Abstract
The ontogenetic development of the mental region still poses a number of unresolved questions in human growth, development and phylogeny. In our study we examine the hypotheses of DuBrul & Sicher (1954) (The Adaptive Chin. Springfield, IL: Charles) and Enlow (1990) (Facial Growth, 3rd edn. Philadelphia, PA: Saunders) to explain the presence of a prominent mental region in anatomically modern humans. In particular, we test whether the prominence of the mental region and the positioning of the teeth are both correlated with the developmental relocation of the tongue and the suprahyoid muscles inserting at the lingual side of the symphysis. Furthermore, we test whether the development of the mental region is associated with the development of the back of the vocal tract. Using geometric morphometric methods, we measured the 3D mandibular and tooth surfaces in a cross-sectional sample of 36 CT-scanned living humans, incorporating the positions of the tongue and the geniohyoid and digastric muscle insertions. The specimens' ages range from birth to the complete emergence of the deciduous dentition. We used multivariate regression and two-block partial least squares (PLS) analysis to study the covariation among the mental region, the muscle insertions, and the teeth both across and within age stages. In order to confirm our results from the 3D cross-sectional sample, and to relate them to facial growth and the position of the cervical column and the hyoid bone, we used 46 lateral radiographs of eight children from the longitudinal Denver Growth Study. The 3D analysis demonstrates that the lingual side of the lower border of the symphysis develops downwards and forwards. These shape changes are significantly correlated with the relocation of muscle insertion sites and also with the vertical reorientation of the anterior teeth prior to emergence. The 2D analysis confirms the idea that as the mental region prominence develops, the space of the laryngopharynx becomes restricted due to upper mid-face retraction and the acquisition of upright body posture. In agreement with the hypotheses of DuBrul & Sicher (1954) and Enlow (1990), our results suggest that the presence of a prominent mental region responds to the space restriction at the back of the vocal tract, and to the packaging of the tongue and suprahyoid muscles in order to preserve the functionality of the laryngopharynx during respiration, feeding and speech.
Keywords: early ontogeny, modern humans, muscles, symphyseal mental region, teeth
Introduction
The prominence of the mental region of the human mandible (Fig. 1) still poses a number of unresolved questions in human growth, development and phylogeny. Some authors have claimed that a prominent mental region is a valid character defining anatomically modern humans (e.g. Stringer et al. 1984). However, a protrusive mental region has been observed in various other extinct hominins (Ascenzi & Sergi, 1971a,b; Wolpoff, 1980; Wolpoff et al. 1981; Smith, 1984; Frayer et al. 1993; Lieberman, 1995; Rosas, 1995; Lam et al. 1996; Mallegni & Trinkaus, 1997), and so remains an ambiguous character in an evolutionary context (Lieberman, 1995, 1999). In contrast to a protrusive mental region, the inverted-T relief (Fig. 1) on the labial side of the symphysis (the chin), which is expressed permanently throughout ontogeny regardless of the degree of anterior mental protrusion, is seen as unique to modern humans (Schwartz & Tattersall, 2000; Tattersall & Schwartz, 2008).
Fig. 1.
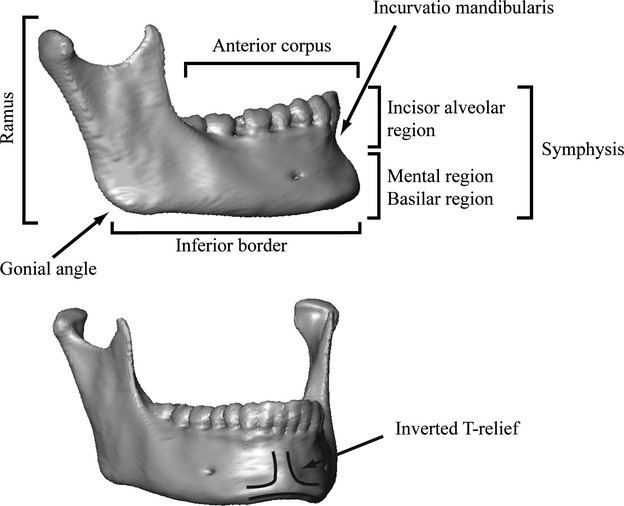
Mandible of an adult modern human with a description of the anatomical regions used in the text.
The factors that might have influenced the presence of a protruding mental region in modern humans have been debated with regard to tooth size reduction (Hrdlicka, 2000; Riesenfeld, 1969), speech articulation (Walkhoff, 1904; DuBrul & Sicher, 1954; Ichim et al. 2007; Daegling, 2012) and masticatory stresses (Howells, 1959; White, 1977; Daegling, 1993, 2012; Dobson & Trinkaus, 2002; Ichim et al. 2006; Gröning et al. 2011).
DuBrul & Sicher (1954) and Enlow (1990) have suggested that the prominence of the mental region is influenced by the development of the vocal tract musculature and the spatial restriction at the back of the oral cavity. However, these hypotheses have received little attention. This is probably because they require advanced techniques not only to study developmental biomechanics, but also to analyze and visualize ontogenetic shape changes. Only recently have methods to visualize shape changes over different developmental stages based on dense metric data become available. For instance, a comparative study between chimpanzee and human fetuses and infants has demonstrated that the development of the symphysis passes through similar stages in both species, but at different ages (Coquerelle et al. 2010a). These developmental steps involve a reorientation of the anterior teeth before eruption and a forward shift of the mental region. Both changes are likely caused by a common factor – the close spatial arrangement of the tongue, the suprahyoid muscles (geniohyoid and digastrics) and the hyoid bone (Coquerelle et al. 2010a). This hypothesis is plausible because: (i) it is bone that undergoes any remodeling required to drift vertically or horizontally the tooth socket before eruption (Enlow, 1990); (ii) changes in load (Carter, 1987; Carter et al. 1991), for instance brought about by modifications of the muscle force orientations (Hohl, 1983; van Spronsen et al. 1997), trigger bone remodeling that can alter the shape of the bone; and (iii) experiments have demonstrated that vocal tract musculature influences the anterior growth of the mandibular symphysis and the anterior teeth drift horizontally to keep pace (Spyropoulos et al. 2002; Liu et al. 2008). This hypothesis and those of DuBrul & Sicher (1954) and Enlow (1990) are examined together in the present study.
The human lower jaw and the appearance of a protrusive mental region coincided with a globularization of the braincase, flexion of the cranial base, a repositioning of the upper mid-face beneath the anterior braincase, a reduction of the anteroposterior dimension of the vocal tract, a marked extension of vertical facial growth, and an upright body posture (Enlow, 1990; Lieberman et al. 2000; McCarthy & Lieberman, 2001; Bastir & Rosas, 2004; Lieberman, 2011). By contrast, the relative size of the human tongue has not been reduced (Negus, 1949; Lieberman, 2011). As in non-human primates, the human tongue is long and flat at birth, occupying almost the entire mouth, and leaving little space for the airway between the back of the oral cavity and the cervical vertebrae. According to DuBrul & Sicher (1954), the prominence of the mental region would compensate the lack of space for the tongue through a labial ‘unrolling’ of the lingual side of the symphyseal lower border (Fig. 2). Such a mechanism may be involved in reorientation of the deciduous and permanent anterior teeth before eruption, as observed by Coquerelle et al. (2010a). The space restriction theory of DuBrul & Sicher (1954) is in accord with that of Enlow (1990), who suggested that the mental prominence is an adaption to cope with facial retraction and the development of an upright body posture. Indeed, the combination of the two is likely to limit the space for the tongue at the back of the vocal tract. The spatial restriction and the packaging of the vocal tract musculature thus appear to be important components of the ontogenetic development of the mandibular symphysis. This study aims to investigate these untested questions.
Fig. 2.
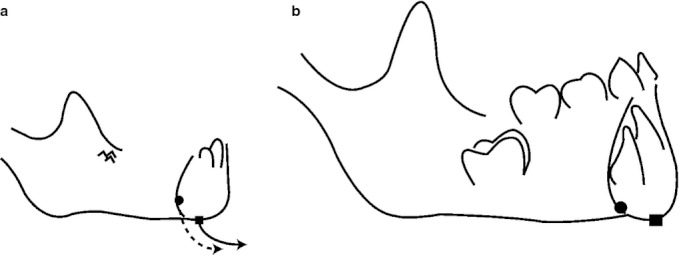
DuBrul & Sicher (1954) hypothesis of symphyseal development. The black circle and square are hypothetical bone markers. From a to b, these markers have been displaced along a downward and forward trajectory (‘labial unrolling’).
In this paper, we apply geometric morphometric methods to test the following hypotheses: (i) in modern humans the prominence of the mental region (as described by DuBrul & Sicher, 1954; Fig. 2) and the reorientation of the anterior teeth prior to eruption (Coquerelle et al. 2010a) are correlated with the labial unrolling of the tongue, the geniohyoid and the anterior digastric muscle insertions; and (ii) the prominence of the mental region is associated with a restriction of the space at the back of the vocal tract during development of the upright body posture and the backward positioning of the face (DuBrul & Sicher, 1954; Enlow, 1990). The aim of testing these hypotheses is to point to possible mechanisms by which the mental region becomes prominent. The unravelling of cause and effect will require experimentation, but this study aims to make an important contribution to the planning of such experiments.
In order to address the ontogenetic development of the mental region prominence, we briefly review growth-related shape changes of the mandible. We particularly focus on how the space at the back of the vocal tract, the ontogenetic changes of the tongue, the suprahyoid muscles and the hyoid bone may be associated with ontogenetic shape changes of the symphysis.
Growth-related shape changes of the mandibular symphysis
The development of the mental region prominence is generally described as a postnatal phenomenon at the labial side of the symphysis that includes resorption of bone at the incisor alveolar process but deposition of bone at the mental region (Kurihara et al. 1980; Enlow, 1990). Forward growth of the symphysis is associated with bone deposition on the labial and lingual side of the symphysis from fetal stages to the emergence of the second deciduous molar (dm2; Enlow & Harris, 1964; Mauser et al. 1975; Kurihara et al. 1980; Enlow, 1990). From the time dm2 emerges, the mental region continues to grow forward, while the incisor alveolar process moves backward as the labial side of this region becomes resorptive. This phenomenon is known as ‘bone remodeling reversal’. The other symphyseal regions remain depository. Even though the mental region becomes more prominent with the bone remodeling reversal at the incisor alveolar process (Kurihara et al. 1980; Enlow, 1990), the prominence of the mental region develops gradually from birth (Coquerelle et al. 2010a), when both the lingual and labial surfaces of the symphysis are fully depository. Differential rates of bone deposition are likely to exist between the mental region and the alveolar process before the initiation of the remodeling reversal, but no quantitative data on these differential growth rates are available.
DuBrul & Sicher (1954) suggested that the prominence of the mental region in modern humans originates from the downward and forward development of the lingual side of the lower border of the symphysis – also described as a labial unrolling (Fig. 2). The location of the depository and resorptive fields is not sufficient to confirm or reject DuBrul & Sicher's (1954) hypothesis, because in the earlier postnatal ages it would be a matter of differential rates of bone deposition along the lingual and labial surface between the mental region and the alveolar process (see above). However, what developmental process could bring about the labial unrolling of the lingual side of the symphyseal lower border (Fig. 2) and lead to the prominence of the mental region? It has been shown experimentally that the suprahyoid muscles (the anterior digastrics, the transverse mandibular, the mylohyoid) and the volume of the tongue (Spyropoulos et al. 2002; Liu et al. 2008) influence the anterior growth of the symphysis and the horizontal drift of the anterior dentition. In humans, during the first 2 years of life, the tongue changes to a rounder shape (Negus, 1949; Lieberman, 1984) via a larger increase in height than length. Although the cause-and-effect relationship has not been clearly demonstrated in the literature, the reshaping of the human tongue may modify the position of the suprahyoid muscle insertions at the lingual side of the mental region by pushing them downward. This is relevant to our first hypothesis, inasmuch as the relocation of the muscle insertions would modify the orientation of the muscular forces influencing the direction of the bone growth (Hohl, 1983; van Spronsen et al. 1997) as well as the drift of the teeth within the jaw. In addition, during the same developmental period, when the symphysis inclines posteriorly and the shape of the tongue becomes rounder, the hyoid bone relocates downwards and forwards relative to the inferior border of the symphysis (King, 1952). This also changes the spatial orientation of the muscular forces and may contribute to the reshaping of the mental region during ontogeny.
During the development of upright body posture in the first few years after birth, the oropharynx and the laryngopharynx are at the crossroads between the forward positioning of the cervical vertebrae alongside the foramen magnum (Aiello & Dean, 1990) and the backward positioning of the ethmomaxillary complex underneath the anterior cranial base, most likely resulting from flexion of the cranial base (Lieberman et al. 2000; McCarthy & Lieberman, 2001). The forward positioning of the cervical column is associated with the forward growth of the mandible, and the forward and downward displacement of the hyoid bone (Solow & Siersbæk-Nielsen, 1986). Hence, during early postnatal growth, the space for the airway may not increase substantially because the mandible, the tongue, the geniohyoid and the anterior digastric muscles are tightly packed, superiorly by the ethmomaxillary complex and posteriorly by the cervical column. The descent of the posterior base of the tongue, the hyoid bone and the larynx down the throat at the time when the shape of the tongue becomes rounder (Negus, 1949) may be related to the contrasting forces of horizontal and vertical growth of the vocal tract, which is essential to maintaining pharyngeal functions such as swallowing and breathing. This spatial restriction at the back of the vocal tract is relevant to the test of our second hypothesis.
Aim of the study
Our study aims to provide new insights into mandibular symphysis development. We explore the developmental pathway of the symphysis by visualizing in 3D the developmental relationship between both the tongue and suprahyoid muscle insertions and the prominence of the mental region in modern humans. We describe the development of human mandibular shape prior to the complete emergence of M1, the period during which the mental region projects forward relative to the alveolar process. We incorporate into our model the positions of the tongue, the geniohyoid and digastric (right and left anterior bellies) muscle insertions, as well as the arrangement of the deciduous and permanent dentition. Based on the geometric morphometric analysis of CT-derived mandibular and tooth surfaces, we visualize average ontogenetic shape changes and individual integration of growth processes to test the first hypothesis that the prominence of the mental region and the orientation of the anterior teeth prior to eruption are correlated with the downward and forward relocation (labial unrolling; Fig. 2) of the insertions of the tongue, the geniohyoid and the anterior digastric muscles. The hypothesis is falsified when the prominence of the mental region and the orientation of the teeth prior to eruption occur independently from the muscle insertion locations.
Comprehensive understanding of the spatial reshaping processes of several interacting anatomical units such as the bone, the teeth and the muscles insertions can only be approached in 3D (e.g. Coquerelle et al. 2010a). For both ethical and technical reasons there are no useful longitudinal datasets of humans (from birth to M1 emergence) available in 3D; this is one of the reasons why so little is known about early postnatal growth and development of this region. Therefore, in order to demonstrate the congruence of our results from cross-sectional data and to discuss them within the context of facial growth and the acquisition of the upright body posture, we include longitudinal cases in 2D (lateral radiographs). We explore ontogenetic shape changes of the mandible in relation to the development of the cranial base, the ethmomaxillary complex, the position of the cervical column and the hyoid bone using 2D geometric morphometric methods. We use regression analysis and thin-plate spline (TPS) deformation grids (Bookstein, 1991) to test our second hypothesis, that the prominence of the mental region is coordinated with a restriction of the space at the back of the vocal tract during development of the upright body posture and the backward positioning of the face. This second hypothesis is falsified when the deformation of the TPS grids does show a compression at the back of the vocal tract while the mental region becomes prominent.
Materials and methods
CT-scanned sample
Our cross-sectional sample consists of 36 soft channel-reconstructed CT scans (15 females, 21 males), with ages ranging from birth to approximately 5.5 years old. This sample is a subset of the sample used in Coquerelle et al. (2010a,b, 2011), which includes soft and hard channel-reconstructed CT scans for the same age range as the present study. In contrast to hard channel CT scan reconstruction, soft channel affords a wide range of gray values for visualization of soft tissues. Pixel size ranges from 0.23 to 0.50 mm, and slice thickness from 0.30 to 0.50 mm. We do not distinguish between males and females in this study because of the relatively small sample size. Also, in Coquerelle et al. (2011) we showed that although postnatal mandibular growth is sexually dimorphic, sex differences are very small compared with age differences and consist mainly of differences in developmental timing.
None of the specimens in the sample has a fully emerged M1. We subdivided the sample into three dental stages according to tooth emergence: DS1, before the complete emergence of the deciduous second incisor (di2; n = 17); DS2, after the emergence of di2 and prior to the full emergence of the deciduous second molar (dm2; n = 10); and DS3, after the emergence of dm2 and prior to the full emergence of M1 (n = 9).
These individuals had been referred for cranial trauma, inflammation of the maxillary sinuses or neonatal distress, but were found to be free of reportable abnormalities. The medical institutes, except for information about age and sex, anonymized the CT scans. French institutional boards approved the use of these data for our present purpose.
Radiographic sample
Our longitudinal sample of lateral radiographs includes eight infants (four males, four females) selected from the Denver Growth Study conducted by the Child Research Council, University of Colorado School of Medicine, between 1931 and 1966 (Maresh & Washburn, 1938; Maresh, 1948; McCammon, 1970; cited by Lieberman et al. 2001). Individuals were radiographed at a distance of 7.5 feet in lateral view. The age of the specimens ranged between 1 month and 5.75 years, and the sample was subdivided into the three dental stages described above. From the age of 1.75 months, the participants were radiographed in a seated position with a radiographic cephalostat, whereas young infants were hand-held (Lieberman et al. 2001).
Reconstruction of the mandibular surfaces, teeth and muscles
The half-maximum height protocol (Spoor et al. 1993) was used to reconstruct each mandibular surface from the CT scans using the software package amira 5.2 (Mercury Computer Systems, Chelmsford, MA, USA). This protocol samples the Hounsfield values on either side of the transition between two adjacent tissues and takes the value halfway between them as the threshold value. The youngest specimens (six infants between 0 and 0.4 years) had areas with different mineralization levels, requiring local adjustments of the threshold value. The reconstructed mandibular halves of the youngest specimens that showed incomplete ossification of the symphysis were fused virtually by cubic interpolation of the surface from each side of the symphyseal cartilage (amira 5.2). In addition to the reconstruction of the mandibular bone and teeth, we reconstructed the muscle of the floor of the mouth using the same protocol (Fig. 3a).
Fig. 3.
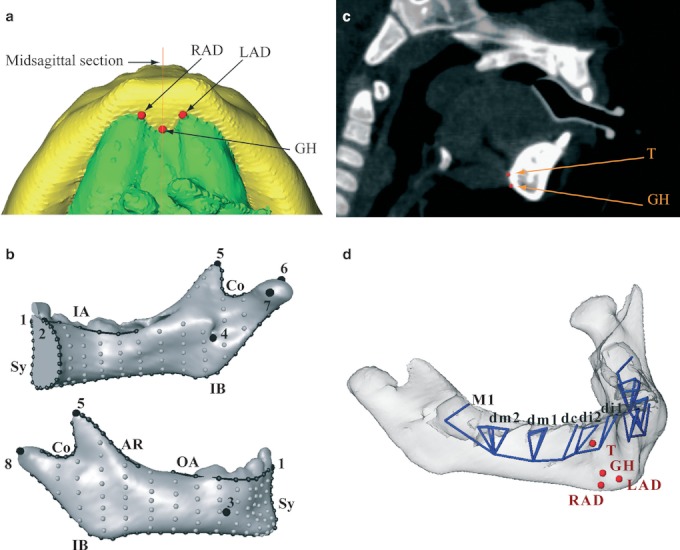
(a) Reconstruction of the mandibular corpus and the suprahyoid muscles (inferior view) together with the landmarks RAD (anterior belly of the right digastric muscle), LAD (anterior belly of the left digastric muscle) and GH (genio-hyoid muscle). (b) Landmark template on the right hemimandible of a 1-year-old specimen. Anatomical landmarks are large black dots, curve semi-landmarks are smaller black dots connected by black lines, and surface semi-landmarks are gray dots. Names of the landmarks and curves are as in Table 1. (c) The landmarks T (tongue) and GH on a sagittal section of a patient. (d) The mandibular template was supplemented with 38 deciduous tooth landmarks (blue vertices) and four muscle landmarks (red dots).
Landmarks and semi-landmarks
The mandibular 3D surface
Using the open-source software edgewarp3d (Bookstein & Green, 2002), a 3D template of 411 landmarks and semi-landmarks was warped onto each mandible to capture the mandibular surface geometry. This mandibular template derives from the template in Coquerelle et al. (2010a,b, 2011; Table 1; Fig. 3b) and was supplemented with four muscle landmarks (the tongue: T; the genio-hyoid: GH; and the right and left anterior bellies of the digastrics: RAD and LAD, respectively), 38 deciduous tooth landmarks (Fig. 3c,d) and 14 permanent tooth landmarks (S1). The points RAD and LAD are digitized at the anteromedial maximum of curvature of the lingual insertion at the symphysis (Fig. 3a). The points T and GH are digitized at the lingual insertion of the symphysis in the sagittal plane (Fig. 3c). Due to the resolution of the CT scans and the density of the muscle in this area, the accuracy of the 3D reconstruction of the muscle insertions varies. Thus, in order to simplify their digitization, the muscular insertions were considered single points as described above. The muscular landmarks are different from bony landmarks, and one has to be cautious when they are located in the immediate vicinity of each other (more details in Oxnard & O'Higgins, 2009). Their relative positions and even their relocations may not be homologous between species or within species, and thus geometric morphometric methods are not applicable. In our geometrical framework, muscular landmarks are located in an area without bony landmarks, and therefore there is no risk of non-homology. Tooth landmarks are the cusp tips and the deepest point of each tooth chamber. Figures 3d and S1 illustrate the landmark positions on the template.
Table 1.
| Landmarks and curves | Label in Figs 3b and 4 |
|---|---|
| Landmark points | |
| Infradentale | 1 |
| Linguale | 2 |
| Right mental foramina | 3 |
| Right mandibular foramina | 4 |
| Tip of the right coronoid | 5 |
| Top of the right condyle | 6 |
| Medial extremity of the right condyle | 7 |
| Lateral extremity of the right condyle | 8 |
| Prosthion | 9 |
| Nasospinale | 10 |
| Posterior nasale spine | 11 |
| Superior tip of the pterygomaxillary fissure | 12 |
| Sella | 13 |
| Clival point | 14 |
| Sphenoidale | 15 |
| Nasion | 16 |
| Superior border of the hyoid bone | 17 |
| Antero-inferior border of the cervical vertebra 2 | 18 |
| Opisthion | 19 |
| Basion | 20 |
| Landmark curves | |
| Midsymphysis | Sy |
| Right outer alveoalar | OA |
| Right inner alveoalar | IA |
| Right anterior ramus | AR |
| Right coronoid | Co |
| Right inferior border | IB |
| Palate | Pa |
| Anterior cranial base | ACB |
| Great sphenoidal wing | SW |
Radiographs of the craniofacial block
A 2D template of 68 landmarks and semi-landmarks (Table 1; Fig. 4) was chosen to capture the shape of the cranial base, the ethmomaxillary complex, the mandible, and the position of the cervical column and the hyoid bone. During digitization, we observed that the hyoid body is located directly below the gonial angle as described by King (1952), but also below the basilar border of the symphysis, whereas it has been described as located above it in the literature (King, 1952; Lieberman & Crelin, 1971). The young infants had been hand-held during the cephalographic acquisitions, consisting in pulling up the menton in order to orient the face as it is in older juveniles and adults with upward body posture. Therefore, the position of the hyoid bone relative to the inferior border of the symphysis was modified.
Fig. 4.
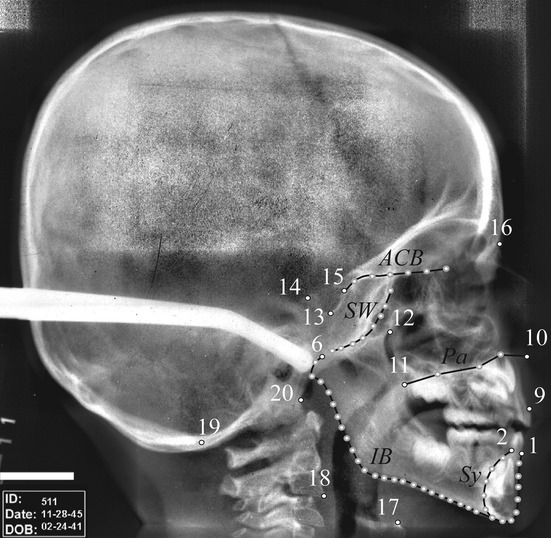
Landmark template on the craniofacial block of specimen 511 (4.75 years old) of the Denver Growth Study. Anatomical landmarks are small white dots with a black outline, curve semi-landmarks are gray dots connected by black lines. Names of the landmarks and curves are as in Table 1.
It was not possible to digitize the coronoid process as we did for the 3D study because its outline was not identifiable for all specimens due to the limited resolution of the cephalographs and the superimposition of tissues in this region. Landmarks and semi-landmarks were digitized using viewbox 4 software (dHAL Software, Kifissia, Greece).
After digitization, 2D and 3D semi-landmarks were allowed to slide along curves and surfaces to minimize the bending energy of the TPS interpolation function computed between each specimen and the sample Procrustes average (Bookstein, 1997; Bookstein et al. 1999; Gunz et al. 2005). After sliding, landmarks and semi-landmarks were treated as homologous points and converted to shape coordinates by Generalized Procrustes Analysis (GPA; Rohlf & Slice, 1990; Bookstein, 1996; Mitteroecker & Gunz, 2009). This involves rescaling the landmark coordinates so that each configuration has a unit centroid size (CS: square root of the summed squared Euclidean distances from all (semi)landmarks to their centroid; Dryden & Mardia, 1998). Then all configurations are translated and rotated to minimize the overall sum of the squared distances between corresponding (semi)landmarks.
3D analyses of the mandibular surfaces and tooth positions
The GPA is based only on the landmarks and semi-landmarks of the mandibular surface because the tooth landmarks are not present in all individuals due to different onsets of mineralization among the teeth. The tooth landmarks are scaled, translated and rotated according to the mandibular (semi)landmarks using the TPS interpolation function. Afterwards, we carried out a principal component analysis (PCA) of the matrix of shape coordinates augmented by a column of the natural logarithm of CS (lnCS) – corresponding to PCA in form space (Mitteroecker et al. 2004; 2005).
A sequence of mandibular surfaces was reconstructed to visualize size-related shape variation throughout growth. We first estimated several sets of mandibular, muscular and dental coordinates via local linear regressions of the Procrustes shape coordinates on chronological age (Bulygina et al. 2006; Coquerelle et al. 2011). Then the mandibular surfaces corresponding to the regression estimates were computed using the triangulated surface of one individual and the TPS as an interpolation function (Bookstein, 1991).
Two-block PLS analysis
The regression estimates visualize the average association between the muscle insertions, the shape of the symphysis and the tooth positions across the age stages in the sample. But this association does not necessarily imply a causal relationship, i.e. actual developmental integration. Statistical associations across individuals of the same developmental stage (i.e. same age range, same dental stage) are more likely to reflect integration due to developmental interactions and common developmental mechanisms (Mitteroecker et al. 2012). We thus use two-block PLS analysis (also called singular warp analysis when applied to Procrustes coordinates; Rohlf & Corty, 2000; Bookstein et al. 2003; Mitteroecker & Bookstein, 2007, 2008; Mitteroecker et al. 2012) to study integration within the same age stages. For our data, the amount of allometric shape variation within the DS1 subsample is similar to that of the combined DS2–DS3 subsample (Fig. 5a). Therefore, we used this subdivision: the DS1 subsample with specimens younger than 1.1 years; and the DS2–DS3 subsample with specimens ranging from 1.4 to 5.5 years old. As growth is substantial in each subsample, the covariance among morphological units will tend to be very high due in large part to that joint ‘dependence’ (Bookstein, 1991). Therefore, growth must be removed because it affects all the developmental units under study, otherwise the observed correlation is unreliable (Mitteroecker & Bookstein, 2007). Thus, we regressed out size (lnCS) from the shape coordinates in each subsample to remove allometric shape variation and variation in developmental timing (Mitteroecker & Bookstein, 2007; Mitteroecker et al. 2012). We used the di2s in the DS1 subsample and the permanent second incisors (I2s) in the DS2–DS3 subsample in order to assess the association between the teeth, the symphyseal bone and the muscle insertions. We decided upon a single superimposition of all landmarks in each subsample instead of three separate ones for each block because the relative position and orientation of these three blocks are important aspects of the biology of the mandibular symphysis.
Fig. 5.
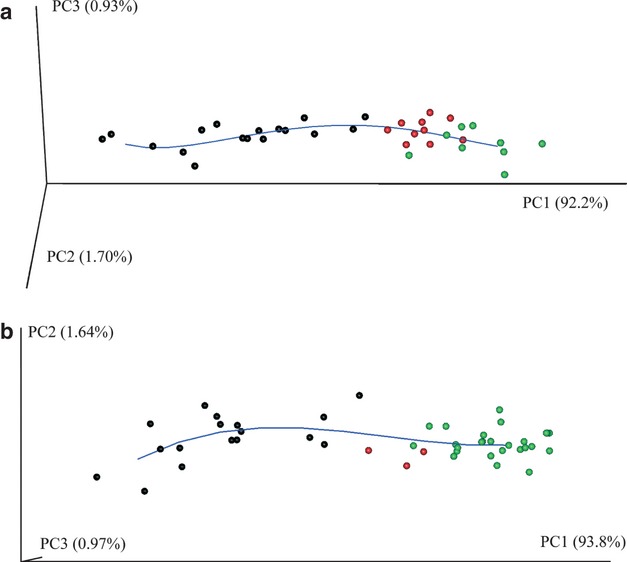
(a) Scatterplot of the first three principal components (PCs) of Procrustes form space of the mandibular surfaces, muscles and tooth coordinates. The blue line is a local linear regression through these points; it represents the average ontogenetic trajectory. (b) Scatterplot of the first three PCs of Procrustes form space of the 2D craniofacial block. The blue line is a local linear regression through these points representing the average ontogenetic trajectory.
We assessed the strength of the developmental association between the first pair of singular warps (SW1s) corresponding to the Procrustes variables of the symphyseal bone, the muscle insertion and the teeth (di2s and I2s) via Pearson product-moment correlation coefficients (r). For the significance of the PLS test, the original labels of the second block of Procrustes coordinates are permuted and the r between the SW1s is calculated. This is done 1000 times. The proportion of r from the permuted PLS that is equal to or larger than the r from the original PLS is equal to the significance level (Good, 2000).
2D analyses of the craniofacial block
The GPA of the craniofacial block is based on all landmarks and semi-landmarks of the longitudinal sample. To remove variation in individual average shape, we subtracted the grand mean of each individual from the Procrustes coordinates of all age stages for that individual and carried out a form space PCA of the residuals. Afterwards, we reconstructed a sequence of craniofacial blocks via local linear regressions of the Procrustes shape coordinates on chronological age. In order to visualize the shape changes of the craniofacial block from each stage to its next older stage, the regression estimates were compared using TPS deformation grids (Bookstein, 1991). Visualizations of shape differences are exaggerated by an appropriate factor to ease interpretation.
Results
The mandibular surface and tooth positioning
The first three principal components (PC) in Procrustes form space account for approximately 95% of the total form variance (Fig. 5a). PC1 alone accounts for 92.2% of total form variance and represents allometric form change (correlation of PC1 and lnCS: r = 0.993). PC2 and PC3 characterize non-size-related shape changes. The scatterplot of the PC scores displays a curvilinear ontogenetic trajectory, indicating a combination of different linear growth processes (Huttergger & Mitteroecker, 2011).
To analyze these different trends, (Fig. 6i,ii) illustrates the ontogenetic shape changes of the mandible throughout different developmental stages along the ontogenetic trajectory (Fig. 5a): a, birth; b, halfway through DS1; c, at the end of DS1; d, halfway through DS2; and e, halfway through DS3. In order to focus on the shape changes over growth, the shapes depicted are regression estimates, scaled to CS = 1.0. This figure considers only the deciduous dentition, as it is not possible to include permanent teeth in this statistical framework. The Supporting Information videos S2–S4 offer a comprehensive visualization of the coordinated symphyseal shape changes, tooth reorientations and muscle insertion displacements. The videos S2 and S3 correspond to linear interpolations between the regression estimates presented in (Fig. 6i,ii). The video S4 includes the permanent dentition (S1) but is constrained to the age range from mid-DS1 to mid-DS3.
Fig. 6.
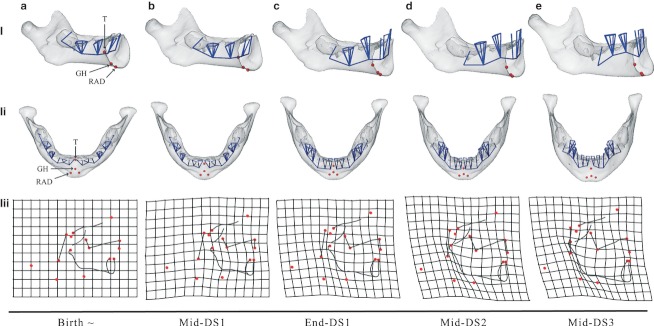
Morphs of the human mandibular surfaces (i, ii) and the craniofacial block (iii) from birth to mid-DS3 based on the regression in Fig. 5a (cross-sectional) and Fig. 5b (longitudinal). They are scaled to constant overall CS. (i, ii) Translucent surfaces enable the visualization of the position and orientation of deciduous teeth and the floor of the tooth chambers (blue), as well as muscle landmarks (red): the tongue (T), the genio-hyoid muscle (GH), and the anterior belly of the right and left digastric muscles (RAD and LAD). (iii) TPS deformation grids of the craniofacial block from each age stage to its next older stage. TPS grids visualize developmental shape change (exaggerated by factor of 2) after birth. Red dots: landmarks as in Fig. 4 and Table 1.
Table 2 provides a detailed description of the mandibular shape changes observed in Fig. 6i,ii and Supporting Information videos S2 and S3. The downward and forward relocation of the muscle insertions is coordinated with: (i) the downward and forward projection of the basal symphysis; (ii) the flattening and the widening medio-laterally of the labial side of the mental region; and (iii) the reorientation of the deciduous incisors to a vertical position prior to eruption.
Table 2.
Description of mandibular shape differences between subsequent age groups. For visualization see Fig. 6i,ii
| Birth | The symphysis is vertical and the anterior teeth have an upward-forward orientation (Fig. 6ai). The floor of the tooth chambers is located below the tongue (T). On the labial side of the symphysis, the mental region has a pointed configuration anteriorly and, on the lingual side, the triangle formed by GH, RAD and LAD is roughly equilateral (Fig. 6aii). |
| From birth to mid-DS1 | The floor of the tooth chambers, the landmarks T, GH, RAD, LAD and the basal symphysis simultaneously move downward and forward (Fig. 6bi) in the Procrustes coordinate system. The deciduous incisors reorient to an approximately vertical position. The distance between the tongue and the genio-hyoid decreases, and the floor of the tooth chambers is now at the same level as T (Fig. 6bi). The triangle GH-RAD-LAD and the mental region retain the same configuration as at birth (Fig. 6bii). The ramus heightens supero-posteriorly; it also broadens antero-posteriorly more than the corpus does. |
| From mid-DS1 to end-DS1 | While RAD and LAD maintain their position, T and GH continue to displace both inferiorly and ventrally (Fig. 6ci,ii). The triangle GH-RAD-LAD is no longer equilateral (Fig. 6cii). Simultaneously, at the labial side of the symphysis, the mental region flattens and widens medio-laterally (Fig. 6cii). The teeth continue to move mesially, and the floor of the incisor, canine and dm1 chambers move upward, away from the inferior border of the mandible, during tooth emergence (Fig. 6ci). The ramus continues to increase supero-posteriorly but becomes relatively narrower antero-posteriorly. The height of the symphysis also increases (Fig. 6ci) and becomes relatively narrower antero-posteriorly when the deciduous incisors emerge (Fig. 6ci). |
| From end-DS1 to mid-DS2 | T and GH continue to displace downward and forward (Fig. 6di), while RAD and LAD remain fixed (Fig. 6dii). The triangle GH-RAD-LAD becomes much more obtuse. Simultaneously, the mental region widens medio-laterally (Fig. 6dii). The deciduous incisors, canines and first molars have now fully erupted. |
| From DS2 to DS3 | When dm2 reaches its full occlusal position, the landmarks GH, RAD and LAD simultaneously project anteriorly as the mental region projects forward (Fig. 6d,ei). The distance between T and GH increases (Fig. 6d,ei.ii). The ramus rotates toward the corpus leading to a more closed gonial angle. Simultaneously, the gonial region becomes less ventrally pointed. The ‘incurvatio mandibularis’ (Hublin & Tillier, 1981), the inward curvature of bone below the labial alveolar margin, becomes well distinguished as the symphyseal alveolar region displaces backward (Fig. 6ei). |
Figure 7 (see also Supporting Information video S2) illustrates the displacement of the muscle insertions from birth to halfway through DS3. These landmarks follow a downward and forward curvilinear trajectory. Simultaneously with the emergence of the deciduous anterior teeth, the floor of the permanent anterior tooth chambers moves downward along with landmarks T and GH (Supporting Information video S4). When dm2 reaches its full occlusal position, the floor of the permanent anterior tooth chamber moves anteriorly along with the forward projection of the mental region and muscle insertions. This brings the permanent anterior teeth underneath the deciduous teeth.
Fig. 7.
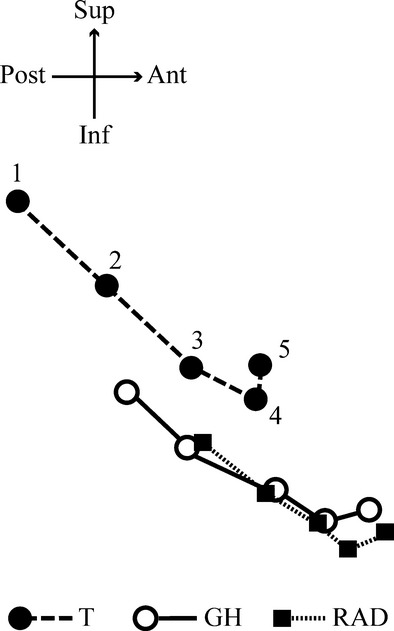
Trajectories of the three muscle landmarks from birth (1), mid-DS1 (2), end-DS1 (3), mid-DS2 (4), to mid-DS3 (5). The represented shape coordinates are based on a superimposition of all landmarks and semi-landmarks. Ant, anterior; Inf, inferior; Post, posterior; Sup, superior.
Table 3 lists the Pearson product-moment correlation coefficients (r) between the first pair of singular warps (SW1s) corresponding to the Procrustes coordinates of the vestibular surface of the symphysis, the muscles and the teeth (di2s and I2s). The first pair of SWs accounts for more than 45% of the summed squared covariances between these Procrustes coordinates (Table 3), and is visualized as single shape deformation in Fig. 8a–e. In the DS1 and the DS2–DS3 subsamples, the high and significant correlations (r > 0.70) confirm that the forward and downward relocation of the muscles insertions, the projection of the mental region and the repositioning of the teeth prior to emergence are tightly linked (Table 3; Fig. 8a–e). Also in both infant groups, once we regressed out the part of tooth positions and orientations explained by the displacement of the muscle insertions, the correlation between the symphyseal variables and the tooth residuals is low (r < 0.42) and non-significant (Table 3).
Table 3.
Correlation coefficients between the first pair of singular warps 1 (SW1), computed for two developmental periods: through the dental stage 1 (DS1) and through the dental stages 2 and 3 (DS2–DS3)
| DS1 | DS2–DS3 | ||||||
|---|---|---|---|---|---|---|---|
| Block 1 | Block 2 | SW1s (%) | r | Figure | SW1s (%) | r | Figure |
| Bone | Muscle | 55.0 | 0.929** | 8a | 59.3 | 0.905** | 8d |
| Muscle | Teeth | 54.0 | 0.703* | 8b | 45.2 | 0.826* | 8e |
| Bone | Teeth | 47.3 | 0.834* | 8c | 57.8 | 0.774* | 8f |
| Bone | TeethMuscle | 33.0 | 0.376 | – | 37.0 | 0.415 | – |
P-value < 0.01.
P-value < 0.001.
For a visualization of the correlated shape changes between the two blocks of shape variables, see Fig. 8.
Fig. 8.
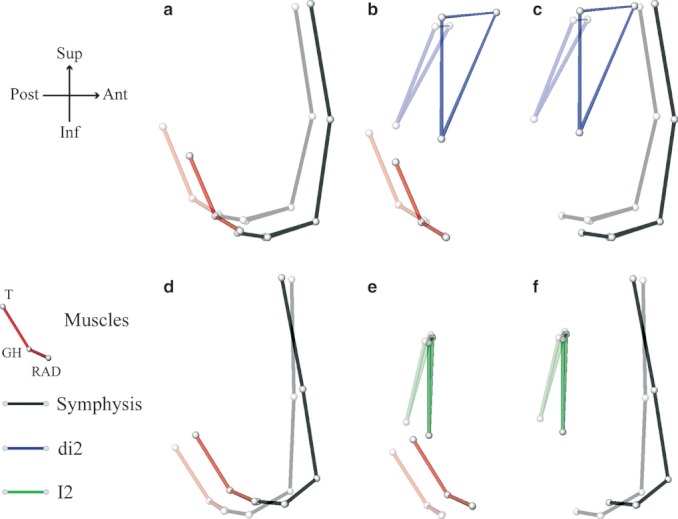
Correlated shape changes along singular warp 1 between: (1) the muscles and the symphyseal bone (a, d); (2) the muscle and the teeth (b, e); and (3) the teeth and the symphyseal bone (c, f).
The craniofacial block
Approximately 96% of the total Procrustes form variance is captured by the first three PCs (Fig. 5b). Similar to the 3D analysis of the mandibular surface, PC1 comprises size-related shape changes (r = 0.996). Likewise, a combination of different linear growth processes characterizes the ontogeny of the craniofacial block prior to M1 emergence.
(Figure 6iii) illustrates the shape changes of the craniofacial block along the ontogenetic trajectory (Fig. 5b) for the same developmental stages as the 3D surface morphs of the mandible. These shapes are regression estimates scaled to CS = 1.0. The 2D mandibular shape changes match those of the 3D analysis: (i) at birth, the symphysis is orthogonal to the inferior border (Fig. 6ai,iii); (ii) from birth to mid-DS1, the ramus enlarges antero-posteriorly more than the corpus does as shown by the squares of the TPS grid that are larger at the ramus than at the corpus (Fig. 6bi,iii); and (iii) from mid-DS2 to mid-DS3, the prominence of the chin becomes more marked with a well-distinguished ‘incurvatio mandibularis’ (Hublin & Tillier, 1981), when the ramus rotates towards the corpus while it grows backwards and upwards (Fig. 6d,ei,iii).
The cranial base flexes and the ethmomaxillary complex rotates backwards relative to the posterior cranial base during the period from birth to mid-DS3 (Fig. 6a–eiii). Once the mandible is no longer retrognathic, i.e. by mid-DS1 (Fig. 6biii), the incisor alveolar process of the mandible follows the lingual displacement of that of the ethmomaxillary complex (Fig. 6c–eiii), in accordance with Enlow's (1990) counterpart principle.
At DS1, the average length of the oropharynx, measured as the distance between posterior nasal spine and basion (l = 25.0 ± 1.9 mm), is larger than its average height, measured between posterior nasal spine and the hyoid bone (h = 22.7 ± 2.6 mm). Conversely at DS3, the average height of the oropharynx (h = 31.3 ± 2.8 mm) is larger than its average length (h = 27.6 ± 1.6 mm). From DS1 to DS3, oropharyngeal length increases 10.4%, while its height increases 37.4% on average. Simultaneously, the angle between the clival plane (basion, clival point) and the back of the ethmomaxillary complex (pterygomaxillary fissure, posterior nasal spine) decreases substantially (DS1: 84.1 ± 5.4 °; DS3: 70.7 ± 5.8 °). Therefore, the oropharynx reduces antero-posteriorly (relatively) during growth due to the conjoint effect of posterior rotation and vertical growth of the ethmomaxillary complex (see Supporting Information S5). By the end of DS1, vertical growth of the ethmomaxillary complex is predominant over the horizontal growth of the oropharynx (Fig. 6c–eiii).
Both in the 2D and the 3D analyses, throughout DS2 and DS3, the ramus rotates towards the corpus as it shows a greater heightening than lengthening, leading to the closure of the gonial angle (Fig. 6d,ei,iii). This discrepancy between vertical and horizontal growth is associated with the greater vertical growth of the ethmomaxillary complex relative to horizontal growth of the pharynx (Fig. 6d,eiii; Enlow, 1990).
As the ethmomaxillary complex rotates backwards and the cervical column is displaced forwards, the length of the oropharynx slightly increases (see above), but that of the laryngopharynx, measured between the second cervical vertebrae (CV2) and the hyoid bone, stagnates (DS1: 20.7 ± 2.2 mm; DS3: 19.6 ± 1.4 mm), in line with other studies (King, 1952; Durzo & Brodie, 1962). In addition to the linear measurements of the laryngopharynx, the compression and shearing of the TPS grid clearly show the horizontal space restriction (Fig. 6c–eiii). Coordinated with this horizontal space restriction, the prominence of the mental region becomes noticeable as the hyoid bone moves downwards and forwards, and the cervical column is displaced forwards.
Discussion
Our 2D and 3D analyses addressed the ontogenetic development of the mental region prominence in modern humans. We tested the two hypotheses: (i) that the prominence of the mental region and the reorientation of the anterior teeth prior to eruption are correlated with the labial unrolling of the tongue, the geniohyoid and the anterior digastric muscle insertions; and (ii) that the prominence of the mental region is associated with a restriction of the space at the back of the vocal tract during development of the upright body posture and the backward positioning of the ethmomaxillary complex.
Our study is based on cross-section 3D data as well as on longitudinal 2D data. The comprehensive visualization of tooth positioning, muscles insertion relocation at the symphysis and their correlation with the prominence of the mental region could only be approached in 3D. However, cross-sectional data can be biased by inter-individual variation, and hence we demonstrated the congruence of the 3D shapes changes observed in lateral view with those obtained from longitudinal 2D data: the 3D and the 2D visualizations of the mandibular shape change (regression estimates) match each other over developmental time (Fig. 6i,iii). We are thus able to discuss the 3D analysis within the context of facial growth and the acquisition of the upright body posture using the 2D longitudinal data.
We found that the prominence of the mental region is significantly correlated with the downward and forward displacements of the tongue (T), the geniohyoid (GH) and the anterior digastric (RAD and LAD) muscle insertions, both along the average ontogenetic trajectory and within age stages. This result is consistent with DuBrul & Sicher's (1954) assumption that the prominence of the mental region originates form the labial unrolling of the lingual side of the symphyseal lower border (Fig. 2). In addition, we found that the prominence of the mental region is associated with the horizontal space restriction at the back of the vocal tract (DuBrul & Sicher, 1954) due to the backward rotation of the ethmomaxillary complex and the forward positioning of the cervical column related to development of the upright body posture. The result is also in agreement with Enlow's (1990) hypothesis that the protrusive mental region is an adaptation to cope with the facial retraction of which the backward rotation of the ethmomaxillary complex is a major component during human evolution and ontogeny (Lieberman et al. 2000; McCarthy & Lieberman, 2001).
In detail, the 3D analysis shows that the prominence of the mental region is correlated with the downward and forward development of the lingual side of the symphyseal inferior border (Figs 6i,ii and 8a,d; Table 3), and that development of the protrusive mental region passes through three stages: (i) from birth to end-DS1, a forward and downward projection; (ii) from end-DS1 to mid-DS2, the widening of the labial base; and (iii) at the transition between DS2 and DS3, a forward projection. Although the symphyseal shape changes seem consistent with bone remodeling reversal initiating during this latter stage (Kurihara et al. 1980), our results show that the mental region starts to project during DS1 (see also Coquerelle et al. 2010a). Hence the appearance of the prominence is likely to involve differential rates of bone deposition at the labial side between the mental region and the alveolar process before the initiation of the remodeling reversal.
These differential rates of bone deposition are particularly plausible when one analyzes the relative positioning of the incisor alveolar process of the mandible with respect to that of the ethmomaxillary complex (Fig. 6iii). Once the mandible is no longer retrognathic by mid-DS1, the incisor alveolar process follows the backward positioning of the ethmomaxillary complex linked to the flexion of the cranial base. Kurihara et al. (1980) and Enlow (1990) mentioned that the positioning of the alveolar process of the mandible is accompanied by bone resorption at the labial surface, and relates to the overbite and overjet incisor relationship. However, this incisor relationship is established during DS1, when these teeth come into occlusion and thus before the initiation of the reversal remodeling. Therefore, while the mental region continues to develop forwards, a particular rate of bone deposition at the incisor alveolar process needs to exist if this area is to keep pace with the ethmomaxillary complex, and assure the establishment of the overbite and overjet relationship of the deciduous incisors. More data, however, are needed to adequately test this possible developmental mechanism.
We further demonstrated that the reorientation of both the deciduous and permanent anterior teeth is highly correlated with the appearance of the mental prominence (Table 3). That covariation was shown to be tightly bound to the relocation of the muscle insertions at the lingual side of the symphysis. Indeed, the correlation between the teeth and the symphyseal bone drops if the part of tooth positions and orientations explained by the displacement of the muscle insertions is regressed out from the tooth coordinates (Table 3). Before emergence, teeth have very little capacity to influence bone remodeling (Enlow, 1990). They can only be moved by displacement processes, either through remodeling of an individual alveolar socket or by displacement of the entire arch as a unit. It is the bone that must undergo any remodeling required (Enlow, 1990). Bone remodeling is triggered by changes in loads (Carter, 1987; Carter et al. 1991). Such changes may be brought about by the relocation of the muscles insertions (Figs 6i and 8; Support Information videos S2, S4), combined with the modifications of the orientation of the muscles forces (Hohl, 1983; van Spronsen et al. 1997) related to the displacement of the hyoid bone relatively to the lingual side of symphysis (Fig. 6iii). Therefore, the tongue and the suprahyoid muscles may be a major component in triggering the position and orientation of the anterior teeth through the bone remodeling that shapes the prominence of the mental region. Such a mechanism may be also essential to the transition from deciduous to permanent occlusion, including exfoliation, by repositioning the permanent anterior teeth underneath the deciduous precursors (Supporting Information video S4). Here too, more data are needed to explore this developmental process.
The 2D analysis demonstrates that the prominence of the mental region is coordinated with the horizontal space restriction at the back of the vocal tract, in agreement with the theories of DuBrul & Sicher (1954) and Enlow (1990). The laryngopharynx and the tongue, the suprahyoid muscles and the hyoid bone – functional matrices of the mandible – are tightly packed by the ethmomaxillary complex and the cervical column as the two displace in opposite directions prior to M1 emergence. While the alveolar region of the symphysis follows the posterior development of the ethmomaxillary complex to afford occlusion, the inferior border develops downwards and forwards via the hyoid bone, the tongue and the forward displacement of the cervical column (Fig. 6iii; Solow & Siersbæk-Nielsen, 1986) during the acquisition of the upright body posture (Enlow, 1990).
The downward and forward relocation of the hyoid bone is associated with the absolute height increase of the oropharynx in addition to the forward positioning of the laryngopharynx (Fig. 6c–eiii). Because they belong to the same counterpart, the absolute height increase of the oropharynx may result from that of the tongue when it changes from a flat ape-like shape to a rounder shape. The base of the tongue descends into the throat, simultaneously pushing the hyoid bone (Negus, 1949; Lieberman, 1984).
Because of the close anatomical and temporal relationship, we suggest that the displacements of the tongue and the suprahyoid muscle insertions are associated with the forward and downward displacement of the hyoid bone (Fig. 6d,eiii) and the reshaping of the tongue (Fig. 9a,b). The lower border of the symphysis would expand outwards via the muscles insertions, contributing to the projection of the mental region in order to provide space for the muscles, prevent obstruction of the laryngopharynx, and to maintain its functionality during breathing, feeding and speech. Data on orientations and forces of the tongue and suprahyoid muscles at different ages from birth onwards are needed in order to test this hypothesis biomechanically.
Fig. 9.
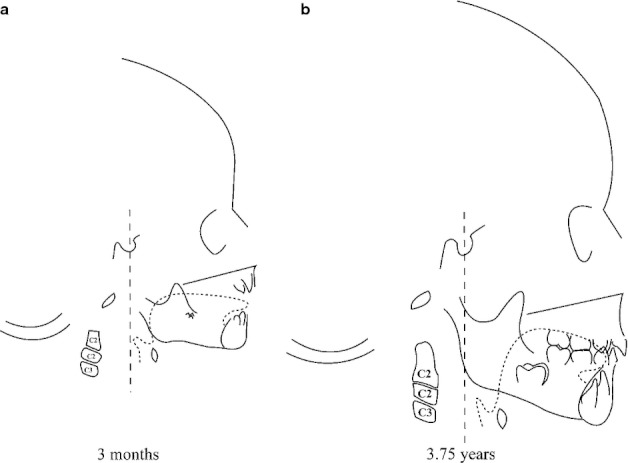
Schemes of specimen 064 of the Denver Growth Study, radiographed at age 3 months (a) and 3.75 years (b). Dashed lined: tongue. C2 and C3: cervical vertebrae 2 and 3.
Recently, Gröning et al. (2011) showed that a vertical symphysis with a protrusive mental region, which characterizes modern humans in contrast to other hominids, improves the resistance to mandibular loads, particularly to coronal bending and dorso-ventral shear. This is interesting because the shortening of the human mandible could be associated with an increase of coronal bending (Daegling, 1993). In particular, Gröning et al. (2011) showed that a vertical symphysis with a protrusive mental region has high resistance to loads during biting at the anterior dentition (incision). The orientation of the teeth has a role in the distribution of the high stresses produced during biting both in the teeth and their supporting structure. Because the deciduous incisors become functional simultaneously with the forward projection of the mental region, and because the orientation of the incisors is tightly bound to the development of the mental region, the biomechanical properties of a protrusive mental region during biting are likely to be rooted very early during postnatal ontogeny. However, our results show that the development of the mental prominence is associated with the displacement of the tongue and suprahyoid muscle insertions, and the repositioning of the laryngopharynx. Therefore, considering the prominence of the mental region as an adaptation to loads during incision and mastication seems too limited with respect to the development and the maintenance of the functions of breathing, feeding and speech supplied by the laryngopharynx and these muscles. Also, the authors did not consider the genioglossus muscle and the muscles of the floor of the mouth as a potentially influencing factor in their simulation study.
In parallel with the development of mastication, articulated language develops once the larynx and the hyoid bone have moved down the neck during postnatal life to reach the adult level at about 2 years old (Negus, 1949; Carlsöö & Leijon, 1960; Roche & Barkla, 1965; Westhorpe, 1987). Consequently, the related shape changes of the mandibular components may accommodate the functional loading increase related to mastication and speech. Ichim et al. (2007) have demonstrated that the limits of strain concentrations produced by the oblique contractions of the genioglossus muscle during speech occur at the outline of the inverted-T relief (chin) over the labial surface of the adult prominent mental region, but have not found convincing results for any other mechanical reasons for a prominent mental region. The extent to which the inverted-T relief is associated with the same factors that leads to the prominence of the mental region is still unknown and requires further study.
In fetal and neonates Pan troglodytes, the development of the symphysis passes through similar stages as those viewed in modern humans after birth (Coquerelle et al. 2010a). Falk (1975) observed that the position of the hyoid bone is anterior to the gonial angles in neonate chimpanzees, as in humans after 2 years. In the present study, we demonstrated that the downward and forward repositioning of the hyoid bone covaries with the forward development of the mental region in modern humans. Still in humans, we showed that this covariation includes other changes related to the cranial base, the cervical column and the ethmomaxillary complex. Further investigations comparing the two species would help our understanding of the evolutionary shape change of the human symphysis.
Conclusions
Our study provides new insights into the development of the mandibular symphysis and demonstrates that the lingual side of the symphyseal border develops labially, as suggested by DuBrul & Sicher (1954), while the tongue and suprahyoid muscle insertions relocate downwards and forwards. Moreover, this integration involves the reorientation of the anterior teeth prior to emergence. Our study is in accord with the hypothesis that the mental region is an adaptation to cope with facial retraction and the acquisition of the upright body posture in order to maintain the laryngopharyngeal space and functionality (DuBrul & Sicher, 1954; Enlow, 1990).
Acknowledgments
The authors thank J. Treil (Clinique Pasteur, Toulouse); J Braga (University Paul Sabatier, Toulouse); F. Brunelle, N. Boddaert, J.-M. Debaets, C. Leroy and D. Gustave (AP-HP Necker, Paris); and V. Dousset, C. Douws, C. Thibaut and E. Gatuing (C.H.U. Pellegrin, Bordeaux) for access to their CT datasets and collections. Thanks also to Dan Lieberman for the access to the Denver Growth Study. The authors are grateful to M. Bastir, J. Braga, M. Frelat and D. Halazonetis for discussions; and thank A.G. Drake for assistance in editing the final edition of the manuscript. Finally, the authors thank Dan Lieberman and the two anonymous reviewers for their helpful comments on an earlier draft of this manuscript. This research has been supported by the Fondation Fyssen and the EU FP6 Marie Curie Actions MRTN-CT-2005-019564 (EVAN; http://www.evan.at), and Cátedra Dental Implants and Biomaterials SA (Prof. JC Prados-Frutos, University Rey Juan Carlos).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Mandibular template as in Fig. 3d with the 38 deciduous tooth landmarks (blue vertices) and the four muscle landmarks (red dots), but augmented with the 14 permanent tooth landmarks (green vertices).
Video S2. Morph of the human mandibular surface from birth to mid-DS3, right lateral view.
Video S3. Morph of the human mandibular surface from birth to mid-DS3, antero-superior view.
Video S4. Morph of the human mandibular surface from mid-DS1 to mid-DS3, right lateral view.
Fig. S5. Illustration of the relative reduction of the oropharynx length (Ba-NPS segment) during dental stages (DS)1, 2 and 3. Usually the ethmomaxillary complex (EMC) is described as growing away from the cranial base (e.g. Enlow, 1990), but the distance between basion (Ba) and sella (S) increases as much as that between basion and the posterior nasal spine (PNS) during the same developmental period (on average 10%).We therefore used Bookstein's (1991) base-line registration method to show that the relative decrease of the oropharynx length results from both the backward rotation and the vertical elongation of the EMC (S-PNS segment).
References
- Aiello L, Dean C. An Introduction to Human Evolutionary Anatomy. New York: Academic Press; 1990. [Google Scholar]
- Ascenzi A, Sergi A. Il giacimento con mandibola neandertaliana di Archi (Reggio Calabria) Accd Naz Lincei. 1971a;50:763–771. [Google Scholar]
- Ascenzi A, Sergi A. A new Neanderthal child mandible from an Upper Pleistocene site in southern Italy. Nature. 1971b;233:280–283. doi: 10.1038/233280a0. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. Facial heights: evolutionary relevance of postnatal ontogeny for facial orientation and skull morphology in humans and chimpanzees. J Hum Evol. 2004;47:359–381. doi: 10.1016/j.jhevol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Bookstein FL. Biometrics, biomathematics and the morphometric synthesis. Bull Math Biol. 1996;58:313–365. doi: 10.1007/BF02458311. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med Image Anal. 1997;1:225–243. doi: 10.1016/s1361-8415(97)85012-8. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Green WDK. 2002. Users Manual, EWSH3.19. http://ftp://brainmap.med.umich.edu/pub/ewsh.3.19.manual.
- Bookstein FL, Schaefer K, Prossinger H, et al. Comparing frontal cranial profiles in archaic and modern Homo by morphometric analysis. Anat Rec (New Anat) 1999;257:217–224. doi: 10.1002/(SICI)1097-0185(19991215)257:6<217::AID-AR7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Gunz P, Mitteroecker P, et al. Cranial integration in Homo: singular warps analysis of the midsagittal plane in ontogeny and evolution. J Hum Evol. 2003;44:167–187. doi: 10.1016/s0047-2484(02)00201-4. [DOI] [PubMed] [Google Scholar]
- Bulygina E, Mitteroecker P, Aiello L. Ontogeny of facial dimorphism and patterns of individual development within one human population. Am J Phys Anthropol. 2006;131:432–443. doi: 10.1002/ajpa.20317. [DOI] [PubMed] [Google Scholar]
- Carlsöö S, Leijon G. A radiographic study of the position of the hyo-laryngeal complex in relation to the skull and the cervical column in man. Trans R Sch Dent Umea (Stockh) 1960;5:13–35. [Google Scholar]
- Carter DR. Mechanical loading history and skeletal biology. 1. Biomechanics. 1987;20:1095–1109. doi: 10.1016/0021-9290(87)90027-3. [DOI] [PubMed] [Google Scholar]
- Carter DR, Wong M, Orr TE. Musculoskeletal ontogeny, phylogeny and functional adaptation. J Biomech. 1991;24(S1):3–16. doi: 10.1016/0021-9290(91)90373-u. [DOI] [PubMed] [Google Scholar]
- Coquerelle M, Bookstein FL, Braga J, et al. Fetal and infant growth patterns of the mandibular symphysis in modern humans and chimpanzees. J Anat. 2010a;217:507–520. doi: 10.1111/j.1469-7580.2010.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquerelle M, Bayle P, Bookstein FL, et al. The association between dental mineralization and mandibular form: a study combining additive conjoint measurement and geometric morphometrics. J Anthropol Sci. 2010b;88:129–150. [PubMed] [Google Scholar]
- Coquerelle M, Bookstein FL, Braga J, et al. Sexual dimorphism of the human mandible and its association with dental development. Am J Phys Anthropol. 2011;145:192–202. doi: 10.1002/ajpa.21485. [DOI] [PubMed] [Google Scholar]
- Daegling DJ. Functional morphology of the human chin. Evol Anthropol. 1993;1:170–177. [Google Scholar]
- Daegling DJ. The human mandible and the origins of speech. J Anthropol. 2012;2012:1–14. [Google Scholar]
- Dobson SD, Trinkaus E. Cross-sectional geometry and morphology of the mandibular symphysis in Middle and Late Pleistocene Homo. J Hum Evol. 2002;43:67–87. doi: 10.1006/jhev.2002.0563. [DOI] [PubMed] [Google Scholar]
- Dryden IL, Mardia KV. Statistical Shape Analysis. New York: Wiley; 1998. [Google Scholar]
- DuBrul LE, Sicher H. The Adaptive Chin. Springfield, IL: Charles; 1954. [Google Scholar]
- Durzo CA, Brodie AG. Growth behavior of the hyoid bone. Angle Orthod. 1962;32:193–204. [Google Scholar]
- Enlow DH. Facial Growth. 3rd edn. Philadelphia, PA: Saunders; 1990. [Google Scholar]
- Enlow DH, Harris DB. A study of the postnatal growth of the human mandible. Am J Orthod. 1964;50:25–50. [Google Scholar]
- Falk D. Comparative anatomy of the larynx in man and the chimpanzee: implications for language in Neanderthal. Am J Phys Anthropol. 1975;43:123–132. doi: 10.1002/ajpa.1330430116. [DOI] [PubMed] [Google Scholar]
- Frayer DW, Wolpoff MH, Thorne AG, et al. Theories of modern human origins: the paleontological test. Am Anthropol. 1993;95:14–50. [Google Scholar]
- Good P. Permutation Tests: A Practical Guide to Resampling Methods for Testing Hypotheses. New York, NY: Springer; 2000. [Google Scholar]
- Gröning F, Liu J, Fagan MJ, et al. Why do humans have chins? Testing the mechanical significance of modern human symphyseal morphology with finite element analysis. Am J Phys Anthropol. 2011;144:593–606. doi: 10.1002/ajpa.21447. [DOI] [PubMed] [Google Scholar]
- Gunz P, Mitteroecker P, Bookstein FL. Semilandmarks in three dimensions. In: Slice DE, editor. Modern Morphometrics in Physical Anthropology. New York: Kluwer Academic; 2005. pp. 73–98. [Google Scholar]
- Hohl T. Masticatory muscle transposition in primates: effects on craniofacial growth. J Max-Fac Surg. 1983;11:149–156. doi: 10.1016/s0301-0503(83)80038-1. [DOI] [PubMed] [Google Scholar]
- Howells WW. Mankind in the Making. Garden City, NY: Doubleday; 1959. [Google Scholar]
- Hrdlicka A. Human dentition and teeth from the evolutionary and racial standpoint. Dominion Dent J. 1911;23:403–417. [Google Scholar]
- Hublin J-J, Tillier A-M. The Mousterian juvenile mandible form Irhoud (Morocco): a phylogenetic interpretation. In: Stringer CB, editor. Aspects of Human Evolution. London: Taylor & Francis; 1981. pp. 167–185. [Google Scholar]
- Huttergger S, Mitteroecker P. Invariance and meaningfulness in phenotype spaces. Evol Biol. 2011;38:335–351. [Google Scholar]
- Ichim I, Swain VM, Kieser J. Mandibular stiffness in humans: numerical predictions. J Biomech. 2006;39:1903–1913. doi: 10.1016/j.jbiomech.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Ichim I, Kieser J, Swain M. Tongue contractions during speech may have led to the development of the bony geometry of the chin following the evolution of human language: a mechanobiological hypothesis for the development of the human chin. Med Hypotheses. 2007;69:20–24. doi: 10.1016/j.mehy.2006.11.048. [DOI] [PubMed] [Google Scholar]
- King E. A roentgenographic study of laryngeal growth. Angle Orthod. 1952;22:23–37. [Google Scholar]
- Kurihara S, Enlow DH, Rangel RD. Remodeling reversals in anterior parts of the human mandible and maxilla. Angle Orthod. 1980;50:98–106. doi: 10.1043/0003-3219(1980)050<0098:RRIAPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lam YM, Pearson OM, Smith CM. Chin morphology and sexual dimorphism in the fossil hominid mandible sample from Klasies River Mouth. Am J Phys Anthropol. 1996;100:545–577. doi: 10.1002/(SICI)1096-8644(199608)100:4<545::AID-AJPA8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Lieberman P. The Biology and Evolution of Language. Cambridge, MA: Harvard University Press; 1984. [Google Scholar]
- Lieberman DE. Testing hypotheses about recent human evolution from skulls: integrating morphology, function, development and phylogeny. Curr Anthropol. 1995;36:159–197. [Google Scholar]
- Lieberman DE. Homology and hominid phylogeny: problems and potential solutions. Evol Anthropol. 1999;7:142–151. [Google Scholar]
- Lieberman DE. The Evolution of the Human Head. Cambridge, MA: Harvard University Press; 2011. [Google Scholar]
- Lieberman P, Crelin ES. On the speech of Neanderthal man. Linguist Inq. 1971;2:203–222. [Google Scholar]
- Lieberman DE, Ross CF, Ravosa MJ. The primate cranial base: ontogeny, function, and integration. Am J Phys Anthropol. 2000;113(S31):117–169. doi: 10.1002/1096-8644(2000)43:31+<117::aid-ajpa5>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McCarthy RC, Hiiemae KM, et al. Ontogeny of postnatal hyoid and larynx descent in humans. Arch Oral Biol. 2001;46:117–128. doi: 10.1016/s0003-9969(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Shcherbatyy V, Gaoman G, et al. Effects of tongue volume reduction on craniofacial growth: a longitudinal study on orofacial skeletons and dental arches. Oral Arch Biol. 2008;53:991–1001. doi: 10.1016/j.archoralbio.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallegni F, Trinkaus E. A reconsideration of the Archi 1 Neanderthal mandible. J Hum Evol. 1997;33:651–668. doi: 10.1006/jhev.1997.0159. [DOI] [PubMed] [Google Scholar]
- Maresh MM. Growth of the heart related to bodily growth during childhood and adolescence. Pediatrics. 1948;2:382–402. [PubMed] [Google Scholar]
- Maresh MM, Washburn AH. Size of the heart in healthy children. Am J Dis Child. 1938;56:33–60. [Google Scholar]
- Mauser C, Enlow DH, Overman DO. Growth and remodeling of the human fetal face and cranium. In: McNamara JA, et al., editors. Determinants of Mandibular Form and Growth. Ann Arbor, MI: Center for Human Growth and Development, University of Michigan; 1975. pp. 243–275. Monograph 5. Craniofacial Growth Series. [Google Scholar]
- McCammon R. Human Growth and Development. Springfield, IL: Thomas; 1970. [Google Scholar]
- McCarthy RC, Lieberman DE. Posterior maxillary (PM) plane and anterior cranial architecture in primates. Anat Rec. 2001;264:247–260. doi: 10.1002/ar.1167. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Bookstein FL. Heterochrony and geometric morphometrics: a comparison of cranial growth in Pan paniscus versus Pan troglodytes. Evol Dev. 2005;7:244–258. doi: 10.1111/j.1525-142X.2005.05027.x. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Bookstein FL. The conceptual and statistical relationship between modularity and morphological integration. Syst Biol. 2007;56:818–836. doi: 10.1080/10635150701648029. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Bookstein FL. The evolutionary role of modularity and integration in the hominoid cranium. Evolution. 2008;62:943–958. doi: 10.1111/j.1558-5646.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P. Advances in geometric morphometrics. Evol Biol. 2009;36:235–247. [Google Scholar]
- Mitteroecker P, Gunz P, Bernhard M, et al. Comparison of cranial ontogenetic trajectories among great apes and humans. J Hum Evol. 2004;46:679–698. doi: 10.1016/j.jhevol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P, Bookstein FL. Heterochrony and geometric morphometrics: a comparison of cranial growth in Pan paniscus versus Pan troglodytes. Evol Dev. 2005;7:244–258. doi: 10.1111/j.1525-142X.2005.05027.x. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P, Neubauer S, et al. How to explore morphological integration in human evolution and development? Evol Biol. 2012 DOI 10.1007/s11692-012-9178-3. [Google Scholar]
- Negus VE. The Comparative Anatomy and Physiology of the Larynx. New York: Hafner; 1949. [Google Scholar]
- Oxnard CE, O'Higgins P. Biological clearly needs morphometrics. Does morphometrics need biology? Biol Theory. 2009;4:84–97. [Google Scholar]
- Riesenfeld A. The adaptative mandible: an experimental study. Acta Anat (Basel) 1969;72:246–262. doi: 10.1159/000143250. [DOI] [PubMed] [Google Scholar]
- Roche AF, Barkla DH. The level of the larynx during childhood. Ann Otol Rhinol Laryngol. 1965;74:645–654. doi: 10.1177/000348946507400307. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Corty M. The use of two-block partial least squares to study covariation in shape. Syst Biol. 2000;49:740–753. doi: 10.1080/106351500750049806. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Slice DE. Extensions of the procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. [Google Scholar]
- Rosas A. Seventeen new mandibular specimens from Atapuerca/Ibeas Middle Pleistocene hominids sample (1985–1992) J Hum Evol. 1995;28:533–559. [Google Scholar]
- Schwartz JH, Tattersall I. The human chin revisited: what is it and who has it? J Hum Evol. 2000;38:367–409. doi: 10.1006/jhev.1999.0339. [DOI] [PubMed] [Google Scholar]
- Smith FH. Fossil hominids from the Upper Pleistocene of Central Europe and the origin of modern Europeans. In: Smith FH, Spencer F, editors. The Origins of Modern Humans: a World Survey of the Fossil Evidence. New York: Alan R. Liss; 1984. pp. 137–209. [Google Scholar]
- Solow B, Siersbæk-Nielsen S. Growth changes in head posture related to craniofacial development. Am J Orthod. 1986;89:132–140. doi: 10.1016/0002-9416(86)90089-8. [DOI] [PubMed] [Google Scholar]
- Spoor F, Zonneveld F, Macho GA. Linear measurements of cortical bone and dental enamel by computed tomography: applications and problems. Am J Phys Anthropol. 1993;91:469–484. doi: 10.1002/ajpa.1330910405. [DOI] [PubMed] [Google Scholar]
- van Spronsen PH, Koolstra JH, van Ginkel FC, et al. Relationships between the orientation and moment arms of the human jaw muscles and normal craniofacial morphology. Eur J Orthod. 1997;19:313–328. doi: 10.1093/ejo/19.3.313. [DOI] [PubMed] [Google Scholar]
- Spyropoulos MN, Tsolakis AI, Alexandridis C, et al. Role of suprahyoid musculature on mandibular morphology and growth orientation in rats. Am J Orthod Dentofacial Orthop. 2002;122:392–400. doi: 10.1067/mod.2002.125992. [DOI] [PubMed] [Google Scholar]
- Stringer CB, Hublin J-J, Vandermeersch B. The origin of anatomically modern humans in western Europe. In: Smith FH, Spencer F, editors. The Origins of Modern Humans: a World Survey from the Fossil Evidence. New York: Alan R. Liss; 1984. pp. 51–135. [Google Scholar]
- Tattersall I, Schwartz JH. The morphological distinctiveness of Homo sapiens and its recognition in the fossil record: clarifying the problem. Evol Anthropol. 2008;38:367–409. [Google Scholar]
- Walkhoff O. Die menschliche Sprache in ihrer Bedeutung für die funktionelle Gestalt des Unterkiefers. Anat Anz. 1904;24:129. [Google Scholar]
- Westhorpe RN. The position of the larynx in children and its relationship to the ease of intubation. Anaesthes Intensive Care. 1987;15:384–388. doi: 10.1177/0310057X8701500405. [DOI] [PubMed] [Google Scholar]
- White TD. Ann Arbor: University Microfilms, University of Michigan; 1977. The anterior mandibular corpus of early African hominidae: functional significance of shape and size. PhD. Dissertation. [Google Scholar]
- Wolpoff MH. Paleoanthropology. New York: Knopf; 1980. [Google Scholar]
- Wolpoff MH, Smith F, Malez M, et al. Upper Pleistocene hominid remains from Vindija Cave, Croatia, Yugoslavia. Am J Phys Anthropol. 1981;54:499–545. doi: 10.1002/ajpa.1330680308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


