Abstract
Radial glial cells serve diverse roles during the development of the central nervous system (CNS). In the embryonic brain, they are recognised as guidance conduits for migrating neuroblasts and as multipotent stem cells, generating both neurons and glia. While their stem cell capacities in the developing spinal cord are as yet not fully clarified, they are classically seen as a population of astrocytes precursors, before gradually disappearing as the spinal cord matures. Although the origins and lineages of CNS radial glial cells are being more clearly understood, the relationships between radial glial cells and growing white matter (WM) tracts are largely unknown. Here, we provide an in-depth description of the distribution and organisation of radial glial cell processes during the peak periods of axonogenesis in the rat spinal cord. We show that radial glial cell distribution is highly ordered in the WM from E14 to E18, when the initial patterning of axon tracts is taking place. We report that the density of radial glial cell processes is tightly conserved throughout development in the dorsal, lateral and ventral WM funiculi along the rostrocaudal axis of the spinal cord. We provide evidence that from E16 the dorsal funiculi grow within and are segregated by fascicles of processes emanating from the dorsomedial septum. The density of radial glial cells declines with the maturation of axon tracts and coincides with the onset of the radial glial cell–astrocyte transformation. As such, we propose that radial glial cells act as structural scaffolds by compartmentalising and supporting WM patterning in the spinal cord during embryonic development.
Keywords: axonogenesis, development, radial glia, white matter patterning
Introduction
The formation of axon tracts is a highly complex developmental process essential for normal neural function. Central to establishing the functional pattern of the expanding nervous system are the interactions between growing axons, adjacent axons, the extracellular matrix and glial cells (see review by Chotard & Salecker, 2004). In some regions, prior to the extension of exploratory growth cones, radial glial cells serve as guidance conduits, directing migrating neurons from their germinal zone origins to their final destinations (see review by Hatten & Mason, 1990). Radial glia are found in the developing nervous system prior to the differentiation of astrocytes and oligodendrocytes, and are usually only present during development; however, some radial glia exist in the adult brain and are likely to function as astrocyte progenitor cells and possibly stem cells (Liuzzi & Miller, 1987; Merkle et al. 2004). In the developing brain, radial glia can generate both neurons and glia (see review by Pinto & Gotz, 2007), whereas in the developing spinal cord most seem to generate glial cells only (Barry & McDermott, 2005). In addition to their role in guiding migrating neurons and their ability to differentiate into neurons and glia, the structural organisation of radial glia in certain regions suggests that they could also potentially direct axon growth and assist in axon tract formation. Indeed, in the developing brain, neuroepithelial cells, radial glial cells and astrocytes have all been shown to create physical boundaries throughout the central nervous system (CNS; see review by Steindler, 1993). These boundaries help maintain normal bilateral brain connections by preventing abnormal axonal decussation (see review by Brunso-Bechtold & Henkel, 1996). Furthermore, many axonal projections within the brain require these glial boundaries for normal development. For instance, the corpus callosum develops with the aid of a glial ‘sling’, which if disrupted inhibits callosal axons projecting across the midline (Silver et al. 1982, 1993; Norris & Kalil, 1991). Other areas in the developing brain that require glial boundaries for their correct formation include the dorsal and ventral domains of the telencephalon (Chapouton et al. 2001), the developing rat ventral root transitional zone (O'Brien et al. 2001) and the rostral migratory stream (RMS; Lois et al. 1996; see review by Cayre et al. 2009).
Despite the well-established structural and functional roles that radial glia play during brain development (see reviews by Brunso-Bechtold & Henkel, 1996; Pinto & Gotz, 2007), it remains unclear whether they act in a similar manner during the formation of the peripherally positioned axon tracts in the white matter (WM) of the spinal cord. The spinal cord WM is a thoroughfare of axon tracts that carries sensory and motor information to and from the peripheral nervous system (PNS) in the ventral, lateral and dorsal funiculi. It has been shown that glial processes may play a physical role in the guidance of the pioneering axons of the corticospinal tract (CST; Joosten & Gribnau, 1989; Schwab & Schnell, 1991). In addition, the dorsal midline glial septum, which in the rat extends dorsally from the dorsal part of the central canal to the pial surface, has been suggested to prevent premature corticospinal axon decussation (Joosten & Gribnau, 1989). Intriguingly, prior even to the appearance of these so-called glial ‘cordones’ (see review by Steindler, 1993), the ‘blueprint hypothesis’, as developed for lower vertebrates, suggests that the paths growth cones recognise are physically formed by neuroepithelial cells and their glial derivatives before axons arrive (Singer et al. 1979). However, evidence for the existence of such a blueprint and, indeed, knowledge of the precise structural interrelationship between the radial glial scaffold and the developing WM of the spinal cord is lacking.
Here, we provide a quantitative and qualitative analysis of the spatial and temporal distribution and regional organisation of radial glial cell processes in the developing rat spinal cord WM during periods of peak axon growth and tract formation. We show that processes emanating from the dorsomedial septum (DMS) form transient boundaries that surround and compartmentalise the emerging dorsal columns. In addition, we report that neuroepithelial radial cell processes are present in the presumptive WM prior to axon tract formation. Furthermore, as these cells transition to radial glial cells, they adopt highly organised patterns in the WM during the formation of the ventral, lateral and dorsal funiculi. These findings provide further insight into how accurate axonal growth and pathfinding during spinal cord development may be facilitated and directed by the intimate structural organisation of the radial glial scaffold and growing axons. At the molecular level, this scaffold may also offer a permissive substrate and, potentially, trophic support for growing spinal cord axons.
Materials and methods
Animals
Embryonic day (E)13 to E20, and postnatal day (P)2 and P6 Sprague–Dawley rats were obtained from the Biological Services Unit, Cork. Embryos were removed by laparotomy following anaesthesia using halothane, and a terminal overdose of sodium pentobarbital was administered to pregnant dams. Embryos were fixed in 4% paraformaldehyde (PFA) for 24 h at 4 °C. They were then cryoprotected in a 30% sucrose solution and snap-frozen in liquid nitrogen-chilled isopentane and stored at −80 °C. All procedures were approved by the Animal Experimentation Ethics Committee of University College Cork.
DiI labelling
E14 embryos were extracted as above and fixed in 4% PFA for 6 h at 4 °C. The whole spinal cord was then dissected out, and a small amount of DiI tissue-labelling paste (NeuroTrace; Molecular Probes, CA, USA) was applied ventrolaterally using the tip of a needle. The spinal cords were then placed in phosphate-buffered saline (PBS) for 30 days at room temperature to allow the DiI to diffuse laterally along axonal lengths (DiI travels anterogradely and retrogradely at approximately 0.2 mm day−1 in fixed tissue; Godement et al. 1987). After 30 days, 200-µm slices were cut with a tissue chopper (McIlwain, CA, USA) and placed in PBS in preparation for immunohistochemistry.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Barry & McDermott, 2005). Briefly, 15-μm cryosections were used for all analyses. Sections were blocked in 10 mm PBS containing 20% normal goat serum (NGS) plus 0.2% Triton-X 100 (Sigma, UK), and then incubated in primary antibody in 10 mm PBS containing 1% NGS plus 0.2% Triton-X 100 (Sigma) for 2 h at room temperature or overnight at 4 °C. The following primary antibodies were used where indicated: 2F7 (undiluted asicites; Z. Kaprelian); GAP43 (1 : 200; Chemicon, CA, USA); nestin (1 : 200; Chemicon); vimentin (1 : 200; Sigma),; and GLAST (1 : 200; Chemicon). Propidium iodide was used to label cell nuclei and served as a topographical marker of spinal cord maturation. Primary antibody binding was detected by incubation with secondary antibodies (1 : 100; Sigma) for 1 h at room temperature, or overnight at 4 °C. Immunofluorescent sections were imaged using confocal microscopy (Bio-Rad, CA, USA).
For 200-μm-thick slices, floating tissue sections were rinsed in PBS and incubated in 10% normal horse serum and 0.4% Triton-X 100 in PBS for 1 h. Slices were then incubated in primary antibodies: nestin (1 : 500; Chemicon) and brain lipid-binding protein (BLBP; 1 : 500; Abcam, UK) for 48 h at 4 °C. Tissue was rinsed with PBS and incubated in secondary antibodies (Alexafluor 488 and Alexafluor 647 conjugated; Invitrogen, CA, USA) overnight at 4 °C. Tissue was then rinsed in PBS and mounted on coverslips with PVA-DABCO mounting media. Slices were imaged using confocal microscopy at 1-μm intervals (Olympus Fluoview 1000, Hamburg, Germany) and projected or reconstructed using Imaris Software (Bitmap, Zurich, Switzerland).
Quantification of process density in the spinal cord WM
For fibre density analysis, 15-μm cryosections were cut from the rostral and caudal regions of the spinal cord in a coronal or longitudinal plane. For qualitative analysis of radial glial cell–axonal interactions, sections were cut at a thickness of 40 μm in a coronal plane. The analysis of radial glial process density in the spinal cord WM was carried out using the line intersect stereological approach (see review by Mayhew, 1992). This involves assessing the number of times nestin-immunoreactive processes intersect with a series of 100-μm reference lines placed centrally in the ventral, lateral and dorsal WM, and oriented in planes parallel to the pial surface. The first reference line was placed lateral to the developing ventral floor plate at E14, E16 and E18, while the last was placed in the lateral WM at E14 or in the dorsal WM at E16 and E18, lateral to the developing growing dorsal funiculus. For quantitative analysis, five embryos from different litters were studied at E14, E16 and E18. Optical sections (3 × 5-μm) through a 15-μm section were imaged, and radial glial cell process density was quantified in each optical section. Two confocal series were examined per spinal cord, one from the rostral level and one from the caudal level. All data are reported as means ± standard error of the mean. A one-way analysis of variance with Tukey's post hoc test was used to determine statistical significance.
Quantification of radial glial cells at E14
To determine the ratio of radial glial cells to radial neuroepithelial cells, 15-μm cryosections were cut from the rostral, middle and caudal regions of the spinal cord at E14 in a coronal plane. Radial glial cells and radial neuroepithelial cells were immunostained for BLBP and nestin, respectively, and imaged using confocal microscopy. Three confocal series were examined from the rostral, middle region and caudal region of three individual spinal cords from separate litters. The total numbers of nestin-immunoreactive radial neuroepithelial cell processes and nestin- and BLBP-immunoreactive radial glial cell processes in each spinal cord were then quantified, pooled and compared.
Western blotting
Western blotting on spinal cord homogenates from the embryonic and postnatal spinal cords were prepared using methods previously described (O'Keeffe et al. 2004). Briefly, whole protein homogenates were prepared from E14, E16, E18, E20, P2 and P6 spinal cord by lysing in RIPA buffer. Protein concentrations were determined using a Bradford assay with bovine serum albumin (BSA) as a standard. Blots were incubated in primary antibodies vimentin (1 : 000; Sigma), GAP-43 (1 : 000; Chemicon) or β-actin (1 : 000; Sigma) in 1% BSA in 10 mm PBS containing 0.1% Tween20 (PBS-T). Following three washes for 10 min in PBS-T, blots were incubated in horseradish peroxidase-labelled anti-rabbit or anti-mouse IgG (Promega, WI, USA). Membranes were rinsed three times for 10 min with PBS-T and developed with ECL Plus (GE Healthcare, USA). Densitometry was performed using Image J software. Individual data sets from three vimentin and two GAP43 Western blots were combined and averaged.
Results
A radial glial scaffold surrounds the developing dorsal funiculi
Firstly, we examined the organisation of radial glial processes in the vicinity of the emerging dorsal funiculi. From E16, vimentin- and nestin-immunoreactive radial glial processes course from the central canal to the pial surface of the dorsal and ventral spinal cord (Fig. 1A,B). These cells express the glial-specific marker GLAST (Fig. 1C). A band of vimentin- and nestin-immunoreactive processes, termed the DMS, extends from the dorsal part of the central canal to the dorsal pial surface (arrow in Fig. 1D). This septum does not express any of the glial markers used to differentiate radial glial cells from their neuroepithelial cell precursors (Barry & McDermott, 2005). As such, the cells composing the DMS can be considered distinct from typical radial glia. The DMS clearly divides the growing GAP43-immunoreactive axons of the emerging dorsal funiculi into right and left halves (Fig. 1D). Nestin- (data not shown) and vimentin-immunoreactive fibres emerge laterally from the DMS ventral to the pioneering GAP43-immunoreactive dorsal funiculi axons in the regions of the presumptive ascending cuneate and gracile fasciculi (arrows in Fig. 1E,F). As with the DMS, these fibres do not express the radial glial markers GLAST or BLBP and are, therefore, in this study classified as similar to DMS-fibres. Considering that the dorsal funiculi develop gradually along the rostrocaudal axis of the spinal cord, the structural arrangement of DMS and its laterally projecting fibres suggests that they may provide a framework along which axons will grow. From early E17, the axons of the dorsal funiculi, as identified by GAP43, begin to segregate into the cuneate fasciculus and the more medial gracile fasciculus (Fig. 1G). This process appears to be facilitated by the dispersed network of laterally projecting DMS-fibres condensing into distinct dorsolaterally projecting fascicles extending from the septum to the dorsal pial surface through the emerging cuneate and gracile fasciculi, thereby physically separating them from one another (arrows in Fig. 1H,I). These boundary-forming fibres are transient and dissipate around the perinatal period once the cuneate and gracile fasciculi have become established (Barry & McDermott, 2005). At late E17, the cuneate fasciculus extends ventrally and is divided clearly along the midline by the DMS (arrow in Fig. 1J). Another network of DMS-derived fibres arch bilaterally from the septum and extend dorsolaterally first and then medially to form an almost closed loop (arrows in Fig. 1K,L). These processes seem to form a tube-like scaffold that surrounds the dorsal funiculus throughout the longitudinal extent of the cord, thereby creating a physical sleeve through which the axons project, potentially helping to prevent misdirection of axons as they extend rostrally.
Fig. 1.
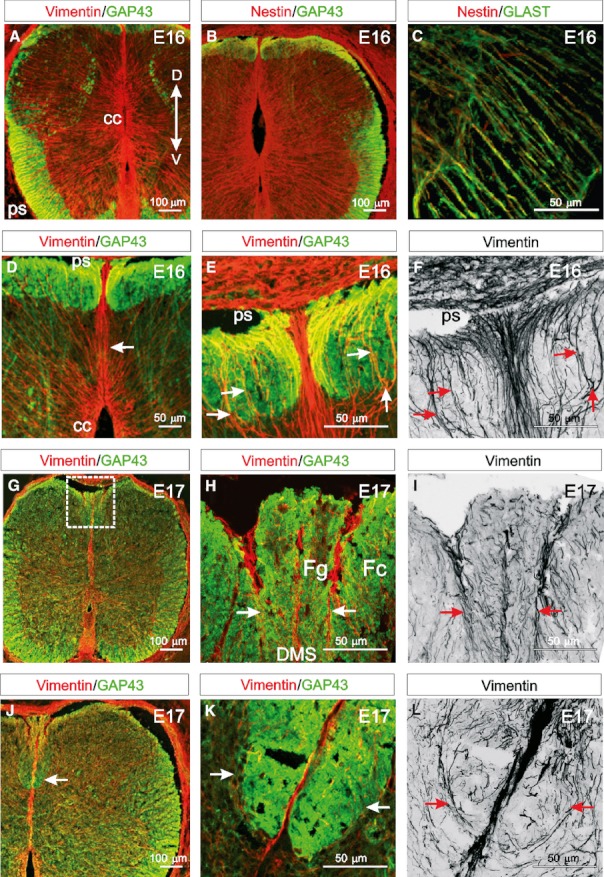
DMS-fibres facilitate the formation of the dorsal funiculi. (A,B) At E16 vimentin- and nestin-immunoreactive radial glial cells are present in dorsal and ventral regions of the spinal cord extending from the central canal (cc) to the pial surface (ps). GAP43 is evident in the growing ventral, lateral, dorsolateral and dorsal funiculi. (C) Nestin is coexpressed with the glial specific marker GLAST in radial glial cell processes. (D) At E16, the vimentin-immunoreactive DMS extends from the dorsal central canal to the dorsal pial surface and divides the emerging GAP43-immunoreactive dorsal funiculi bilaterally. (E) Vimentin-immunoreactive collaterals (DMS-fibres) emanating from the DMS appear to provide a scaffold around which the dorsal funiculi grow (arrows). (F) Panel (E) converted to grey-scale, which clearly highlights vimentin-immunoreactive processes emanating laterally from the DMS on both sides of the spinal cord and then extending dorsally (arrows) to the pial surface. (G) Early at E17, the GAP43-immunoreactive cuneate and gracile fasciculi begin to separate in the dorsal spinal cord (box). (H) A higher magnification image of a region similar to that boxed in (J) showing vimentin-immunoreactive DMS-fibres appearing to segregate the more medial gracile fasciculus (Fg) from the cuneate fasciculus (Fc) (arrows). (I) Panel (H) converted to grey-scale, highlighting how fascicles of vimentin-immunoreactive DMS-fibres extend from the DMS to the dorsal pial surface (arrows). (J) At late E17, the cuneate fasciculus enlarges ventrally (arrow). (K) A higher magnification image of a similar region indicated by the arrow in (J) showing a band of vimentin-immunoreactive processes radiating from the DMS bilaterally to surround the growing cuneate fasciculus, seemingly forming a framework through which axons grow (arrows). (L) Panel (K) converted to grey-scale, showing vimentin-immunoreactive DMS-fibres extending laterally from the DMS first then extending dorsally (arrows). (Images A–C, D, G and J are confocal projections of 15–20 images captured at 0.5-μm intervals. Images E, F, H–I and K and L are confocal projections of 65–70 images captured at 0.5-μm intervals. Scale bars are in microns as indicated. Dorsoventral orientations are consistent for all images as indicated in A).
Radial glial cell processes display a highly conserved structural pattern in the developing spinal cord WM
To determine how radial glial cells or their precursors relate to and perhaps segregate fascicles of nascent axons throughout the spinal cord WM, we examined their distribution and organisational pattern in the ventral and lateral funiculi throughout the rostrocaudal axis of the spinal cord, during the periods of peak WM growth from E13 to E18. Firstly, we examined the scaffold formed by radial neuroepithelial cells (radial glia precursors) in the WM at E13, which is approximately the time of onset of axon ingrowth in the rat spinal cord (Oudega et al. 1995). At this stage, 2F7-immunoreactive mature and immature neuronal cell bodies and axons are present in the presumptive grey matter (GM) ventrally, and in axon fascicles emerging in the subpial regions of the ventral and lateral spinal cord (Fig. 2A). At this stage, the WM here consists largely of the pioneering axons of the rubrospinal, reticulospinal and vestibulospinal tracts. Neuroepithelial cells in the ventral ventricular zone have radially arranged nestin-immunoreactive processes, many of which course fully from the ventricular zone to the pial surface. These neuroepithelial processes frequently change direction, crossover and branch (Fig. 2B). In the ventrolateral WM, some of these neuroepithelial radial fibres appear to exit the spinal cord and pass through the presumptive ventral root (Fig. 2C).
Fig. 2.
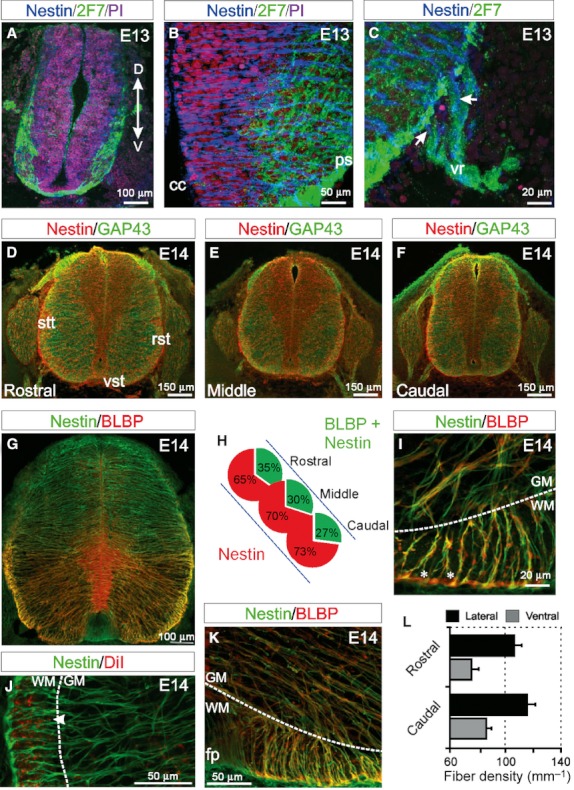
The organisation of radial neuroepithelial and radial glial cells at the onset of white matter (WM) formation in the spinal cord. (A) At E13, nestin-immunoreactive neuroepithelial cells are present ventrally and dorsally. 2F7-immunoreactive mature and immature neurons and axons are present in the presumptive ventral horn and WM, respectively, in the presumptive lateral WM and crossing over the ventral midline. (B) Nestin-immunoreactive radial cells extend from the central canal to the pial surface through the emerging ventral grey matter (GM) and WM. (C) In the ventrolateral spinal cord, some nestin-immunoreactive processes extend past the pial surface and appear to enter the ventral root (vr; arrows), possibly guiding motor axons exiting the spinal cord. (D–F) At E14, the emerging ventral and lateral WM tracts are present in the rostral, middle and caudal spinal cord. These tracts correspond to the growing vestibulospinal tracts (vst) ventrally, the spinothalamic tracts (stt) and the reticulospinal tracts (rtt) laterally. A band of axons entering from the dorsal root are evident dorsally. Nestin-immunoreactive processes radiate from the central canal to the pial surface in the rostral, middle and caudal spinal cord. (G) At E14, brain lipid-binding protein (BLBP) is coexpressed with nestin in the ventral portion of the spinal cord in radial glial processes. (H) The proportion of BLBP and nestin expressing radial glial cells compared with nestin expressing radial neuroepithelial cells decreases along a rostral to caudal gradient. (I) Two-hundred-micrometer-thick slices show how nestin- and BLBP-immunoreactive radial glial cells ventrally adopt a uniform orientation as they pass through the GM/WM interface (dotted line) and radiate toward the pial surface. Occasionally, spaces are observed between radial glial cell processes that persist through the thickness of the slice (asterisks). (I–K) Radial glial cells often branch at the GM/WM interface (dotted lines) resulting in an increased number of processes entering the presumptive WM. DiI labelling of axons shows how discrete fascicles seem to be contained within channels of nestin-immunoreactive processes in the WM (arrowhead in J). (K) Near the ventral floor plate (fp), nestin- and BLBP-immunoreactive radial glial cell processes display a striking change of direction as the transition from the GM/WM interface (dotted line) to the pial surface. (L) The densities of radial glial cell processes in the dorsal and lateral WM are maintained throughout the rostrocaudal axis of the spinal cord. (Images A–F are confocal projections of 15–20 images captured at 0.5-μm intervals, images G, I–K are confocal projections of 400 images captured at 0.5-μm intervals. Scale bars are in microns as indicated. Dorsoventral orientations are consistent for all images as indicated in A).
By E14, the GAP43-immunoreactive ventral and lateral funiculi of the spinal cord have increased in size in the rostral and caudal spinal cord (Fig. 2D–F). Dorsally, neurons are present in the GM, and a band of axons entering from the dorsal rootlets is present both rostrally and caudally. Nestin-immunoreactive processes extend from the central canal to the pial surface. Ventrally, these processes now also express BLBP, identifying them as classical radial glial cells of astroglial lineage (Fig. 2G; Feng et al. 1994; Shibata et al. 1997). The ratio of nestin- and nestin/BLBP-immunoreactive cells along the rostrocaudal axis was quantified. In rostral regions of the cord, nestin/BLBP-immunoreactive cells represent 35% of the entire radial cell population, while 65% remain nestin-immunoreactive-only radial cells (Fig. 2H) and do not express BLBP. At mid spinal cord levels, 30% of radial processes have transformed to radial glial cells at E14, while in caudal regions of the spinal cord, 27% are radial glial cells.
We next analysed 200-µm slices of immunostained spinal cords using confocal microscopy to visualize BLBP- and nestin-immunoreactive radial glial cells, and more accurately assess their three-dimensional structural organisation. Using this approach, BLBP- and nestin-immunoreactive radial glial processes appeared uniformly organised as they radiate from the ventricular zone to the pial surface (Fig. 2G). Ventrally, the processes often change direction once they encounter the GM/WM interface, and adopt an orientation more perpendicular to the pial surface (Fig. 2I,K). BLBP expression along these processes appears upregulated along the portion of the radial glial cell process that extends through the WM (Fig. 2G,I–K). We regularly observed ‘corridors’ in these 200-μm slices formed by a periodic spatial arrangement of radial glial processes (asterisks in Figs 2I and S1). This was particular strikingly in the ventral and lateral WM, and these corridors extended through the longitudinal axis of the spinal cord. Additionally, radial glial processes branched extensively at the GM/WM interface (dotted line in Figs 2I–K and S1), such that significantly more processes passed through the WM than through the GM. Furthermore, we used Dil to label axons in smaller numbers than conventional antibody-based immunohistochemistry, and to visualise the structural relationship between developing axonal fasciculi and glial processes more clearly. Using this approach the segregation of axons in the WM by the finely branched glial processes was clearly evident, and frequently axons appeared to be bundled between the corridors formed by radial glial processes (arrowhead in Fig. 2J). To determine the spatial distribution of radial glial processes in the WM, we quantified the radial glial fibre density per mm of the ventral and lateral WM through the rostrocaudal axis of the E14 spinal cord (Fig. 2L). At E14, radial glial fibre density is similar in the ventral WM of the rostral (75.3 ± 4.1 fibres mm−1) and caudal (85.1 ± 4.6 fibres mm−1) regions of the spinal cord. At this stage, the descending axons of the rubrospinal tract, reticulospinal tract and vestibulospinal tract are present in the ventral and lateral WM in rostral and caudal regions (Fig. 2D–F). The conservation of the pattern of radial glial processes through these growing axon tracts suggests that careful regulation of process density along the rostrocaudal axis of the spinal cord may be required for correct tract formation. Fibre densities are also conserved in the rostral (106.4 ± 5.0 fibres mm−1) and caudal (115.2 ± 5.7 fibres mm−1) lateral WM (Fig. 2L).
At E16, a longitudinal section through the dorsolateral spinal cord showed that the GAP43-immunoreactive WM, presumably containing ascending spinothalamic and dorsal spinocerebellar tracts, is present the entire length of the spinal cord (arrows in Fig. 3A). As at E14, nestin-immunoreactive radial glial processes course radially from the central canal to the pial surface both ventrally and dorsally in rostral, middle and caudal regions (Fig. 3B–D). At this age, in contrast to E14, these cells express GLAST and BLBP in both dorsal and ventral regions of the spinal cord (Barry & McDermott, 2005). As at E14, these processes appear uniformly arranged as they pass from the GM/WM junction through the developing WM to the pial surface (Fig. 3E,F). Moreover, the density of these radial glial processes was constant through the rostrocaudal axis of the cord both dorsally (rostral: 77.5 ± 3.2 fibres mm−1; caudal: 74.81 ± 3.3 fibres mm−1) and ventrally (rostral: 71.6 ± 2.4 fibres mm−1; caudal: 69.3 ± 2.61 fibres mm−1; Fig. 3G).
Fig. 3.
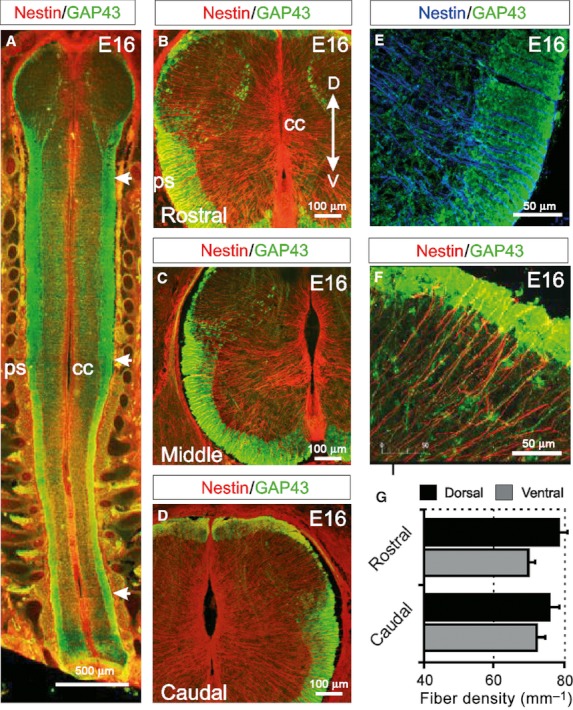
Radial glial cells coursing through the WM display a high degree of organisation in all regions of the E16 spinal cord. (A) Longitudinal section of an E16 embryo showing how GAP43-immunoreactive axon tracts are present the entire length of the spinal cord (arrows). Nestin-immunoreactive radial glial cells radiate from the central canal to the pial surface at all levels. (B–D) At E16, GAP43 expression is increasing in the ventral and lateral WM of the rostral, middle and caudal spinal cord. The presumptive WM of the dorsal horn and the dorsal funiculi is also emerging at all levels of the spinal cord. Nestin-immunoreactive radial glial cells radiate from the central canal to the pial surface both dorsally and ventrally. (E,F) Radial glial processes appear evenly distributed in the ventral (E) and dorsal (F) WM. (G) The densities of radial glial processes in the ventral and dorsal WM are maintained throughout the rostrocaudal axis of the spinal cord. (All images are confocal projections of 15–20 images captured at 0.5-μm intervals. Scale bars are in microns as indicated. Dorsoventral orientations are consistent for images B–F as indicated in B).
At E18, GAP43-immunoreactive axons were extensive in ventral, lateral and dorsal WM funiculi and in the GM (Fig. 4A). The pattern resembles that of the mature spinal cord (data not shown), indicating that much of the structural organisation of the adult WM is already in place by this stage of development. The radial glial cell–astrocyte transformation is underway in most regions of the spinal cord by E18 (Barry & McDermott, 2005). As a consequence, many radial glial cells are beginning to lose their ventricular zone attachment and appear to branch at the GM/WM junction (arrows in Fig. 4B). However, the density of radial glial processes in the WM is still relatively constant within the E18 spinal cord. There is no statistical variation in fibre density evident in the ventral WM of the rostral (70.3 ± 2.2 fibres mm−1) and caudal (69.2 ± 2.1 fibres mm−1) spinal cord, or in the dorsal WM of the rostral (61.6 ± 3.3 fibres mm−1) and caudal spinal cord (61.8 ± 2.8 fibres mm−1; Fig. 4C).
Fig. 4.

(A) GAP43 is expressed in the ventral, lateral and dorsal funiculi and in the GM. Nestin-immunoreactive radial glial cells still radiate from the central canal to the pial surface. (B) Radial glial cells exhibit a high degree of branching at the GM/WM interface (arrows), but remain evenly distributed in the WM. (C) The densities of radial glial cell processes in the ventral and dorsal WM are maintained throughout the rostrocaudal axis of the spinal cord. (D) The mean densities of radial glial cells in both the rostral (*P < 0.05) and caudal (+P < 0.05) WM are significantly reduced from E14 to E16, and from E16 to E18, in line with axon tract formation. (E) The combined mean densities of radial glial cell processes in all regions of the WM decline significantly from E14 to E16, and from E16 to E18 (*,**P < 0.05). (F) Western blot showing vimentin and GAP43 expression from E14 to P6. β-actin was used as a control. (G) Densitometrical analysis showing how GAP43-immunoreactive axons increase postnatally, while vimentin-immunoreactive radial glial cells decline. (Both images are confocal projections of 15–20 images captured at 0.5-μm intervals. Scale bars are in microns as indicated, the dorsoventral orientation is consistent for B as indicated in A).
Overall, the density of radial glial processes in the WM decreases substantially throughout the spinal cord with increasing age (Fig. 4D,E; P < 0.05). To confirm the loss of radial glial cell processes from the spinal cord during development, Western blots were carried out on spinal cord lysates isolated from E14, when radial glial cells first differentiate from radial precursor neuroepithelial cells, to P6 when WM radial glia are no longer present in the cord (Fig. 4F). Levels of vimentin are greatest at E14 and decrease with increasing age. Vimentin becomes lost from the postnatal spinal cord, in accordance with the loss of the radial glial cell phenotype during development and the postnatal restriction of vimentin to some WM astrocytes (Fig. 4F,G). The concomitant level of maturation of axons and consequently of the spinal cord was revealed by Western blot analysis of GAP43 expression in lysates ranging in age from E14 to P6 (Fig. 4F,G). Levels of GAP43 increase with increasing age, reflecting the establishment of axon pathways in WM regions during development.
Discussion
During brain development, radial glial cells generate neurons and glia, and facilitate neuronal migration and axon tract formation (see review by Pinto & Gotz, 2007). Despite the well-established roles of radial glia in the developing brain, little is known regarding their roles in the developing spinal cord. One potential key difference seems to be that spinal cord radial glial cells lack the neurogenic potential of those in the cerebral cortex (Barry & McDermott, 2005). Furthermore, the development of the structural relationship between the radial glia scaffold and growing axons in the spinal cord is largely unknown. This study describes the precise spatial and temporal development of the radial glial scaffold, and shows that it is conserved along the rostrocaudal and dorsoventral gradients of the spinal cord. We show that certain specialised radial processes compartmentalise developing WM axon tracts, and once these tracts are established the radial glial scaffold disappears, as development proceeds. We found that vimentin- and nestin-immunoreactive processes surrounded the emerging dorsal funiculi, while they grow through the spinal cord. It seems plausible that this scaffold, which disappears once the dorsal funiculi have matured (Barry & McDermott, 2005) forms a permissive corridor through which axons are temporally directed and partitions the tract from the surrounding WM. Such ‘guidance conduits’ have been reported elsewhere in the CNS; in the RMS (Lois & Alvarez-Buylla, 1994; Lois et al. 1996), in the developing optic nerve (Silver, 1984) and in the corpus callosum (Silver et al. 1982). Joosten & Gribnau (1989) also described a vimentin- and glial fibrillary acidic protein (GFAP)-expressing glial scaffold, which they suggested plays a physical role in the guidance of CST axons, via a channel-like routing system. It is noteworthy that in our study these processes do not express GLAST or BLBP, two markers classically associated with a maturing astroglial phenotype (Feng et al. 1994; Shibata et al. 1997). It seems plausible that the scaffold surrounding the dorsal funiculi is derived from a specialised type of radial neuroepithelial cell, which does not acquire a strictly radial glial phenotype. We and others have previously demonstrated that GFAP is not expressed in the dorsal midline until the postnatal period (Oudega & Marani, 1991; Barry & McDermott, 2005), suggesting that an earlier non-GFAP-expressing radial phenotype is responsible for the segregating processes seen in this study.
We have also demonstrated that the cuneate and gracile fasciculi of the dorsal columns are separated from one another by a specialised network of fibres that arise from the DMS. These fibres may act as boundaries, potentially ensuring that growing axons within these tracts do not intermingle or cross to the other side of the spinal cord. As with the glial corridor that envelops the cuneate fasciculus more ventrally, this network of boundary-forming fibres dissipates around the perinatal period once the dorsal horns are fully established (Barry & McDermott, 2005).
Prior to differentiation of radial glial cells in the spinal cord, neuroepithelial cells extend processes from the ventricular zone to the pial surface of the spinal cord (see review by McDermott et al. 2005). As well as acting as neuronal and glial precursors, it is possible that the radial orientation of these processes facilitates the earliest migration of axons through the emerging ventral WM. We also observed some radial fibres crossing the pial surface and entering the developing ventral root. These particular fibres may act as a substrate facilitating the growth and subsequent exit of the earliest lower motor neuron axons from the ventral horn through the CNS–PNS transitional zone and into the PNS. These radial cells begin to express BLBP and GLAST ventrally from E14 (Shibata et al. 1997; Barry & McDermott, 2005), identifying them as radial glial cells. From E14 onwards, there is a highly organised and uniform scaffold of radial glial fibres along the entire rostrocaudal axis of the spinal cord. As these fibres transition from the GM into the WM they change course such that their processes become more perpendicularly oriented to the long axis of the WM, often intertwining with one another and appear periodically spaced. Occasionally, spaces appear between these processes in the WM, and this spacing is maintained throughout the depth of a 200-μm slice of spinal cord. It seems reasonable to suggest that these channels are bundling groups of axons together into optimally spaced compartments; ensuring that axons within such units receive sufficient and appropriate growth stimuli and do not diverge from their predefined pathways. Increased branching and an increase in BLBP expression is observed in the portion of the radial glial cell process distal to the GM/WM interface. BLBP is a member of the fatty-acid-binding family of cytoplasmic proteins, and is thought to play roles in metabolism and the trafficking of a ligand to a nuclear receptor (Feng et al. 1994; see review by Zimmerman & Veerkamp, 2002). BLBP is a target of notch in radial glia (Anthony et al. 2005), is required for the establishment of the radial glial scaffold system (Feng et al. 1994) and is involved in establishing cortical layering (Kurtz et al. 1994; Anton et al. 1997). It has also been reported to function in mediating the interactions between axons and Schwann cells in the PNS (Miller et al. 2003). BLBP is expressed in radial glial cells in the spinal cord along the rostrocaudal gradient (Feng et al. 1994), concurrent with the appearance of axon tracts as described by Altman & Bayer (1984). Its substantial upregulation in radial glial processes in the WM during the expansion of the ventral and lateral funiculi suggests it may be important for specific neuronal–glial interactions that regulate the pathfinding properties of growth cones.
The density of radial glial cell processes passing through either the ventral or dorsal WM is similar through the rostrocaudal axis of the spinal cord, during the periods of axonogenesis. The highest density of spinal cord radial glial cells processes was observed in the lateral WM at E14 (10 300 fibres mm−2). Interestingly, a similar density of Muller glial cells processes has been reported in the adult rabbit retina (10–15 000 fibres mm−2; Robinson & Dreher, 1990). Given the uniformity of fibre density along the rostrocaudal axis, it is possible that that there is a necessary minimum ratio of glial processes to axons to ensure appropriate axon growth.
As well as providing potential physical routes for growing axonal tracts to follow, glial cells also contribute to this directional growth by providing growth-promoting and growth-inhibiting signals. Indeed, semaphorins, netrins, slits and ephrins are highly conserved families of guidance molecules expressed by glial cells that are already known to provide attractive and repulsive guidance cues to facilitate axon pathfinding and circuit formation (Dottori et al. 1998; Kidd et al. 1999; Shu & Richards, 2001; Manitt & Kennedy, 2002; Wang et al. 2008). Further research is required to elucidate the spectrum of guidance molecules employed by conduit-forming radial glia in the developing spinal cord.
Over the past two decades our understanding of radial glial cell function, particularly in the cortex, has expanded from what was once seen as merely a migratory guidance role to now being key protagonists in populating the nervous system. While their migratory and neuronal progenitor roles are as yet not clarified in the spinal cord, our findings suggest a function for radial glial cells and DMS-fibres there, namely facilitating axon guidance and WM patterning. Evidence is accumulating showing how transplanted adult and embryonic radial glial cells sustain axons as they attempt to grow through a spinal cord contusion site (Hasegawa et al. 2005; Parr et al. 2007), providing structural and metabolic support to the regenerating axons. Henceforth, it will be important to further elucidate these roles, by identifying the factors necessary to regulate the high degree of radial glial cell organisation evident during axon extension and determining if similar degrees of organisation exist in all the regions of the CNS.
Acknowledgments
The authors wish to acknowledge funding from the following sources: The Health Research Board of Ireland; The Programme for Research in Third-Level Institutions 3; The Natural Sciences and Engineering Research Council of Canada; and The Irish Research Council for Science, Engineering & Technology.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Image sequence showing the periodic spatial arrangement of nestin-immunoreactive radial glial processes in the WM matter.
References
- Altman J, Bayer SA. The development of the rat spinal cord. Adv Anat Embryol Cell Biol. 1984;85:1–164. doi: 10.1007/978-3-642-69537-7. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Mason HA, Gridley T, et al. Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes Dev. 2005;19:1028–1033. doi: 10.1101/gad.1302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Marchionni MA, Lee KF, et al. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50:187–197. doi: 10.1002/glia.20166. [DOI] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Henkel CK. Axon decussation and midline glia in the developing ferret auditory hindbrain. Prog Brain Res. 1996;108:165–181. doi: 10.1016/s0079-6123(08)62539-x. [DOI] [PubMed] [Google Scholar]
- Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapouton P, Schuurmans C, Guillemot F, et al. The transcription factor neurogenin 2 restricts cell migration from the cortex to the striatum. Development. 2001;128:5149–5159. doi: 10.1242/dev.128.24.5149. [DOI] [PubMed] [Google Scholar]
- Chotard C, Salecker I. Neurons and glia: team players in axon guidance. Trends Neurosci. 2004;27:655–661. doi: 10.1016/j.tins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Dottori M, Hartley L, Galea M, et al. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci USA. 1998;95:13 248–13 253. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Godement P, Vanselow J, Thanos S, et al. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987;101:697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Chang YW, Li H, et al. Embryonic radial glia bridge spinal cord lesions and promote functional recovery following spinal cord injury. Exp Neurol. 2005;193:394–410. doi: 10.1016/j.expneurol.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Mason CA. Mechanisms of glial-guided neuronal migration in vitro and in vivo. Experientia. 1990;46:907–916. doi: 10.1007/BF01939383. [DOI] [PubMed] [Google Scholar]
- Joosten EA, Gribnau AA. Astrocytes and guidance of outgrowing corticospinal tract axons in the rat. An immunocytochemical study using anti-vimentin and anti-glial fibrillary acidic protein. Neuroscience. 1989;31:439–452. doi: 10.1016/0306-4522(89)90386-2. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnutgen F, et al. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- Liuzzi FJ, Miller RH. Radially oriented astrocytes in the normal adult rat spinal cord. Brain Res. 1987;403:385–388. doi: 10.1016/0006-8993(87)90081-3. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Manitt C, Kennedy TE. Where the rubber meets the road: netrin expression and function in developing and adult nervous systems. Prog Brain Res. 2002;137:425–442. doi: 10.1016/s0079-6123(02)37034-1. [DOI] [PubMed] [Google Scholar]
- Mayhew TM. A review of recent advances in stereology for quantifying neural structure. J Neurocytol. 1992;21:313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- McDermott KW, Barry DS, McMahon SS. Role of radial glia in cytogenesis, patterning and boundary formation in the developing spinal cord. J Anat. 2005;207:241–250. doi: 10.1111/j.1469-7580.2005.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101:17 528–17 532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SJ, Li H, Rizvi TA, et al. Brain lipid binding protein in axon-Schwann cell interactions and peripheral nerve tumorigenesis. Mol Cell Biol. 2003;23:2213–2224. doi: 10.1128/MCB.23.6.2213-2224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CR, Kalil K. Guidance of callosal axons by radial glia in the developing cerebral cortex. J Neurosci. 1991;11:3481–3492. doi: 10.1523/JNEUROSCI.11-11-03481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien D, Dockery P, McDermott K, et al. Early development of rat ventral root transitional zone: an immunohistochemical and morphometric study. J Neurocytol. 2001;30:11–20. doi: 10.1023/a:1011961106703. [DOI] [PubMed] [Google Scholar]
- O'Keeffe GW, Dockery P, Sullivan AM. Effects of growth/differentiation factor 5 on the survival and morphology of embryonic rat midbrain dopaminergic neurones in vitro. J Neurocytol. 2004;33:479–488. doi: 10.1007/s11068-004-0511-y. [DOI] [PubMed] [Google Scholar]
- Oudega M, Marani E. Expression of vimentin and glial fibrillary acidic protein in the developing rat spinal cord: an immunocytochemical study of the spinal cord glial system. J Anat. 1991;179:97–114. [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Touri F, Deenen MG, et al. Immunocytochemical localisation of microtubule-associated proteins 1b and 2 in the developing rat spinal cord. J Anat. 1995;187(Pt 3):723–737. [PMC free article] [PubMed] [Google Scholar]
- Parr AM, Kulbatski I, Tator CH. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. 2007;24:835–845. doi: 10.1089/neu.2006.3771. [DOI] [PubMed] [Google Scholar]
- Pinto L, Gotz M. Radial glial cell heterogeneity–the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Dreher Z. Muller cells in adult rabbit retinae: morphology, distribution and implications for function and development. J Comp Neurol. 1990;292:178–192. doi: 10.1002/cne.902920203. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Schnell L. Channeling of developing rat corticospinal tract axons by myelin-associated neurite growth inhibitors. J Neurosci. 1991;11:709–721. doi: 10.1523/JNEUROSCI.11-03-00709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Yamada K, Watanabe M, et al. Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci. 1997;17:9212–9219. doi: 10.1523/JNEUROSCI.17-23-09212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Richards LJ. Cortical axon guidance by the glial wedge during the development of the corpus callosum. J Neurosci. 2001;21:2749–2758. doi: 10.1523/JNEUROSCI.21-08-02749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J. Studies on the factors that govern directionality of axonal growth in the embryonic optic nerve and at the chiasm of mice. J Comp Neurol. 1984;223:238–251. doi: 10.1002/cne.902230207. [DOI] [PubMed] [Google Scholar]
- Silver J, Lorenz SE, Wahlsten D, et al. Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J Comp Neurol. 1982;210:10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- Silver J, Edwards MA, Levitt P. Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J Comp Neurol. 1993;328:415–436. doi: 10.1002/cne.903280308. [DOI] [PubMed] [Google Scholar]
- Singer M, Nordlander RH, Egar M. Axonal guidance during embryogenesis and regeneration in the spinal cord of the newt: the blueprint hypothesis of neuronal pathway patterning. J Comp Neurol. 1979;185:1–21. doi: 10.1002/cne.901850102. [DOI] [PubMed] [Google Scholar]
- Steindler DA. Glial boundaries in the developing nervous system. Annu Rev Neurosci. 1993;16:445–470. doi: 10.1146/annurev.ne.16.030193.002305. [DOI] [PubMed] [Google Scholar]
- Wang J, Chan CK, Taylor JS, et al. Localization of Nogo and its receptor in the optic pathway of mouse embryos. J Neurosci Res. 2008;86:1721–1733. doi: 10.1002/jnr.21626. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW, Veerkamp JH. New insights into the structure and function of fatty acid-binding proteins. Cell Mol Life Sci. 2002;59:1096–1116. doi: 10.1007/s00018-002-8490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


