Abstract
The aim of this study was to analyze the effects of cryotherapy on the biochemical and morphological changes in ischemic and reperfused (I/R) gastrocnemius muscle of rats. Forty male Wistar rats were divided into control and I/R groups, and divided based on whether or not the rats were submitted to cryotherapy. Following the reperfusion period, biochemical and morphological analyses were performed. Following cryotherapy, a reduction in thiobarbituric acid-reactive substances and dichlorofluorescein oxidation levels were observed in I/R muscle. Cryotherapy in I/R muscle also minimized effects such as decreased cellular viability, levels of non-protein thiols and calcium ATPase activity as well as increased catalase activity. Cryotherapy also limited mitochondrial dysfunction and decreased the presence of neutrophils in I/R muscle, an effect that was corroborated by reduced myeloperoxidase activity in I/R muscle treated with cryotherapy. The effects of cryotherapy are associated with a reduction in the intensity of the inflammatory response and also with a decrease in mitochondrial dysfunction.
Keywords: cryotherapy, inflammatory response, ischemia and reperfusion, mitochondrial dysfunction, oxidative stress
Introduction
Ischemia and reperfusion (I/R) injury is a common circulatory disorder characterized by a reduction in blood flow through the arterial vessels of an injured area. This phenomenon is considered a crucial factor in the generation of oxidative damage that follows a skeletal muscle lesion, such as those associated with sports injuries. Because sports injuries are generally characterized as traumatic injuries (Beiner & Jokl, 2001), it can be assumed that the pathophysiology of skeletal muscle tissues at the site of the injury involves factors, such as capillary rupture and impairment of blood flow through the site of the lesion. On the other hand, common skeletal muscle lesions, such as muscle strains (Carvalho et al. 2010) and skeletal muscle contusions (Puntel et al. 2010), also have been recently reported to show significant oxidative damage at the site of the lesion and also in the blood components.
The pathophysiology of I/R injury is well characterized by the impairment of oxidative metabolism of the involved tissues (Carden & Granger, 2000). As a result, an excessive reactive oxygen species (ROS) generation could be observed in I/R tissues (Harris et al. 1986; Choudhury et al. 1991). In these conditions elevated ROS levels could exceed cellular antioxidant defense system capacity to scavenge these molecules, resulting in a condition knew as oxidative stress. Besides, important biological systems, such as those that depend on the sulfhydryl groups (–SH) integrity for their normal functioning, could be impaired (Sun et al. 2001). Moreover, some important enzymes activities, such as the lactate dehydrogenase (LDH; Pamp et al. 2005) and delta aminolevunilate dehydratase (Δ-ALA-D) activities (Folmer et al. 2003), and the non-enzymatic antioxidant glutathione (GSH) levels could be affected in these conditions (Sies, 1997).
The development of therapies with the potential to effectively modulate the oxidative damage resulting from skeletal muscle injury is of interest. In this context, cryotherapy has been reported to effectively modulate the oxidative damage that follows skeletal muscle strain (Carvalho et al. 2010) and contusion injuries (Puntel et al. 2010). Furthermore, the use of cryotherapy to minimize the impairment of oxidative metabolism in I/R muscle has been previously described in the literature (Presta & Ragnotti, 1981). However, although cryotherapy has been considered to be one of the most efficient strategies to treat different types of skeletal muscle lesions (Bleakley et al. 2004), the biochemical mechanisms involved in the protective effects of cryotherapy have not been elucidated. At least in part, the benefits of cryotherapy may be related to its capacity to reduce the intensity of the inflammatory response, to attenuate mitochondrial dysfunction and to preserve the morphology of skeletal muscle (Carvalho et al. 2010; Puntel et al. 2010).
Therefore, the aim of this study was to analyze the effects of cryotherapy under biochemical and morphological changes in I/R gastrocnemius muscle of rats.
Materials and methods
Ethical approval
The animals were maintained and used in accordance with the guidelines of the Committee on Care and Use of Experimental Animal Resources of the Federal University of Santa Maria, Brasil.
Chemical reagents
The reagents thiobarbituric acid (TBA), dichlorofluorescein diacetate (DCF-DA), methyltetrazolium (MTT), ethylene glycol tetraacetic acid (EGTA), Ellman's reagent (DTNB), N,N,N′,N′-tetramethylbenzidine and ouabaine were supplied by Sigma-Aldrich Chemical (St Louis, MO, USA). The other reagents used were obtained from local suppliers.
Animals
Forty adult male Wistar rats weighing 270–320 g from our own breeding colony were kept in cages of five animals each, with food and water ad libitum in a room with controlled temperature (22 ± 3 °C), and on a 12 h light/dark cycle with lights on at 07:00 hours. The animals were divided into four main groups.
Control non-treated and non-lesioned animals – animals not submitted to the standard I/R injury (n = 10).
Control cold treated and non-lesioned animals – animals not submitted to the standard I/R injury and treated with cryotherapy (n = 10).
Lesioned non-treated animals – animals submitted to the standard I/R injury without any treatment (n = 10).
Lesioned and cold treated animals – animals submitted to the standard I/R injury and treated with cryotherapy (n = 10).
I/R injury
The skeletal muscle I/R injury was developed according to the method proposed by Strock & Majno (1969), with few modifications. Firstly, the animals were anesthetized with ketamine (50 mg kg−1; i.p.) and xylazine (10 mg kg−1; i.p.). The ischemia was performed using an external tourniquet that was tensioned in the proximal portion of the thigh near to the hip junction. The ischemia was maintained during 3 h. Thereafter, the tourniquet was removed in order to start the reperfusion period. The reperfusion period was maintained during 2 h and then the animals were killed in order to remove the muscle to perform the biochemical and morphological analysis.
Cryotherapy
The treatment of the animals with cryotherapy was performed with ice pieces placed into a malleable bag of ice that covered the entire hind limb submitted to the I/R injury (Presta & Ragnotti, 1981). The treatment section was developed for 3 h during all the ischemia period.
Biochemical analysis
Tissue preparation
Skeletal muscle homogenates: the animals were killed by decapitation and the right gastrocnemius muscle was removed, quickly homogenized in NaCl (150 mm) and kept in ice. After the homogenization, the skeletal muscle samples were centrifuged at 4000 g at 4 °C for 10 min to yield a low-speed supernatant fraction. The obtained supernatant fraction was used for TBA-reactive substances (RS), DCF-RS, non-protein –SH and MTT reduction levels, and also for the CAT, superoxide dismutase (SOD), Ca2+ ATPase and LDH enzymes activities determination.
For the myeloperoxidase (MPO) enzyme activity measurement, the muscle samples were homogenized in potassium phosphate buffer (20 mm, pH 7.4) containing ethylenediaminetetraacetic acid (EDTA; 0.1 mm). After the homogenization, the skeletal muscle samples were centrifuged at 2000 g at 4 °C for 10 min to yield a low-speed supernatant fraction. Then, the supernatant fraction was centrifuged again at 20 000 g at 4 °C for 15 min to yield a final pellet that was resuspended in potassium phosphate buffer (50 mm, pH 6.0) containing hexadecyltrimethylammonium bromide (0.5%). The samples were finally freeze-thawed twice for the subsequent enzymatic MPO assay.
Isolation of skeletal muscle mitochondria: rat skeletal muscle mitochondria were isolated as described by Tonkonogi & Salhin (1997), with some modifications. The isolated mitochondria were used to determine the mitochondrial DCF-RS, ΔΨ, MnSOD enzyme activity, and the mitochondrial GSH/glutathione disulfide (GSSG) levels.
Oxidative stress markers and cell viability determination
TBA-RS levels: the TBA-RS levels were determined in skeletal muscle supernatant fraction samples according to the method described by Ohkawa et al. (1979). TBA-RS levels were measured at 532 nm using a standard curve of malondialdehyde and corrected by the protein content.
DCF-RS levels: for DCF-RS levels determination, the skeletal muscle supernatant fraction samples (50 μL) were added to a medium containing Tris-HCl buffer (0.01 mm; pH 7.4) and DCF-DA (7 μm; Puntel et al. 2010). DCF-RS levels were determined using a standard curve of DCF and the results were corrected by the protein content (Pérez-Severiano et al. 2004).
Non protein –SH levels: levels of non protein –SH were determined in skeletal muscle supernatant fraction samples according to the method proposed by Ellman (1952) with some modifications (Puntel et al. 2010). Results were calculated in relation to a standard curve constructed with GSH and also corrected by the protein content (Ellman, 1952).
MTT reduction levels: for MTT reduction levels determination, the skeletal muscle supernatant fraction samples (500 μL) were added to a medium containing 0.5 mg mL−1 of MTT and were incubated in the dark for 1 h at 37 °C. The MTT reduction reaction was stopped by the addition of 1 mL of dimethylsulfoxide. The formed formazan levels were determined spectrophotometrically at 570 nm, and the results were corrected by the protein content (Mosmann, 1983).
Enzymes activity determination
CAT activity: the CAT enzyme activity was determined in skeletal muscle supernatant fraction according to the method proposed by Aebi (1984).
SOD activity: the SOD enzyme activity was determined in skeletal muscle supernatant fraction according to the method proposed by Misra & Fridovich (1972).
Ca2+ ATPase activity: the Ca2+ ATPase enzyme activity was determined in skeletal muscle supernatant fraction samples according to the method proposed by Zaidi & Michaelis (1999), with some modifications (Puntel et al. 2010).
LDH activity: the LDH enzyme activity was determined spectrophotometrically in skeletal muscle supernatant fraction samples using diagnosis kits (LDH Liquiform, Labtest, MG, Brasil).
MPO activity: the MPO enzyme activity was determined in skeletal muscle according to the method proposed by Grisham et al. (1986), with some modifications (Puntel et al. 2010).
Indicators of the skeletal muscle mitochondria function
Mitochondrial DCF-RS level determination: the mitochondrial DCF-RS generation was assayed according to Garcia-Ruiz et al. (1997). Briefly, the skeletal muscle mitochondria samples (150 μg protein mL−1) were incubated in a medium containing KCl (65 mm), sucrose (100 mm), EGTA (0.05 mm), bovine serum albumin (BSA; 0.2%), HEPES (10 mm, pH 7.2), and the respiratory substrates glutamate (5 mm) and succinate (5 mm). The reaction was started with the DCFA-DA (1 μm) addition, and the medium was kept at constant stirring during the assay period. The fluorescence analysis was performed at 488 nm for excitation and 525 nm for emission, with slit widths of 5 nm.
Mitochondrial ΔΨ determination: the mitochondrial ΔΨ determination was assayed according to Akerman & Wikstron (1976). Briefly, the skeletal muscle mitochondria samples (150 μg protein mL−1) were incubated in a medium containing KCl (65 mm), sucrose (100 mm), EGTA (0.05 mm), BSA (0.2%), HEPES (10 mm, pH 7.2), safranine O (10 μm), and the respiratory substrates glutamate (5 mm) and succinate (5 mm). The reaction was started with the mitochondria addition, and the medium was kept at constant stirring during the assay period. The fluorescence analysis was performed at 495 nm for excitation and 586 nm for emission, with slit widths of 5 nm.
Mitochondrial MnSOD activity: the mitochondrial MnSOD enzyme activity was determined in skeletal muscle-isolated mitochondria according to the method proposed by Misra & Fridovich (1972). Briefly, aliquots of 100 μL of isolated mitochondria were added to a medium containing sodium bicarbonate-carbonate buffer (50 mm; pH 10.2), EDTA (2 mm) and adrenaline (0.4 mm). The kinetic analysis of SOD was started after adrenaline addition, and the color reaction was measured at 480 nm.
Mitochondrial GSH and GSSG levels: the mitochondrial GSH and GSSG levels were determined according to Hissin & Hilf (1976) with some modifications. Briefly, the skeletal muscle-isolated mitochondria (150 μg protein mL−1) were resuspended in 1.5 mL sodium-phosphate buffer (100 mm NaH2PO4, 5 mm EDTA, pH 8.0) and 500 μL phosphoric acid (H3PO4) 4.5%, and were centrifuged at 100 000 g for 30 min. For GSH determination, 100 μL of the supernatant resulting from the centrifugation was added to 1.8 mL phosphate buffer and 100 μL o-phthalaldehyde (OPT). After thorough 15 min, the solution was transferred to a quartz cuvette and the fluorescence was measured at 420 nm for emission and 350 nm for excitation, with slit widths of 3 nm. For GSSG determination, 250 μL of the supernatant resulting from the centrifugation was added to 100 μL of N-ethylmaleimide and incubated at room temperature for 30 min. After the incubation, 140 μL of the mixture was added to 1.76 mL NaOH (100 mm) solution and 100 μL OPT. After thorough 15 min, the solution was transferred to a quartz cuvette and the fluorescence was measured at 420 nm for emission and 350 nm for excitation, with slit widths of 3 nm. The mitochondrial GSH and GSSG levels were determined from comparisons with a linear GSH or GSSG standard curve, respectively.
Protein determination
The protein content was determined according to Lowry et al. (1951) using BSA as standard.
Histopathological analysis
One sample of the skeletal muscle tissue was used for the histopathological analysis in order to investigate the loss of the normal skeletal muscle cells architecture in the I/R muscle. Besides, the presence of neutrophils was examined as an indicator of the inflammatory response. After being excised, the skeletal muscle was maintained in buffered formaldehyde solution (10%) until the microscopic preparation and staining. The muscle samples were sectioned longitudinally along its proximal and distal origins. The histological slides were stained with hematoxylin and eosin in order to analyze the main morphological changes in the skeletal muscle tissue architecture. Moreover, we performed the Giemsa's staining in order to underline the presence of inflammatory cells infiltrated among the skeletal muscle cells.
Statistical analysis
Data were analyzed by one-way anova followed by Duncan test. Differences between groups were considered significant when P < 0.05.
Results
Oxidative damage and cell viability in I/R muscle
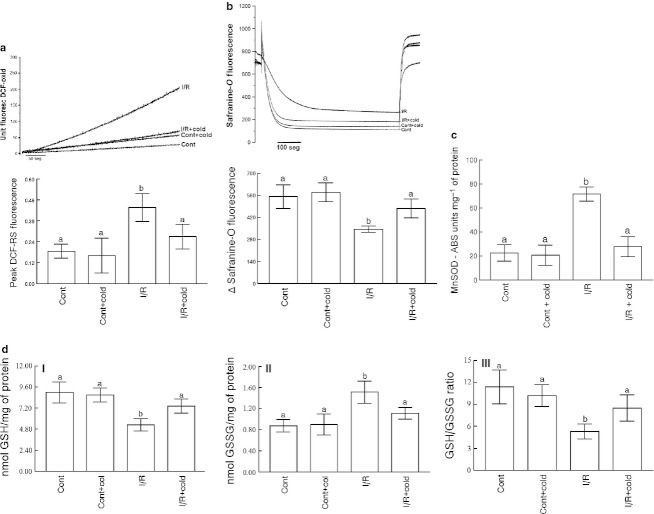
Cryotherapy reduced the increase in DCF-RS and TBA-RS levels that was observed in the I/R muscle (Fig. 1a,b, respectively; P ≤ 0.05). Moreover, the decrease in MTT reduction observed in I/R muscle was limited by cryotherapy (Fig. 1c; P ≤ 0.05).
Fig. 1.

Oxidative damage in ischemic and reperfused (I/R) muscle: (a) dichlorofluorescein (DCF)-RS levels; (b) TBA-RS levels; (c) methyltetrazolium (MTT) reduction levels. (a) The DCF-RS levels are expressed in μmol of DCF-oxidized per mg of protein; (b) the TBA-RS levels are expressed in μmol of malondialdehyde (MDA) per mg of protein; (c) the MTT reduction levels are expressed in % of the control values. Data are expressed as mean ± SE (n = 5) and were analyzed by anova, followed by Duncan's test when appropriate. Differences were considered significant when P ≤ 0.05. Significant differences are marked as a or b.
Figure 2a shows that the cryotherapy counteracted a significant decrease in non-protein –SH groups levels depicted in the I/R muscle (Fig. 2a; P ≤ 0.05).
Fig. 2.

Non-enzymatic and enzymatic antioxidants in ischemic and reperfused (I/R) muscle: (a) non-protein –SH levels; (b) CAT activity; (c) SOD activity. (a) The non-protein –SH levels are expressed in nmol of SH per mg of protein; (b) the CAT activity is expressed in μmol H2O2 per min per mg of protein; (c) the SOD activity is expressed in units of absorbance per mg of protein. Data are expressed as mean ± SE (n = 5) and were analyzed by anova, followed by Duncan's test when appropriate. Differences were considered significant when P ≤ 0.05. Significant differences are marked as a or b.
Enzymes activities in I/R muscle
Increased CAT activity observed in I/R muscle was significantly diminished by cryotherapy (Fig. 2b; P ≤ 0.05). On the other hand, the SOD activity was not significantly changed by I/R injury or by cryotherapy (Fig. 2c).
Cryotherapy also neutralized the significant decrease in Ca2+ ATPase activity (Fig. 3a; P ≤ 0.05) and a significant increase in LDH activity (Fig. 3b; P ≤ 0.05) that were observed in I/R muscle.
Fig. 3.

Enzymes activities in ischemic and reperfused (I/R) muscle: (a) Ca2+ ATPase activity levels; (b) lactate dehydrogenase (LDH) activity levels. (a) Ca2+ ATPase activity levels are expressed in nmol of Pi per min per mg of protein; (b) the LDH activity is expressed in % of the control values (the mean value of control LDH activity was 46.9 ± 10.7 units mg−1 of protein). Data are expressed as mean ± SE (n = 5) and were analyzed by anova, followed by Duncan's test when appropriate. Differences were considered significant when P ≤ 0.05. Significant differences are marked as a or b.
Skeletal muscle MPO activity was significantly increased in I/R muscle (Fig. 4; P ≤ 0.05), and cryotherapy reduced this increase to values similar to those observed in control muscle (Fig. 4).
Fig. 4.
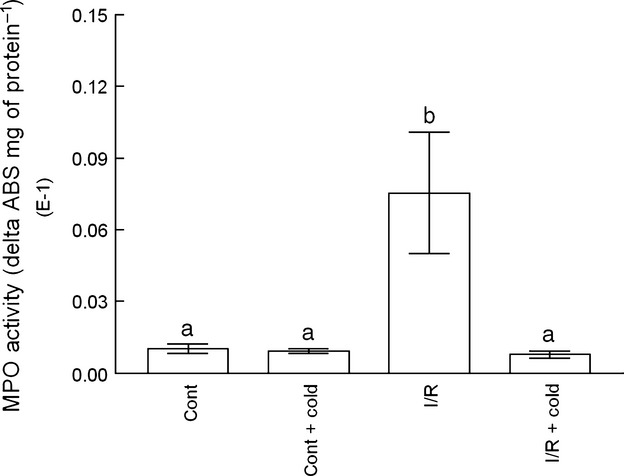
Myeloperoxidase (MPO) activity in ischemic and reperfused (I/R) muscle. The MPO activity is expressed in absorbance variation units (delta ABS) per mg of protein. Data are expressed as mean ± SE (n = 5) and were analyzed by anova, followed by Duncan's test when appropriate. Differences were considered significant when P ≤ 0.05. Significant differences are marked as a or b.
Mitochondrial function in I/R muscle
Significant increase in mitochondrial DCF-RS generation (Fig. 5a; P ≤ 0.05) and also a significant decrease in the mitochondrial ΔΨ (Fig. 5b; P ≤ 0.05), observed in I/R muscle, were limited by cryotherapy.
Fig. 5.
Mitochondrial function in ischemic and reperfusion (I/R) muscle: (a) mitochondrial dichlorofluorescein-reactive substances (DCF-RS) generation; (b) mitochondrial Δψ; (c) mitochondrial MnSOD activity; (d) mitochondrial glutathione (GSH) (I), glutathione disulfide (GSSG) (II) and GSH/GSSG ratio (III) levels. (a and b) The values are presented in fluorescence units (F.U.) according to that described in Materials and methods. (a and b) A representative graph illustrating a single experimental trial and a graph showing the results of six–eight independent experiments are presented. (c) The values are presented in absorbance variation units (delta ABS) per mg of protein. (d) The GSH (I) levels were expressed in nmol GSH per mg of protein, the GSSH (II) levels were expressed in nmol GSSG per mg of protein. For each experimental trial, an independent and fresh mitochondrial preparation was performed. (a–d) The data are expressed as mean ± SE (n = 5) and were analyzed by anova, followed by Duncan's test when appropriate. Differences were considered significant when P ≤ 0.05. Significant differences are marked as a or b.
Mitochondrial MnSOD activity was also significantly increased in I/R muscle (Fig. 5c; P ≤ 0.05), and this increase was adequately neutralized by cryotherapy. Besides, mitochondrial GSH levels were significantly decreased in I/R muscle, as the GSH/GSSG ratio was also significantly decreased (Fig. 5d; P ≤ 0.05). Cryotherapy maintained mitochondrial GSH and GSSG levels near to the observed in control muscle (Fig. 5d).
Histopathological analysis in I/R muscle
Ischemic and reperfused injury depicted a marked presence of neutrophils cells in skeletal muscle (Fig. 6aIII,bIII). Moreover, a swelling of the skeletal muscle tissue, with several vacuoles spaces formation among the muscular fasciculus, was also evident (Fig. 6aIII,bIII). Figure 6aIV and bIV shows that both neutrophils infiltration and swelling in I/R muscle were appropriately counteracted by cryotherapy.
Fig. 6.
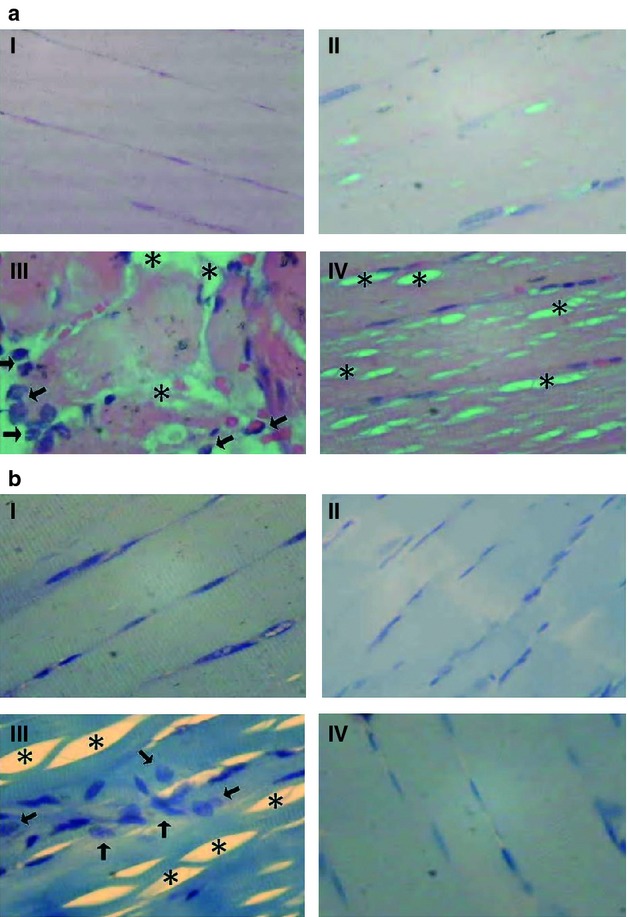
Histopathologic analysis in I/R muscle. The neutrophils infiltrations areas (arrow), as well as the impaired skeletal muscle cells striations areas (sharp) were identified. (a) The skeletal muscle slides were prepared with the hematoxiline/eosine staining; and in (b) with the Giemsa's staining. In all cases, I – control non-treated and non-I/R lesioned muscle; II – control cold-treated and non-I/R lesioned muscle; III – I/R-lesioned and non-treated skeletal muscle; and III – I/R-lesioned and cold-treated skeletal muscle. In all cases the images were 400 times increased and all the histological sections were longitudinal.
Discussion
We observed that cryotherapy had the potential to counteract the increased oxidative damage and to limit the histopathological changes in I/R muscle. Thus, our results are in agreement with our previously published studies, which reported that therapeutic cold effectively minimizes the oxidative damage that follows a skeletal muscle strain lesion (Carvalho et al. 2010), as well as the morphological changes and oxidative damage that follows a skeletal muscle contusion lesion (Puntel et al. 2010).
An important phenomenon that appears to be involved in the pathophysiology of an acute I/R injury is the mitochondrial dysfunction that seems to be associated with the oxidative damage in skeletal muscle. The significant increase in mitochondrial MnSOD activity (Fig. 5c), as well as the significant decrease in mitochondrial GSH levels (Fig. 5d), can be interpreted as a mitochondrial mechanism to counteract the increased superoxide anion (O−2) production. Therefore, a change in the level of hydrogen peroxide (H2O2) might be expected with the consequent increased consumption of GSH via glutathione peroxidase enzyme activity, thus changing the mitochondrial GSH/GSSG ratio. These oxidative changes may be associated with the increased generation of DCF-RS generation (Fig. 5a), and also with the decreased mitochondrial ΔΨ (Fig. 5b) observed in I/R muscle. In general, the mitochondrial dysfunction in I/R muscle was similar to that observed following a skeletal muscle contusion (Puntel et al. 2010). Therefore, we hypothesize that an acute event of I/R injury may be a suitable condition present in the pathophysiology of skeletal muscle disorders, such as contusion lesions.
The morphological changes in the structure of skeletal muscle cells following I/R injury, such as excessive inflammatory cell infiltration among the muscular fasciculus (Fig. 6bIII), are in agreement with the increase in MPO enzyme activity (Fig. 4). Therefore, is possible to infer that the intensity of the inflammatory response is an important phenomenon involved in the pathophysiology of an acute I/R injury. Similarly, we previously demonstrated the relationship between the presence of a large number of infiltrating neutrophils and increased MPO enzyme activity in the skeletal muscle tissue after a skeletal muscle contusion (Puntel et al. 2010).
Therefore, both an uncontrolled inflammatory response and mitochondrial dysfunction could be related to the increased DCF-RS (Fig. 1a) and TBA-RS (Fig. 1b) levels observed in I/R muscle. Previous studies have demonstrated that both an uncontrolled inflammatory response (Supinski & Callahan, 2007) and the mitochondrial dysfunction (Kowaltowski et al. 2001; Andreyev et al. 2005; Murfy, 2009) can result in increased ROS formation. Although we did not specifically measure the formation of ROS formation in I/R muscle, it is well known that increased levels of ROS can result in increased formation of DCF-RS and TBA-RS. As a result, some important cellular enzyme systems, which are sensitive to oxidative damage, may be impaired in I/R muscle. We observed a significant decrease in Ca2+ ATPase activity (Fig. 3a), which is an enzyme that becomes functionally impaired due to the oxidation of critical –SH groups located in its active site (Pamp et al. 2005). The involvement of –SH oxidation in I/R muscle was corroborated by an observed decrease in the levels of non protein –SH (GSH as major compound) (Fig. 2a). It is interesting to note that I/R injury caused a significant increase in the skeletal muscle CAT enzyme activity (Fig. 2b).
In addition, a significant decrease in MTT reduction was observed in I/R muscle (Fig. 1d). MTT reduction depends on adequate functionality of the oxidoreductase enzyme family, such as dehydrogenase enzymes (Muszbek, 1997). Because the majority of these enzymes are located in the mitochondria, their functional impairment may be related to the mitochondria dysfunction. Furthermore, the level of MTT reduction is typically used as an index of cellular viability (Liu et al. 1997). Similarly, an evident loss of integrity of the muscular fasciculi and significant formation of vacuole spaces was observed in I/R muscle (Fig. 6aIII,bIII). Because the integrity of skeletal muscle cell structure is important to cell survival, the morphological damage induced by I/R injury may also compromise the cell viability (Brancaccio et al. 2007). Taken together, these results are in accordance with previous observations in skeletal muscle tissue following a skeletal muscle contusion (Puntel et al. 2010).
The capacity of cryotherapy to minimize the oxidative and morphological damage resulting from an acute I/R injury is in agreement with previous published studies (Presta & Ragnotti, 1981). Similar benefits of cryotherapy were observed in the treatment of skeletal muscle strain (Carvalho et al. 2010) and contusion lesions (Puntel et al. 2010). We believe that these effects of cryotherapy are related, at least in part, to the physiological decrease in blood flow in the treated tissue, which may determine a reduction in the intensity of the inflammatory response to an acute I/R injury (Bleakley et al. 2004). Moreover, the reduced blood flow could minimize oxygen metabolism in I/R muscle treated with cryotherapy, thus minimizing the secondary damage that follows an acute I/R injury (Knight, 1976). Another possibility is the direct effect of cryotherapy on the skeletal muscle cells oxidative metabolism without change in the blood flow in treated areas. More studies are necessary to elucidate the occurrence of such changes and also to correlate them with the oxidative metabolism through the cryotherapy-treated areas.
Concluding remarks
In conclusion, we observed that cryotherapy minimized both the high inflammatory response intensity and mitochondrial dysfunction in I/R muscle. These effects of cryotherapy seem to be the major mechanisms to explain a reduced oxidative and morphological damage that follows an acute I/R injury. Finally, our results contribute to the understanding of benefits and also mechanisms by which the cryotherapy acts in the treatment of skeletal muscle lesions, such as those observed in sportive practice. Moreover, cryotherapy is usually applied only in the injured areas and generally not immediately after the occurrence of lesions. Therefore, more studies are necessary to evaluate the morphological and biochemical implications of retards to start the cryotherapeutic treatment in conditions that mimic I/R injuries in skeletal muscle.
Acknowledgments
The financial support by FAPERGS, CAPES, CNPq and FINEP research grant ‘Rede Instituto Brasileiro de Neurociência (IBN-Net)’ # 01.06.0842-00 is gratefully acknowledged. N.V.B., F.A.A.S and J.B.T.R are recipients of CNPq fellowships, and N.R.C. receives fellowships from CAPES.
Author contributions
The first author G.O.P. and the corresponding author F.A.A.S. contributed in all the steps of this study construction: concept/design, acquisition of data, data analysis/interpretation, drafting of the manuscript, critical revision and approval of this article. The authors N.R.C., F.D. and A.C.F.S. contributed for data analysis/interpretation and approval of this article. The authors R.L.P., V.F, N.B.V.B., L.F.F.R. and J.B.T.R. contributed for data analysis/interpretation, drafting of the manuscript, critical revision and approval of this article.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Akerman KEO, Wikstron KF. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976;68:191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–224. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Beiner JM, Jokl P. Muscle contusion injuries: current treatment options. J Am Acad Orthop Surg. 2001;9:227–237. doi: 10.5435/00124635-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Bleakley C, Mcdonough S, Macauley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32:251–261. doi: 10.1177/0363546503260757. [DOI] [PubMed] [Google Scholar]
- Brancaccio P, Maffulli N, Limogelli FM. Creatine kinase monitoring in sports medicine. Br Med Bull. 2007;81–82:209–230. doi: 10.1093/bmb/ldm014. [DOI] [PubMed] [Google Scholar]
- Carden DL, Granger DN. Pathophysiology of ischemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Carvalho NR, Puntel GO, Correa PS, et al. Protective effects of the therapeutic cold and heat against the oxidative damage induced by a muscle strain injury in rats. J Sports Sci. 2010;28:923–935. doi: 10.1080/02640414.2010.481722. [DOI] [PubMed] [Google Scholar]
- Choudhury NA, Sakagughi MBBS, Koyano K, et al. Free radical injury in skeletal muscle ischemia and reperfusion. J Surg Res. 1991;51:392–398. doi: 10.1016/0022-4804(91)90139-d. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1952;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Folmer V, Soares JMC, Gabriel D, et al. A high fat diet inhibits δ aminolevulinate dehydratase and increases lipid peroxidation in mice (Mus musculus) J Nutr. 2003;133:2165–2170. doi: 10.1093/jn/133.7.2165. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, et al. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11 369–11 377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- Grisham MB, Hernandez LA, Granger LN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Harris K, Walker PM, Mickle DAG, et al. Metabolic response of skeletal muscle to ischemia. Am J Physiol. 1986;250:H213–H220. doi: 10.1152/ajpheart.1986.250.2.H213. [DOI] [PubMed] [Google Scholar]
- Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Knight KL. Effects of hypothermia on inflammation and swelling. Athl Train J Natl Athl Train Assoc. 1976;11:7–10. [Google Scholar]
- Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peterson DA, Kimura H, et al. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Meth. 1983;16:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murfy MO. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszbek L. A highly sensitive method for the measurement of the ATP-ase activity. Anal Biochem. 1997;77:286–288. doi: 10.1016/0003-2697(77)90315-3. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagy K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pamp K, Bramey T, Kirch M, et al. NAD(H) enhances the Cu(II)-mediated inactivation of lactate dehydrogenase by increasing the accessibility of sulfhydryl groups. Free Radic Res. 2005;39:31–40. doi: 10.1080/10715760400023671. [DOI] [PubMed] [Google Scholar]
- Pérez-Severiano F, Rodrígues-Pérez M, Pedraza-Chaverrí J, et al. S-Allylcysteine, a garlic-derived antioxidant, ameliorates quinolinic acid-induced neurotoxicity and oxidative damage in rats. Neurochem Int. 2004;45:1175–1183. doi: 10.1016/j.neuint.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Presta M, Ragnotti G. Quantification of damage to striated muscle after normothermic or hypothermic ischemia. Clin Chem. 1981;27:297–302. [PubMed] [Google Scholar]
- Puntel GO, Carvalho NR, Amaral GP, et al. Therapeutic cold: an effective kind to modulate the oxidative damage resulting of a skeletal muscle contusion. Free Radic Res. 2010;45:133–146. doi: 10.3109/10715762.2010.517252. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Strock PE, Majno G. Vascular responses to experimental tourniquet ischemia. Surg Gynecol Obstet. 1969;129:309–318. [PubMed] [Google Scholar]
- Sun J, Xu L, Eu JP, et al. Class of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem. 2001;276:15 625–15 630. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol. 2007;102:2056–2063. doi: 10.1152/japplphysiol.01138.2006. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Salhin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiol Scand. 1997;161:345–353. doi: 10.1046/j.1365-201X.1997.00222.x. [DOI] [PubMed] [Google Scholar]
- Zaidi A, Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca2+ -ATPase. Free Radic Biol Med. 1999;27:810–821. doi: 10.1016/s0891-5849(99)00128-8. [DOI] [PubMed] [Google Scholar]