Abstract
The WTX gene is frequently lost or mutated in Wilms’ tumor. In this issue of Molecular Cell, Kim et al., identifies WTX modulation of the p53 tumor suppressor activity through regulating p53 acetylation. Therefore, WTX differentially regulates the oncogenic β-catenin pathway and the tumor suppressing p53 pathway.
Wilms’ tumor is an embryonic kidney tumor that affects 1 in 10,000 of live births and represents the most common solid organ tumor diagnosed in children (Huff 2011). Genetic analysis of patients and their tumors reveal several important gene alterations in Wilms’ tumor (Huff 2011), including deletion or loss of function mutations of the Wilms tumor 1 (WT1) gene that encodes a tumor suppressing transcription factor, activating mutations of the β-catenin (CTNNB1) oncogene which is a key effector of the WNT signaling pathway, epigenetic upregulation of insulin-like growth factor 2 (IGF2), and inactivation of a newly discovered Wilms tumor gene on the X chromosome (WTX)(Rivera et al., 2007). Unlike autosomal tumor suppressors, WTX is inactivated by a “single-hit” that targets the X chromosome in males or the active X chromosome in females.
Somatic inactivation of WTX is found in up to 1/3 of sporadic Wilms’ tumor (Rivera et al., 2007), while germline mutation of WTX is linked to the OSCS syndrome characterized by sclerozing bone dysplasia and developmental abnormalities in females and embryonic lethality in males (Jenkins et al. 2009). Several studies have reported distinct functions of WTX in different cellular compartments. In the cytoplasm, WTX downregulates the Wnt signaling pathway through promoting ubiquitination and degradation of β-catenin (Major et al., 2007); at the plasma membrane, WTX stimulates phosphorylation of the LRP6 Wnt receptor (Tanneberger et al., 2011); in the nucleus WTX modulates nuclear WT1 transcription activation (Huff 2011). In this issue of Molecular Cell, Kim et al. (2012) reports WTX modulation of p53 activity through enhancing CBP/p300-mediated p53 acetylation, linking WTX to the classic p53 tumor suppressor pathway and sheds light on a role for WTX in tumorigenesis through regulating p53 coactivators.
The authors made use of an inducible human embryonic kidney cell (HEK) 293 line, in which the expression of full length WTX is regulated by doxycycline concentration. HEK293 cells tolerate high levels of endogenous p53 owing to inactivation by the adenoviral E1B 55K oncoprotein in part through sequestration in the characteristic cytoplasmic E1B body. 9 out of 13 upregulated pathways identified by the authors using microarray expression profiling following induction of WTX were functionally related to p53, suggesting a strong correlation between WTX and p53 activation. Further, the unexpected observation that ectopic expression of WTX in HEK293 disrupts the cytoplasmic E1B body and releases p53 for accumulation in the nucleus led to proposition of WTX-p53 interaction. Indeed, using a co-transfection system, the authors demonstrated distinct pairwise protein interactions involving WTX-p53 and WTX-E1B 55K. Using a series of deletion mutants to map the interaction domains, the authors discovered that WTX and p53 compete for E1B 55K binding.
Having established the interaction between WTX and p53, the authors then focused on elucidating the functional consequences of WTX expression or depletion on p53 activation. To this end, the authors measured several well studied post-translational modifications that positively regulate p53 transcription activity. Ectopic expression of WTX enhances p53 acetylation at Lys373/382 and depletion of WTX strikingly reduces p53 acetylation at Lys373/382 both at the basal level and following DNA damage. These observations were consistent in HEK293 and non-adenovirus-transformed cells, confirming a role for WTX in positively regulating p53 acetylation independently of the E1B 55K oncoprotein.
In an effort to dissect the molecular mechanisms underlying the upregulation of p53 acetylation by WTX, the authors investigated whether WTX might modulate acetyl transferases (CBP/p300) and deacetylases (HDAC1, HDAC2 and Sirt1) that target p53 at Lys373/382. While the steady state levels of the deacetylases were not affected by depletion of WTX, reduction of CBP and less consistently p300 protein expression is quite prominent. The reduced protein level of CBP/p300 following WTX inactivation is a result of accelerated protein turnover as demonstrated by half-life measurement. Inactivation of the respective E3 ubiquitin ligases for CBP and p300 abolish the effect of WTX depletion on CBP/p300 stability. The authors further showed that WTX is capable of binding CBP and enhancing CBP-p53 interaction. These data supports that WTX enhances p53 acetylation through stabilizing the CBP/p300 acetyl transferases.
Finally the authors investigated the physiological relevance of WTX upregulation of p53 acetylation by looking at typical p53 responses. Using flow cytometry analysis, the authors show that inactivation of WTX significantly suppresses the p53-mediated apoptotic response and moderately reduces growth arrest.
The study by Kim and coworkers broadens our understanding of the functional properties of WTX, and raises several interesting points. The authors provide evidence of WTX regulation of the p53 transcription activity. Thus on one hand WTX negatively regulates the oncogenic Wnt/β-catenin signaling pathway, on the other hand WTX positively regulates the classic p53 tumor suppressor pathway (Fig. 1). Notably activating mutations of the β-catenin gene and inactivation of the TP53 gene occur in Wilms’ tumor at a frequency of 15% and 5% respectively (Huff 2011). However these mutations rarely co-occur with loss of WTX, suggesting a functional redundancy in which WTX inactivation has a similar effect to directly upregulating β-catenin activity or downregulating p53 activity.
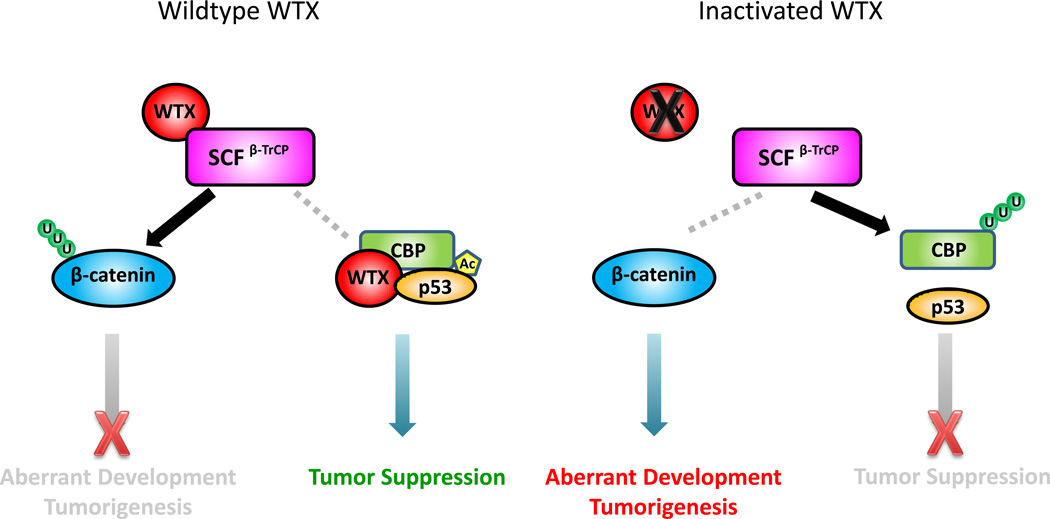
Figure 1. Schematic presentation of WTX differential regulation of the β-catenin pathway and the p53 pathway.
WTX interacts with the SCFβ-TrCP E3 ubiquitin ligase complex. This interaction promotes β-catenin degradation in the cytoplasm, therefore antagonizes β-catenin accumulation and subsequent coactivation of oncogenic target genes. On the other hand, WTX stabilizes CBP and enhances p53 acetylation, allowing transcription activation of tumor-suppressing target genes that are involved in various responses to cellular stress. Genetic inactivation of WTX leads to constitutive activation of the β-catenin oncogene, eventually resulting in abberent development and tumorigenesis. Loss of WTX accelerates CBP turnover, therefore reducing p53 acetylation and transcription activity.
The compromised p53 activity due to reduced acetylation in the context of WTX inactivation is consistent with previous studies that demonstrate acetylation is indispensable for p53 activation (Tang et al., 2008). The collective mutation of p53 acetylation sites completely abolishes p53-mediated cell cycle arrest and apoptosis. Moreoever, CBP/p300 are recently identified as haploinsufficient tumor suppressors because monoallelic inactivation leads to lymphomagenesis (Pasqualucci et al., 2011). Therefore it will be interesting to test the CBP/p300 status in Wilms’ tumor, particularly in those with wildtype p53. One may speculate that CBP/p300 mutation, inactivation of WTX, and loss of p53 are mutually exclusive.
Interestingly, β-catenin and CBP, whose stability is differentially regulated by WTX, share the same β-TrCP containing E3 ubiquitin ligase complex (Major et al., 2007, Kim et al., 2012). How might WTX interact with an E3 ubiquitin ligase adaptor yet achieve opposite regulatory effects on its substrates? The molecular mechanisms that govern these differential effects await elucidation. Furthermore, domain mapping reveals that the C-terminal domain of WTX protein is indispensable for WTX- β-catenin and WTX-p53 interaction, which might explain why WTX inactivation in Wilms’ tumor is most commonly achieved through whole gene deletion or N-terminal nonsense point mutations.
The high incidence of WTX inactivation in Wilms’ tumor predicts that WTX is a tumor suppressor gene. However, OSCS patients with germline inactivation of WTX are not tumor-prone (Jenkins 2009); Wtx deletion in mice leads to defective mesenchymal differentiation without predisposition to tumor development (Moison et al., 2011). These support a critical yet complex role of WTX in development, but how this turn links to tumor suppression awaits further revelation. Additional confounding factors may be required for WTX-mediated tumor suppression. Such factors may include presence of other gene alterations, the differentiation status of the cell, and the microenvironment in cells harboring WTX mutations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Huff V. Nat. Rev. Cancer. 2011;11:111–121. doi: 10.1038/nrc3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins ZA, van Kogelenberg M, Morgan T, Jeffs A, Fukuzawa R, Pearl E, Thaller C, Hing AV, Porteous ME, Garcia-Minaur S, et al. Nat. Genet. 2009;41:95–100. doi: 10.1038/ng.270. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Rivera MN, Coffman EJ, Haber DA. Mol. Cell. 2012 doi: 10.1016/j.molcel.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse JP, Gu W. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major MB, Camp ND, Berndt JD, Yi XH, Goldenberg SJ, Hubbert C, Biechele T, Gingras AC, Zheng N, MacCoss MJ, Angers S, Moon RT. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- Moisan A, Rivera MN, Lotinun S, Akhavanfard S, Coffman EJ, Cook EB, Stoykova S, Mukherjee S, Schoonmaker JA, Burger A, et al. Dev. Cell. 2011;20:583–596. doi: 10.1016/j.devcel.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, Kasper LH, Lerach S, Tang HY, Ma J, et al. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera MN, Kim WJ, Wells J, Driscoll DR, Brannigan BW, Han M, Kim JC, Feinberg AP, Gerald WL, Vargas SO, et al. Science. 2007;315:642–645. doi: 10.1126/science.1137509. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Cell. 2008;133:612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanneberger K, Pfister AS, Brauburger K, Schneikert J, Hadjihannas MV, Kriz V, Schulte G, Bryja V, Behrens J. EMBO J. 2011;30:1433–1443. doi: 10.1038/emboj.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]