Abstract
Successful fertilization heralds the onset of development and requires both gamete recognition and a definitive block to polyspermy. Sperm initially bind and penetrate the extracellular zona pellucida (ZP) that surrounds ovulated eggs, but are unable to bind the zona surrounding preimplantation embryos. The ZP of humans is composed of four (ZP1–4) and that of mouse three (ZP1–3) glycoproteins. Models for gamete recognition developed in mice had proposed that sperm bind to ZP3 glycans. However, phenotypes observed in genetically engineered mice are not consistent with this widely accepted model. More recently, taking advantage of the observation that human sperm do not bind to mouse eggs, human ZP2 was defined as the zona ligand in transgenic mouse models using gain-of-function assays. The sperm-binding site is an N-terminal domain of ZP2 that is cleaved by ovastacin, a metalloendoprotease released from egg cortical granules following fertilization. Proteolysis of this docking site provides a definitive block to polyspermy as sperm bind to uncleaved, but not cleaved ZP2 even after fertilization and cortical granule exocytosis. While progress has been made in defining the ZP ligand, less headway has been made in identifying the cognate sperm receptor. Although a number of sperm receptor candidates have been documented to interact with specific proteins in the ZP in vitro, continued fertility after genetic ablation of the cognate gene indicates that none are essential for gamete recognition. These on-going investigations inform reproductive medicine and suggest new therapies to improve fertility and/or provide contraception, thus expanding reproductive choices for human couples.
Keywords: gamete recognition, zona pellucida, ZP3 glycan-release models, ZP2 cleavage model, sperm surface receptor
Introduction
Human infertility, defined as the inability to conceive after 6–12 months of unprotected sexual intercourse, affects ∼14% of couples (Boivin et al., 2007) and is recognized by the World Health Organization as a major public health issue (http://www.who.int/reproductivehealth/publications/infertility/progress63.pdf). In one-third of patients, the cause of infertility is unknown. While some struggle to have children, others seek better control over their reproductive choices to prevent unwanted pregnancies. The world population is growing at unprecedented rates and the 6.9 billion people present in 2010 are likely to increase to 9.6 billion over the next 50 years (http://esa.un.org/wpp/Excel-Data/population.htm). Initiated by seminal descriptions of human IVF (Steptoe and Edwards, 1978) and ICSI (Palermo et al., 1992) significant advances continue to be reported in reproductive biology and medicine. However, there remains a compelling need to better understand the molecular basis of fertilization to develop more comprehensive therapies for infertility and improved means of family planning.
At coitus, tens of millions of sperm are deposited in the lower female reproductive tract, but relatively few progress to the ampulla of the oviduct and encounter ovulated eggs. During this transit, functionally mature sperm become capacitated and gain the ability to bind to the extracellular zona pellucida (ZP), undergo acrosome exocytosis and fertilize eggs (Bailey, 2010; Buffone et al., 2012). The ovulated egg is encased in a cumulus oophorus composed of a hyaluronan interspersed with cumulus cells from ovarian follicles. Fertilization reflects a cascade of events that include (1) relative taxon-specific gamete recognition; (2) sperm penetration of the egg coat followed by gamete fusion and (3) a post-fertilization block to polyspermy. The ZP not only plays pivotal roles in these events, but also serves to protect the early embryo as it passes through the oviduct prior to implantation in the uterus (Yanagimachi, 1994).
Over the last several decades, investigators seeking to understand the role of the ZP in gamete recognition have proposed models based on biochemistry, cell biology and, more recently, mouse genetics. Depending on the vertebrate model, each component of the zona matrix has been proposed as a ligand for sperm binding (Bleil and Wassarman, 1980a; Tian et al., 1997; Ganguly et al., 2010; Yonezawa et al., 2012) which have been summarized in a number of recent reviews (Hedrick, 2008; Shur, 2008; Ikawa et al., 2010; Monne and Jovine, 2011; Gupta et al., 2012). While acknowledging that it is likely that multiple proteins play supporting roles, we focus on those that are essential for fertilization. Although continued fertility of null mutants indicates that the cognate protein is not essential for gamete recognition, the reverse (infertility after genetic ablation) may not provide insight into gamete recognition if the absence of the protein disrupts other requirements for fertilization (e.g. sperm mobility, formation of a zona matrix and mating behaviour). We seek to review current models to discuss their strengths and weaknesses with the hope of galvanizing research to ensure a better molecular understanding of gamete recognition at the ZP.
Composition and structure of mouse and human zonae pellucidae
Four genetic loci encode mouse and human ZP genes. However, the mouse ZP (∼7 μm thick) that surrounds ovulated eggs (∼80 μm diameter) is composed of only three glycoproteins, ZP1 (526 aa, 120 kD), ZP2 (599 aa, 120 kD) and ZP3 (329 aa, 83 kD) (Bleil and Wassarman, 1980b; Shimizu et al., 1983) because Zp4 is a pseudogene and does not express the cognate protein (Lefievre et al., 2004). A thicker (∼16 μm) human ZP surrounds the larger human egg (120 μm diameter) and is composed of four secreted glycoproteins, ZP1 (528 aa, 100 kD), ZP2 (602 aa, 75 kD), ZP3 (328 aa, 55 kD) and ZP4 (444 aa, 65 kD) (Shabanowitz and O'Rand, 1988; Lefievre et al., 2004). In mice, each zona protein has a signal peptide to direct it into the secretory system with a transmembrane domain near the carboxyl terminus that tethers it to the endomembrane and similar motifs are present in the homologous human zona proteins (Ringuette et al., 1988; Chamberlin and Dean, 1990; Liang et al., 1990; Liang and Dean, 1993; Harris et al., 1994; Epifano et al., 1995; Boja et al., 2003; Lefievre et al., 2004). Within the endomembrane system, individual mouse zona proteins are glycosylated, mostly with N-glycans with a smaller mass of O-glycans (Tulsiani et al., 1992; Nagdas et al., 1994; Easton et al., 2000; Boja et al., 2003). After trafficking as individuals through the oocyte (Hoodbhoy et al., 2006; Jimenez-Movilla and Dean, 2010), each zona protein is cleaved upstream of a di-basic motif (Boja et al., 2003) and is released from the oocyte's plasma membrane to form the insoluble ZP (Jovine et al., 2002, 2004; Jimenez-Movilla and Dean, 2010).
The three secreted mouse proteins interact non-covalently to form the three-dimensional zona matrix. Each protein has a ZP domain of ∼260 aa that contains eight conserved cysteine residues (Bork and Sander, 1992) and is divided into N-terminal ‘ZP-N’ and C-terminal ‘ZP-C’ subdomains. The zona domain, along with the cytoplasmic tails, control final assembly of the zona proteins into the extracellular matrix (Jovine et al., 2004; Han et al., 2010; Jimenez-Movilla and Dean, 2010). Upstream of the zona domain, a trefoil domain (http://pfam.sanger.ac.uk/family/trefoil) has been identified in ZP1 (Sommer et al., 1999) and ZP4 (Bork, 1993), but its function remains unknown. Intramolecular disulfide linkages stabilize the native conformation of the secreted zona glycoproteins, but only ZP1 has inter-molecular disulfide bonds that form homodimers within the zona matrix (Bleil and Wassarman, 1980b; Epifano et al., 1995). Although the zona proteins have been well defined, including the three-dimensional structure of chicken ZP3 (Han et al., 2010), there remains considerable controversy over the nature of the zona ligand and the cognate sperm surface receptor necessary for gamete recognition.
Following fertilization, there is an effective block to polyspermy. First, within minutes, the egg plasma membrane is modified so that no additional sperm fuse with the egg. This block is not associated with changes in electrical potential observed in the other species, but its molecular basis remains unknown (Jaffe et al., 1983; Horvath et al., 1993). Also within minutes, additional sperm do not penetrate the ZP (Sato, 1979; Stewart-Savage and Bavister, 1988), but whether this reflects changes in the zona matrix or in sperm remains to be determined. Over several hours, the zona is further modified to preclude sperm binding. Although other changes have been inferred, the only documented biochemical modification of the ZP is the proteolytic cleavage of ZP2 after which the two fragments remain disulfide bonded (Bleil et al., 1981). This cleavage provides a definitive block to polyspermy by preventing sperm binding; if sperm do not bind, they cannot penetrate the zona matrix or fuse with the egg plasma membrane. However, prevention of post-fertilization ZP2 cleavage does not preclude live births, although decreased fecundity is observed (Gahlay et al., 2010; Burkart et al., 2012; Sachdev et al., 2012). Thus, the early blocks to polyspermy appear particularly important to ensure monospermic fertilization and the successful onset of development.
The puzzling role of ZP glycan ligands in gamete recognition
Using soluble, SDS-PAGE purified and re-natured zona proteins in an in vitro competitive sperm-binding assay with ovulated eggs, ZP3 initially was reported as the zona ligand for mouse sperm binding. No inhibitory effect of sperm binding to ovulated eggs was observed with re-natured ZP1 or ZP2, or with ZP3 isolated from the zona surrounding 2-cell embryos (Bleil and Wassarman, 1980a). These observations introduced the widely accepted glycan release model for gamete recognition which, with time, became increasingly precise to implicate O-glycans attached to Ser332 and Ser334 of ZP3 as zona ligands for sperm binding (Fig. 1). Following fertilization, these glycan ligands would be released by a putative glycosidase exocytosed from egg cortical granules to prevent sperm binding to 2-cell embryos (Florman and Wassarman, 1985; Chen et al., 1998). The nature of the carbohydrate ligand has remained controversial with α1,3 galactose and N-acetylglucosamine being proposed in mouse (Bleil and Wassarman, 1988; Miller et al., 1992) and the sialyl-LewisX antigen in humans (Pang et al., 2011). However, mice lacking either the proposed zona ligand (α1,3 galactose) or the putative sperm receptor for N-acetylglucosamine are fertile (Thall et al., 1995; Asano et al., 1997; Lu and Shur, 1997). Furthermore, not only are Ser332 and Ser334 unadorned with carbohydrate in native mouse ZP (Boja et al., 2003), but mutating the sites to prevent glycosylation does not adversely affect fertility in transgenic mice (Liu et al., 1995), even in the absence of endogenous normal ZP3 (Gahlay et al., 2010).
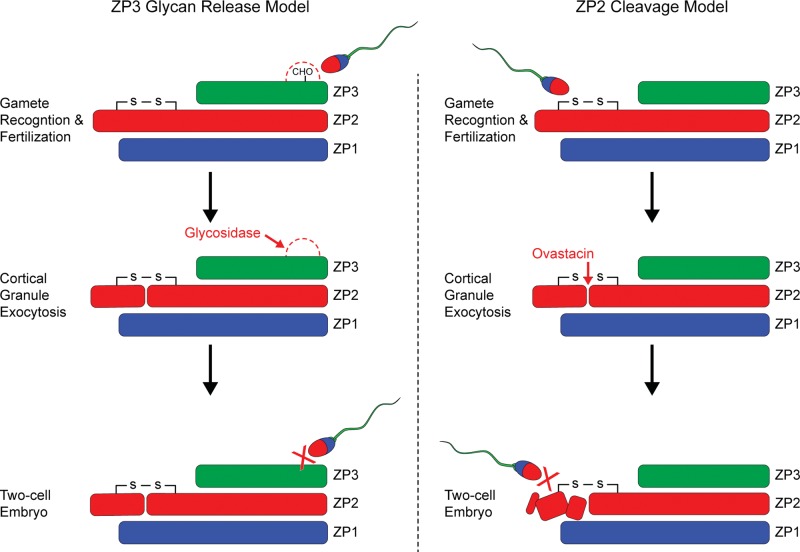
Figure 1.
Models of gamete recognition. A specific glycan-release model (left panel) proposes that O-glycans on ZP3 Ser332 and Ser334 act as ligands for a sperm surface receptor. Following fertilization, cortical granules would release a glycosidase that would remove the O-glycans and account for the inability of sperm to bind to 2-cell embryos. The ZP2 cleavage model (right panel) proposes that sperm bind to an N-terminal domain of ZP2. Following fertilization, ovastacin (encoded by Astl), a cortical granule metalloendoprotease is exocytosed and the proteolytic destruction of the ZP2 docking site prevents sperm from binding to 2-cell embryos. Each model has been tested using mouse transgenesis. Mouse zonae pellucidae containing human rather than mouse ZP3 do not support human sperm binding and Zp3S332A;S334A mutant mice have normal fertility. These results are not consistent with the ZP3 glycan-release model as currently articulated. In contrast, mouse zonae pellucidae containing human rather than mouse ZP2 support human sperm binding/penetration and Zp2Mut and AstlNull mice in which ZP2 remains uncleaved in 2-cell embryos support de novo sperm binding despite fertilization and cortical granule exocytosis. These results support the ZP2 cleavage model of gamete recognition (Gahlay et al., 2010; Baibakov et al., 2012; Burkart et al., 2012).
An additional complexity for glycan-release models is the heterogeneity of zona glycans, which implies a need for a range of different sperm surface receptors or that only subsets of zona ligands are biologically active. The appeal of glycan-release models is also diminished with continued fertility of mice lacking specific glycosyl transferases which restricts the pool of possible glycan ligands (Asano et al., 1997; Lu and Shur, 1997; Lowe and Marth, 2003; Shi et al., 2004; Williams et al., 2007). More recently, it has been reported that if ZP2 remains intact, sperm bind to the ZP independent of fertilization and cortical granule exocytosis (Rankin et al., 2003; Baibakov et al., 2007; Gahlay et al., 2010; Burkart et al., 2012). This observation is not consistent with the post-fertilization exocytosis of a cortical granule glycosidase that would prevent sperm binding by release of a ligand, an axiom integral to the glycan-release model. For sperm to bind to the ZP after fertilization and cortical granule exocytosis, the candidate glycan would have to remain accessible for sperm binding and yet have been inaccessible for cleavage by a cortical granule glycosidase.
The cause of the discrepancy between the initial biochemical- and more recent genetic-based assays in the assessment of the ZP3 glycan-release models remains unclear. It may be that soluble zona proteins isolated by SDS-PAGE and then re-natured do not provide a realistic proxy for the same protein in the insoluble ZP. Of note, these assays never achieved 100% inhibition of sperm binding and only one sperm is necessary for fertilization. Alternatively, it has been reported that solubilized ZP3 triggers the acrosome reaction and acrosome-reacted sperm do not bind to the ZP (Saling et al., 1979). However, this possibility was considered less likely (Bleil and Wassarman, 1980a, 1983) and would not explain the observation that ZP3 glycopeptides inhibit sperm binding (Florman and Wassarman, 1985), but do not induce the acrosome reaction (Florman et al., 1984; Leyton and Saling, 1989). Because of these aforementioned experimental results and caveats, other models of sperm–egg recognition have been sought.
The ZP2 cleavage model of gamete recognition
Mouse genetics has been used to systematically test the role of individual zona proteins in gamete recognition. Each single-copy mouse gene has been successfully targeted in embryonic stem cells which were then used to establish mouse lines lacking ZP1, ZP2 or ZP3. In the absence of ZP1, mice form a more loosely woven zona matrix, but remain fertile albeit with decreased fecundity (Rankin et al., 1999). A more dramatic phenotype is observed in the absence of either ZP2 or ZP3 (Liu et al., 1996; Rankin et al., 1996, 2001). In both instances, there is no ZP surrounding ovulated eggs which are quickly resorbed into the epithelial lining of the oviduct, a phenomenon previously reported following biochemical removal of the zona matrix (Bronson and McLaren, 1970; Modlinski, 1970). Both Zp3Null and Zp3Null mouse lines are uniformly sterile. Thus, ZP1 is not essential for gamete recognition and fertility, but the absence of a ZP matrix in the null mice precludes the assessment of either ZP2 or ZP3 in gamete recognition.
Eutherian mammals have syntenic loci encoding single copies of ZP1, ZP2, ZP3 and ZP4 (Spargo and Hope, 2003; Boja et al., 2005; Goudet et al., 2008), although no mouse ZP4 protein is present because of multiple stop and missense codons (Lefievre et al., 2004). The primary structure of secreted mouse and human ZP1 (71%), ZP2 (62%) and ZP3 (71%) are well conserved, but gamete recognition is relatively taxon-specific and human sperm will not bind to the mouse ZP (Bedford, 1977) nor fuse with mouse eggs (Quinn, 1979; Yanagimachi, 1984). Therefore, to investigate further the roles of ZP2 and ZP3, transgenic mouse lines in which human ZP1, ZP2 and ZP3 replaced the endogenous mouse proteins were established and human sperm were used to interrogate the human–mouse chimeric zonae pellucidae. Human sperm bound to the ZP in the presence of human ZP2, but not in the presence of human ZP1, ZP3 or ZP4 (Yauger et al., 2011; Baibakov et al., 2012). This was a dominant genetic effect and required that human sperm be capacitated to bind to the surface of the ZP (Fig. 2). In mice with a ZP formed by four human proteins and lacking the three endogenous mouse proteins, human sperm bound and penetrated the zona matrix. Acrosome-reacted human sperm accumulated in the perivitelline space unable to fuse or fertilize mouse eggs (Baibakov et al., 2012). Taken together, it appears that ZP2 rather than ZP3 is the zona ligand to which human sperm bind.
Figure 2.
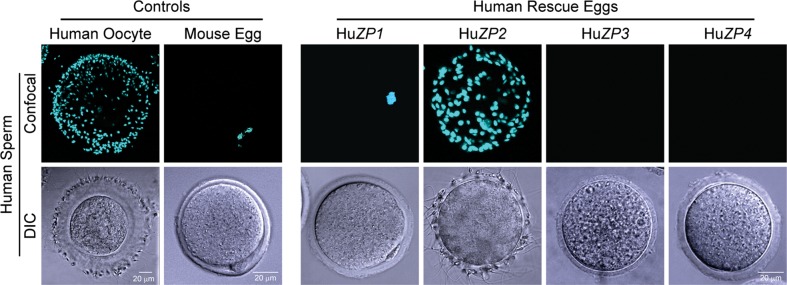
Human sperm bind to transgenic mice expressing human ZP2 in the ZP. Confocal and differential interference contrast images of capacitated human sperm binding to the ZP of mice expressing human ZP1, human ZP2, human ZP3 or human ZP4, in the absence of the corresponding mouse protein. Human oocytes and mouse eggs serve as positive and negative controls, respectively. Modified from ref. Baibakov et al. (2012).
Following fertilization, ZP2 is cleaved and sperm do not bind to 2-cell mouse embryos (Bleil et al., 1981). The ZP2 cleavage site was defined biochemically (166LA↓DE169) as that recognized by the astacin family of metalloendoproteases (Gahlay et al., 2010). Ovastacin, an oocyte-specific astacin (Quesada et al., 2004) encoded by Astl, is released from cortical granules and is responsible for the post-fertilization cleavage of ZP2 (Burkart et al., 2012). Mutation of the cleavage site or ablation of the gene encoding ovastacin leaves ZP2 intact following fertilization and supports sperm binding to the ZP despite cortical granule exocytosis (Gahlay et al., 2010; Burkart et al., 2012) (Fig. 3). Using truncated recombinant ZP2 peptides in a bead-binding assay, the sperm-binding site was further refined to ∼115 aa at the N-terminus of ZP2 (Baibakov et al., 2012).
Figure 3.
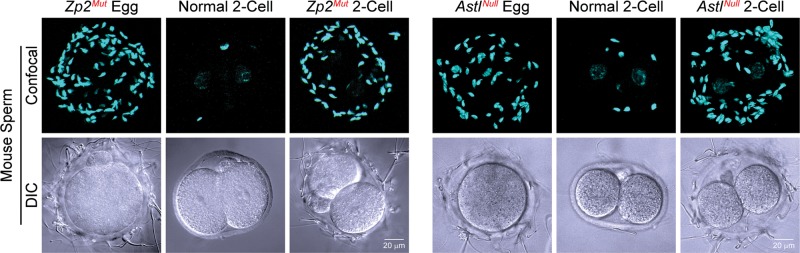
Mouse sperm bind to 2-cell embryos, if ZP2 remains uncleaved. Capacitated mouse sperm binding to 2-cell embryos in which ZP2 remains intact because ZP2 was mutated to prevent post-fertilization cleavage (Zp2Mut, left panel) or because of the absence of ovastacin (AstlNull, right panel). AstlNull and Zp2Mut eggs serve as positive controls and normal 2-cell embryos as negative controls. Modified from refs Gahlay et al. (2010) and Burkart et al. (2012).
These observations led to a simple, unifying formulation for the molecular basis of gamete recognition in which capacitated sperm attach to an N-terminal domain of ZP2 prior to zona penetration and gamete fusion. Following fertilization, ovastacin is released from egg cortical granules and cleaves extracellular ZP2 (Burkart et al., 2012). This effectively destroys the sperm-binding domain (Greenhouse et al., 1999; Gahlay et al., 2010; Burkart et al., 2012) and accounts for the inability of sperm to bind to 2-cell embryos (Fig. 1). There is a single N-glycan at Asn83, but no O-glycans in this region of native mouse ZP235–149 (Boja et al., 2003), but whether or not this glycan plays a role in gamete recognition has not been determined.
Although earlier reports implicated ZP2 as a secondary ligand for acrosome-reacted sperm (Bleil et al., 1988; Tsubamoto et al., 1999; Kerr et al., 2002; Chakravarty et al., 2008; Chiu et al., 2008), the Xenopus laevis homolog, gp69/64, inhibits primary sperm binding to eggs in vitro (Tian et al., 1997). Following fertilization and cleavage at the conserved 155FD↓DD158 site, the C-terminal native gp69/64 glycopeptide does not affect sperm binding. Although a short recombinant peptide gp69/64130–156 did not inhibit sperm binding, the longer gp69/6434–156 N-terminal domain (homologous to mouse ZP235–149) was not tested. Following fertilization Xenopus laevis ZP2 is cleaved by a zinc metalloprotease (Lindsay and Hedrick, 2004) and post-fertilization proteolysis of the N-terminal domain of gp69/64 could account for the inability of sperm to bind to early Xenopus laevis embryos as it does in mice. Thus, the ZP2 cleavage model of gamete recognition may pertain more broadly among vertebrates.
Sperm receptor candidates mediating fertilization
It has long been presumed that gamete recognition of a ZP ligand requires a cognate sperm receptor, but its identity and localization have defied definition. The acrosome is a subcellular organelle that underlies the anterior surface of the sperm. During fertilization, its outer membrane fuses with the plasma membrane releasing macromolecules, the functions of which are incompletely understood. Although the sperm receptor traditionally has been considered to reside in the plasma membrane, acrosome-reacted sperm lacking the anterior plasma membrane are reported to fertilize eggs in vitro (Fleming and Yanagimachi, 1982; Kuzan et al., 1984; Inoue et al., 2011; Jin et al., 2011) and the site of acrosome exocytosis remains under active investigation (Avella and Dean, 2011). There is a surprising surfeit of sperm surface receptor candidates for the seemingly simple organization of the mammalian ZP. Several strong candidates have arisen from biochemical and cell biological analyses that documented their importance in gamete recognition using in vitro assays. Unexpectedly, genetic ablation of individual candidates in transgenic mice did not prevent fertility. This suggests that the in vitro assays used to support their candidacy may not have captured the physiological complexity inherent in in vivo gamete recognition. Alternatively, it may be that multiple gene products are required to form a sperm surface complex that interacts with the ligand in the ZP in vivo. However, it is not clear why a need for additional proteins would not have been noted in the in vitro assays initially used to propose the candidate protein. Redundancy of sperm surface receptors has also been invoked to account for the difficulty in forming a consensus on the correct sperm surface receptor. Here we briefly review sperm surface candidates that have been tested by genetic ablation of the cognate gene in transgenic mice (Table I).
Table I.
Sperm receptor candidates for zona binding ablated in transgenic mice.
| Sperm protein | Gene | Expression | Localization | Zona ligand | Null phenotype |
|---|---|---|---|---|---|
| GalTase: UDP-Gal:betaGlcNAc beta 1,4-galactosyltransferase, polypeptide 1 | B4galt1 | Ubiquitous | Plasma membrane | ZP3 | Fertile |
| SED1 (MFG-E8): milk fat globule-EGF factor 8 | Mfge8 | Ubiquitous | Plasma membrane | ZP3, ZP2 | Subfertile or fertile |
| SPAM1 (PH20): sperm adhesion molecule 1 | Spam1 | Testis specific | Inner acrosomal membrane | Not specified | Fertile |
| ZP3R (SP56): ZP 3 receptor | Zp3r | Testis specific | Acrosomal matrix | ZP3 | Fertile |
| Proacrosin acrosin prepropeptide | Acr | Testis specific | Acrosomal matrix | ZP2 | Fertile |
| Zonadhesin | Zan | Testis specific | Outer acrosomal membrane | Not specified | Fertile |
| ADAM3 (cyritestin): a disintegrin and metallopeptidase domain 3 | Adam3 | Testis specific | Plasma membrane | Not specified | Sterile |
Sperm surface receptor candidates for binding to the ZP
β1,4-galactosyltransferase (GalTase) was one of the first sperm surface molecule proposed to play a role in sperm–egg binding. Male mice with mutant alleles of the T/t complex exhibit transmission distortion ratios in which the mutant t-allele is preferentially passed to the next generation (Fraser and Dudley, 1999). Based on a reported 4-fold increase of enzymatic activity in the mutant allele, a role was proposed for GalTase in gamete recognition (Shur, 1981). GalTase is a member of the galactosyltransferase superfamily of enzymes that are responsible for the synthesis of glycoside residues of glycoproteins, glycolipids and glycosaminoglycans (Joziasse, 1992). GalTase is localized in the endoplasmic reticulum, the Golgi apparatus and on the cell surface of a variety of cells. It has been proposed to mediate cell–cell interactions by binding to the glycoconjugate substrates on the adjacent cell surface and extracellular matrix (Roseman, 1970; Hathaway and Shur, 1988). GalTase was reported to interact directly with O-linked oligosaccharides on ZP3 (Miller et al., 1992) and inhibiting GalTase activity or blocking its recognition site on the ZP greatly diminished sperm binding (Shur and Hall, 1982). Both affinity-purified GalTase and anti-GalTase antibodies disrupted sperm–zona binding in a dose-dependent manner (Lopez et al., 1985). However, genetic ablation of GalTase did not significantly affect male fertility (Asano et al., 1997; Lu and Shur, 1997).
A second candidate sperm receptor, ZP3R (sp56), was initially localized on the plasma membrane of acrosome-intact mouse sperm (Cheng et al., 1994; Suzuki-Toyota et al., 1995). ZP3R was reported to possess affinity for ZP3 glycans (Bleil and Wassarman, 1990; Cheng et al., 1994). Purified native or recombinant ZP3R inhibited sperm binding to the ZP surrounding mouse eggs, but not to embryos in vitro (Bookbinder et al., 1995; Buffone et al., 2008). Subsequent studies relocated ZP3R within the acrosomal matrix (Foster et al., 1997; Kim et al., 2001) and genetic ablation of the cognate gene did not affect male fertility (Muro et al., 2012).
Using native, particulate pig ZP as an affinity matrix, two proteins were isolated from pig sperm membranes and identified as candidate proteins for adherence of sperm to the ZP. One was the aforementioned ZP3R and the other was designated zonadhesin (Hardy and Garbers, 1994, 1995). Zonadhesin is expressed uniquely in the testes and contains multiple cell adhesion molecule domains, including MAM (meprin/A5 antigen/mu receptor tyrosine phosphatase), mucin and von Willebrand D domains (Hardy and Garbers, 1995; Gao and Garbers, 1998). Initially present in the outer acrosomal membrane and outer aspect of the acrosomal matrix (Bi et al., 2003), zonadhesin becomes exposed on the sperm surface during capacitation (Tardif et al., 2010). As with other candidates, native isoforms and recombinant zonadhesin bound to the ZP (Hickox et al., 2001; Lea et al., 2001) and anti-zonadhesin antibody significantly reduced sperm binding and IVF (Tardif et al., 2010). However, zonadhesin null males were fertile (Tardif et al., 2010).
SED1 (secreted protein that contains notch-like epidermal growth factor (EGF) repeats and discoidin/F5/8 type C domains) is the mouse homolog of a boar sperm surface protein (p47) isolated by affinity chromatography with porcine zona proteins (Ensslin et al., 1995, 1998). Mouse SED1 is secreted by the epididymal epithelium and attaches to the sperm plasma membrane overlying the acrosome when sperm progress through the epididymis. SED1 binds to ZP3 on immunoblots and to a lesser extent to ZP2 (Ensslin and Shur, 2003). The protein is encoded by Mfge8 (milk fat globule-EGF 8) and has been genetically ablated by four groups of investigators. In each case, male mice were fertile, with either decreased (Ensslin and Shur, 2003; Silvestre et al., 2005) or normal (Hanayama et al., 2004; Neutzner et al., 2007) fecundity.
Among the field of sperm receptor candidates, ADAM3 (a disintegrin and metalloprotease 3) is noteworthy in providing an infertile phenotype in mice lacking the protein (Shamsadin et al., 1999; Nishimura et al., 2001). In addition, multiple genes including Ace, Clgn, Adam2, Adam1a, Calr3, Tpst2, Rnasse10, Pdilt and Pmis2, when disrupted, display defective zona-binding ability to cumulus-free eggs and impaired migration into the oviduct similar to that observed in Adam3 null males (Krege et al., 1995; Ikawa et al., 1997, 2011; Cho et al., 1998; Nishimura et al., 2004; Marcello et al., 2011; Krutskikh et al., 2012; Tokuhiro et al., 2012; Yamaguchi et al., 2012). All of these additional null mutations lack ADAM3 which suggests a common denominator for their observed phenotype. However, mutant sperm lacking ADAM3 fertilized eggs in vivo following injection into the oviduct and in vitro, in the presence, but not in the absence, of the cumulus oophorus (Tokuhiro et al., 2012; Yamaguchi et al., 2012). Thus, rather than gamete recognition, the major role for ADAM3 may be in mediating passage of sperm through the utero-tubal junction. Of note, the orthologous gene in humans is two non-functional pseudogenes (Grzmil, 2001) and men are fertile in the absence of ADAM3 protein.
Acrosome-reacted sperm receptor candidates for binding to the ZP
A second group of sperm receptor candidates has been reported to interact with the ZP after sperm acrosome exocytosis. PH20, later renamed SPAM1 (sperm adhesion molecule 1), is a glycosyl phosphatidylinositol-anchored protein first identified in guinea pigs. This protein is located on both the plasma membrane and the inner acrosomal membrane of the sperm head and contains a ZP binding domain near the C-terminus and a hyaluronidase domain on the N-terminus with enzymatic activity (Hunnicutt et al., 1996). Monoclonal antibodies to SPAM1 inhibited the binding of acrosome-reacted, but not acrosome intact, guinea pig sperm to the ZP (Primakoff et al., 1985; Myles et al., 1987) and immunization resulted in 100% effective, but reversible contraception (Primakoff et al., 1988). Although native and recombinant SPAM1 have hyaluronidase activity (Gmachl et al., 1993; Lin et al., 1994; Hunnicutt et al., 1996), Spam1 null male mice were fertile. Mouse sperm lacking SPAM1 penetrated, albeit with decreased efficiency, the hyaluronan of the cumulus oophorus and fertilized eggs in vitro (Baba et al., 2002).
Acrosin, another well-investigated sperm molecule implicated in sperm–zona interaction, is present ubiquitously in the acrosome matrix. It was initially detected in detergent extracts of boar sperm as a binding partner of the pig ZP (Brown and Jones, 1987; Jones and Brown, 1987; Topfer-Petersen and Henschen, 1987) and subsequently identified as proacrosin (Jones, 1991; Urch and Patel, 1991; Richardson and O'Rand, 1996). Cleavage of proacrosin activates acrosin, a major serine protease of mouse sperm (Stambaugh and Buckley, 1972; Huang-Yang and Meizel, 1975; Baba et al., 1989). Due to its potential roles in zona binding and proteolysis, acrosin was inferred to play a role in gamete recognition and zona matrix penetration (Stambaugh and Buckley, 1972; Topfer-Petersen and Henschen, 1987, 1988). Although acrosin exhibited an affinity with ZP2 (Urch and Patel, 1991; Howes et al., 2001) and may convey a selective advantage to sperm, male mice lacking acrosin remained fertile (Baba et al., 1994; Yamagata et al., 1998).
The difficulty in identifying a sperm surface protein as essential for gamete recognition has been puzzling. One heretical thought is that sperm might not need to bind to the ZP prior to penetration and fusion with eggs. This might account for observations that acrosome-reacted sperm can penetrate and fertilize zona-intact eggs in vitro (Fleming and Yanagimachi, 1982; Kuzan et al., 1984; Inoue et al., 2011; Jin et al., 2011). However, a non-binding paradigm for gamete recognition would be difficult to reconcile with the observed taxon specificity of gamete recognition reported in vitro (Baibakov et al., 2012). Thus, the search for the elusive sperm surface protein involved in gamete recognition continues.
Perspectives
Mouse genetics have proved useful in testing established models of gamete recognition and in proposing new paradigms for further investigation. The widely accepted ZP3 glycan-release models first introduced more than 30 years ago are not consistent with recent results in transgenic mice. However, the newly introduced ZP2 cleavage model of gamete recognition is supported by the taxon-specificity of human sperm binding to zonae pellucidae in which human ZP2 replaces endogenous mouse ZP2 and the discovery of ovastacin as the cortical granule metalloendoprotease that cleaves ZP2 to prevent post-fertilization sperm binding. This paradigm-shifting model is falsifiable and predicts that human sperm will bind to mouse ZP2 containing a human ZP2 N-terminal domain and that female mice lacking the mouse ZP2 N-terminal domain will be sterile. Both predictions can be evaluated in transgenic mice expressing the appropriate mutant form of ZP2 in the Zp2 null background. However, identification of the zona ligand is only half the story for understanding the molecular basis of gamete recognition at the surface of the ZP.
If prior focus on ZP3 was misplaced, the current molecular definition of ZP2 as the zona ligand offers a path forward in biochemically identifying the cognate sperm surface receptor. Once nominated, an essential sperm receptor candidate should meet particular criteria including in vivo and in vitro infertility after genetic ablation in transgenic mice. This phenotype should be rescued by expressing the human orthologue in transgenic mice and crossing them into the Zp2 null background so that they express only the human sperm receptor. Sperm from these mice should bind and penetrate the zonae pellucidae containing human ZP2. If fertile, these mice would provide compelling evidence for the candidacy of the identified sperm receptor. Conversely, taxon-specificity should be preserved and the ‘humanized’ transgenic sperm should not bind or fertilize normal mouse eggs. We note that the putative sperm receptor need not be a single protein, but could be a complex which would significantly complicate its identification.
A ‘humanized’ model with male mice expressing the human sperm receptor and female mice with human ZP2 in their ZP would establish a robust system for further investigation of human gamete interactions. For example, the ability of human sperm to bind to the ‘humanized’ ZP could prove useful in identifying human sperm most suitable for ICSI and contraceptive strategies aimed at pre-fertilization cleavage of ZP2 to prevent sperm binding to ovulated eggs could be experimentally addressed in this model system. While significant progress has been made in our understanding of the molecular basis of fertilization and the post-fertilization block to polyspermy, many investigative challenges remain.
As the field progresses in providing a detailed explanation of gamete recognition, these advances should rapidly empower reproductive medicine and inform on the diverse etiologies of human infertility as well as provide improved strategies for family planning.
Acknowledgements
We apologize to colleagues whose work could not be cited due to space limitations and we thank Drs Stephanie Beall and Keizo Tokuhiro for critical reading of the manuscript.
Authors' roles
M.A.A. and B.X. wrote the manuscript which was edited by J.D. who also contributed to the figures.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, NIDDK.
Conflict of interest
None declared.
References
- Asano M, Furukawa K, Kido M, Matsumoto S, Umesaki Y, Kochibe N, Iwakura Y. Growth retardation and early death of beta-1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J. 1997;16:1850–1857. doi: 10.1093/emboj/16.8.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avella MA, Dean J. Fertilization with acrosome-reacted mouse sperm: implications for the site of exocytosis. Proc Natl Acad Sci USA. 2011;108:19843–19844. doi: 10.1073/pnas.1118234109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Michikawa Y, Kawakura K, Arai Y. Activation of boar proacrosin is effected by processing at both N- and C-terminal portions of the zymogen molecule. FEBS Lett. 1989;244:132–136. doi: 10.1016/0014-5793(89)81178-0. [DOI] [PubMed] [Google Scholar]
- Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem. 1994;269:31845–31849. [PubMed] [Google Scholar]
- Baba D, Kashiwabara S, Honda A, Yamagata K, Wu Q, Ikawa M, Okabe M, Baba T. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J Biol Chem. 2002;277:30310–30314. doi: 10.1074/jbc.M204596200. [DOI] [PubMed] [Google Scholar]
- Baibakov B, Gauthier L, Talbot P, Rankin TL, Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development. 2007;134:933–943. doi: 10.1242/dev.02752. [DOI] [PubMed] [Google Scholar]
- Baibakov B, Boggs NA, Yauger B, Baibakov G, Dean J. Human sperm bind to the N-terminal domain of ZP2 in humanized zonae pellucidae in transgenic mice. J Cell Biol. 2012;197:897–905. doi: 10.1083/jcb.201203062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JL. Factors regulating sperm capacitation. Syst Biol Reprod Med. 2010;56:334–348. doi: 10.3109/19396368.2010.512377. [DOI] [PubMed] [Google Scholar]
- Bedford JM. Sperm/egg interaction: The specificity of human spermatozoa. Anat Rec. 1977;188:477–488. doi: 10.1002/ar.1091880407. [DOI] [PubMed] [Google Scholar]
- Bi M, Hickox JR, Winfrey VP, Olson GE, Hardy DM. Processing, localization and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome. Biochem J. 2003;375:477–488. doi: 10.1042/BJ20030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Mammalian sperm–egg interaction: Identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980a;20:873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev Biol. 1980b;76:185–202. doi: 10.1016/0012-1606(80)90371-1. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Sperm–egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Galactose at the nonreducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein's sperm receptor activity. Proc Natl Acad Sci USA. 1988;85:6778–6782. doi: 10.1073/pnas.85.18.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Identification of a ZP3-binding protein on acrosome-intact mouse sperm by photoaffinity crosslinking. Proc Natl Acad Sci USA. 1990;87:5563–5567. doi: 10.1073/pnas.87.14.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil JD, Beall CF, Wassarman PM. Mammalian sperm–egg interaction: fertilization of mouse eggs triggers modification of the major zona pellucida glycoprotein, ZP2. Dev Biol. 1981;86:189–197. doi: 10.1016/0012-1606(81)90329-8. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Greve JM, Wassarman PM. Identification of a secondary sperm receptor in the mouse egg zona pellucida: role in maintenance of binding of acrosome-reacted sperm to eggs. Dev Biol. 1988;128:376–385. doi: 10.1016/0012-1606(88)90299-0. [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- Boja ES, Hoodbhoy T, Fales HM, Dean J. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J Biol Chem. 2003;278:34189–34202. doi: 10.1074/jbc.M304026200. [DOI] [PubMed] [Google Scholar]
- Boja ES, Hoodbhoy T, Garfield M, Fales HM. Structural conservation of mouse and rat zona pellucida glycoproteins. Probing the native rat zona pellucida proteome by mass spectrometry. Biochemistry. 2005;44:16445–16460. doi: 10.1021/bi051883f. [DOI] [PubMed] [Google Scholar]
- Bookbinder LH, Cheng A, Bleil JD. Tissue- and species-specific expression of sp56, a mouse sperm fertilization protein. Science. 1995;269:86–89. doi: 10.1126/science.7604284. [DOI] [PubMed] [Google Scholar]
- Bork P. A trefoil domain in the major rabbit zona pellucida protein. Protein Sci. 1993;2:669–670. doi: 10.1002/pro.5560020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Sander C. A large domain common to sperm receptors (Zp2 and Zp3) and TGF-beta type III receptor. FEBS Lett. 1992;300:237–240. doi: 10.1016/0014-5793(92)80853-9. [DOI] [PubMed] [Google Scholar]
- Bronson RA, McLaren A. Transfer to the mouse oviduct of eggs with and without the zona pellucida. J Reprod Fertil. 1970;22:129–137. doi: 10.1530/jrf.0.0220129. [DOI] [PubMed] [Google Scholar]
- Brown CR, Jones R. Binding of zona pellucida proteins to a boar sperm polypeptide of Mr 53,000 and identification of zona moieties involved. Development. 1987;99:333–339. doi: 10.1242/dev.99.3.333. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Zhuang T, Ord TS, Hui L, Moss SB, Gerton GL. Recombinant mouse sperm ZP3-binding protein (ZP3R/sp56) forms a high order oligomer that binds eggs and inhibits mouse fertilization in vitro. J Biol Chem. 2008;283:12438–12445. doi: 10.1074/jbc.M706421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Ijiri TW, Cao W, Merdiushev T, Aghajanian HK, Gerton GL. Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev. 2012;79:4–18. doi: 10.1002/mrd.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart AD, Xiong B, Baibakov B, Jimenez-Movilla M, Dean J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J Cell Biol. 2012;197:37–44. doi: 10.1083/jcb.201112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty S, Kadunganattil S, Bansal P, Sharma RK, Gupta SK. Relevance of glycosylation of human zona pellucida glycoproteins for their binding to capacitated human spermatozoa and subsequent induction of acrosomal exocytosis. Mol Reprod Dev. 2008;75:75–88. doi: 10.1002/mrd.20726. [DOI] [PubMed] [Google Scholar]
- Chamberlin ME, Dean J. Human homolog of the mouse sperm receptor. Proc Natl Acad Sci USA. 1990;87:6014–6018. doi: 10.1073/pnas.87.16.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Litscher ES, Wassarman PM. Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proc Natl Acad Sci USA. 1998;95:6193–6197. doi: 10.1073/pnas.95.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Le T, Palacios M, Bookbinder LH, Wassarman PM, Suzuki F, Bleil JD. Sperm–egg recognition in the mouse: Characterization of sp56, a sperm protein having specific affinity for ZP3. J Cell Biol. 1994;125:867–878. doi: 10.1083/jcb.125.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PC, Wong BS, Lee CL, Pang RT, Lee KF, Sumitro SB, Gupta SK, Yeung WS. Native human zona pellucida glycoproteins: purification and binding properties. Hum Reprod. 2008;23:1385–1393. doi: 10.1093/humrep/den047. [DOI] [PubMed] [Google Scholar]
- Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG. Fertilization defects in sperm from mice lacking fertilin beta. Science. 1998;281:1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- Easton RL, Patankar MS, Lattanzio FA, Leaven TH, Morris HR, Clark GF, Dell A. Structural analysis of murine zona pellucida glycans. Evidence for the expression of core 2-type O-glycans and the Sd(a) antigen. J Biol Chem. 2000;275:7731–7742. doi: 10.1074/jbc.275.11.7731. [DOI] [PubMed] [Google Scholar]
- Ensslin MA, Shur BD. Identification of mouse sperm SED1, a bi-motif EGF repeat and discoidin-domain protein involved in sperm–egg binding. Cell. 2003;114:405–417. doi: 10.1016/s0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- Ensslin M, Calvete JJ, Thole HH, Sierralta WD, Adermann K, Sanz L, Topfer-Petersen E. Identification by affinity chromatography of boar sperm membrane-associated proteins bound to immobilized porcine zona pellucida. Mapping of the phosphorylethanolamine-binding region of spermadhesin AWN. Biol Chem Hoppe Seyler. 1995;376:733–738. doi: 10.1515/bchm3.1995.376.12.733. [DOI] [PubMed] [Google Scholar]
- Ensslin M, Vogel T, Calvete JJ, Thole HH, Schmidtke J, Matsuda T, Topfer-Petersen E. Molecular cloning and characterization of P47, a novel boar sperm-associated zona pellucida-binding protein homologous to a family of mammalian secretory proteins. Biol Reprod. 1998;58:1057–1064. doi: 10.1095/biolreprod58.4.1057. [DOI] [PubMed] [Google Scholar]
- Epifano O, Liang L-F, Dean J. Mouse Zp1 encodes a zona pellucida protein homologous to egg envelope proteins in mammals and fish. J Biol Chem. 1995;270:27254–27258. doi: 10.1074/jbc.270.45.27254. [DOI] [PubMed] [Google Scholar]
- Fleming AD, Yanagimachi R. Fertile life of acrosome-reacted guinea pig spermatozoa. J Exp Zool. 1982;220:109–115. doi: 10.1002/jez.1402200114. [DOI] [PubMed] [Google Scholar]
- Florman HM, Wassarman PM. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985;41:313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florman HM, Bechtol KB, Wassarman PM. Enzymatic dissection of the functions of the mouse egg's receptor for sperm. Dev Biol. 1984;106:243–255. doi: 10.1016/0012-1606(84)90079-4. [DOI] [PubMed] [Google Scholar]
- Foster JA, Friday BB, Maulit MT, Blobel C, Winfrey VP, Olson GE, Kim K-S, Gerton GL. AM67, a secretory component of the guinea pig sperm acrosomal matrix, is related to mouse sperm protein sp56 and the complement component 4-binding proteins. J Biol Chem. 1997;272:12714–12722. doi: 10.1074/jbc.272.19.12714. [DOI] [PubMed] [Google Scholar]
- Fraser LR, Dudley K. New insights into the t-complex and control of sperm function. Bioessays. 1999;21:304–312. doi: 10.1002/(SICI)1521-1878(199904)21:4<304::AID-BIES6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Gahlay G, Gauthier L, Baibakov B, Epifano O, Dean J. Gamete recognition in mice depends on the cleavage status of an egg's zona pellucida protein. Science. 2010;329:216–219. doi: 10.1126/science.1188178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Bukovsky A, Sharma RK, Bansal P, Bhandari B, Gupta SK. In humans, zona pellucida glycoprotein-1 binds to spermatozoa and induces acrosomal exocytosis. Hum Reprod. 2010;25:1643–1656. doi: 10.1093/humrep/deq105. [DOI] [PubMed] [Google Scholar]
- Gao Z, Garbers DL. Species diversity in the structure of zonadhesin, a sperm-specific membrane protein containing multiple cell adhesion molecule-like domains. J Biol Chem. 1998;273:3415–3421. doi: 10.1074/jbc.273.6.3415. [DOI] [PubMed] [Google Scholar]
- Gmachl M, Sagan S, Ketter S, Kreil G. The human sperm protein PH-20 has hyaluronidase activity. FEBS Lett. 1993;336:545–548. doi: 10.1016/0014-5793(93)80873-s. [DOI] [PubMed] [Google Scholar]
- Goudet G, Mugnier S, Callebaut I, Monget P. Phylogenetic analysis and identification of pseudogenes reveal a progressive loss of zona pellucida genes during evolution of vertebrates. Biol Reprod. 2008;78:796–806. doi: 10.1095/biolreprod.107.064568. [DOI] [PubMed] [Google Scholar]
- Greenhouse S, Castle PE, Dean J. Antibodies to human ZP3 induce reversible contraception in transgenic mice with ‘humanized’ zonae pellucidae. Hum Reprod. 1999;14:593–600. doi: 10.1093/humrep/14.3.593. [DOI] [PubMed] [Google Scholar]
- Grzmil P. Human cyritestin genes (CYRN1 and CYRN2) are non-functional. Biochem J. 2001;357:551–556. doi: 10.1042/0264-6021:3570551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Bhandari B, Shrestha A, Biswal BK, Palaniappan C, Malhotra SS, Gupta N. Mammalian zona pellucida glycoproteins: structure and function during fertilization. Cell Tissue Res. 2012;349:665–778. doi: 10.1007/s00441-011-1319-y. [DOI] [PubMed] [Google Scholar]
- Han L, Monne M, Okumura H, Schwend T, Cherry AL, Flot D, Matsuda T, Jovine L. Insights into egg coat assembly and egg-sperm interaction from the X-ray structure of full-length ZP3. Cell. 2010;143:404–415. doi: 10.1016/j.cell.2010.09.041. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- Hardy DM, Garbers DL. Species-specific binding of sperm proteins to the extracellular matrix (zona pellucida) of the egg. J Biol Chem. 1994;269:19000–19004. [PubMed] [Google Scholar]
- Hardy DM, Garbers DL. A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor. J Biol Chem. 1995;270:26025–26028. doi: 10.1074/jbc.270.44.26025. [DOI] [PubMed] [Google Scholar]
- Harris JD, Hibler DW, Fontenot GK, Hsu KT, Yurewicz EC, Sacco AG. Cloning and characterization of zona pellucida genes and cDNAs from a variety of mammalian species: ZPA, ZPB, and ZPC gene families. DNA Seq. 1994;4:361–393. doi: 10.3109/10425179409010186. [DOI] [PubMed] [Google Scholar]
- Hathaway HJ, Shur BD. Novel cell surface receptors during mammalian fertilization and development. Bioessays. 1988;9:153–158. doi: 10.1002/bies.950090504. [DOI] [PubMed] [Google Scholar]
- Hedrick JL. Anuran and pig egg zona pellucida glycoproteins in fertilization and early development. Int J Dev Biol. 2008;52:683–701. doi: 10.1387/ijdb.082580jh. [DOI] [PubMed] [Google Scholar]
- Hickox JR, Bi M, Hardy DM. Heterogeneous processing and zona pellucida binding activity of pig zonadhesin. J Biol Chem. 2001;276:41502–41509. doi: 10.1074/jbc.M106795200. [DOI] [PubMed] [Google Scholar]
- Hoodbhoy T, Aviles M, Baibakov B, Epifano O, Jimenez-Movilla M, Gauthier L, Dean J. ZP2 and ZP3 traffic independently within oocytes prior to assembly into the extracellular zona pellucida. Mol Cell Biol. 2006;26:7991–7998. doi: 10.1128/MCB.00904-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath PM, Kellom T, Caulfield J, Boldt J. Mechanistic studies of the plasma membrane block to polyspermy in mouse eggs. Mol Reprod Dev. 1993;34:65–72. doi: 10.1002/mrd.1080340111. [DOI] [PubMed] [Google Scholar]
- Howes E, Pascall JC, Engel W, Jones R. Interactions between mouse ZP2 glycoprotein and proacrosin; a mechanism for secondary binding of sperm to the zona pellucida during fertilization. J Cell Sci. 2001;114:4127–4136. doi: 10.1242/jcs.114.22.4127. [DOI] [PubMed] [Google Scholar]
- Huang-Yang YH, Meizel S. Purification of rabbit testis proacrosin and studies of its active form. Biol Reprod. 1975;12:232–238. doi: 10.1095/biolreprod12.2.232. [DOI] [PubMed] [Google Scholar]
- Hunnicutt GR, Primakoff P, Myles DG. Sperm surface protein PH-20 is bifunctional: one activity is a hyaluronidase and a second, distinct activity is required in secondary sperm–zona binding. Biol Reprod. 1996;55:80–86. doi: 10.1095/biolreprod55.1.80. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Wada I, Kominami K, Watanabe D, Toshimori K, Nishimune Y, Okabe M. The putative chaperone calmegin is required for sperm fertility. Nature. 1997;387:607–611. doi: 10.1038/42484. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm's journey to and interaction with the oocyte. J Clin Invest. 2010;120:984–994. doi: 10.1172/JCI41585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa M, Tokuhiro K, Yamaguchi R, Benham AM, Tamura T, Wada I, Satouh Y, Inoue N, Okabe M. Calsperin is a testis-specific chaperone required for sperm fertility. J Biol Chem. 2011;286:5639–5646. doi: 10.1074/jbc.M110.140152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci USA. 2011;108:20008–20011. doi: 10.1073/pnas.1116965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LA, Sharp AP, Wolf DP. Absence of an electrical polyspermy block in the mouse. Dev Biol. 1983;96:317–323. doi: 10.1016/0012-1606(83)90168-9. [DOI] [PubMed] [Google Scholar]
- Jimenez-Movilla M, Dean J. ZP2 and ZP3 cytoplasmic tails prevent premature interactions and ensure incorporation into the zona pellucida. J Cell Sci. 2010;124:940–950. doi: 10.1242/jcs.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. Interaction of zona pellucida glycoproteins, sulphated carbohydrates and synthetic polymers with proacrosin, the putative egg-binding protein from mammalian spermatozoa. Development. 1991;111:1155–1163. doi: 10.1242/dev.111.4.1155. [DOI] [PubMed] [Google Scholar]
- Jones R, Brown CR. Identification of a zona-binding protein from boar spermatozoa as proacrosin. Exp Cell Res. 1987;171:503–508. doi: 10.1016/0014-4827(87)90182-0. [DOI] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, Litscher E, Wassarman PM. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat Cell Biol. 2002;4:457–461. doi: 10.1038/ncb802. [DOI] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, Litscher ES, Wassarman PM. A duplicated motif controls assembly of zona pellucida domain proteins. Proc Natl Acad Sci USA. 2004;101:5922–5927. doi: 10.1073/pnas.0401600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joziasse DH. Mammalian glycosyltransferases: genomic organization and protein structure. Glycobiology. 1992;2:271–277. doi: 10.1093/glycob/2.4.271. [DOI] [PubMed] [Google Scholar]
- Kerr CL, Hanna WF, Shaper JH, Wright WW. Characterization of zona pellucida glycoprotein 3 (ZP3) and ZP2 binding sites on acrosome-intact mouse sperm. Biol Reprod. 2002;66:1585–1595. doi: 10.1095/biolreprod66.6.1585. [DOI] [PubMed] [Google Scholar]
- Kim KS, Cha MC, Gerton GL. Mouse sperm protein sp56 is a component of the acrosomal matrix. Biol Reprod. 2001;64:36–43. doi: 10.1095/biolreprod64.1.36. [DOI] [PubMed] [Google Scholar]
- Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- Krutskikh A, Poliandri A, Cabrera-Sharp V, Dacheux JL, Poutanen M, Huhtaniemi I. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. 2012;26:4198–4209. doi: 10.1096/fj.12-205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzan FB, Fleming AD, Seidel GE., Jr Successful fertilization in vitro of fresh intact oocytes by perivitelline (acrosome-reacted) spermatozoa of the rabbit. Fertil Steril. 1984;41:766–770. doi: 10.1016/s0015-0282(16)47847-7. [DOI] [PubMed] [Google Scholar]
- Lea IA, Sivashanmugam P, O'Rand MG. Zonadhesin: characterization, localization, and zona pellucida binding. Biol Reprod. 2001;65:1691–1700. doi: 10.1095/biolreprod65.6.1691. [DOI] [PubMed] [Google Scholar]
- Lefièvre L, Conner SJ, Salpekar A, Olufowobi O, Ashton P, Pavlovic B, Lenton W, Afnan M, Brewis IA, Monk M, et al. Four zona pellucida glycoproteins are expressed in the human. Hum Reprod. 2004;19:1580–1586. doi: 10.1093/humrep/deh301. [DOI] [PubMed] [Google Scholar]
- Leyton L, Saling P. Evidence that aggregation of mouse sperm receptors by ZP3 triggers the acrosome reaction. J Cell Biol. 1989;108:2163–2168. doi: 10.1083/jcb.108.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L-F, Dean J. Conservation of the mammalian secondary sperm receptor genes results in promoter function of the human homologue in heterologous mouse oocytes. Dev Biol. 1993;156:399–408. doi: 10.1006/dbio.1993.1087. [DOI] [PubMed] [Google Scholar]
- Liang L-F, Chamow SM, Dean J. Oocyte-specific expression of mouse Zp-2: developmental regulation of the zona pellucida genes. Mol Cell Biol. 1990;10:1507–1515. doi: 10.1128/mcb.10.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Mahan K, Lathrop WF, Myles DG, Primakoff P. A hyaluronidase activity of the sperm plasma membrane protein PH—20 enables sperm to penetrate the cumulus cell layer surrounding the egg. J Cell Biol. 1994;125:1157–1163. doi: 10.1083/jcb.125.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay LL, Hedrick JL. Proteolysis of Xenopus laevis egg envelope ZPA triggers envelope hardening. Biochem Biophys Res Commun. 2004;324:648–654. doi: 10.1016/j.bbrc.2004.09.099. [DOI] [PubMed] [Google Scholar]
- Liu C, Litscher S, Wassarman PM. Transgenic mice with reduced numbers of functional sperm receptors on their eggs reproduce normally. Mol Biol Cell. 1995;6:577–585. doi: 10.1091/mbc.6.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Litscher ES, Mortillo S, Sakai Y, Kinloch RA, Stewart CL, Wassarman PM. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci USA. 1996;93:5431–5436. doi: 10.1073/pnas.93.11.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LC, Bayna EM, Litoff D, Shaper NL, Shaper JH, Shur BD. Receptor function of mouse sperm surface galactosyltransferase during fertilization. J Cell Biol. 1985;101:1501–1510. doi: 10.1083/jcb.101.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe JB, Marth JD. A genetic approach to mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- Lu Q, Shur BD. Sperm from β1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development. 1997;124:4121–4131. doi: 10.1242/dev.124.20.4121. [DOI] [PubMed] [Google Scholar]
- Marcello MR, Jia W, Leary JA, Moore KL, Evans JP. Lack of tyrosylprotein sulfotransferase-2 activity results in altered sperm–egg interactions and loss of ADAM3 and ADAM6 in epididymal sperm. J Biol Chem. 2011;286:13060–13070. doi: 10.1074/jbc.M110.175463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Macek MB, Shur BD. Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm–egg binding. Nature. 1992;357:589–593. doi: 10.1038/357589a0. [DOI] [PubMed] [Google Scholar]
- Modlinski JA. The role of the zona pellucida in the development of mouse eggs in vivo. J Embryol Exp Morphol. 1970;23:539–547. [PubMed] [Google Scholar]
- Monne M, Jovine L. A structural view of egg coat architecture and function in fertilization. Biol Reprod. 2011;85:661–669. doi: 10.1095/biolreprod.111.092098. [DOI] [PubMed] [Google Scholar]
- Muro Y, Buffone MG, Okabe M, Gerton GL. Function of the acrosomal matrix: zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization. Biol Reprod. 2012;86:1–6. doi: 10.1095/biolreprod.111.095877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles DG, Hyatt H, Primakoff P. Binding of both acrosome-intact and acrosome-reacted guinea pig sperm to the zona pellucida during in vitro fertilization. Dev Biol. 1987;121:559–567. doi: 10.1016/0012-1606(87)90191-6. [DOI] [PubMed] [Google Scholar]
- Nagdas SK, Araki Y, Chayko CA, Orgebin-Crist M-C, Tulsiani DRP. O-linked trisaccharide and N-Linked poly-N-acetyllactosaminyl glycans are present on mouse ZP2 and ZP3. Biol Reprod. 1994;51:262–272. doi: 10.1095/biolreprod51.2.262. [DOI] [PubMed] [Google Scholar]
- Neutzner M, Lopez T, Feng X, Bergmann-Leitner ES, Leitner WW, Udey MC. MFG-E8/lactadherin promotes tumor growth in an angiogenesis-dependent transgenic mouse model of multistage carcinogenesis. Cancer Res. 2007;67:6777–6785. doi: 10.1158/0008-5472.CAN-07-0165. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Cho C, Branciforte DR, Myles DG, Primakoff P. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin beta. Dev Biol. 2001;233:204–213. doi: 10.1006/dbio.2001.0166. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Kim E, Nakanishi T, Baba T. Possible function of the ADAM1a/ADAM2 Fertilin complex in the appearance of ADAM3 on the sperm surface. J Biol Chem. 2004;279:34957–34962. doi: 10.1074/jbc.M314249200. [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- Pang PC, Chiu PC, Lee CL, Chang LY, Panico M, Morris HR, Haslam SM, Khoo KH, Clark GF, Yeung WS, et al. Human sperm binding is mediated by the sialyl-Lewis(x) oligosaccharide on the zona pellucida. Science. 2011;333:1761–1764. doi: 10.1126/science.1207438. [DOI] [PubMed] [Google Scholar]
- Primakoff P, Hyatt H, Myles DG. A role for the migrating sperm surface antigen PH-20 in guinea pig sperm binding to the egg zona pellucida. J Cell Biol. 1985;101:2239–2244. doi: 10.1083/jcb.101.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P, Lathrop W, Woolman L, Cowan A, Myles D. Fully effective contraception in male and female guinea pigs immunized with the sperm protein PH-20. Nature. 1988;335:543–546. doi: 10.1038/335543a0. [DOI] [PubMed] [Google Scholar]
- Quesada V, Sanchez LM, Alvarez J, Lopez-Otin C. Identification and characterization of human and mouse ovastacin: a novel metalloproteinase similar to hatching enzymes from arthropods, birds, amphibians, and fish. J Biol Chem. 2004;279:26627–26634. doi: 10.1074/jbc.M401588200. [DOI] [PubMed] [Google Scholar]
- Quinn P. Failure of human spermatozoa to penetrate zona free mouse and rat ova in vitro. J Exp Zool. 1979;210:497–505. doi: 10.1002/jez.1402100312. [DOI] [PubMed] [Google Scholar]
- Rankin T, Familari M, Lee E, Ginsberg AM, Dwyer N, Blanchette-Mackie J, Drago J, Westphal H, Dean J. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development. 1996;122:2903–2910. doi: 10.1242/dev.122.9.2903. [DOI] [PubMed] [Google Scholar]
- Rankin T, Talbot P, Lee E, Dean J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development. 1999;126:3847–3855. doi: 10.1242/dev.126.17.3847. [DOI] [PubMed] [Google Scholar]
- Rankin TL, O'Brien M, Lee E, Wigglesworth KEJJ, Dean J. Defective zonae pellucidae in Zp2 null mice disrupt folliculogenesis, fertility and development. Development. 2001;128:1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- Rankin TL, Coleman JS, Epifano O, Hoodbhoy T, Turner SG, Castle PE, Lee E, Gore-Langton R, Dean J. Fertility and taxon-specific sperm binding persist after replacement of mouse 'sperm receptors' with human homologues. Dev Cell. 2003;5:33–43. doi: 10.1016/s1534-5807(03)00195-3. [DOI] [PubMed] [Google Scholar]
- Richardson RT, O'Rand MG. Site-directed mutagenesis of rabbit proacrosin. Identification of residues involved in zona pellucida binding. J Biol Chem. 1996;271:24069–24074. [PubMed] [Google Scholar]
- Ringuette MJ, Chamberlin ME, Baur AW, Sobieski DA, Dean J. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev Biol. 1988;127:287–295. doi: 10.1016/0012-1606(88)90315-6. [DOI] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970;5:270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Sachdev M, Mandal A, Mulders S, Digilio LC, Panneerdoss S, Suryavathi V, Pires E, Klotz KL, Hermens L, Herrero MB, et al. Oocyte specific oolemmal SAS1B involved in sperm binding through intra-acrosomal SLLP1 during fertilization. Dev Biol. 2012;363:40–51. doi: 10.1016/j.ydbio.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling PM, Sowinski J, Storey BT. An ultrastructural study of epididymal mouse spermatozoa binding to zonae pellucidae in vitro: sequential relationship to the acrosome reaction. J Exp Zool. 1979;209:229–238. doi: 10.1002/jez.1402090205. [DOI] [PubMed] [Google Scholar]
- Sato K. Polyspermy-preventing mechanisms in mouse eggs fertilized in vitro. J Exp Zool. 1979;210:353–359. doi: 10.1002/jez.1402100219. [DOI] [PubMed] [Google Scholar]
- Shabanowitz RB, O'Rand MG. Characterization of the human zona pellucida from fertilized and unfertilized eggs. J Reprod Fertil. 1988;82:151–161. doi: 10.1530/jrf.0.0820151. [DOI] [PubMed] [Google Scholar]
- Shamsadin R, Adham IM, Nayernia K, Heinlein UA, Oberwinkler H, Engel W. Male mice deficient for germ-cell cyritestin are infertile. Biol Reprod. 1999;61:1445–1451. doi: 10.1095/biolreprod61.6.1445. [DOI] [PubMed] [Google Scholar]
- Shi S, Williams SA, Seppo A, Kurniawan H, Chen W, Zhengyi Y, Marth JD, Stanley P. Inactivation of the MgatI gene in oocytes impairs oogenesis, but embryos lacking complex and hybrid N-glycans develop and implant. Mol Cell Biol. 2004;24:9920–9929. doi: 10.1128/MCB.24.22.9920-9929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Tsuji M, Dean J. In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J Biol Chem. 1983;258:5858–5863. [PubMed] [Google Scholar]
- Shur BD. Galactosyltransferase activities on mouse sperm bearing multiple t-lethal and t-viable haplotypes of the T/t-complex. Genet Res. 1981;38:225–236. doi: 10.1017/s0016672300020577. [DOI] [PubMed] [Google Scholar]
- Shur BD. Reassessing the role of protein-carbohydrate complementarity during sperm–egg interactions in the mouse. Int J Dev Biol. 2008;52:703–715. doi: 10.1387/ijdb.082571bs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shur BD, Hall NG. A role for mouse sperm surface galactosyltransferase in sperm binding to the egg zona pellucida. J Cell Biol. 1982;95:574–579. doi: 10.1083/jcb.95.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Théry C, Hamard G, Boddaert J, Aguilar B, Delcayre A, Houbron C, Tamarat R, Blanc-Brude O, Heeneman S, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- Sommer P, Blin N, Gott P. Tracing the evolutionary origin of the TFF-domain, an ancient motif at mucous surfaces. Gene. 1999;236:133–136. doi: 10.1016/s0378-1119(99)00243-7. [DOI] [PubMed] [Google Scholar]
- Spargo SC, Hope RM. Evolution and nomenclature of the zona pellucida gene family. Biol Reprod. 2003;68:358–362. doi: 10.1095/biolreprod.102.008086. [DOI] [PubMed] [Google Scholar]
- Stambaugh R, Buckley J. Studies on acrosomal proteinase of rabbit spermatozoa. Biochim Biophys Acta. 1972;284:473–477. doi: 10.1016/0005-2744(72)90145-3. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RC. Birth after reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- Stewart-Savage J, Bavister BD. A cell surface block to polyspermy occurs in golden hamster eggs. Dev Biol. 1988;128:150–157. doi: 10.1016/0012-1606(88)90277-1. [DOI] [PubMed] [Google Scholar]
- Suzuki-Toyota F, Maekawa M, Cheng A, Bleil JD. Immuno-colloidal gold labeled surface replica, and its application to detect sp56, the egg recognition and binding protein, on the mouse spermatozoon. J Electron Microsc (Tokyo) 1995;44:135–139. [PubMed] [Google Scholar]
- Tardif S, Wilson MD, Wagner R, Hunt P, Gertsenstein M, Nagy A, Lobe C, Koop BF, Hardy DM. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J Biol Chem. 2010;285:24863–24870. doi: 10.1074/jbc.M110.123125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thall AD, Maly P, Lowe JB. Oocyte gal alpha 1,3gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem. 1995;270:21437–21440. doi: 10.1074/jbc.270.37.21437. [DOI] [PubMed] [Google Scholar]
- Tian J, Gong H, Thomsen GH, Lennarz WJ. Gamete interactions in Xenopus laevis: identification of sperm binding glycoproteins in the egg vitelline envelope. J Cell Biol. 1997;136:1099–1108. doi: 10.1083/jcb.136.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhiro K, Ikawa M, Benham AM, Okabe M. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility. Proc Natl Acad Sci USA. 2012;109:3850–3855. doi: 10.1073/pnas.1117963109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topfer-Petersen E, Henschen A. Acrosin shows zona and fucose binding, novel properties for a serine proteinase. FEBS Lett. 1987;226:38–42. doi: 10.1016/0014-5793(87)80546-x. [DOI] [PubMed] [Google Scholar]
- Topfer-Petersen E, Henschen A. Zona pellucida-binding and fucose-binding of boar sperm acrosin is not correlated with proteolytic activity. Biol Chem Hoppe Seyler. 1988;369:69–76. doi: 10.1515/bchm3.1988.369.1.69. [DOI] [PubMed] [Google Scholar]
- Tsubamoto H, Hasegawa A, Nakata Y, Naito S, Yamasaki N, Koyama K. Expression of recombinant human zona pellucida protein 2 and its binding capacity to spermatozoa. Biol Reprod. 1999;61:1649–1654. doi: 10.1095/biolreprod61.6.1649. [DOI] [PubMed] [Google Scholar]
- Tulsiani DR, Nagdas SK, Cornwall GA, Orgebin-Crist MC. Evidence for the presence of high-mannose/hybrid oligosaccharide chain(s) on the mouse ZP2 and ZP3. Biol Reprod. 1992;46:93–100. doi: 10.1095/biolreprod46.1.93. [DOI] [PubMed] [Google Scholar]
- Urch UA, Patel H. The interaction of boar sperm proacrosin with its natural substrate, the zona pellucida, and with polysulfated polysaccharides. Development. 1991;111:1165–1172. doi: 10.1242/dev.111.4.1165. [DOI] [PubMed] [Google Scholar]
- Williams SA, Xia L, Cummings RD, McEver RP, Stanley P. Fertilization in the mouse does not require terminal galactose or N-acetylglucosamine on the zona pellucida glycans. J Cell Sci. 2007;120:1341–1349. doi: 10.1242/jcs.004291. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Keitaro M, Okabe M, Toshimori K, Nakanishi T, Kashiwabara S, Baba T. Acrosin accelerates the dispersal of sperm acrosomal proteins during acrosome reaction. J Biol Chem. 1998;273:10470–10474. doi: 10.1074/jbc.273.17.10470. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Fujihara Y, Ikawa M, Okabe M. Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa. Mol Biol Cell. 2012;23:2671–2679. doi: 10.1091/mbc.E11-12-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R. Zona-free hamster eggs: their use in assessing fertilizing capacity and examining chromosomes of human spermatozoa. Gamete Res. 1984;10:187–232. [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neil J, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 189–317. [Google Scholar]
- Yauger B, Boggs N, Dean J. Human ZP4 is not sufficient for taxon-specific sperm binding to the zona pellucida in transgenic mice. Reproduction. 2011;141:313–319. doi: 10.1530/REP-10-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa N, Kanai-Kitayama S, Kitayama T, Hamano A, Nakano M. Porcine zona pellucida glycoprotein ZP4 is responsible for the sperm-binding activity of the ZP3/ZP4 complex. Zygote. 2012;20:1–9. doi: 10.1017/S0967199411000608. [DOI] [PubMed] [Google Scholar]