Abstract
The estimation of bite force and bite performance in fossil and extinct animals is a challenging subject in palaeontology and is highly dependent on the reconstruction of the cranial myology. Furthermore, the morphology and arrangement of the adductor muscles considerably affect feeding processes and mastication and thus also have important dietary and ecological ramifications. However, in the past, the reconstruction of the (cranial) muscles was restricted to the identification of muscle attachment sites or simplified computer models. This study presents a detailed reconstruction of the adductor musculature of the Cretaceous therizinosaur Erlikosaurus andrewsi based on a stepwise and iterative approach. The detailed, three-dimensional models of the individual muscles allow for more accurate measurements of the muscle properties (length, cross-section, attachment angle and volume), from which muscle and bite force estimates are calculated. Bite force estimations are found to be the lowest at the tip of the snout (43–65 N) and respectively higher at the first (59–88 N) and last tooth (90–134 N) position. Nevertheless, bite forces are comparatively low for E. andrewsi, both in actual numbers as well as in comparison with other theropod dinosaurs. The results further indicate that the low bite performance was mainly used for leaf-stripping and plant cropping, rather than active mastication or chewing processes. Muscle and thus bite force in E. andrewsi (and most likely all therizinosaurs) is considerably constrained by the cranial anatomy and declines in derived taxa of this clade. This trend is reflected in the changes of dietary preferences from carnivory to herbivory in therizinosaurs.
Keywords: 3D modelling, adductor muscles, functional morphology, Therizinosauria
Introduction
The cranial musculature – in particular the adductor or jaw closing muscles – plays a fundamental role in an animal's life. Associated primarily with feeding, it strongly influences not only mastication and feeding processes, but ultimately also diet and habitat selection, and thus reproduction strategies and population ecology. Furthermore, feeding behaviour is closely linked to large-scale evolutionary patterns, trophic interactions and coevolutionary processes (Barrett & Rayfield, 2006). Although muscle tissue can be preserved in rare cases (Kellner, 1996; Briggs et al. 1997), knowledge on the gross muscle anatomy is not readily available in fossils and is therefore heavily dependent on reconstructions. Whereas extant taxa can offer valuable insights on muscle anatomy and topology, actualistic principles are only of limited use or may be prone to errors in fossil groups, such as dinosaurs, which have no direct living equivalent. A phylogenetically bracketed approach (Witmer, 1995), however, can help to minimize wrong assumptions and corroborate reconstructions.
The earliest reconstructions of the musculature in fossils date back to more than a century (Lull, 1908). However, in the past, muscle reconstructions were mainly described and depicted in two dimensions, illustrated as simple line drawings (Adams, 1919; Anderson, 1936; Haas, 1955, 1963, 1969), or restricted to the general identification of attachment sites (Barghusen, 1973). Given the complexity of the cranial muscular system, these reconstructions are limited in conveying information on the orientation, the (mediolateral) extent or the arrangement of the respective muscles. With the advance and wider availability of computed tomography (CT) and computer-aided imaging, a lot of these problems were resolved in more recent muscle reconstructions (Snively & Russell, 2007a; Werneburg, 2011). However, the majority of the works on three-dimensional modelling of muscles represent visualisations of the respective structures in extant taxa based on CT scanning (Jones et al. 2009) or dissections (Huysentruyt et al. 2009), rather than actual reconstructions. Other visualisation techniques are restricted to simplified models, using single or multiple strands or cylinders (Curtis et al. 2008, 2009). Although these models provide a clear picture of the muscle origins and insertions, as well as their three-dimensional position, they only approximate the full muscle anatomy. This limits their accuracy and their usefulness for measurements (cross-sections, volume), the evaluation of spatial arrangement and the information as to which extent the muscles attached to the osteological structures. However, the exact knowledge of the position, the orientation, as well as qualitative and quantitative details, is essential for biomechanical questions and inferences on functional morphology.
Apart from a few exceptions (Greaves, 1991; McAfee, 2011), the research on cranial muscle reconstructions and quantitative measurements of bite performance in extinct animals has focussed nearly exclusively on carnivorous taxa (Therrien, 2005; Anderson & Westneat, 2007; McHenry et al. 2007; Degrange et al. 2010) – notoriously so on large theropod dinosaurs (Erickson et al. 1996; Rayfield et al. 2001; Bates & Falkingham, 2012). Although bite force and performance critically determine prey size (Wroe et al. 2005; Christiansen, 2007), hunting success (Meers, 2002; D'Amore et al. 2011) and feeding style (Van Valkenburgh & Ruff, 1987) in carnivores, these factors are not less important in herbivorous taxa. Still, only a few studies have addressed the relationship between bite performance and plant-processing capabilities (e.g. Reichel, 2010). Furthermore, in theropods, herbivory is more widespread among the different clades than previously thought, leading to major ramifications for the evolution of dietary specialisations (Zanno & Makovicky, 2011). Therizinosauria represents one of these groups and their highly unusual anatomy has been regarded as an adaptation to an herbivorous life style (Weishampel & Norman, 1989; Russell & Russell, 1993; Zanno et al. 2009). Nevertheless, this assumption has not been subjected to a more rigorous, biomechanical approach. This study provides a full, three-dimensional reconstruction of the adductor musculature of Erlikosaurus andrewsi – the only therizinosaur with nearly complete and well preserved cranial remains (Clark et al. 1994) – in order to visualise the respective muscle anatomy and topology. This not only allows for a higher accuracy of measurements in comparison with traditional two-dimensional approaches, but also provides a rigorous test of hypotheses on muscle arrangement and topology. Based on this reconstruction, bite forces and functional and mechanical properties are evaluated.
Institutional abbreviations
AZMNH (Arizona Museum of Natural History, Mesa, Arizona, USA); IGM (Geological Institute of the Mongolian Academy of Sciences, Ulaan Bataar, Mongolia); UMNH;(Utah Museum of Natural History, Salt Lake City, UT, USA).
Materials and methods
The skull of E. andrewsi (IGM 100/111) was CT-scanned at X-Tek Systems Ltd. (now Nikon Metrology), Tring, Hertfordshire, using a XT-H-225ST CT scanner fitted with a special steel honeycomb support to minimise object movement. Scan parameters were set at 180 kV and 145 μA. The resulting rotational projections were processed with custom-build software creating a.vgi and a.vol files, containing 1998 slices with a slice thickness of 145 μm. The CT image files were subsequently imported into avizo (Version 6.3.1 & 7.0.0, Visualization Science Group). The individual elements of the skull were highlighted and separately labelled using the avizo segmentation editor to produce surface models and volumes. Based upon these, a complete reconstruction of the skull of E. andrewsi was created (a full description of which will be published separately), using reflection and superimposition of (more) complete elements to restore breaks or missing portions. Although, the skull of E. andrewsi is well preserved in terms of its completeness, it still shows notable signs of deformation, which was largely corrected for by the superimposition of symmetric elements. Instead of taking the measurements from the original specimen (Sakamoto et al. 2010) or its digital representation, the reconstructed skull model was used as a foundation for the creation of the individual muscles. Thus measurement and scaling errors, which would be caused by the deformed anatomy, could largely be avoided.
For the identification of the individual muscle attachments (Fig. 1) and the subsequent reconstruction process, a stepwise approach was established:
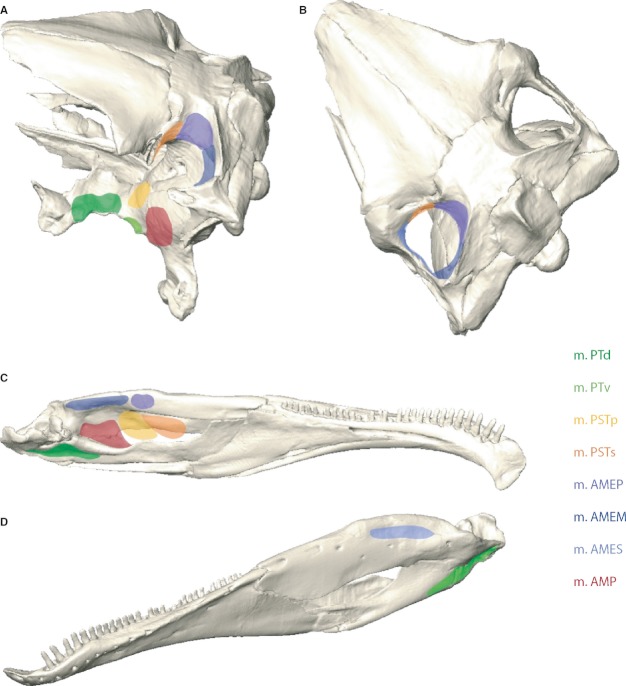
Fig. 1.
Locations of muscle attachments in the skull and lower jaws of Erlikosaurus andrewsi. Muscle origin sites in (A) dorsolateral and (B) dorsal view. Muscle insertions in (C) medial and (D) lateral view. m. AMEM, m. adductor mandibulae externus medialis; m. AMEP, m. adductor mandibulae externus profundus; m. AMES, m. adductor mandibulae externus superficialis; m. AMP, m. adductor mandibulae posterior; m. PSTp, m. pseudotemporalis profundus; m. PSTs, m. pseudotemporalis superficialis; m. PTd, m. pterygoideus dorsalis; m. PTv, m. pterygoideus ventralis.
In a first step, muscle origin (Fig. 1A,B) and insertion (Fig. 1C,D) sites were mapped for each muscle based on osteological correlates and obvious surface features, such as muscle scars, depressions, ridges, crests or other bony protrusions. Distinctive surface rugosities or roughened muscles scars on the bone may stem from tendinous and aponeurotic attachments, whereas fleshy and direct muscle insertions will leave a smooth or only weakly pronounced surface (Bryant & Seymour, 1990).
Where the exact locations for attachment sites could not be identified by surface structures, topological criteria were applied. The extent of one (clearly identified) muscle attachment site constrains the location of an adjacent muscle, so that inferences on its position can be drawn. The available space imposed by the general structure of the respective bones (especially in the cranium) further constrains the possible origin and insertion sites.
In a next step, neurovascular criteria were consulted. The jaw adductor muscles and the individual branches of the trigeminal nerve are closely intertwined. Thus, the trigeminal topology and the relative positions of the ophthalmic (V1), maxillary (V2), and mandibular (V3) divisions of the trigeminal nerve can be used to distinguish separate muscle groups, such as the adductor mandibulae internus from the adductor mandibulae externus group (Holliday & Witmer, 2007).
If the attachment sites for an individual muscle could not be identified in steps 1–3, homological criteria and inferences established by Holliday (2009) for non-avian dinosaurs in general were consulted.
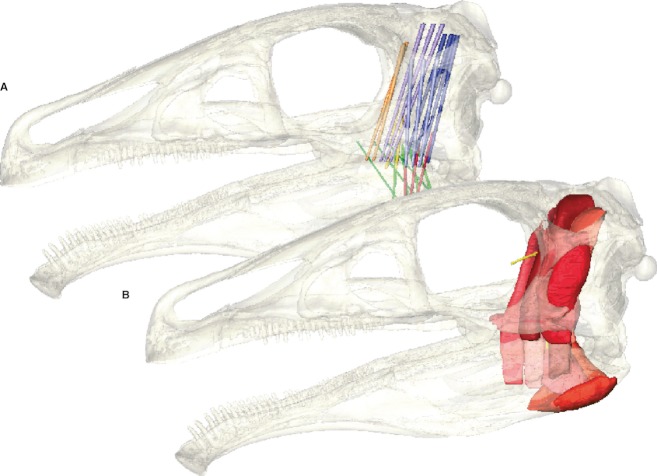
After the origins and insertions were established and defined for each individual muscle, the respective attachment sites were connected by simplified cylinders (Fig. 2A), following a similar approach to Curtis et al. (2009). This approximation allows the gross muscle topology and the orientation to be assessed. Steps 2 and 3 were repeated, this time taking into account the three-dimensional morphology. Where necessary, the creation of cylinder models was repeated to ensure that the individual muscles were not intersecting each other or with osteological structures.
In a final step, the full (‘fleshed out’) muscles (Fig. 2B) were reconstructed based on the spatial arrangement of the simplified cylinder model and the constraints imposed by the surrounding bones (in particular the adductor chamber). Muscle outlines were constructed successively in the coronal, sagittal and horizontal plane (Fig. 3) to obtain the maximal possible detail. This step was repeatedly applied in an iterative process until the muscle models showed a consistent pattern in all views. Where the extent between two or more individual muscles was unclear, an equal distance between each was constructed. The voxel-based approach of this methodology has the advantage that two individual materials, in this case muscles, represented by voxels (volumetric pixels in three-dimensional space) cannot occupy the same space in any of the three spatial planes. This effectively prevents intersections (‘collisions’ in 3D modelling jargon) of two objects and simulates the real-life condition of muscle arrangements more accurately. However, it still has to be ascertained visually that the muscles are not interrupted or discontinuous between origin and insertion site.
Fig. 2.
Reconstructed adductor muscles of Erlikosaurus andrewsi as (A) simplified cylinder model and (B) full reconstruction with the skull rendered semi-transparent.
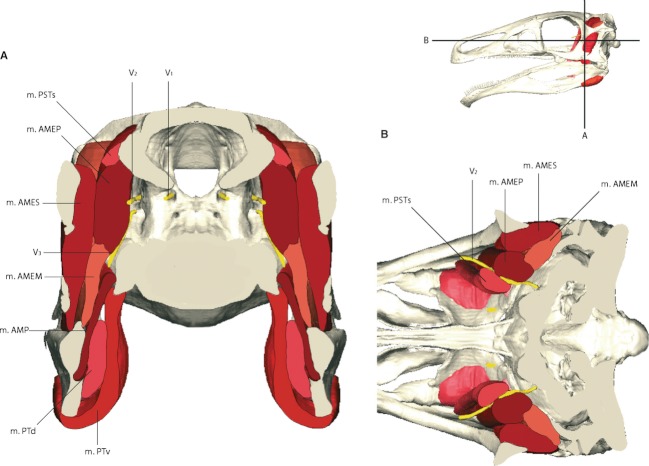
Fig. 3.
Cross-section through the reconstructed skull and adductor muscles of Erlikosaurus andrewsi in (A) coronal and (B) horizontal section. m. AMEM, m. adductor mandibulae externus medialis; m. AMEP, m. adductor mandibulae externus profundus; m. AMES, m. adductor mandibulae externus superficialis; m. AMP, m. adductor mandibulae posterior; m. PSTp, m. pseudotemporalis profundus; m. PSTs, m. pseudotemporalis superficialis; m. PTd, m. pterygoideus dorsalis; m. PTv, m. pterygoideus ventralis; V1, ophthalmic; V2, maxillary; V3 mandibular branch of the trigeminal nerve.
Although it is possible to visualise a variety of different muscle groups, this work focusses on the adductor musculature proper, as this complex has the most significance for bite performance and feeding behaviour. Reconstructions are further restricted to preserved osteological elements. Therefore the hypoglossal or glossopharyngeal musculature was not included here.
Muscle force estimates were calculated after the dry skull method following Thomason (1991). The necessary muscle cross-sectional areas (CSA) were obtained using the avizo material statistics module, which lists the respective CSA values for each individual material (in this case the muscles) and each individual CT slice. Muscle force was calculated (Eq. 1) for each muscle individually, assuming an isometric muscle stress value of 0.3 N mm−2 following Thomason (1991) and Wroe et al. (2005).
| (1) |
Whereas the muscle topology and geometric properties (volumes, CSA) can be deduced from osteological correlates, some parts of the muscle architecture itself cannot be reconstructed in fossils. One particular factor is the pennation or lack thereof. In pennate muscles, the fascicles attach obliquely to the tendon, allowing for higher forces within a smaller range of motion (Alexander, 1983). To compensate for such uncertainties in the muscle force calculations, Thomason (1991) experimentally derived a correction factor. Following this approach, muscle forces in this study were additionally increased by a factor of 1.5 to obtain a minimum and maximum value (see Table 1). Muscle insertion angles from the vertical were measured directly in the three-dimensional model in both the sagittal (α) and the coronal plane (β) on the three-dimensional model by using the avizo measurement module. Resulting force vectors acting at the attachment point on the lower jaw were calculated after Eq. 2:
Table 1.
Cross-sectional area, muscle force and bite force estimates calculated for the individual muscles. Minimum and maximum values are given for the muscle and bite forces (for details see Materials and methods). m. PTd (alternate) refers to the measurements based on an alternative muscle arrangement as shown in Fig. 7
| Muscle | Cross-sectional area [mm2] | Muscle force (N) | Bite force, tip of snout (N) | Bite force, rostral tooth position (N) | Bite force caudal tooth position (N) | Ratio of muscle contribution (%) |
|---|---|---|---|---|---|---|
| m. PTd | 124.30 | 37.29/55.94 | 3.85/5.77 | 5.16/7.74 | 7.89/11.84 | 8.86 |
| m. PTv | 404.76 | 121.43/182.15 | 12.59/18.89 | 16.89/25.34 | 25.82/38.74 | 28.99 |
| m. PSTp | 29.96 | 8.99/13.49 | 0.74/1.11 | 1.68/2.52 | 2.57/3.85 | 2.88 |
| m. PSTs | 79.45 | 23.84/44.76 | 4.56/6.83 | 6.11/9.17 | 9.34/14.02 | 10.49 |
| m. AMEP | 173.56 | 52.07/78.11 | 8.22/12.34 | 11.04/16.55 | 16.87/25.30 | 18.93 |
| m. AMEM | 170.66 | 51.20/76.80 | 4.81/7.22 | 6.46/9.69 | 9.88/14.81 | 11.09 |
| m. AMES | 184.79 | 55.48/83.22 | 5.83/8.74 | 7.82/11.73 | 11.95/17.93 | 13.41 |
| m. AMP | 77.48 | 23.24/34.86 | 2.32/3.48 | 3.11/4.67 | 4.76/7.14 | 5.34 |
| Sum | 1244.96 | 373.54/569.33 | 42.92/64.38 | 58.28/87.41 | 89.08/133.62 | |
| m. PTd (alternate) | 129.70 | 38.91/58.37 | 4.01/6.02 | 5.39/8.09 | 8.23/12.35 | – |
| (2) |
The final bite force estimates were calculated for three points acting at the skull of E. andrewsi: The edentulous tip of the snout, the first tooth position and the last tooth position. Calculations were performed using the relationship (Eq. 3) between the length of the outlever (distance between the bite point and the jaw joint) and the length of the inlever (distance between the insertion point of the respective muscle and the jaw joint).
| (3) |
All muscles were reconstructed as parallel-fibered. It was further assumed that all adductor muscles participated equally and contracted maximally during biting to obtain the maximal possible bite force estimates.
Results
Musculature
m. pterygoideus dorsalis (m. PTd)
The m. pterygoideus dorsalis originates from a pronounced depression on the dorsal surface of the pterygoid (Fig. 1A). Whether the muscle also attached rostrally onto the palatine, as suggested for other theropods (Holliday, 2009), cannot be clearly discerned. The caudal portion of the palatine bears a similar depression dorsally, which could serve as an additional origin of the m. PTd. The enclosed space in the maxillary and nasal region and the inclined palatal process make it unlikely that the muscle extended further rostrally, though. However, the medioventral surface of the jugal bears a shallow muscle scar, which could serve as an additional, possibly tendinous, attachment site for the m. PTd (Fig. 4A).
Fig. 4.

Individual adductor muscles of Erlikosaurus andrewsi, each in left lateral and caudal view, skull rendered semi-transparent. (A) m. PTd, (B) m. PTv, (C) m. PSTp, (D) m. PSTs.
On the lower jaw, the m. PTd inserts along the medial wall of the angular and articular, below and rostral to the jaw joint (a condition also found in Neornithes; Holliday & Witmer, 2007) (Fig. 1C). The respective surface bears an elongated depression, clearly marking the attachment site.
m. pterygoideus ventralis (m. PTv)
The m. pterygoideus ventralis originates from the caudoventral and possibly partly from the caudolateral surface of the pterygoid (Fig. 1A) via a fleshy and/or tendinous insertion. Holliday (2009) discussed the possibility of a jugal attachment, supported by osteological correlates in Brachylophosaurus and Nanotyrannus. Although the ventromedial surface of the jugal bears a muscle scar in E. andrewsi, an attachment of the m. PTv seems unlikely, as the muscle would then cross the path of either the m. AMES or the m. AMEM and m. AMEP (Fig. 4B).
The m. PTv attaches to the ventral and lower lateral surface of the angular, wrapping ventrally around both the angular and the m. PTd (Fig. 1D). The extent of the muscle on the lateral side of the mandible is clearly marked by an elongated depression on the angular and articular, respectively. The m. PTv bulges medially to accommodate the m. PTd and m. AMP laterally (Fig. 3A).
m. pseudotemporalis profundus (m. PSTp)
Holliday (2009) inferred the origination of the m. pseudotemporalis profundus from the lateral surface of the epipterygoid for all non-avian dinosaurs in general. However, the lack of an (preserved?) epipterygoid in E. andrewsi makes the identification of the cranial attachment site of the m. PSTp difficult to discern. Homological criteria (Holliday & Witmer, 2007) would dictate that this muscle passed the trigeminal foramen and the maxillary ramus of the trigeminal nerve rostrally. An origination from the region bounded by the lateral surfaces of the laterosphenoid, the basisphenoid and the pterygoid seems thus likely (Fig. 1A). However, there are no distinct depressions, which would clearly mark the attachment site (Fig. 4C).
As with the m. PSTs, the morphology of the surangular and the general muscle topology make an insertion of the m. PSTp into the mandibular fossa rather than the coronoid eminence likely. An analogue to the m. PSTs, the m. PSTp possibly inserts via the same tendon caudally to it. The rostral extent of both muscles is bounded by the location of the pterygoid and the m. PTd.
m. pseudotemporalis superficialis (m. PSTs)
The m. pseudotemporalis superficialis most likely attached to the rostral wall of the supratemporal fenestra (Fig. 1A). Its position is again inferred from the location of the surrounding muscles (in particular the m. AMEP and the m. AMES) and the trigeminal foramen (Fig. 3B). Following the plesiomorphic condition for archosaurs (Holliday & Witmer, 2007), the maxillary ramus of the trigeminal nerve would have to pass laterally to this muscle. The potential attachment site, formed by the respective processes of the postorbital, the laterosphenoid and the parietal, as well as the caudalmost portion of the frontal, is large. It is amplified by a vertical ridge of the parietal/postorbital bar. In Plateosaurus engelhardti, the caudolateral portion of the frontal and lateral parietal bear muscle scar for the m. PSTs (Fairman, 1999) (Fig. 4D).
The large size of the mandibular fossa indicates that the m. PSTs inserted within the fossa (Fig. 1C). Furthermore, the only moderately developed coronoid eminence, the lack of a prominent coronoid process and the insertion of the m. AMEM and m. AMEP, make an attachment on the surangular surface unlikely. Given that the presence and the morphology of the mandibular fossa are more similar to crocodilians than birds, it infers the insertion of the m. PSTs on its rostromedial surface. Comparable to the mandibular adductor tendon in crocodilians (Busbey, 1989), the m. PSTs had possibly a tendinous rather than a fleshy insertion. The laterally compressed mandibular fossa indicates that the space within the fossa is limited, giving further support for a tendinous insertion. In crocodiles the m. PSTs and the m. intramandibularis (m. IRA) attach to the cartilago transiliens (Iordansky, 1964). As a homologous structure is absent in birds, the extant phylogenetic approach is ambiguous in this regard. However, Tsai & Holliday (2011) discussed the presence of a fibrocartilaginous intertendon between the m. PSTs and m. IRA in birds. The phylogenetically close proximity to birds, the lack of a pronounced pterygoid buttress and the vertical muscle arrangement, would therefore suggest that a similar structure could have been present in theropods.
m. adductor mandibulae externus profundus (m. AMEP)
The m. adductor mandibulae externus profundus occupies a large part of the supratemporal fenestra, originating from its caudomedial surface. The muscle's rostral extent is marked by a sharp vertical crest on the parietal (Figs 1A and 3A). A corresponding structure has been described for various theropods, such as Carcharodontosaurus, Daspletosaurus (Holliday, 2009) or Allosaurus (Rayfield, 2001), but also Corythosaurus casuarius (Ostrom, 1961). The caudal extent of the muscle origin is not as well defined and probably is indicated by the depression of the squamosal process described above, where the m. AMEP contacts the m. AMEM. Unlike in other theropods (Barsbold & Osmólska, 1999; Norell & Makovicky, 2004; Norell et al. 2009), E. andrewsi does not have a pronounced sagittal crest on the parietals. Instead, the dorsal part of the parietals forms a flat surface separated from the adductor chamber/supratemporal fenestra by a sharp lateral edge. The m. AMEP is thus unlikely to have extended onto the parietals beyond this edge dorsally (Fig. 5A).
Fig. 5.
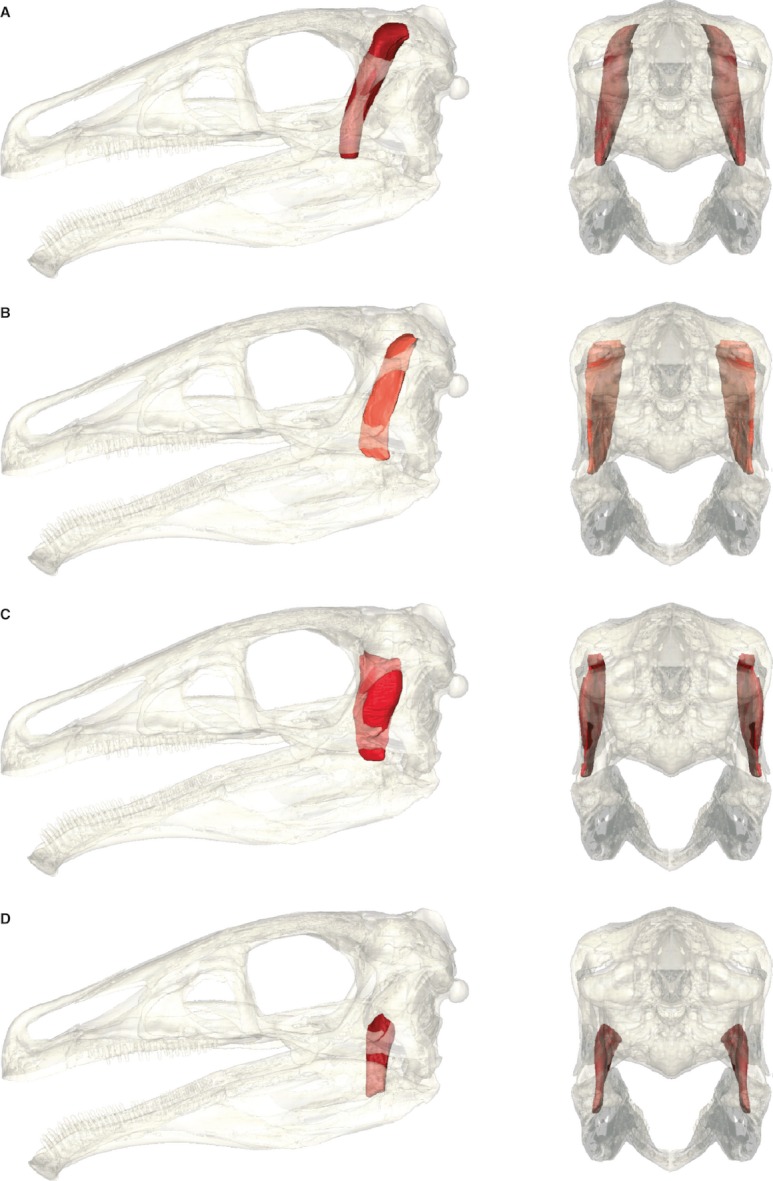
Individual adductor muscles of Erlikosaurus andrewsi, each in left lateral and caudal view skull rendered semi-transparent. (A) m. AMEP, (B) m. AMEM, (C) m. AMES, (D) m. AMP.
In most non-avian dinosaurs and birds, the m. AMEP inserts on the coronoid eminence. As is the case in most theropods, the coronoid is only weakly developed in E. andrewsi and shows no distinct surface features. The rostral extent of this muscle is bounded by the pterygoid and ectopterygoid, respectively, whereas the medial extent is limited by the pseudotemporalis muscle group.
m. adductor mandibulae externus medialis (m. AMEM)
As in most dinosaurs (Holliday, 2009) the m. adductor mandibulae externus medialis most likely originates from the caudal border of the supratemporal fenestra, respectively the rostral surface of the squamosal/parietal bar (Fig. 1A). This is indicated by a faint depression on the squamosal process of the parietal. Fairman (1999) described a similar muscle scar on the parietal on the caudal border of the supratemporal fenestra in P. engelhardti for the origin of the m. AMEM. Dorsally, the parietal bears a sharp ridge clearly separating the rostral and caudal surfaces and thus the adductor and neck muscle groups (Fig. 4B).
The insertion of m. AMEM on the lower jaw is less clear. Identification of the insertion site is mainly based on the surrounding muscle topology and the overall bone structure, limiting the available space in the adductor chamber severely. The surangular is very narrow and dorsally rounded. Unlike the pronounced depression on the lateral side, the medial side of the surangular is smooth. Given the position of the m. AMES and the limited space of the adductor chamber, the m. AMEM most likely inserted to the dorsomedial side of the surangular between the articular and the coronoid eminence. A comparable location has been suggested by Holliday (2009) for P. engelhardti. As found in crocodilians (Holliday & Witmer, 2007), the m. AMEM might merge laterally with the m AMES.
m. adductor mandibulae externus superficialis (m. AMES)
The m. adductor mandibulae externus superficialis originates from the supratemporal bar, formed by the postorbital and the squamosal (Figs 1B and 3A). However, there are no clear osteological correlates, whether the muscle attached to the lateral or medial surface of the supratemporal bar. The lateral surface is smooth and straight, whereas the medial surface bears a longitudinal, convex ridge along most of its entire length. Ventral to this ridge, the postorbital bears an elongated depression. Thus it seems to be more plausible that the m. AMES has a fleshy origination from the medial surface. However, the squamosal bears a pronounced depression on its lower lateral surface, at the junction of the postorbital and quadrate process. It is likely that part of the muscle also attached to this surface, wrapping around the ventral margin of the supratemporal bar. The ventral and dorsal surfaces, though, are thin and tapering and thus too small to form a muscle attachment site (Fig. 4C).
The insertion of the m. AMES on the lower jaw is marked by an elongated depression or fossa on the dorsolateral surface of the surangular, rostral to the articular and jaw joint (Fig. 1D). This fossa is only moderately developed in E. andrewsi and lacks a prominent shelf on the lateral edge of the surangular found in other (carnivorous) theropods (Holliday, 2009). In its morphology, it is more similar to Plateosaurus erlenbergiensis (Prieto-Marquez & Norell, 2011). The coronoid eminence is only weakly developed and dorsally tapering in E. andrewsi and unlikely to form a part of the muscle insertion.
m. adductor mandibulae posterior (m. AMP)
Although the m. adductor mandibulae posterior does not provide clearly discernible muscle scars on the bone surface, data provided by extant phylogenetic bracketing (Holliday, 2009) indicates an origin from the lateral surface of the quadrate (Fig. 1A). Taking the inflated and bulbous morphology of the basisphenoid in E. andrewsi into account, the muscle attachment site extended possibly ventrally and onto the pterygoid. A more dorsally located origination, covering the entire lateral surface of the quadrate flange as in other theropods, seems unlikely, as the basisphenoid expansion limits the space in the adductor chamber severely (Fig. 5D).
A pronounced depression on the medial wall of the mandibular fossa indicates a fleshy insertion of the m. AMP at this point (Figs 1C and 3A), although a tendinous insertion shared with the m. PSTs and m. PSTp cannot be ruled out.
Bite force estimates
Muscle and bite force estimations were calculated for each muscle individually and in total (Table 1) for three different biting positions (Fig. 6). Although the total muscle force generated by the adductor muscles produces a maximum force between 374 and 570 N on each side, only a fraction of it is actually used for biting. The skull and lower jaws of E. andrewsi essentially operate as a third-class lever, with the input force acting at the muscle insertion and the output force acting at the bite point. This arrangement offers no mechanical advantage (= force amplification) but permits jaw opening and closing with a minimum length of the muscle fibres. In the case of E. andrewsi, the inforce and outforce levers are defined by the skull length. The adductor muscles insert close to the jaw joint, reducing the leverage and the adductive force. The available bite force, on the other hand, declines with the distance of the bite point to the jaw joint. Bite force is thus the lowest at the tip of the snout, with 43–65 N, and the highest at the last tooth position, with 90–134 N on each side. Although bite forces do not depend on tooth position alone and can also be influenced by the gape angle between the skull and the lower jaws (Bourke et al. 2008), the nature of the jaw joint in E. andrewsi permits only a moderate maximum gape of 25–30°. Variations in bite forces can thus be expected to be only minimal.
Fig. 6.
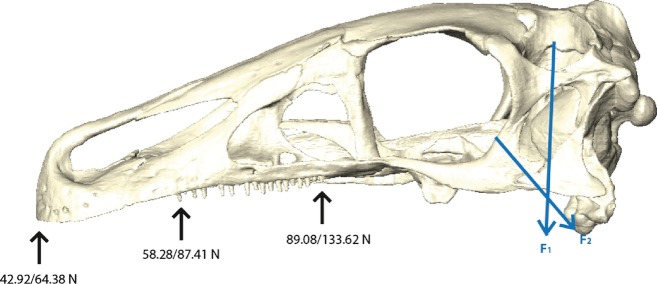
Calculated bite forces (minimum/maximum values) for the tip of the snout, the rostral tooth position and the caudal tooth position. Force vectors representing the respective extremes of muscle directions F1: m. PTd, F2: AMEM.
In comparison with traditional reconstruction methods, the digital reconstruction approach offers the advantage that different scenarios, in this case muscle topologies, can be simulated relatively easily. As mentioned above, the osteological correlates offer no unambiguous indication whether the m. PTd had an additional origin on the palatine and the medioventral surface of the jugal. To explore the influence such a muscle arrangement would have on the muscle and bite force estimates, an alternate muscle version of the m. PTd was reconstructed (Fig. 7). This arrangement results in a marginally larger cross-sectional area due to the additional attachment sites. However, the main portion of the muscle is constrained by the space of the mandibular adductor chamber and the surrounding muscles so that the final effect on the bite force is only insignificant (increase of 4.3%, compare Table 1). This effect is also due to the fact that the m. PTd contributes only moderately to the bite force.
Fig. 7.
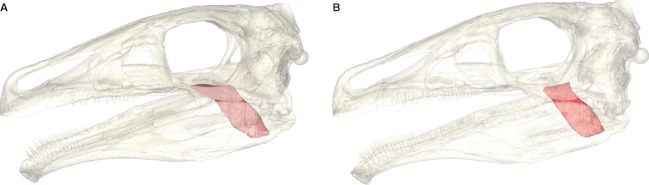
Different topological arrangements of the m. PTd with (A) and without (B) additional origin sites of the muscle on the palatine and medioventral surface of the jugal.
Discussion
Implications for feeding mechanisms in E. andrewsi
The bite force estimates for E. andrewsi are relatively low, both in comparison with other theropods (Erickson et al. 1996; Rayfield et al. 2001; Mazetta et al. 2009; Gignac et al. 2010) and in relation to its body mass (ranging between approximately 160 and 400 kg, following Russell & Dong, 1993). However, comparative values for bite force estimations in theropods nearly exclusively focus on carnivorous taxa (Rayfield et al. 2001; Henderson, 2002; Therrien et al. 2005), whereas estimates for non-carnivorous theropods or even dinosaurs in general are rare (Tanoue et al. 2009; Reichel, 2010). A multitude of dietary assumptions for therizinosaurs has been discussed, ranging from piscivory (Barsbold & Perle, 1980), insectivory (Nessov, 1995; Gillette, 2007) to various forms of herbivory (Gillette, 2007; Zanno et al. 2009; Zanno & Makovicky, 2011). The comparably weak bite forces of E. andrewsi strongly support the latter assumption. The lack of wear facets (Clark et al. 1994), tooth occlusion and the possible presence of a large gut to process plant matter (Zanno et al. 2009; Zanno, 2010b), however, suggest that the available bite force would not have been used for extensive mastication and chewing processes. This would further imply that the tip of the snout (possibly equipped with a keratinous sheath) or for some parts the rostral teeth were used to crop plants or strip foliage of branches. Reichel (2010) calculated the ability of Stegosaurus to crop and crush vegetation of various strengths. Applied to E. andrewsi, her results suggest that it would be able to process only thin (4–8 mm diameter) branches and plant matter. Leaf-stripping behaviour is known to produce characteristic microwear patterns in sauropod teeth (Whitlock, 2011), the absence of which would suggest that E. andrewsi used only the tip of the snout and the premaxillary region to procure soft plant matter or fruits. The comparably narrow width of the snout would further infer a certain degree of feeding selectivity (Carrano et al. 1999; Tanoue et al. 2009).
It is noteworthy that the Komodo dragon Varanus komodoensis, the largest living lizard, has a demonstrably weak bite compared with its size (Moreno et al. 2008). This lack in bite force is compensated by the incorporation of the postcranial muscles, resulting in caudally and ventrocaudally directed pulling (D'Amore et al. 2011). A similar feeding mechanism and interaction between the jaw adductor and cervical musculature has been suggested for several (carnivorous) theropods (Rayfield, 2004; Snively & Russell, 2007b). It seems plausible that E. andrewsi, phylogenetically constrained by the general theropod bodyplan, may have similarly harnessed the postcranial musculature for branch-stripping.
The three-dimensionally modelled adductor muscles and respective cross-sections further demonstrate that the main contributors of the muscle force and thus bite force are the m. AMES, m. AMEM, m. AMEP and m. AMP, all of which are located within the adductor chamber. The possibly achievable muscle volume is therefore significantly constrained and limited by the surrounding bone structure, most noticeably by the laterally expanded and inflated basisphenoid (‘basisphenoidal bulla’). The only possibility to increase adductor muscle volume would be by the extension of the pterygoideus muscles (in particular the m. PTv) onto the palatines and or the caudal part of the maxilla. However, the acute insertion angle of these muscles reduces the actual force output and contribution of these muscles severely, whereas the more extensive configuration of the m. PTd has been shown to have only little effect on the muscle force. In the basal therizinosaur Falcarius utahensis (UMNH VP 15000, 15001) the basisphenoid is only moderately expanded (Zanno, 2010a). It becomes successively more pneumatic and inflated in derived taxa, such as Nothronychus mckinleyi (AZMNH-2117) (Smith et al. 2011) or, as shown above, E. andrewsi. The space in the adductor chamber, available for the respective muscle groups, thus became more restricted in derived therizinosaurs. This would suggest that bite force was gradually reduced – a trend that parallels the suggested deviation from obligatory predation to herbivory in this group (Zanno, 2010b).
Conclusions
The three-dimensional reconstruction of the adductor myology of Erlikosaurus andrewsi demonstrates the capability of this method to visualise and measure muscle anatomy in high detail and provides a rigorous test of assumptions on the spatial arrangement of the individual muscles which have to be accommodated in the adductor chamber. The presented methods exploit the possibility to model and access information in three dimensions. The individual muscles can be traced in all three planes of space, allowing for precise orientation and scaling of each structure. Different topological scenarios can be tested and evaluated. The model further facilitates the quick and accurate collection of measurements (attachment angles, length, cross-section, volume) compared with traditional methods, such as photographs (Sakamoto et al. 2010) or clay models (Rayfield, 2001). This factor is especially important to obtain a solid estimate of muscle and bite force. The digital models can further be easily exported and used to define muscle loads and locations in finite element models.
Based on the presented methods, the bite force estimates for E. andrewsi are comparably low, both in actual numbers as well as in relation to its size and phylogenetic position among other theropods. Taking further evidence, such as the lack of wear facets or tooth occlusion, into account, the results of this study infer that feeding mechanisms in E. andrewsi might have mainly consisted of cropping and branch-stripping, possibly in interaction with the postcranial musculature, whereas active mastication and chewing processes are more unlikely. Furthermore, the osteological constraints imposed by the skull morphology indicate that bite force declined in derived therizinosaurs. This is consistent with dietary adaptations and the conversion from (hyper-)carnivory to herbivory in this group.
Acknowledgments
Special thanks are due to Perle Altangerel (Mongolian Academy of Sciences, Ulaan Bataar) for making the specimen of Erlikosaurus andrewsi available for scanning, and to Andrew Ramsey (Nikon Metrology) for his support with the scanning of the specimen and the provision of software to process the raw scan data. Mike Getty (Utah Museum of Natural History) and Larry Witmer (Ohio University) made comparative material available for study. Stefanie Klug (University of Bristol) and Richard Butler (GeoBio-Center, Ludwig-Maximilians-Universität, Munich) are thanked for advice and insightful discussions. Phil Anderson and Emily Rayfield (University of Bristol) carefully reviewed an earlier version of the manuscript and suggested useful improvements. This research is supported by a doctoral fellowship to Stephan Lautenschlager by the Volkswagen Foundation.
References
- Adams LA. A memoir of the phylogeny of the jaw muscles in recent and fossil vertebrates. Ann N Y Acad Sci. 1919;28:51–166. [Google Scholar]
- Alexander PM. Animal Mechanics. Oxford: Blackwell Scientific; 1983. p. 301. [Google Scholar]
- Anderson HT. The jaw musculature of the phytosaur Machaeroprosopus. J Morphol. 1936;59:549–587. [Google Scholar]
- Anderson PSL, Westneat MW. Feeding mechanics and bite force modelling of the skull of Dunkleosteus terrelli, an ancient apex predator. Biol Lett. 2007;3:77–80. doi: 10.1098/rsbl.2006.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghusen HR. The adductor jaw musculature of Dimetrodon (Reptilia, Pelycosauria) J Paleontol. 1973;47:823–834. [Google Scholar]
- Barrett PM, Rayfield E. Ecological and evolutionary implications of dinosaur feeding behaviour. Trends Ecol Evol. 2006;21:217–224. doi: 10.1016/j.tree.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Barsbold R, Osmólska H. The skull of Velociraptor (Theropoda) from the Late Cretaceous of Mongolia. Acta Palaeontol Pol. 1999;44:189–219. [Google Scholar]
- Barsbold R, Perle A. Segnosauria, a new infraorder of carnivorous dinosaurs. Acta Palaeontol Pol. 1980;25:187–195. [Google Scholar]
- Bates KT, Falkingham PL. Estimating maximum bite performance in Tyrannosaurus rex using multi-body dynamics. Biol Lett. 2012;8:660–664. doi: 10.1098/rsbl.2012.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke J, Wroe S, Moreno K, et al. Effects of gape and tooth position on bite force and skull stress in the dingo (Canis lupus dingo) using a 3-dimensional finite element approach. PLoS ONE. 2008;3:e2200. doi: 10.1371/journal.pone.0002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DEG, Wilby PR, Perez-Moreno BP, et al. The mineralization of dinosaur soft tissue in the Lower Cretaceous of Las Hoyas, Spain. J Geol Soc. 1997;154:587–588. [Google Scholar]
- Bryant HN, Seymour KL. Observations and comments on the reliability of muscle reconstruction in fossil vertebrates. J Morphol. 1990;206:109–117. doi: 10.1002/jmor.1052060111. [DOI] [PubMed] [Google Scholar]
- Busbey AB. Form and function of the feeding apparatus of Alligator mississippiensis. J Morphol. 1989;202:99–127. doi: 10.1002/jmor.1052020108. [DOI] [PubMed] [Google Scholar]
- Carrano MT, Janis CM, Sepkoski JJ., Jr Hadrosaurs as ungulate parallels: lost lifestyles and deficient data. Acta Palaeontol Pol. 1999;44:237–261. [Google Scholar]
- Christiansen P. Comparative bite forces and canine bending strength in feline and sabretooth felids: implications for predatory ecology. Zool J Linn Soc. 2007;151:423–437. [Google Scholar]
- Clark JM, Perle A, Norell MA. The skull of Erlicosaurus andrewsi, a Late Cretaceous ‘Segnosaur’ (Theropoda: Therizinosauridae) from Mongolia. Am Mus Novit. 1994;3115:1–39. [Google Scholar]
- Curtis N, Kupczik K, O'Higgins P, et al. Predicting skull loading: applying multibody dynamics analysis to a macaque skull. Anat Rec. 2008;291:491–501. doi: 10.1002/ar.20689. [DOI] [PubMed] [Google Scholar]
- Curtis N, Jones MEH, Evans SE, et al. Visualising muscle anatomy using three-dimensional computer models – an example using the head and neck muscles of Sphenodon. Palaeontol Electronica. 2009;12:18. [Google Scholar]
- D'Amore DC, Moreno K, McHenry CR, et al. The effects of biting and pulling on the forces generated during feeding in the Komodo Dragon (Varanus komodoensis. PLoS ONE. 2011;6:e26226. doi: 10.1371/journal.pone.0026226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrange FJ, Tambussi CP, Moreno K, et al. Mechanical analysis of feeding behavior in the extinct ‘Terror Bird’ Andalgalornis steulleti (Gruiformes: Phorusrhacidae) PLoS ONE. 2010;5:e11856. doi: 10.1371/journal.pone.0011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson GM, Van Kirk SD, Su J, et al. Bite-force estimation for Tyrannosaurus rex from tooth-marked bones. Nature. 1996;382:706–708. [Google Scholar]
- Fairman JE. Prosauropod and Iguanid Jaw Musculature: A Study of the Evolution of Form and Function, Unpublished Master's thesis, Baltimore: Johns Hopkins University; 1999. pp. 1–94. [Google Scholar]
- Gignac PM, Makovicky PJ, Erickson GM, et al. A description of Deinonychus antirrhopus bite marks and estimates of bite force using tooth indentation simulations. J Vertebr Paleontol. 2010;30:1169–1177. [Google Scholar]
- Gillette DD. Therizinosaur: mystery of the sickle-clawed dinosaur. Plateau: Land and People of the Colorado Plateau, 4/2. 2007;4:1–69. [Google Scholar]
- Greaves WS. Differences in bite force at the cheek teeth in fossil and recent selenodont artiodactyls. J Vertebr Paleontol. 1991;11:32A. [Google Scholar]
- Haas G. The jaw musculature in Protoceratops and in other ceratopsians. Am Mus Novit. 1955;1729:1–24. [Google Scholar]
- Haas G. A proposed reconstruction of the jaw musculature of Diplodocus. Ann Carnegie Mus. 1963;36:139–157. [Google Scholar]
- Haas G. On the jaw muscles of ankylosaurs. Am Mus Novit. 1969;2399:1–12. [Google Scholar]
- Henderson DM. The eyes have it: the sizes, shapes, and orientations of theropod orbits as indicators of skull strength and bite force. J Vertebr Paleontol. 2002;22:766–778. [Google Scholar]
- Holliday CM. New insights into dinosaur jaw muscle anatomy. Anat Rec. 2009;292:1246–1265. doi: 10.1002/ar.20982. [DOI] [PubMed] [Google Scholar]
- Holliday CM, Witmer LM. Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. J Morphol. 2007;268:457–484. doi: 10.1002/jmor.10524. [DOI] [PubMed] [Google Scholar]
- Huysentruyt F, Brunain M, Adriaens D. Ontogeny of the cranial musculature in Corydoras aeneus Callichthyidae, Siluriformes. J Fish Biol. 2009;75:1601–1614. doi: 10.1111/j.1095-8649.2009.02386.x. [DOI] [PubMed] [Google Scholar]
- Iordansky NN. The jaw muscles of the crocodiles and some relating structures of the crocodilian skull. Anat Anz. 1964;115:256–280. [PubMed] [Google Scholar]
- Jones MEH, Curtis N, O'Higgins P, et al. The head and neck muscles associated with feeding in Sphenodon (Reptilia: Lepidosauria: Rhynchocephalia) Palaeontol Electronica. 2009;12:1–56. [Google Scholar]
- Kellner AWA. Fossilized theropod soft tissue. Nature. 1996;379:32. [Google Scholar]
- Lull RS. The cranial musculature and the origin of the frill in the ceratopsian dinosaurs. Am J Sci. 1908;25:387–399. [Google Scholar]
- Mazetta GV, Cisilino AP, Blanco RE, et al. Cranial mechanics and functional interpretations of the horned carnivorous dinosaur Carnotaurus sastrei. J Vertebr Paleontol. 2009;29:822–830. [Google Scholar]
- McAfee RK. Feeding mechanics and dietary implications in the fossil sloth Neocnus (Mammalia: Xenarthra: Megalonychidae) from Haiti. J Morphol. 2011;272:1204–1216. doi: 10.1002/jmor.10976. [DOI] [PubMed] [Google Scholar]
- McHenry CR, Wroe S, Clausen PD, et al. Supermodeled sabercat, predatory behavior in Smilodon fatalis revealed by high-resolution 3D computer simulation. PNAS. 2007;104:16010–16015. doi: 10.1073/pnas.0706086104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meers MB. Maximum bite force and prey size of Tyrannosaurus rex and their relationships to the inference of feeding behavior. Hist Biol. 2002;16:1–12. [Google Scholar]
- Moreno K, Wroe S, McHenry C, et al. Cranial performance in the Komodo dragon (Varanus komodoensis) as revealed by high-resolution 3-D finite element analysis. J Anat. 2008;212:736–746. doi: 10.1111/j.1469-7580.2008.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessov LA. Dinosaurs of Northern Eurasia: New Data about Assemblages, Ecology and Paleobiogeography. St. Petersburg, Russia: Scientific Research Institute of the Earth's Crust, St. Petersburg State University; 1995. [in Russian] [Google Scholar]
- Norell MA, Makovicky PJ. Dromeosauridae. In: Weishampel DB, Dodson P, Osmólska H, editors. The Dinosauria. 2nd edn. Berkeley: University of California Press; 2004. pp. 196–209. [Google Scholar]
- Norell MA, Makovicky PJ, Bever GS, et al. A review of the Mongolian Cretaceous dinosaur Saurornithoides (Troodontidae: Theropoda) Am Mus Novit. 2009;3654:1–63. [Google Scholar]
- Ostrom JH. Cranial morphology of the hadrosaurian dinosaurs of North America. Bull Am Mus Nat Hist. 1961;122:33–186. [Google Scholar]
- Prieto-Marquez A, Norell MA. Redescription of a nearly complete skull of Plateosaurus (Dinosauria: Sauropodomorpha) from the Late Triassic of Trossingen (Germany) Am Mus Novit. 2011;3727:1–58. [Google Scholar]
- Rayfield EJ. Cranial design and function in a large theropod dinosaur: a study using finite element analysis. Unpublished PhD thesis, University of Cambridge, UK, pp. 300. 2001. [DOI] [PubMed]
- Rayfield EJ. Cranial mechanics and feeding in Tyrannosaurus rex. Proc R Soc Lond B. 2004;271:1451–1459. doi: 10.1098/rspb.2004.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayfield EJ, Norman DB, Horner CC, et al. Cranial design and function in a large theropod dinosaur. Nature. 2001;409:1033–1037. doi: 10.1038/35059070. [DOI] [PubMed] [Google Scholar]
- Reichel M. A model for the bite mechanics in the herbivorous dinosaur Stegosaurus (Ornithischia, Stegosauridae) Swiss J Geosci. 2010;103:235–240. [Google Scholar]
- Russell DA, Dong Z. The affinities of a new theropod from the Alxa-Desert, Inner Mongolia, People's Republic of China. Can J Earth Sci. 1993;30:2107–2127. [Google Scholar]
- Russell DA, Russell DE. Mammal-dinosaur convergence – evolutionary convergence between a mammalian and dinosaurian clawed herbivore. Natl Geogr Res Explor. 1993;9:70–79. [Google Scholar]
- Sakamoto M, Lloyd GT, Benton MJ. Phylogenetically structured variance in felid bite force: the role of phylogeny in the evolution of biting performance. J Evol Biol. 2010;23:463–478. doi: 10.1111/j.1420-9101.2009.01922.x. [DOI] [PubMed] [Google Scholar]
- Smith DK, Zanno LE, Sanders RK, et al. New information on the braincase of the North American therizinosaurian (Theropoda, Maniraptora) Falcarius utahensis. J Vertebr Paleontol. 2011;31:387–404. [Google Scholar]
- Snively E, Russell AP. Functional morphology of neck musculature in the Tyrannosauridae (Dinosauria, Theropoda) as determined via a hierarchical inferential approach. Zool J Linn Soc. 2007a;151:759–808. [Google Scholar]
- Snively E, Russell AP. Functional variation of neck muscles and their relation to feeding style in Tyrannosauridae and other large theropod dinosaurs. Anat Rec. 2007b;290A:934–957. doi: 10.1002/ar.20563. [DOI] [PubMed] [Google Scholar]
- Tanoue K, Grandstaff BS, You H, et al. Jaw mechanics in basal Ceratopsia (Ornithischia, Dinosauria) Anat Rec. 2009;292:1352–1369. doi: 10.1002/ar.20979. [DOI] [PubMed] [Google Scholar]
- Therrien F. Feeding behaviour and bite force of sabretoothed predators. Zool J Linn Soc. 2005;145:393–426. [Google Scholar]
- Therrien F, Henderson DM, Ruff CB. Bite me: biomechanical models of theropod mandibles and implications for feeding behaviour. In: Carpenter K, editor. The Carnivorous Dinosaurs. Bloomington: Indiana University Press; 2005. pp. 179–237. [Google Scholar]
- Thomason JJ. Cranial strength in relation to estimated biting forces in some mammals. Can J Zool. 1991;69:2326–2333. [Google Scholar]
- Tsai HP, Holliday CM. Ontogeny of the alligator cartilago transiliens and its significance for sauropsid jaw muscle evolution. PLoS ONE. 2011;6:e24935. doi: 10.1371/journal.pone.0024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Valkenburgh B, Ruff CB. Canine tooth strength and killing behaviour in large carnivores. J Zool. 1987;212:379–397. [Google Scholar]
- Weishampel DB, Norman DB. Vertebrate herbivory in the Mesozoic; jaws, plants, and evolutionary metrics. In: Farlow JO, editor. Paleobiology of the Dinosaurs. Boulder: Geological Society of America; 1989. pp. 87–100. [Google Scholar]
- Werneburg I. The cranial musculature of turtles. Palaeontol Electronica. 2011;14:1–99. [Google Scholar]
- Whitlock JA. Inferences of diplodocoid (Sauropoda: Dinosauria) feeding behavior from snout shape and microwear analyses. PLoS ONE. 2011;6:e18304. doi: 10.1371/journal.pone.0018304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer LM. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: Thomason JJ, editor. Functional Morphology in Vertebrate Paleontology. Cambridge: Cambridge University Press; 1995. pp. 19–33. [Google Scholar]
- Wroe S, McHenry CR, Thomason JJ. Bite club: comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa. Proc R Soc Lond B. 2005;272:619–625. doi: 10.1098/rspb.2004.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanno LE. A taxonomic and phylogenetic re-evaluation of Therizinosauria (Dinosauria: Maniraptora) J Syst Paleontol. 2010a;8:503–543. [Google Scholar]
- Zanno LE. Osteology of Falcarius utahensis (Dinosauria: Theropoda): characterizing the anatomy of basal therizinosaurs. Zool J Linn Soc. 2010b;158:196–230. [Google Scholar]
- Zanno LE, Makovicky PJ. Herbivorous ecomorphology and specialization patterns in theropod dinosaur evolution. PNAS. 2011;108:232–237. doi: 10.1073/pnas.1011924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanno LE, Gillette DD, Albright LB, et al. A new North American therizinosaurid and the role of herbivory in ‘predatory’ dinosaur evolution. Proc R Soc Lond B. 2009;276:3505–3511. doi: 10.1098/rspb.2009.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]