polak JF, Goff DC., Jr Association between carotid intima-media thickness and pericardial fat in the Multi-Ethnic Study of Atherosclerosis (MESA). J Stroke Cerebrovasc Dis 2010;19:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorter PM, Visseren FL, Moll FL, van der Graaf Y; SMART Study Group Intra-abdominal fat and metabolic syndrome are associated with larger infrarenal aortic diameters in patients with clinically evident arterial disease. J Vasc Surg 2008;48:114–120 [DOI] [PubMed] [Google Scholar]
- 40.Chaowalit N, Somers VK, Pellikka PA, Rihal CS, Lopez-Jimenez F. Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis 2006;186:354–359 [DOI] [PubMed] [Google Scholar]
- 41.Eroglu S, Sade LE, Yildirir A, et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis 2009;19:211–217 [DOI] [PubMed] [Google Scholar]
- 42.Jeong JW, Jeong MH, Yun KH, et al. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J 2007;71:536–539 [DOI] [PubMed] [Google Scholar]
- 43.Greif M, Becker A, von Ziegler F, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:781–786 [DOI] [PubMed] [Google Scholar]
- 44.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 2009;30:850–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol 2008;102:1602–1607 [DOI] [PubMed] [Google Scholar]
- 46.Konishi M, Sugiyama S, Sugamura K, et al. Association of pericardial fat accumulation rather than abdominal obesity with coronary atherosclerotic plaque formation in patients with suspected coronary artery disease. Atherosclerosis 2010;209:573–578 [DOI] [PubMed] [Google Scholar]
- 47.Gorter PM, de Vos AM, van der Graaf Y, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol 2008;102:380–385 [DOI] [PubMed] [Google Scholar]
- 48.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2009;90:499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding J, Kritchevsky SB, Harris TB, et al. ; Multi-Ethnic Study of Atherosclerosis The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarin S, Wenger C, Marwaha A, et al. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol 2008;102:767–771 [DOI] [PubMed] [Google Scholar]
- 51.Lehman SJ, Massaro JM, Schlett CL, O’Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis 2010;210:656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Vos AM, Prokop M, Roos CJ, et al. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J 2008;29:777–783 [DOI] [PubMed] [Google Scholar]
- 53.Mahabadi AA, Reinsch N, Lehmann N, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis 2010;211:195–199 [DOI] [PubMed] [Google Scholar]
- 54.Saam T, Rominger A, Wolpers S, et al. Association of inflammation of the left anterior descending coronary artery with cardiovascular risk factors, plaque burden and pericardial fat volume: a PET/CT study. Eur J Nucl Med Mol Imaging 2010;37:1203–1212 [DOI] [PubMed] [Google Scholar]
- 55.Sade LE, Eroglu S, Bozbaş H, et al. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis 2009;204:580–585 [DOI] [PubMed] [Google Scholar]
- 56.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab 2008;295:E313–E322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol 2005;25:2594–2599 [DOI] [PubMed] [Google Scholar]
- 58.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460–2466 [DOI] [PubMed] [Google Scholar]
- 59.Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol 2005;289:H1807–H1813 [DOI] [PubMed] [Google Scholar]
- 60.Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054–2061 [DOI] [PubMed] [Google Scholar]


