Abstract
Articular cartilage and subchondral bone act together, forming a unit as a weight-bearing loading-transmitting surface. A close interaction between both structures has been implicated during joint cartilage degeneration, but their coupling during normal growth and development is insufficiently understood. The purpose of the present study was to examine growth-related changes of cartilage mechanical properties and to relate these changes to alterations in cartilage biochemical composition and subchondral bone structure. Tibiae and femora of both hindlimbs from 7- and 13-week-old (each n = 12) female Sprague-Dawley rats were harvested. Samples were processed for structural, biochemical and mechanical analyses. Immunohistochemical staining and protein expression analyses of collagen II, collagen IX, COMP and matrilin-3, histomorphometry of cartilage thickness and COMP staining height were performed. Furthermore, mechanical testing of articular cartilage and micro-CT analysis of subchondral bone was conducted. Growth decreased cartilage thickness, paralleled by a functional condensation of the underlying subchondral bone due to enchondral ossification. Cartilage mechanical properties seem to be rather influenced by growth-related changes in the assembly of major ECM proteins such as collagen II, collagen IX and matrilin-3 than by growth-related alterations in its underlying subchondral bone structure. Importantly, the present study provides a first insight into the growth-related structural, biochemical and mechanical interaction of articular cartilage and subchondral bone. Finally, these data contribute to the general knowledge about the cooperation between the articular cartilage and subchondral bone.
Keywords: development, extracellular matrix, maturation, micro CT, subchondral bone, viscoelastic properties
Introduction
The cartilage extracellular matrix (ECM) is mainly composed of collagens, proteoglycans, and non-collagenous glycoproteins, which interact with each other and are organized in a complex molecular network. Mechanical loading during growth and development influences ECM composition and structure and lays the basis for tissue quality and function at later age, thereby shaping a rather homogeneous ECM to a very loading-optimized and loading-specialized tissue (Brama et al. 2000; Brommer et al. 2005). One of the main components of the cartilage ECM are the collagens, which provide the tensile strength of the tissue through their highly organized fibrillar structure. During growth and maturation, changes within the collagen network architecture take place, altering its orientation and organization, finally forming the arcade-like ‘Benninghoff network’ (Rieppo et al. 2009; Julkunen et al. 2010). Proteoglycans form the other major group of macromolecules in the ECM. Their interspersed position between the collagen fibrils and their hydrophilic character creates a swelling pressure within the cartilage, which mainly provides the compressive stiffness of the articular cartilage. It was shown that growth-related changes of the proteoglycans in articular cartilage are of minor importance compared with changes in collagen content and orientation (Simunek & Muir, 1972; Thonar & Sweet, 1981). On the other hand, several glycoproteins and non-glycoproteins such as cartilage oligomeric matrix protein (COMP) and matrilin-3 are known for their adaptor function within the ECM network. COMP is suggested to have a structural role in enchondral ossification as well as in the assembly and stabilization of the ECM by its interaction with collagens, matrilins and aggrecan (Thur et al. 2001; Mann et al. 2004; Chen et al. 2007). COMP has been shown to influence the formation of collagen I and II fibrils by encouraging early association of collagen molecules and thereby accelerating fibrillogenesis, with a distinct organization of the fibrils (Halasz et al. 2007). Matrilin-3 expression was found during development and gradually ceased in healthy cartilage after birth (Klatt et al. 2000). This may indicate its less important role in the functional maintenance of fully differentiated cartilage. Moreover, the reactivation of both COMP and matrilin-3 synthesis described in osteoarthritis supposes a function during attempted repair (Pullig et al. 2002; Koelling et al. 2006). Collagen IX, in combination with collagen II and collagen XI, is a key component in the cartilage collagen fibril (Eyre et al. 2004) that interconnects fibrillar collagens via other ECM components such as matrilin-3 and COMP (Budde et al. 2005; Zaucke & Grassel, 2009). The fact that mice lacking collagen IX or matrilin-3 show osteoarthritis-like alterations (Fassler et al. 1994; van der Weyden et al. 2006) suggests that both proteins are involved in cartilage formation during growth and emphasizes their important role in fibrillar network stabilization and organization. However, at present we do not fully understand how growth influences the composition of the major ECM stabilizing proteins and how these changes affect the mechanical behaviour of articular cartilage.
The mechanical integrity of cartilage and its resistance to injury does not depend solely on the biochemical composition of its ECM, but also on its interaction with the underlying subchondral bone (Burr, 1998). Both structures act in concert with complementary mechanical functions, the former as a bearer and the latter as a structural girder and shock absorber (Layton et al. 1988). According to Radin & Rose (1986) the subchondral bone absorbs most of the mechanical load transmitted by diarthodial joints. Due to the relatively greater stiffness and strength of the subchondral bone in comparison with the overlying articular cartilage (Choi et al. 1990), it is generally accepted that the subchondral bone plays an important role in intraarticular load transmission. There is evidence that the initiation and progression of cartilage degeneration involves a disruption of the normal mechanical equilibrium between both of these structures. It has been suggested that increased subchondral bone stiffness reduces the ability to dissipate the load within the joint. This is related to higher peak dynamic forces in the overlying articular cartilage, which can accelerate cartilage degeneration (Radin & Rose, 1986). However, at present it remains unexplained whether cartilage degeneration is initiated in the cartilage layer, in the bone layer or in both layers simultaneously (Burr & Schaffler, 1997). Many studies have focused on trauma- and osteoarthritis-induced subchondral bone alterations (Boyd et al. 2005; Botter et al. 2006; Sniekers et al. 2008), but its general adaptation behaviour during growth and development is insufficiently investigated. Whereas subchondral bone loss in mild degenerated cartilage and bone sclerosis in late-stage osteoarthritis has been reported (Dedrick et al. 1993), subchondral plate volume fraction was shown to either increase or decrease in response to training in young racing horses (Boyde & Firth, 2005; Muir et al. 2006). Due to the irrevocable connection of the subchondral bone to the overlying articular cartilage, both structures are thought to interact directly with each other and have to be considered therefore as a functional bone–cartilage unit. However, to the best of our knowledge, there is a lack of studies investigating growth-related changes in articular cartilage and subchondral bone simultaneously, considering both structures as a functional unit.
Taken together, integrative studies connecting growth-related changes in cartilage mechanical properties with alterations in cartilage biochemical composition and subchondral bone structure are rare. Therefore, one objective of our study was to assess biochemical and mechanical changes of rat knee articular cartilage in relation to growth, specifically by focusing on collagens and major ECM network stabilizing proteins, which are crucial for the unique mechanical properties of the tissue. Another aim was to relate the changes observed in the mechanical properties of the cartilage to changes in the morphological/structural properties of subchondral bone, which is of fundamental importance for understanding the functional cooperation of the bone–cartilage unit during growth.
Materials and methods
Samples
Tibiae and femora of both hindlimbs from 7- and 13-week-old (each n = 12) female Sprague–Dawley rats were harvested. Right tibiae were frozen in liquid nitrogen and stored at −80° C until protein expression analyses. Right femora were fixed in 4% paraformaldehyde (PFA) for 24 h and prepared for immunohistochemical analyses. Left tibiae were wrapped in saline-soaked gauze and stored at −20° C until mechanical testing. Left femora were used for structural and mechanical bone properties analyses (Hamann et al. 2012a). Further, the 13-week-old animals were used as an unloaded age-matched control group in a previously performed independent cartilage adaptation experiment (Hamann et al. 2012b). The study protocol and all animal procedures were in compliance with the principles of laboratory animal care and the German Laws on the Protection of Animals. The study has been approved by an ethical committee and has been authorized by the North Rhine-Westphalian State Office for Environment, Health and Consumer Protection.
Immunohistochemistry
Immunohistochemical staining of collagen II, collagen IX, COMP and matrilin-3 was carried out as described earlier (Hamann et al. 2012b). Briefly, right femora were decalcified in 20% ethylenediaminetetraacetic acid (EDTA) for 4 weeks, embedded in paraffin, and cut in 8-μm-thick sections in the sagittal plane. Sections of the mid-medial femoral condyle were incubated with the following primary antibodies: mouse monoclonal antibody against human collagen II (1 : 100 dilution; Calbiochem, La Jolla, CA, USA), affinity-purified rabbit polyclonal antibody against the NC4 domain of mouse collagen IX (1 : 1000 dilution) (Budde et al. 2005), rabbit polyclonal antibody against bovine COMP (1 : 1000 dilution) (Schmitz et al. 2008) and affinity-purified rabbit polyclonal antibody against mouse matrilin-3 (1 : 1000 dilution) (Klatt et al. 2000). As secondary antibodies, horse radish-peroxidase (HRP)-conjugated swine anti-rabbit IgG and HRP-conjugated rabbit anti-mouse IgG (both Dako, Copenhagen, Denmark) were used. Sections were stained using 3-amino-9-ethylcarbazole (AEC; Sigma, Germany).
Histomorphometry
Staining for collagen II and COMP revealed a restricted localization and there was a clear demarcation between stained and unstained tissue. The area of the stained tissue was analyzed quantitatively. Staining patterns of collagen II were used to measure the mean cartilage thickness. Intense COMP staining is referred to as the mean COMP staining height. Three sections of each mid-medial femoral condyle were analyzed. In each section, eight images were captured at a 200-fold magnification, starting posteriorly and moving in an anterior direction. For histomorphometric analysis, five measurements in each image were conducted using the image analysis software nis elements d 3.0 (Nikon GmbH, Düsseldorf, Germany). The total values of 120 individual measurements were averaged to obtain the mean femoral cartilage thickness and mean femoral COMP staining height. As staining for collagen IX and matrilin-3 did not display clearly defined positively stained areas, the expression of these proteins was not evaluated quantitatively.
Protein extraction and immunoblotting
To quantify total protein levels, collagen II, collagen IX, COMP and matrilin-3 were extracted from tibial cartilage. After detachment from the underlying bone, each proximal part of the frozen right tibiae was weighed and pulverized in liquid nitrogen using mortar and pestle. Non-collagenous proteins were sequentially extracted in three fractions by adding 10 volumes (mL g–1 of wet tissue) of buffer I [Tris-buffered saline (TBS), buffer II (TBS containing 10 mm EDTA], and buffer III (4 m GuHCl) as previously described (Nicolae et al. 2007). For the detection of COMP and matrilin-3 by immunoblotting, identical volumes of fraction I, II and III were electrophoresed for both groups on 3–12% sodium dodecyl sulphate (SDS) polyacrylamide gels under non-reducing conditions (Laemmli, 1970). Collagenous proteins were extracted by pepsin digestion at 4° C for 48 h at pH ∼ 2.4 using the previously described procedure (Hamann et al. 2012b). For the detection of collagen II, in both groups identical volumes of pepsin extracts were reduced with 5% β-mercaptoethanol and run on 3–12% SDS polyacrylamide gels. For the detection of collagen IX, samples were run under non-reducing conditions on a 15% SDS polyacrylamide gel. Detection of collagen II, COMP and matrilin-3 was performed with the same primary antibodies used for the immunohistochemistry. In the case of collagen IX, a mouse monoclonal antibody against the low molecular weight fragment of collagen IX (1 : 200 dilution) (Hybridoma Bank, Iowa City, IA, USA) (Ye et al. 1991) was used. The same secondary antibodies as for immunohistochemistry were used. Antibody detection was conducted using 2.5 mm luminol, 0.4 mm ρ-coumaric acid, 0.01% H2O2 as luminescent agent (Fluka, Buchs, Switzerland) visualized by exposure on x-ray films. To evaluate the amounts of the proteins extracted quantitatively, samples from two independent animals of each group were evaluated using the image processing program imagej 1.45s (National Institutes of Health, Bethesda, MD, USA).
Mechanical testing
The compressive mechanical properties of tibial cartilage in the weight-bearing area of the medial plateau were tested under stress–relaxation as described previously (Hamann et al. 2012b). Prior to mechanical testing, thickness of the samples was measured by the needle technique as described by Hoch et al. (1983). Three measurements were performed and always conducted in the same chronological order. The mean value acted as initial cartilage thickness for the stress–relaxation test. For the step-wise stress–relaxation tests, a porous plane-ended indenter with a diameter of 0.5 mm (Wang et al. 2006) was utilized, placed between the three thicknesses. A mechanical testing device (Zwick Z2.5/TN1S, Zwick GmbH & Co. KG, Ulm, Germany) equipped with a 10 N load-cell was used to induce a constant strain. Before the experiments, the samples were equilibrated under 0.002 N for 5 min. Total step length was 400 s. Three steps of strain (10, 15 and 20%) were applied (Fig. 1A). The (compression) aggregate modulus HA was calculated from the linear slope of the 10–20% equilibrium stress–strain curves (Fig. 1B) using the equation of Korhonen et al. (2002):
Fig. 1.
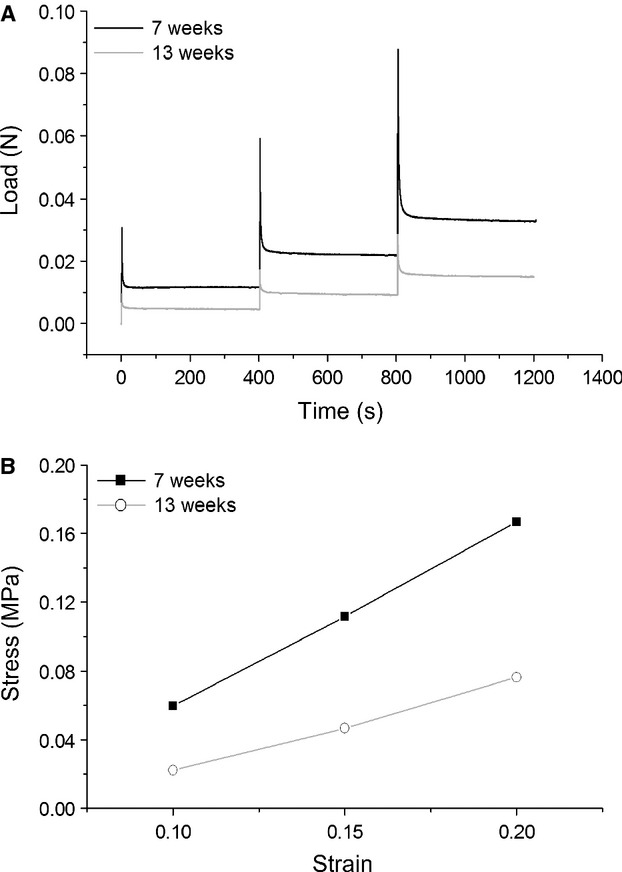
Mechanical testing of tibial articular cartilage by stress–relaxation using indentation. (A) Representative stepwise stress–relaxation curve. (B) Equilibrium stress–strain curve at 10, 15 and 20% strain with an indenter 0.5 mm in diameter.
where νS is the Poisson ratio [value of 0.3 (Wang et al. 2006)] and ES the Young's modulus described by the equation according to Hayes et al. (1972):
where a is the radius of the indenter, h the cartilage thickness, κ the theoretical scaling factor (Hayes et al. 1972), and E the slope of the equilibrium stress–strain curve.
Micro-CT analysis
After mechanical testing, each tibia was sawed off the sample holder, placed perpendicularly in a specimen tube, and submerged with 70% EtOH. A high-resolution micro-CT scanner (μCT 35, Scanco Medical AG, Bassersdorf, Switzerland) was used to determine structural parameters of the subchondral bone of the medial tibial plateau. All scans were performed with an isotropic voxel size of 6 μm, 55 kVp tube voltage, 145 mA tube current, and 400 ms integration time. Grey-scale CT images were filtered using a constrained Gaussian filter (support = 2, sigma = 1.2) to remove noise in the original volume data. For analysis, individual volumes of interest (VOI) were created within the subchondral bone of each medial plateau. The VOIs were positioned in the weight-bearing area of each tibial plateau (100 slices) using anatomical landmarks, underneath the region where the stress–relaxation test of the cartilage was performed before. The VOIs were segmented in a cortical (subchondral plate) and a trabecular region using a fully automatic image analysis algorithm (Scanco Medical) based on the dual threshold technique by Buie et al. (2007) with a value corresponding to 25% for the 7-week-old and 34% for the 13-week-old animals of the maximal grey-scale value. Due to obvious variations in the degree of bone mineralization between the two age groups, thresholds were adjusted and optimized per group by visual inspection of the quality of segmentation. For the trabecular analysis, bone volume fraction (BV/TV,%), which describes the ratio of bone volume over tissue volume, trabecular thickness (Tb.Th, μm) (Hildebrand & Ruegsegger, 1997a; Hildebrand et al. 1999), trabecular number (Tb.N, 1 mm–3), trabecular separation (Tb.Sp, μm), connectivity density (Conn.D, 1 mm–3), which provides a measure of the number of the connections per volume (Odgaard & Gundersen, 1993), and the structure model index (SMI), a quantification of the trabecular bone with a rod-like or plate-like structure (Hildebrand & Ruegsegger, 1997b), were determined. For the subchondral plate, plate thickness (Pl.Th, μm) (Hildebrand & Ruegsegger, 1997a), and plate porosity (Pl.Por, %), describing the ratio of the volume of the pores over the total volume, were calculated.
Statistical analysis
The statistical evaluations were performed with spss for Windows (IBM SPSS Statistics 19.0, IBM Deutschland GmbH, Ehningen, Germany). Results are presented as mean values ± SD. Normal distributions and homogeneity of variances were checked by the Kolmogorov–Smirnov test and Levene's test, respectively. An unpaired Student's t-test was used to test for significant differences between the two groups. Interrelations between the subchondral bone parameters, cartilage thickness, and aggregate modulus of tibial cartilage were analyzed using Pearson's bivariate correlation analysis. Data were considered to be significant at α < 0.05.
Results
Body mass, femur length and wet mass
The body mass was significantly different (P < 0.001) between the two age groups, with a mean body mass of 181 ± 12 g for the 7-week-old and 277 ± 20 g for the 13-week-old animals. Moreover, both the femur length and the femur wet mass were significantly greater (both P < 0.001) in the 13-week-old group. The femur length was 30.0 ± 0.7 mm for the 7-week-old and 35.0 ± 0.7 mm for the 13-week-old animals. The femur wet mass was 0.67 ± 0.05 g for the 7-week-old and 0.95 ± 0.05 g for the 13-week-old animals.
Immunohistochemistry
Due to technical problems, one femur of the 7-week-old animals could not be evaluated. Collagen II was present throughout all zones of articular cartilage in the 7- and 13-week-old animals (Fig. 2A). In both groups, strong staining was seen around all chondrocytes. In addition, in the younger animals a strong pericellular and interterritorial staining in the superficial and transitional zone could be observed. Collagen IX was present throughout the articular cartilage in the 7-week-old animals (Fig. 2B). Both the ECM and pericellular matrix were stained in all zones. In the 13-week-old animals, collagen IX was confined to the ECM of the deep zone and calcified cartilage. In the superficial and transitional zone, collagen IX was slightly expressed in the pericellular matrix. Moderate superficial and a strong pericellular staining of COMP within the lower transitional and deep zone was shown in the 7-week-old animals. In the 13-week-old animals, COMP could be detected in the superficial, upper transitional, and deep zone, whereas the lower transitional zone remained unstained. In the superficial and upper transitional zone, the staining was mainly pericellular. Matrilin-3 was detected in the ECM of the whole articular cartilage of the younger animals. In contrast, only the ECM of the deep zone and calcified cartilage was stained in the 13-week-old animals.
Fig. 2.
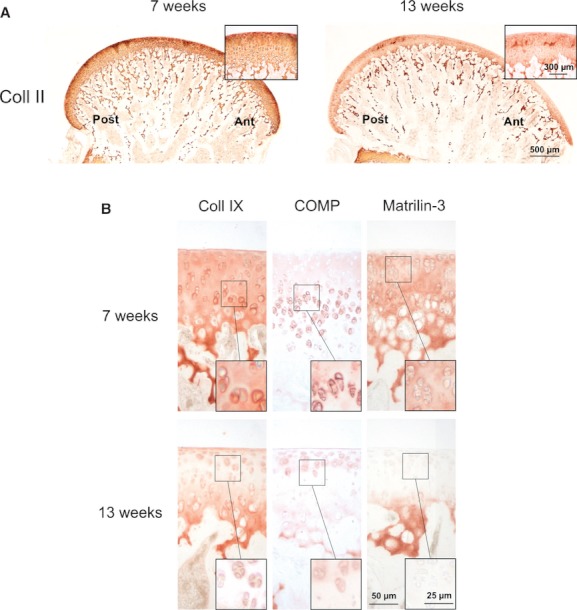
Representative immunohistochemical staining of collagen II, collagen IX, COMP and matrilin-3 in the distal mid-medial femoral articular cartilage from 7- and 13-week-old animals. (A) Cartilage surface stained with collagen II. Magnification ×20, inserts ×200. ant, anterior; post, posterior. (B) Staining for collagen IX, COMP and matrilin-3. Magnification ×200, inserts ×400.
Histomorphometry
Mean femoral cartilage thickness and mean femoral COMP staining height were significantly reduced in the 13-week-old compared with the 7-week-old animals (thickness: −24.5%, P < 0.001; COMP: −67.2%, P < 0.001) (Fig. 3A,B). Additionally, the COMP height/cartilage thickness ratio was significantly (−41.7%, P < 0.001) reduced in the 13-week-old compared with the younger animals (Fig. 3C).
Fig. 3.
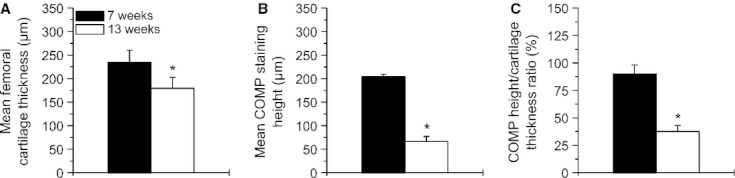
Histomorphometry of cartilage thickness and COMP staining height in the distal mid-medial femoral articular cartilage. (A) Mean cartilage thickness of 7-week-old (n = 11) and 13-week-old (n = 12) animals. Values are means ± SD. (B) Mean COMP staining height of the 7-week-old (n = 11) and 13-week-old (n = 12) animals. Values are means ± SD. (C) COMP height/cartilage thickness ratio of the 7-week-old (n = 11) and 13-week-old (n = 12) animals. Values are means ± SD. Significant differences are indicated: *P < 0.001.
Immunoblotting
Proteins were sequentially extracted with neutral salt buffer (fraction I), high salt buffer with 10 mm EDTA (fraction II), and 4 m GuHCl (fraction III). The amounts and extractability of COMP were similar in both age groups, with the largest amount of COMP being detected in fraction III (Fig. 4A). Matrilin-3 showed only good extractability in fraction III and gave an obviously weaker (−19.4%) signal in the 13-week-old compared with the 7-week-old animals (Fig. 3A). Collagens were extracted by pepsin digestion of the remaining pellets after GuHCl extraction. The total amounts of the extracted collagen II and collagen IX were reduced (−23.4 and −24.0%) in the older compared with the younger animals (Fig. 4B).
Fig. 4.
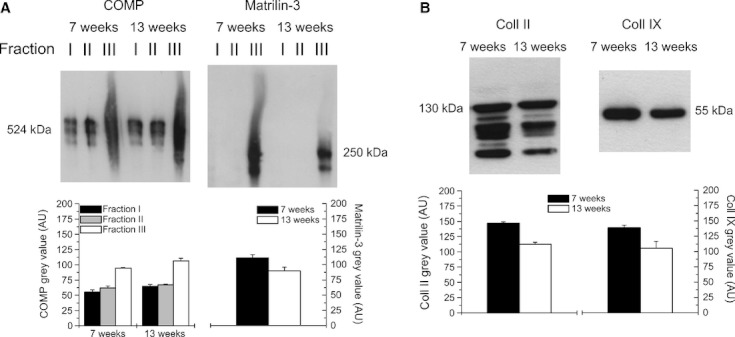
Representative immunoblot of tibial articular cartilage extracts. Proteins were sequentially extracted in Tris-buffered saline (TBS) (fraction I), TBS containing 10 mm EDTA (fraction II) and 4 m GuHCl (fraction III). (A) Western blot analysis of fractions I, II and III were performed for COMP and matrilin-3 of the 7- and 13-week-old animals. Bands of both COMP (fraction I, II, III separately) and matrilin-3 (fraction III) were used for quantification. While COMP showed similar amounts in both age groups, a higher total amount of matrilin-3 was detected in the younger animals. (B) Collagens were extracted by pepsin digestion. Specific bands of collagen II (total) and collagen IX were evaluated quantitatively. Higher total amounts were found for both collagen II and IX in the younger animals. Values in (A) and (B) are means ± SD (n = 2 samples each).
Mechanical testing
The mean tibial cartilage thickness of the 13-week-old animals was significantly (−35.3%, P < 0.001) reduced compared with the 7-week-old animals (Fig. 5A). The (compression) aggregate modulus HA obtained from the equilibrium stress–strain curves at 10–20% strain was significantly (−31.6%, P = 0.041) reduced in the 13-week-old animals compared with the younger animals (Fig. 5B). One tibia of the 7-week-old animals showed abnormal structural defects and was therefore excluded from the mechanical testing. The mean tibial cartilage thickness did not correlate with the aggregate modulus (r² = 0.12, P = 0.10) (Fig. 7A).
Fig. 5.
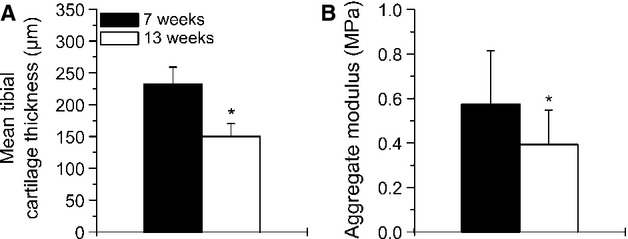
Mean cartilage thickness (A) and aggregate modulus (B) of proximal tibial articular cartilage in the weight-bearing area from the 7-week-old (n = 11) and 13-week-old (n = 12) animals. Values are means ± SD. Significant differences are indicated: *P < 0.05.
Fig. 7.
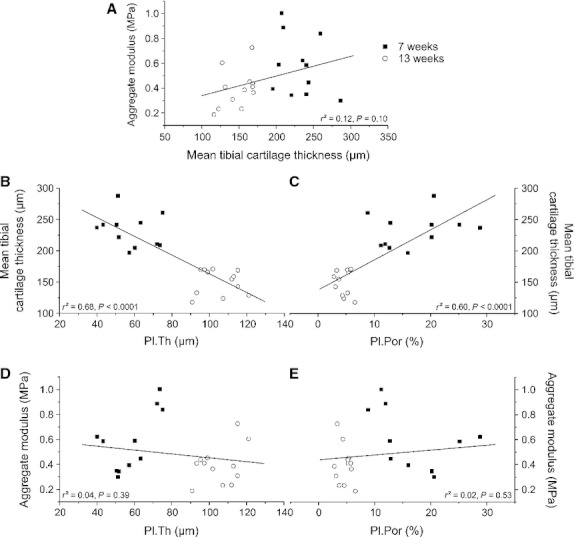
Correlations between the mean tibial cartilage thickness, aggregate moduli at 10–20% strain, and subchondral bone parameters of the medial tibial plateau. (A) Correlation between cartilage thickness and aggregate modulus (n = 23). (B) Correlation between plate thickness (Pl.Th) and cartilage thickness (n = 23). (C) Correlation between plate porosity (Pl.Por) and cartilage thickness (n = 23). (D) Correlation between plate thickness (Pl.Th) and aggregate modulus (n = 23). (E) Correlation between plate porosity (Pl.Por) and aggregate modulus (n = 23).
Micro-CT analysis
The subchondral trabecular BV/TV and Tb.Th was significantly (+23.5 and +28.1%, both P < 0.001) increased in the 13-week-old animals compared with the younger animals (Fig. 6B). In addition, in Tb.N and Conn.D, significant changes (P < 0.001–0.01) between the two age groups could be detected, whereas no changes were found in Tb.Sp (Table 1). The subchondral Pl.Por was significantly (−72.1%, P < 0.001) decreased and the Pl.Th was significantly (+81.1%, P < 0.001) increased in the 13-week-old animals compared with the younger animals (Fig. 6C). The subchondral Pl.Th and Pl.Por were correlated with the mean tibial cartilage thickness (Fig. 7B,C) and aggregate modulus (Fig. 7D,E). Pl.Th showed a negative correlation with the cartilage thickness at 10–20% strain (r² = 0.68, P < 0.0001), whereas Pl.Por correlated positively with the cartilage thickness at 10–20% strain (r² = 0.60, P < 0.0001). In contrast, neither Pl.Th nor Pl.Por showed any correlation with the aggregate modulus.
Fig. 6.
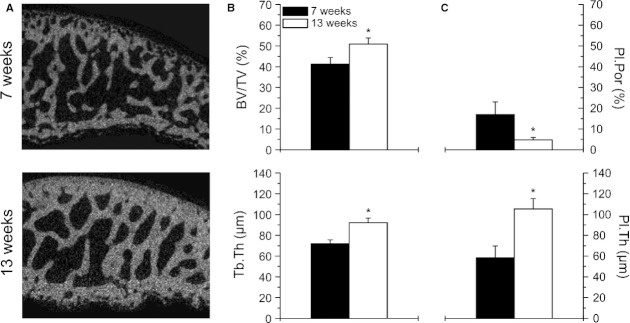
Micro-CT analysis of the subchondral bone in the weight-bearing area of the tibia. (A) Structural differences of the subchondral plate and subchondral trabecular bone between the two age groups. (B) Bone volume fraction (BV/TV) and trabecular thickness (Tb.Th) of the subchondral trabecular bone of the 7-week-old (n = 12) and 13-week-old (n = 12) animals. Values are means ± SD. (C) Plate porosity (Pl.Por) and plate thickness (Pl.Th) of the subchondral plate of the 7-week-old (n = 12) and 13-week-old (n = 12) animals. Values are means ± SD. Significant differences are indicated: *P < 0.001.
Table 1.
Subchondral bone parameters from the 7- and 13-week-old animals determined by micro-CT analysis in the weight-bearing area of the medial tibial plateau
| 7 weeks (n = 12) | 13 weeks (n = 12) | Δrel. (%) | |
|---|---|---|---|
| BV/TV (%) | 41.2 ± 3.2 | 50.9 ± 3.0** | +23.5 |
| Tb.Th (μm) | 72.0 ± 3.6 | 92.2 ± 4.3** | +28.1 |
| Tb.N (1 mm–3) | 6.19 ± 0.45 | 6.90 ± 0.59* | +11.5 |
| Tb.Sp (μm) | 142 ± 14 | 133 ± 14 | −6.3 |
| Conn.D (1 mm–3) | 347 ± 47 | 263 ± 47** | −24.2 |
| SMI | −0.46 ± 0.28 | −0.63 ± 0.33 | +37.0 |
| Pl.Th (μm) | 58.2 ± 11.5 | 105.4 ± 10.1** | +81.1 |
| Pl.Por (%) | 16.94 ± 6.12 | 4.73 ± 1.21** | −72.1 |
BV/TV, bone volume fraction; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation; Conn.D, connectivity density; SMI, structure model index; Pl.Th, plate thickness; Pl.Por,%, plate porosity. Values are means ± SD.
Significant at:
P < 0.01,
P < 0.001.
Discussion
In the present study, we examined the effect of growth of the cartilage–bone unit. To relate growth-related changes in cartilage mechanical properties to changes in biochemical composition and the subchondral bone structure, we performed multiple analyses at different levels. Our results indicate a high modelling activity during growth based on changes in cartilage mechanical properties and biochemical composition as well as subchondral bone structure. In articular cartilage a decrease in thickness as well as both changes in localization and amounts of major ECM stabilizing proteins such as collagen II, collagen IX and matrilin-3 were detected accompanied by reduced mechanical properties. The subchondral trabecular bone and subchondral plate were found to condense with increasing age. Our correlation analyses led us to speculate that cartilage mechanical properties might not be influenced by these growth-related alterations in subchondral bone structure.
As demonstrated by the significant increase in body mass, femur length and wet mass, and according to Ellender et al. (1988) the rats used in the present study were in a rapid growth phase, reaching skeletal maturity at the age of 13 weeks. Our results confirmed the notion that articular cartilage thickness decreases during growth (Brommer et al. 2005; Turnhout et al. 2010). It has been suggested that this reduction in cartilage thickness is caused by an advancement of the enchondral ossification front and the subsequent development of the subchondral bone (Marks & Hermey, 1996). This conclusion is corroborated by our correlation analyses showing that cartilage thickness correlates negatively with subchondral bone plate thickness (r² = 0.68, P < 0.0001) and positively with subchondral bone plate porosity (r² = 0.60, P < 0.0001). It is interesting to note that the percentage reduction of the mean cartilage thicknesses measured by histomorphometry (−24.5%) and needle indentation testing (−35.3%) were similar in relation to the amounts of collagen II evaluated by immunoblots (−23.4%). This is likely due to the fact that collagen II is the major cartilage constituent that exists in all zones, as demonstrated in our immunohistochemical stainings independent of the age of the animals analyzed. Due to the uniform staining pattern of collagen II in cartilage of both age groups, it is difficult to estimate the amount in the respective cartilage zone. We cannot say whether collagen II is substantially redistributed within the cartilage during growth and speculate that the reduction in collagen II determined by our immunoblots is mainly due to the reduction in cartilage thickness.
Further analyses of tibial cartilage extracts by immunoblots revealed that the expression of other structural matrix proteins such as matrilin-3 and collagen IX also decreased with age. Absence of matrilin-3 was shown to reduce chondrocyte differentiation during embryonic development and accounts for a higher incidence of osteoarthritis during ageing (van der Weyden et al. 2006). Moreover, a certain amount of matrilin-3 seems to be essential for an optimal activation of chondrocytes by mechanical signals, illustrating its significance especially with regard to mechanical adaptation (Kanbe et al. 2007). In our study, the amount of matrilin-3 decreased (−19.4%) during growth as demonstrated by both immunoblots and immunohistochemistry. Due to the reduction in cartilage thickness and the loss of staining of matrilin-3 in the superficial and transitional zone in the older animals, a more pronounced reduction in the immunoblots was expected. This apparent contradiction could be explained by a concomitant redistribution of matrilin-3 to the deeper zones and calcified cartilage. Nevertheless, our results confirm that matrilin-3 has a less important role in the functional maintenance of differentiated cartilage, as speculated by Klatt et al. (2000). Similar to our observations Klatt et al. (2002) reported in a follow-up study that deposition of matrilin-3 at the articular surface is not as pronounced as in the deeper cartilage layers in late development.
The total amount of COMP determined by immunoblots remained largely unaffected from the developmental stage. In contrast to this, immunohistochemical analyses of femoral cartilage showed a massive reduction in COMP staining height (−67.2%) during growth. In rapidly growing rats, a moderate superficial and a pericellular staining of the lower transitional and deep zone could be observed, whereas with ageing the presence of COMP is restricted mainly to a pericellular staining in the superficial and upper transitional zone and a slight staining in the deep zone. The profound redistribution and perhaps re-expression of COMP in distinct zones might compensate for the expected reduction due to decreased cartilage thickness during ageing. As a consequence, the total extractable amount of COMP remains rather unchanged. Our findings are in accordance with earlier studies showing that COMP expression is decreased in early developing rat femoral head cartilage (Shen et al. 1995). Shen et al. (1995) proposed that COMP may be involved in regulating cell growth and proliferation, important events in the growing cartilage. Further, one can speculate that COMP plays a specific role during rat cartilage development, as it has been reported to regulate collagen fibrillogenesis (Halasz et al. 2007). This does not solely underline its importance and high occurrence during growth but may explain its reactivation during degenerative cartilage diseases suggesting an important function in tissue repair (Koelling et al. 2006).
Collagen IX is thought to be involved in maintaining the structural integrity of the ECM (Cremer et al. 1998; Blaschke et al. 2000) due to its bridging function between fibrillar collagens and other ECM components. Lack of collagen IX resulted in age-related OA-like changes in mice knee joint articular cartilage (Fassler et al. 1994; Hu et al. 2006) suggesting an important role in fibrillar network stabilization and organization (Eikenberry & Bruckner, 1999). In our study, in healthy growing rats collagen IX expression and amount decreased (−24.0%) with increasing age, with minor changes and/or degradation in collagen IX localization as visualized in our immunohistochemical stainings. Besides the reduction in cartilage thickness, the fact that collagen IX has been shown to form covalent cross-links with collagen II (Eyre et al. 2004) could explain its lower levels. Interestingly, collagen IX has been associated with the regulation of the collagen fibril diameter (Hagg et al. 1998) and it is known that the fibril diameter in mouse cartilage changes with age (Hagg et al. 1997). This could explain a differential expression and/or localization also in cartilage of growing rats. Moreover, collagen IX is suggested to be one of the most important binding partners of matrilin-3 (Budde et al. 2005) and COMP (Thur et al. 2001) for connecting with fibrillar collagens. Therefore, the marked decrease in expression and the strong redistribution of matrilin-3 as well as of COMP could be related to the reduction in collagen IX. One can speculate that, similar to matrilin-3 and COMP, the existence and localization of collagen IX is strongly related to the developmental stage of articular cartilage. The distribution of collagen IX expression showed less obvious alterations between the two age groups, confirming its important role as a fibril-stabilizing and -organizing component (Eikenberry & Bruckner, 1999), not only in the rapid-growth phase. However, the cartilage ECM is a complex alloy of a considerable number of proteins that are organized in a dense and highly organized network and subjected to enormous alterations during development. The role and function of the different proteins analyzed in our study seem to be very complex but highly interconnected. Some of the investigated proteins seem to undergo redistribution, some degradation.
The mechanical properties of articular cartilage depend on its biochemical composition, ultrastructural organization (Armstrong & Mow, 1982; Jurvelin et al. 1988) and are related to its experienced mechanical loading (Brama et al. 1999; Helminen et al. 2000). The aggregate modulus of adult cartilage has been positively correlated with proteoglycan content (Mow et al. 2005) but also with collagen content (Sah et al. 1996). Interestingly, growth decreased the aggregate modulus (compressive modulus) in our study. This is contrary to previous studies in which bovine cartilage aggregate modulus was found to increase with increasing stage of development (fetal, calf, adult) (Williamson et al. 2001). This finding was explained by the higher collagen content found in older cartilage and is in agreement with other studies analyzing the collagen content in bovine cartilage explants (Pal et al. 1981; Sah et al. 1994). In accordance with earlier studies (Williamson et al. 2001; Kiviranta et al. 2006), we speculate that collagen does not solely determine cartilage tensile properties and strength, but also essentially influences the compressive properties of articular cartilage. In our study, increasing age decreased the collagen content, which would explain the reduced compressive properties. In accordance with this, Julkunen et al. (2009) further demonstrated that the mechanical properties of articular cartilage do not follow a linear increase during development, indicating that they are highly dependent on the developmental stage and the time at which they are investigated. Moreover, it should be taken into account that the regions analyzed in the study of Williamson et al. (2001) are different from the regions analyzed in our study. Articular cartilage has been shown to vary in its composition and mechanical properties between anatomical sites (Froimson et al. 1997) depending on the local mechanical loads. Whereas Williamson et al. (2001) used patellofemoral groove and femoral condyle cartilage, we analyzed tibial cartilage, which is likely to differ in its composition and therefore mechanical properties.
The characterization of growth-related variations in articular cartilage compressive properties and biochemical composition provides a first insight into the complexity of cartilage development. Our results revealed no strong correlation between the aggregate modulus and cartilage thickness (r² = 0.12, P = 0.10), suggesting that cartilage quality determined by its organization and composition influences the mechanical performance rather than thickness itself. Moreover, the degradation or redistribution of several other important matrix proteins, such as matrilin-3, COMP and collagen IX, supports the assumption that alterations in the matrix composition cause changes in and determine mechanical properties. Unfortunately, there is no study available that has systemically assessed the relationship between cartilage composition and mechanical properties of rat articular cartilage during development. Nevertheless, our calculated aggregate moduli are in good agreement with the values for rat femoral cartilage during ageing ranging from 0.35 to 0.53 MPa (Wang et al. 2006). It should be noted that besides the proteins analyzed in our study, a variety of other ECM components might contribute to the cartilage mechanical integrity and function.
It has been speculated that the mechanical competence of articular cartilage is also modulated by the underlying subchondral bone. Several studies investigated how subchondral bone alterations are related to osteoarthritic cartilage. Thereby, loss of thickness and increased porosity in the subchondral plate in early stages of osteoarthritis, followed by a plate thickening at later phase, could be demonstrated (Dedrick et al. 1993; Botter et al. 2006; Sniekers et al. 2008). This might be related to increased stiffness, making the bone less deformable to vulnerable loads (Radin & Rose, 1986). Thus, it can be assumed that health and integrity of the overlying articular cartilage depends on the mechanical properties of its bony bed. Early training was shown to induce conflicting results regarding subchondral bone changes in horses (Boyde & Firth, 2005; Muir et al. 2006). However, growth-related habitual subchondral bone changes are insufficiently described and understood. In our study, both subchondral trabecular and plate parameters increased. This indicates that condensation of the subchondral bone does not solely occur during degenerative cartilage diseases, but also reflects a physiological process during development. In other studies, it was found that increasing bone volume fraction in association with mild cartilage damage was not accompanied by an increase in stiffness (Day et al. 2001). This indicates that the relationship between stiffness and volume fraction can be disrupted and is not clearly correlated, making changes in subchondral bone structure and microarchitecture difficult to interpret. Unfortunately, mechanical testing of the subchondral bone could not be performed in our study.
Although it appears that growth decreased cartilage aggregate modulus with condensing of the underlying subchondral bone in our study, correlation analyses revealed no association between these parameters. This could indicate that growth-related condensation of subchondral plate structure did not influence cartilage compressive properties, but this needs to be elucidated further. Future studies should therefore focus on quantifying and connecting subchondral mechanical properties with cartilage mechanical properties to understand the synergistic effects of the bone–cartilage unit during growth.
We provide one of the most profound studies available to date investigating the influence of growth on subchondral bone and its overlying articular cartilage simultaneously; however, there are a few limitations. Due to differences in cartilage morphology, biochemical composition and mechanical properties between different articulating surfaces, tibial cartilage may develop differently from femoral cartilage, making a direct comparison of them difficult. However, tibial cartilage was chosen for mechanical testing and immunoblot analyses due to its less curved surface in contrast to femoral cartilage, which allows a more precise positioning of the indenter as well as a more accurate separation of the cartilage from the underlying bone, respectively. Moreover, in a sample as small as the rat tibial plateau, the determination of the cartilage thickness of the entire tibial plateau as well as of the cartilage tissue volume is methodologically challenging. Therefore, the sample loading for the immunoblots performed in the present study was not normalized and complete extracts from two independent samples per treatment were quantified. Although clear differences in extractable amounts were detected for three out of four proteins, conclusions that can be drawn are limited unless additional data using independent methods confirm these changes. However, to the best of our knowledge, our study is the first to investigate the influence of growth on cartilage morphology, biochemical composition and mechanical properties, as well as on the structure of subchondral bone in such a systematic and integrative manner.
In conclusion, the present study relates for the first time growth-related changes in cartilage mechanical properties to changes in cartilage major network proteins as well as to changes in subchondral bone structure. A high modelling activity of both structures comprising the bone–cartilage unit could be demonstrated. Growth decreased cartilage thickness by a subsequent functional condensation of the underlying subchondral bone due to enchondral ossification. Cartilage mechanical properties seem to be influenced by growth-related changes in the assembly of major ECM stabilizing proteins such as collagen II, collagen IX and matrilin-3 rather than by the growth-related alterations in subchondral bone structure. The present study provides a first insight into the growth-related structural, biochemical and mechanical interaction of articular cartilage with subchondral bone. Finally, these data contribute to the general knowledge about the cooperation between the articular cartilage and subchondral bone.
Acknowledgments
We wish to acknowledge Jan Laschinger for his assistance in immunohistochemical analyses. The study was supported by a grant of the German Sport University Cologne and by the Deutsche Forschungsgemeinschaft (grant ZA561/2-1 to FZ). The sponsors had no involvement in study design, collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Authors' contributions
All authors have made substantial contributions to the following: (i) the conception and design of the study, or acquisition of data, or analysis and interpretation of data; (ii) drafting or revising the article critically for important intellectual content; (iii) final approval of the version to be submitted. Authors′ responsibility for the integrity of the work undertaken: Nina Hamann and Anja Niehoff.
Disclosures
None of the authors has anything to disclose for this manuscript and there are no conflicts of interest.
References
- Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64:88–94. [PubMed] [Google Scholar]
- Blaschke UK, Eikenberry EF, Hulmes DJ, et al. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000;275:10370–10378. doi: 10.1074/jbc.275.14.10370. [DOI] [PubMed] [Google Scholar]
- Botter SM, Van Osch GJ, Waarsing JH, et al. Quantification of subchondral bone changes in a murine osteoarthritis model using micro-CT. Biorheology. 2006;43:379–388. [PubMed] [Google Scholar]
- Boyd SK, Muller R, Leonard T, et al. Long-term periarticular bone adaptation in a feline knee injury model for post-traumatic experimental osteoarthritis. Osteoarthritis Cartilage. 2005;13:235–242. doi: 10.1016/j.joca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Boyde A, Firth EC. Musculoskeletal responses of 2-year-old Thoroughbred horses to early training. 8 Quantitative back-scattered electron scanning electron microscopy and confocal fluorescence microscopy of the epiphysis of the third metacarpal bone. N Z Vet J. 2005;53:123–132. doi: 10.1080/00480169.2005.36489. [DOI] [PubMed] [Google Scholar]
- Brama PA, Tekoppele JM, Bank RA, et al. Influence of different exercise levels and age on the biochemical characteristics of immature equine articular cartilage. Equine Vet J Suppl. 1999;31:55–61. doi: 10.1111/j.2042-3306.1999.tb05314.x. [DOI] [PubMed] [Google Scholar]
- Brama PA, Tekoppele JM, Bank RA, et al. Functional adaptation of equine articular cartilage: the formation of regional biochemical characteristics up to age one year. Equine Vet J. 2000;32:217–221. doi: 10.2746/042516400776563626. [DOI] [PubMed] [Google Scholar]
- Brommer H, Brama PA, Laasanen MS, et al. Functional adaptation of articular cartilage from birth to maturity under the influence of loading: a biomechanical analysis. Equine Vet J. 2005;37:148–154. doi: 10.2746/0425164054223769. [DOI] [PubMed] [Google Scholar]
- Budde B, Blumbach K, Ylostalo J, et al. Altered integration of matrilin-3 into cartilage extracellular matrix in the absence of collagen IX. Mol Cell Biol. 2005;25:10465–10478. doi: 10.1128/MCB.25.23.10465-10478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie HR, Campbell GM, Klinck RJ, et al. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41:505–515. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Burr DB. The importance of subchondral bone in osteoarthrosis. Curr Opin Rheumatol. 1998;10:256–262. doi: 10.1097/00002281-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Burr DB, Schaffler MB. The involvement of subchondral mineralized tissues in osteoarthrosis: quantitative microscopic evidence. Microsc Res Tech. 1997;37:343–357. doi: 10.1002/(SICI)1097-0029(19970515)37:4<343::AID-JEMT9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chen FH, Herndon ME, Patel N, et al. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J Biol Chem. 2007;282:24591–24598. doi: 10.1074/jbc.M611390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kuhn JL, Ciarelli MJ, et al. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J Biomech. 1990;23:1103–1113. doi: 10.1016/0021-9290(90)90003-l. [DOI] [PubMed] [Google Scholar]
- Cremer MA, Rosloniec EF, Kang AH. The cartilage collagens: a review of their structure, organization, and role in the pathogenesis of experimental arthritis in animals and in human rheumatic disease. J Mol Med (Berl) 1998;76:275–288. doi: 10.1007/s001090050217. [DOI] [PubMed] [Google Scholar]
- Day JS, Ding M, Van der Linden JC, et al. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J Orthop Res. 2001;19:914–918. doi: 10.1016/S0736-0266(01)00012-2. [DOI] [PubMed] [Google Scholar]
- Dedrick DK, Goldstein SA, Brandt KD, et al. A longitudinal study of subchondral plate and trabecular bone in cruciate-deficient dogs with osteoarthritis followed up for 54 months. Arthritis Rheum. 1993;36:1460–1467. doi: 10.1002/art.1780361019. [DOI] [PubMed] [Google Scholar]
- Eikenberry EF, Bruckner P. Supramolecular structure of cartilage matrix. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Bone and Cartilage Metabolism. Academic Press: San Diego; 1999. pp. 289–300. [Google Scholar]
- Ellender G, Feik SA, Carach BJ. Periosteal structure and development in a rat caudal vertebra. J Anat. 1988;158:173–187. [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Pietka T, Weis MA, et al. Covalent cross-linking of the NC1 domain of collagen type IX to collagen type II in cartilage. J Biol Chem. 2004;279:2568–2574. doi: 10.1074/jbc.M311653200. [DOI] [PubMed] [Google Scholar]
- Fassler R, Schnegelsberg PN, Dausman J, et al. Mice lacking alpha 1 (IX) collagen develop noninflammatory degenerative joint disease. Proc Natl Acad Sci U S A. 1994;91:5070–5074. doi: 10.1073/pnas.91.11.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froimson MI, Ratcliffe A, Gardner TR, et al. Differences in patellofemoral joint cartilage material properties and their significance to the etiology of cartilage surface fibrillation. Osteoarthritis Cartilage. 1997;5:377–386. doi: 10.1016/s1063-4584(97)80042-8. [DOI] [PubMed] [Google Scholar]
- Hagg R, Hedbom E, Mollers U, et al. Absence of the alpha1(IX) chain leads to a functional knock-out of the entire collagen IX protein in mice. J Biol Chem. 1997;272:20650–20654. doi: 10.1074/jbc.272.33.20650. [DOI] [PubMed] [Google Scholar]
- Hagg R, Bruckner P, Hedbom E. Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX. J Cell Biol. 1998;142:285–294. doi: 10.1083/jcb.142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz K, Kassner A, Morgelin M, et al. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282:31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- Hamann N, Kohler T, Muller R, et al. The effect of level and downhill running on cortical and trabecular bone in growing rats. Calcif Tissue Int. 2012a;90:429–437. doi: 10.1007/s00223-012-9593-6. [DOI] [PubMed] [Google Scholar]
- Hamann N, Zaucke F, Heilig J, et al. Effect of different running modes on the morphological, biochemical, and mechanical properties of articular cartilage. Scand J Med Sci Sports. 2012b doi: 10.1111/j.1600-0838.2012.01513.x. doi: 10.1111/j.1600-0838.2012.01513.x. [DOI] [PubMed] [Google Scholar]
- Hayes WC, Keer LM, Herrmann G, et al. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- Helminen HJ, Hyttinen MM, Lammi MJ, et al. Regular joint loading in youth assists in the establishment and strengthening of the collagen network of articular cartilage and contributes to the prevention of osteoarthrosis later in life: a hypothesis. J Bone Miner Metab. 2000;18:245–257. doi: 10.1007/pl00010638. [DOI] [PubMed] [Google Scholar]
- Hildebrand T, Ruegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997a;185:67–75. [Google Scholar]
- Hildebrand T, Ruegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin. 1997b;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- Hildebrand T, Laib A, Muller R, et al. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- Hoch DH, Grodzinsky AJ, Koob TJ, et al. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1:4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- Hu K, Xu L, Cao L, et al. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum. 2006;54:2891–2900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Harjula T, Iivarinen J, et al. Biomechanical, biochemical and structural correlations in immature and mature rabbit articular cartilage. Osteoarthritis Cartilage. 2009;17:1628–1638. doi: 10.1016/j.joca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Iivarinen J, Brama PA, et al. Maturation of collagen fibril network structure in tibial and femoral cartilage of rabbits. Osteoarthritis Cartilage. 2010;18:406–415. doi: 10.1016/j.joca.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Jurvelin J, Saamanen AM, Arokoski J, et al. Biomechanical properties of the canine knee articular cartilage as related to matrix proteoglycans and collagen. Eng Med. 1988;17:157–162. doi: 10.1243/emed_jour_1988_017_042_02. [DOI] [PubMed] [Google Scholar]
- Kanbe K, Yang X, Wei L, et al. Pericellular matrilins regulate activation of chondrocytes by cyclic load-induced matrix deformation. J Bone Miner Res. 2007;22:318–328. doi: 10.1359/jbmr.061104. [DOI] [PubMed] [Google Scholar]
- Kiviranta P, Rieppo J, Korhonen RK, et al. Collagen network primarily controls Poisson's ratio of bovine articular cartilage in compression. J Orthop Res. 2006;24:690–699. doi: 10.1002/jor.20107. [DOI] [PubMed] [Google Scholar]
- Klatt AR, Nitsche DP, Kobbe B, et al. Molecular structure and tissue distribution of matrilin-3, a filament-forming extracellular matrix protein expressed during skeletal development. J Biol Chem. 2000;275:3999–4006. doi: 10.1074/jbc.275.6.3999. [DOI] [PubMed] [Google Scholar]
- Klatt AR, Paulsson M, Wagener R. Expression of matrilins during maturation of mouse skeletal tissues. Matrix Biol. 2002;21:289–296. doi: 10.1016/s0945-053x(02)00006-9. [DOI] [PubMed] [Google Scholar]
- Koelling S, Clauditz TS, Kaste M, et al. Cartilage oligomeric matrix protein is involved in human limb development and in the pathogenesis of osteoarthritis. Arthritis Res Ther. 2006;8:R56. doi: 10.1186/ar1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen RK, Laasanen MS, Toyras J, et al. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002;35:903–909. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layton MW, Goldstein SA, Goulet RW, et al. Examination of subchondral bone architecture in experimental osteoarthritis by microscopic computed axial tomography. Arthritis Rheum. 1988;31:1400–1405. doi: 10.1002/art.1780311109. [DOI] [PubMed] [Google Scholar]
- Mann HH, Ozbek S, Engel J, et al. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem. 2004;279:25294–25298. doi: 10.1074/jbc.M403778200. [DOI] [PubMed] [Google Scholar]
- Marks SC, Hermey DC. The structure and development of bone. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. New York: Academic Press; 1996. pp. 3–14. [Google Scholar]
- Mow VC, Gu WY, Chen FH. Structure and function of articular cartilage and meniscus. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics & Mechano-Biology. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 181–258. [Google Scholar]
- Muir P, McCarthy J, Radtke CL, et al. Role of endochondral ossification of articular cartilage and functional adaptation of the subchondral plate in the development of fatigue microcracking of joints. Bone. 2006;38:342–349. doi: 10.1016/j.bone.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Nicolae C, Ko YP, Miosge N, et al. Abnormal collagen fibrils in cartilage of matrilin-1/matrilin-3-deficient mice. J Biol Chem. 2007;282:22163–22175. doi: 10.1074/jbc.M610994200. [DOI] [PubMed] [Google Scholar]
- Odgaard A, Gundersen HJ. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone. 1993;14:173–182. doi: 10.1016/8756-3282(93)90245-6. [DOI] [PubMed] [Google Scholar]
- Pal S, Tang LH, Choi H, et al. Structural changes during development in bovine fetal epiphyseal cartilage. Coll Relat Res. 1981;1:151–176. doi: 10.1016/s0174-173x(81)80017-9. [DOI] [PubMed] [Google Scholar]
- Pullig O, Weseloh G, Klatt AR, et al. Matrilin-3 in human articular cartilage: increased expression in osteoarthritis. Osteoarthritis Cartilage. 2002;10:253–263. doi: 10.1053/joca.2001.0508. [DOI] [PubMed] [Google Scholar]
- Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;3:4–40. [PubMed] [Google Scholar]
- Rieppo J, Hyttinen MM, Halmesmaki E, et al. Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation. Osteoarthritis Cartilage. 2009;17:448–455. doi: 10.1016/j.joca.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Sah RL, Chen AC, Grodzinsky AJ, et al. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- Sah RL, Trippel SB, Grodzinsky AJ. Differential effects of serum, insulin-like growth factor-I, and fibroblast growth factor-2 on the maintenance of cartilage physical properties during long-term culture. J Orthop Res. 1996;14:44–52. doi: 10.1002/jor.1100140109. [DOI] [PubMed] [Google Scholar]
- Schmitz M, Niehoff A, Miosge N, et al. Transgenic mice expressing D469Delta mutated cartilage oligomeric matrix protein (COMP) show growth plate abnormalities and sternal malformations. Matrix Biol. 2008;27:67–85. doi: 10.1016/j.matbio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Shen Z, Heinegard D, Sommarin Y. Distribution and expression of cartilage oligomeric matrix protein and bone sialoprotein show marked changes during rat femoral head development. Matrix Biol. 1995;14:773–781. doi: 10.1016/s0945-053x(05)80020-4. [DOI] [PubMed] [Google Scholar]
- Simunek Z, Muir H. Changes in the protein-polysaccharides of pig articular cartilage during prenatal life, development and old age. Biochem J. 1972;126:515–523. doi: 10.1042/bj1260515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniekers YH, Intema F, Lafeber FP, et al. A role for subchondral bone changes in the process of osteoarthritis; a micro-CT study of two canine models. BMC Musculoskelet Disord. 2008;9:20. doi: 10.1186/1471-2474-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonar EJ, Sweet MB. Maturation-related changes in proteoglycans of fetal articular cartilage. Arch Biochem Biophys. 1981;208:535–547. doi: 10.1016/0003-9861(81)90542-7. [DOI] [PubMed] [Google Scholar]
- Thur J, Rosenberg K, Nitsche DP, et al. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J Biol Chem. 2001;276:6083–6092. doi: 10.1074/jbc.M009512200. [DOI] [PubMed] [Google Scholar]
- Van Turnhout MC, Schipper H, Engel B, et al. Postnatal development of collagen structure in ovine articular cartilage. BMC Dev Biol. 2010;10:62. doi: 10.1186/1471-213X-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kalu DN, Banu J, et al. Effects of ageing on the biomechanical properties of rat articular cartilage. Proc Inst Mech Eng H. 2006;220:573–578. doi: 10.1243/09544119H04404. [DOI] [PubMed] [Google Scholar]
- van der Weyden L, Wei L, Luo J, et al. Functional knockout of the matrilin-3 gene causes premature chondrocyte maturation to hypertrophy and increases bone mineral density and osteoarthritis. Am J Pathol. 2006;169:515–527. doi: 10.2353/ajpath.2006.050981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19:1113–1121. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- Ye XJ, Terato K, Nakatani H, et al. Monoclonal antibodies against bovine type IX collagen (LMW fragment): production, characterization, and use for immunohistochemical localization studies. J Histochem Cytochem. 1991;39:265–271. doi: 10.1177/39.3.1704390. [DOI] [PubMed] [Google Scholar]
- Zaucke F, Grassel S. Genetic mouse models for the functional analysis of the perifibrillar components collagen IX, COMP and matrilin-3: implications for growth cartilage differentiation and endochondral ossification. Histol Histopathol. 2009;24:1067–1079. doi: 10.14670/HH-24.1067. [DOI] [PubMed] [Google Scholar]


