Abstract
Background and Purpose
Previous structure–activity relationship studies with analogues of the CB1 receptor antagonist rimonabant have demonstrated that a subset of these analogues with 3-substituent replacements of rimonabant's pyrazole core displayed cannabimimetic profiles seemingly independent of CB1 receptors. We sought to further evaluate these analogues in several behavioural models sensitive to detecting THC-like abuse liability.
Experimental Approach
Selected analogues were tested in a battery of tests in mice to replicate previous findings. Cross-generalization tests were conducted in mice trained to discriminate either THC or O-6629 from vehicle. Rimonabant and its analogues were also evaluated in substitution and challenge tests. Finally, development of cross-tolerance between THC and O-6211 in the mouse test battery was assessed.
Key Results
O-6629 and O-6658 produced dose-dependent acute cannabimimetic activity in mice, but neither substituted for nor antagonized THC's discriminative stimulus. Cross-substitution was observed with O-6658 in mice discriminating O-6629, whereas rimonabant neither substituted for nor attenuated the O-6629 discriminative stimulus. THC and morphine did not generate O-6629-like responding. Cross-tolerance did not develop in mice repeatedly treated with THC when tested with O-6211 in the mouse test battery.
Conclusions and Implications
While some overlap exists between the pharmacological profiles of THC and these 3-substituent rimonabant analogues, the effects are mediated by distinct neural targets. Notably, these analogues are unlikely to possess marijuana-like abuse liability in humans, but general abuse liability has not yet been determined. Efforts to determine the mechanism(s) of action of this seemingly unique class of compounds are underway.
Keywords: cannabinoids, rimonabant, pyrazole, THC, cross-tolerance, drug discrimination
Introduction
The endocannabinoid system, comprised of two known receptors (CB1 and CB2), their endogenous ligands (e.g. anandamide, 2-arachidonoylglycerol), and respective synthetic and metabolic enzymes of these ligands, is implicated in many physiological processes, including appetite, reward, and cognition (Howlett et al., 2004). Development of the prototypic CB1 receptor antagonist rimonabant (Rinaldi-Carmona et al., 1994) and subsequent analogues (Gatley et al., 1996) as mechanistic tools to explore the endocannabinoid system has been responsible for many critical findings that form the underpinnings of cannabinoid (CB) pharmacology. Briefly prescribed in the UK as an anti-obesity medication, rimonabant was withdrawn from the market due to adverse psychiatric effects (Christopoulou and Kiortsis, 2011). In addition to its known consequences on body weight and lipid markers, a number of studies have noted the potential utility of CB1 receptor antagonists for treating nicotine, opiate and alcohol abuse (Beardsley et al., 2009).
Rimonabant is comprised of a central pyrazole core surrounded by 2,4-dichlorophenyl, carboxyaminopiperidine, methyl and 4-chlorophenyl substituents at the 1-, 3-, 4- and 5-positions respectively (Figure 1). Investigation of the structure–activity relationship (SAR) of rimonabant analogues has revealed that the 1-substituent is most unique and may be related to the antagonist properties of rimonabant (Thomas et al., 1998). While the 3-substituent was initially suggested to be involved in receptor recognition (Wiley et al., 2001) and the inverse agonist effects of rimonabant (Hurst et al., 2006), more recent research has indicated a more complex SAR for this substituent. For instance, we reported on a subset of 3-substituted rimonabant analogues with the following paradoxical actions (Wiley et al., 2012): (i) selective CB1 receptor affinity with minimal CB2 receptor binding; (ii) no activity or inverse agonism in [35S]GTPγS functional assay; (iii) agonist-like effects in a tetrad of tests in mice in which Δ9-tetrahydrocannabinol (THC) produces a characteristic profile of hypolocomotion, antinociception, hypothermia and catalepsy; (iv) poor correlation between CB1 receptor affinity and potency in the mouse tetrad tests; (v) lack of blockade of these effects by rimonabant; and (vi) pharmacological activity in CB1 -/- mice. These results suggest that this novel class of pyrazole analogues produce their CB agonist-like effects through a non-CB1, non-CB2 mechanism. Since the abuse liability of CB agonists like THC is most likely mediated by CB1 receptor activation (Huestis et al., 2001), the unique profile of effects occasioned by these analogues raises the possibility that they may produce some of the beneficial effects of CB1 receptor agonists (e.g. antinociception) without the same abuse liability. In terms of drug discovery, this profile would be advantageous over medications developed from phytocannabinoids or other CB agonist templates, in that these analogues are structurally distinct and would not be classified a priori as analogues of THC or abused synthetic CBs under drug control provisions (e.g. US Drug Enforcement Administration schedule I).
Figure 1.
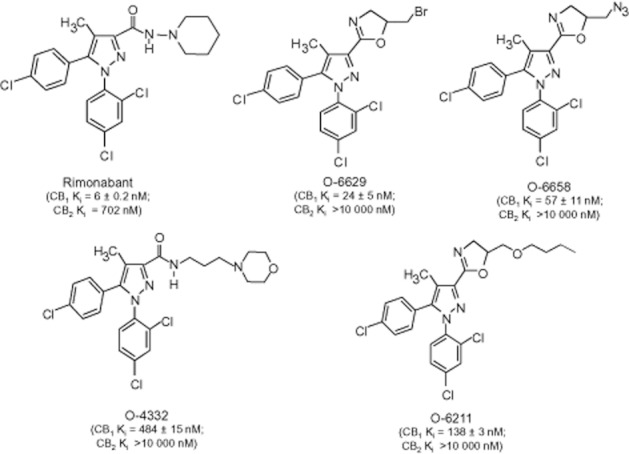
Chemical structures of rimonabant and selected 3-substituted analogues (O-6629, O-6658, O-4332 and O-6211). CB1 and CB2 receptor-binding affinities (nM), as determined by a [3H]CP55940 displacement assay, are also shown (from Wiley et al., 2012).
To investigate this hypothesis, we evaluated the potential of selected 3-substituted rimonabant analogues with this profile (O-6629, O-6658, O-4332 and O-6211; Figure 1) to produce THC-like abuse liability in several mouse models. Assessment of abuse liability in rodents typically involves determination of pharmacological similarity to a known substance of abuse (e.g. THC), self-administration, drug discrimination and evaluation of tolerance/dependence (Balster, 1991). Since THC self-administration has been demonstrated in non-human primates (Tanda et al., 2000), but not rodents, this procedure was omitted. Pharmacological similarity was assessed in a tetrad of tests in mice in which THC and other CB agonists produce characteristic effects, including hypolocomotion, antinociception, hypothermia and catalepsy (Martin et al., 1991). Next, the compounds were evaluated for generalization to and antagonism of THC in drug discrimination, a highly pharmacologically selective preclinical model of marijuana's subjective effects in humans (Balster and Prescott, 1992). In addition, mice were trained to discriminate O-6629, one of the most potent analogues with CB1 receptor affinity comparable to THC, and cross-generalization to THC was examined. Finally, cross-tolerance was assessed in THC-tolerant mice in the tetrad of tests.
Methods
Subjects
Adult male, C57BL/6J mice (20–25 g) obtained from Jackson Laboratories (Bar Harbor, ME, USA) served as subjects for the acute tetrad of tests and drug discrimination experiments. For cross-tolerance experiments, adult male Institute for Cancer Research (ICR) mice (Harlan, VA, USA) were used. Mice tested in the tetrad were experimentally naïve and group-housed (n = 4–5 group−1) in clear plastic cages with fitted tops and corncob bedding. Subjects had unlimited access to food (Teklad chow; Harlan, Indianapolis, IN, USA) and water in the home cage except during testing. The evening before acute (or first repeated) tests, mice were randomly assigned to treatment conditions (n = 6 treatment condition−1) and transported to the laboratory to allow them to adjust to ambient room temperatures. Mice in the discrimination experiments were housed individually under identical conditions, except that subjects were food-deprived to 85–90% of their free feeding body weight to promote food-reinforced operant responding. Subjects were housed in a light (12 h light–dark cycle, lights on at 0600) and temperature-controlled (22–24°C) vivarium. THC generalization data and results of substitution tests with non-pyrazole CBs from a subset of the THC-trained mice used here (n = 5–11) have been published previously (Wiley et al., 2011). The Guide for Care and Use of Laboratory Animals (National Research Council, 1996) was followed and the Institutional Animal Care and Use Committee at Virginia Commonwealth University approved all procedures. ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010) were consulted when writing the manuscript.
Drugs
Structures of rimonabant and each of the rimonabant analogues tested herein are shown in Figure 1. Rimonabant analogues (Organix Inc., Woburn, MA, USA) were dissolved in a vehicle consisting of ethanol, Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ, USA) and saline at a ratio of 1:1:18. THC [National Institute on Drug Abuse (NIDA), Rockville, MD, USA] was also dissolved in the 1:1:18 vehicle for the cross-tolerance tetrad experiments. For the drug discrimination experiments, THC and rimonabant (NIDA) were dissolved in 0.78% Tween-80 (Fischer Scientific, Pittsburgh, PA, USA) and 99.22% saline. Morphine sulfate (NIDA) was dissolved in physiological saline. All doses were administered at a volume of 10 mL kg−1 body weight. Routes of administration and pre-session injection intervals are described in the Methods sections for each procedure. Rimonabant analogues tested here were chosen from a series of analogues that produced similar pharmacological profiles based upon demonstrated potency in the tetrad tests and availability of sufficient quantity of the compound for behavioural studies. Compounds with a range of CB1 receptor affinities (Ki = 24–484 nM), with no or minimal CB2 receptor affinity were selected. All drug/molecular target nomenclature is in accordance with the guidelines established by Alexander et al. (2011).
Apparatus
For tetrad experiments, assessment of spontaneous activity (SA) in mice occurred in standard activity chambers containing eight photocell beams enclosed in sound- and light-attenuating cubicles (Med Associates, St. Albans, VT, USA). A standard tail flick apparatus and a digital thermometer (Fisher Scientific, Pittsburgh, PA, USA) were used to measure antinociception and rectal temperature respectively. Catalepsy was evaluated using an apparatus fabricated in house comprised of a metal ring (5.5 cm in diameter) attached to a stand at a height of 16 cm.
Drug discrimination experiments were conducted in standard computer-interfaced operant conditioning chambers (Med Associates) with two nose poke apertures in the left and right positions (8 cm apart) on the front panel. Each aperture contained an infrared beam that was interrupted when a mouse inserted their snout, counting as one response. centered between the apertures was a recessed food receptacle connected to a food hopper that delivered reinforcement (14 mg sweetened pellets; Bio-Serv, Frenchtown, NJ, USA). Test chambers were housed in sound-attenuated chambers and ventilation fans provided masking noise. MED-PC software (Med Associates) controlled session parameters and recorded data.
Experimental design overview
The goal of these studies was to determine whether representative compounds from a novel series of 3-substituent rimonabant analogues share pharmacological activity with THC in behavioural assays predictive of CB abuse potential. In the first set of experiments, selected analogues were tested in a tetrad of in vivo tests in mice, in which psychoactive CB agonists produce a characteristic profile of effects, including hypolocomotion, hypothermia, antinociception and catalepsy (Martin et al., 1991). While we had demonstrated previously that these (and other similar) compounds produced these effects (Wiley et al., 2012), the previous tests used a different mouse strain and route of administration than was planned for the drug discrimination experiments reported here. After this dose range study, selected analogues were tested for substitution and antagonism of the THC discriminative stimulus. Another group of mice were trained to discriminate O-6629 (one of the most potent analogues in the series) from vehicle. Subsequent to acquisition of the discrimination, substitution tests were conducted with other 3-substituent rimonabant analogues, rimonabant and THC, as well as the non-CB, morphine. Finally, the 3-substituent rimonabant analogue, O-6211, was evaluated for cross-tolerance in THC-tolerant mice in the tetrad tests. Specific procedures are described in the following statements.
Tetrad tests in mice
Each mouse was tested in all of the tetrad assays: locomotor activity, tail flick, rectal temperature and ring immobility. Prior to drug administration, rectal temperature and baseline tail flick latency were determined. The latter procedure involved placing the mouse's tail on an ambient heat source (i.e. bright light) and latency (in seconds) for tail removal served as the dependent variable. Typical control latencies were 2–4 s. A 10 s maximal latency was used in order to minimize tissue damage. After measuring temperature and baseline tail flick latency, mice were administered vehicle or drug. Following the designated pretreatment time (see following statements), they were placed into individual activity chambers for 10 min. SA was measured as the total number of beam interruptions during the entire session. Immediately thereafter, mice were re-tested in the tail flick procedure. Antinociception was expressed as the percent maximum possible effect (%MPE) as follows: [(test latency – baseline latency) / (10 – baseline latency) × 100]. Rectal temperature was reassessed next and expressed as the difference between pre- and post-injection temperatures. Finally, mice were placed on the ring apparatus, and the amount of time the animals remained cataleptic (i.e. motionless except for respiration; whisker movements were scored as movement) during a 5 min period was recorded. Percent immobility was expressed as [(time immobile / 300) × 100], whereby 300 represented the total session time (in seconds). For the acute tetrad experiments, O-6629 and O-6658 were administered s.c. 60 min prior to the start of the experiments.
Drug discrimination: training procedure
Mice were trained to nose poke on one aperture under a fixed ratio (FR) 1 schedule of food reinforcement, as every response resulted in delivery of a food pellet. The value of the FR requirement was gradually increased from FR1 to FR10 over several sessions until subjects responded readily under FR10 conditions on each aperture. Next, drug discrimination training commenced whereby subjects were trained to discriminate either 5.6 mg kg−1 THC versus vehicle or 5.6 mg kg−1 O-6629 versus vehicle. During acquisition, responding was restricted to one aperture following THC/O-6629 administration and to the opposite aperture following vehicle administration. This errorless training persisted for 16 sessions according to a double alternation schedule of drug–vehicle injection (i.e. DDVVDDVV). The position of the drug-associated aperture (left or right) was assigned randomly for all subjects. Following errorless training, mice were allowed to respond on either aperture, but only responses on the injection-appropriate aperture prompted reinforcement. Responses on the incorrect aperture reset the ratio requirement on the correct aperture. The double alternation schedule of drug administration was used throughout the remainder of the studies.
Successful acquisition of the training drug discriminative stimulus for each discrimination group was demonstrated when subjects met the following three criteria for seven out of eight consecutive sessions: (i) first completed FR10 occurred on the appropriate aperture; (ii) ≥ 80% of total responding occurred on the appropriate aperture; and (iii) response rate ≥0.17 responses s–1. Control tests with the training drug (5.6 mg kg−1 THC or O-6629) and vehicle were conducted prior to each dose effect determination. During control and test sessions, responses on both apertures were reinforced according to the FR10 schedule and the FR counter was reset if an animal interrupted responding on one aperture to respond on the other. The three training criteria also had to be met during the most recent training sessions with the training drug and vehicle immediately prior to all test sessions. Testing typically occurred on Tuesdays and Fridays with a minimum of 2 days between tests, provided these criteria were met.
THC discrimination testing procedure
After successful acquisition of the discrimination and completion of control tests, a generalization curve was conducted with THC (1–30 mg·kg−1) in all subjects. Then, rimonabant was tested with the training dose to assess CB1 receptor mediation of THC's discriminative stimulus. Next, O-4332, O-6629 and O-6658 were tested alone to determine whether they occasioned THC-like responding and in combination with the THC training dose to determine whether they modified THC's discriminative stimulus. Rimonabant analogues were administered s.c. 60 min prior to the start of the experiments for both generalization and antagonism test sessions. THC and its vehicle were administered s.c. 30 min pre-session, while rimonabant was given s.c. 40 min pre-session.
O-6629 discrimination testing procedure
After successful acquisition of the discrimination and completion of control tests, a generalization curve was conducted with O-6629 (1–10 mg·kg−1) in all subjects. Subsequently, mice were randomly assigned to one of two discrimination groups. In group 1, substitution tests were conducted with rimonabant and O-6658. A challenge test with rimonabant was also conducted against the O-6629 training dose. In group 2, substitution tests with THC and O-6629 were performed. Morphine was assessed as a negative control. Morphine was administered s.c. 30 min pre-session. All other drug-dosing parameters were the same as the THC discrimination experiments.
Cross-tolerance dosing regimen
To assess the development of cross-tolerance to the characteristic tetrad of in vivo effects produced by THC in mice, 10 mg kg−1 THC was administered s.c. twice daily for 3 days. Separate cohorts were treated with vehicle in the same manner. On day 4, mice were injected i.v. with their assigned test compound (vehicle, 10 mg kg−1 THC, 3 mg kg−1 O-6211, 10 mg kg−1 O-6211) 5 min prior to the start of tetrad testing. Testing procedure parameters were as described earlier for the acute tetrad tests.
Data analysis
Tetrad data for O-6629 and O-6658 were analysed using a separate one-way ANOVA for each dependent measure. Significant differences between means were followed by Dunnett's post hoc test to identify differences in treatment condition relative to vehicle controls (P < 0.05). In drug discrimination experiments, the number of responses on each aperture was converted into percent drug lever responding (%DLR) by dividing the number of responses on the drug aperture by total responses and multiplying by 100. Full substitution for the THC or O-6629 discriminative stimulus was defined as ≥80% DLR and partial substitution ranged from 50–79% DLR. Responses per second for each session were calculated and analysed using repeated-measures ANOVA, followed by Dunnett's post hoc test when appropriate to identify response rate differences relative to vehicle control (P < 0.05). For challenge tests, ANOVA and Dunnett's post hoc tests were also used to compare %DLR between drug doses and THC/O-6629 control. %DLR data for subjects that failed to make 10 or more responses during the course of a test session was excluded from analysis. Response rate analyses included data for all mice. These statistical analyses were conducted with GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). Cross-tolerance tetrad data were analysed using factorial ANOVA (pretreatment × test treatment), followed by Tukey–Kramer post hoc tests (when appropriate) to further examine main effects and interactions using NCSS software (NCSS LLC, Kaysville, UT, USA).
Results
Cannabimimetic effects of O-6629 and O-6658
Data from the evaluation of O-6629 and O-6658 in the tetrad tests are presented in Figure 2. O-6629 significantly decreased SA [F(3,20) = 88.38, P < 0.05] and body temperature [F(3,20) = 78.16, P < 0.05], and produced significant antinociception [F(3,20) = 3.26, P < 0.05] and catalepsy [F(2,15) = 17.06, P < 0.05]. Post hoc analyses indicated that, relative to vehicle control, significant differences in each test occurred following administration of either 10 and/or 30 mg kg−1 O-6629.
Figure 2.
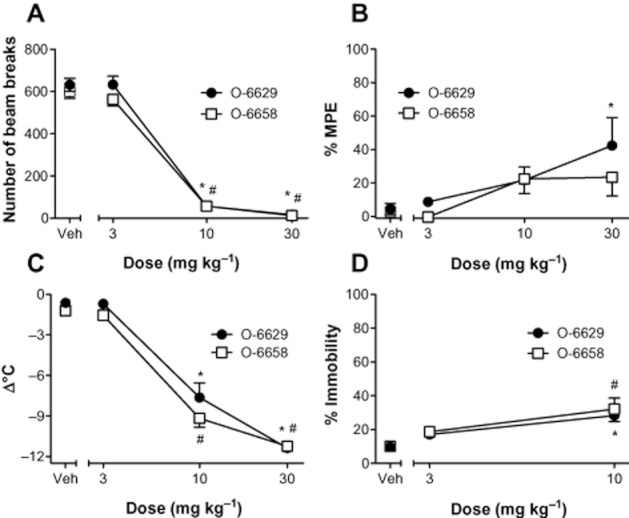
Cannabimimetic activity of the 3-substituent rimonabant analogues, O-6629 and O-6658, in the mouse tetrad. Mice were treated with O-6629 or O-6658 (s.c.), then assessed in the tetrad tests 1 h later. Locomotor data (A) are expressed as number of beam breaks in a 10 min open field test. Antinociception data (B) are presented as % maximal possible effect (MPE). Hypothermia data (C) are expressed as change in body temperature (pre – post; °C). Catalepsy (D) is expressed as percent time immobile. Data represent the mean (±SEM) of six mice per treatment. Significant differences from vehicle are denoted by * (O-6629) or # (O-6658), P < 0.05.
O-6658 significantly decreased SA [F(3,20) = 149.00, P < 0.05], and body temperature [F(3,20) = 164.00, P < 0.05] and produced catalepsy [F(2,15) = 7.71, P < 0.05]. In contrast to O-6629, O-6658 did not produce significant antinociception. Ataxia-like motor impairment was observed in all subjects treated with 30 mg kg−1 of O-6629 or O-6658 and catalepsy assessment at this dose ceased following five attempts to place each mouse back on the ring. Thus, no data are presented for these compounds at the high dose. Similar to O-6629, post hoc tests revealed that significant differences were produced by 10 and/or 30 mg kg−1 O-6658 doses.
Evaluation of 3-substitutent rimonabant analogues in THC discrimination
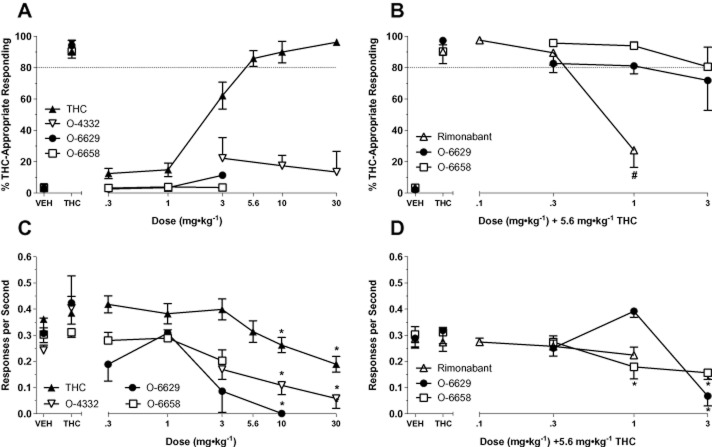
A total of 13 out of 16 subjects met acquisition criteria (correct FFR, ≥80% condition-appropriate responding, response rate ≥0.17 responses s−1) in a mean of 29.50 (±2.18) sessions. Subjects that failed to meet acquisition criteria after 100 training sessions were excluded from the study. Generalization testing results with THC in all subjects meeting acquisition criteria are presented in Figure 3. Dose-dependent generalization with THC was observed, with full substitution seen with 5.6, 10 and 30 mg kg−1 doses of THC (panel A). Analysis of response rates revealed a significant effect of treatment, [F(7,84) = 10.42, P < 0.05]. Compared to vehicle, response rates were significantly decreased by 10 and 30 mg kg−1 doses, P < 0.05 (panel C).
Figure 3.
Generalization (left panels) and challenge (right panels) test data with rimonabant and 3-substituent pyrazole analogues in mice trained to discriminate 5.6 mg kg−1 THC from vehicle. Top panels depict percentage of THC-appropriate responding and bottom panels depict response rate (responses s−1). Data represent the mean (+SEM) of 4–13 mice group−1. Significant differences in response rate relative to vehicle control are denoted by *, P < 0.05. Significant differences in THC-appropriate responding during challenge tests compared to THC control are denoted by #, P < 0.05.
Generalization testing: 3-substituent rimonabant analogues
Results from generalization testing with O-4332, O-6629 and O-6658 are presented in Figure 3 (left panels). None of these analogues generated THC-like responding (panel A). Analysis of response rates revealed significant main effects for O-4332 [F(4,32) = 16.02, P < 0.05] and O-6629 [F(5,15) = 5.39, P < 0.05]. Compared to vehicle controls, response rates were significantly decreased by 10 mg kg−1 doses of both compounds, and 30 mg kg−1 O-4332, P < 0.05. At this dose, all subjects responded fewer than 10 times during the test session; thus, no generalization data are presented for this dose. O-6658 failed to significantly alter response rates, although a trend towards a decrease in responding was observed [F(4,20) = 2.57, P = 0.07].
Challenge testing: rimonabant and 3-substitutent analogues
Results from challenge tests with rimonabant and selected 3-substituent analogues against the training dose of THC (5.6 mg kg−1) are presented in Figure 3 (right panels). Rimonabant dose-dependently attenuated THC's discriminative stimulus, with a significant decrease in THC-like responding occurring with 1 mg kg−1 rimonabant pretreatment (panel B). Analysis of response rate data revealed a significant effect of treatment, [F(5,25) = 3.46, P < 0.05]. Compared to vehicle, response rates were significantly decreased during control tests with 1 mg kg−1 rimonabant, P < 0.05 (data not shown). Pretreatment with either O-6629 or O-6658 did not effectively block the THC discriminative stimulus. While the 3 mg kg−1 dose of O-6629 resulted in a reduction of THC-appropriate responding below full substitution criteria (71.8% DLR), this coincided with significant rate suppression [F(4,28) = 14.91, P < 0.05]. Out of eight mice tested, full substitution was observed in three out of four mice that responded. Challenge with O-6658 significantly altered response rates [F(4,28) = 5.90, P < 0.05] at doses of 1.0 and 3.0 mg kg−1, P < 0.05.
Discriminative stimulus effects of O-6629
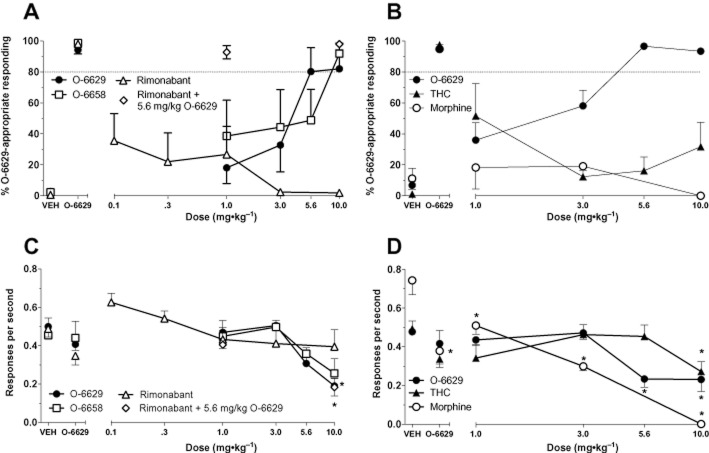
Two separate groups of mice were trained to successfully discriminate 5.6 mg kg−1 O-6629 from vehicle in a mean of 61.53 (±6.78) sessions. Results from generalization testing in these mice are presented in Figure 4. In the first cohort of mice (n = 6; left panels), administration of the training drug or the similar analogue O-6658 resulted in dose-dependent substitution, whereas the parent compound rimonabant did not prompt O-6629-like responding (panel A). Challenge tests with 1 and 10 mg kg−1 rimonabant did not attenuate the O-6629 discriminative stimulus. Significant decreases in response rate were produced by 10 mg kg−1 O-6629 and challenge with 10 mg kg−1 rimonabant, P < 0.05 (panel C).
Figure 4.
Generalization and challenge test data with rimonabant, 3-substitutent pyrazole analogues, THC and morphine in mice trained to discriminate 5.6 mg kg−1 O-6629 from vehicle. Data represent the mean of 5–13 mice group−1. All other details are the same as Figure 3.
In the second cohort of mice (n = 13; right panels), dose-dependent generalization to the training drug was observed, with significant rate suppression [F(5,60) = 7.49, P < 0.05] occurring with 5.6 and 10 mg kg−1 doses (panels B, D). Partial substitution occurred with the lowest dose of THC tested (1 mg kg−1), but this effect was not present at higher doses (panel B). As expected, behaviourally active doses of morphine did not substitute for O-6629. The 10 mg kg−1 dose of THC [F(5,25) = 3.05, P < 0.05] and all tested doses of morphine [F(4,25) = 25.05, P < 0.05] significantly decreased responding relative to respective vehicle control rates (panel D).
Cross-tolerance to the cannabimimetic profile of THC
Data from the cross-tolerance experiments with THC and O-6211 in the tetrad are presented in Figure 5. Here, we evaluated differences in subjects treated repeatedly with either vehicle or THC, then tested with vehicle, THC or O-6211 (3, 10 mg kg−1). A two-way ANOVA revealed significant main effects for pretreatment [F(1,39) = 4.79, P < 0.05] and treatment conditions [F(3,39) = 28.56, P < 0.05] on SA (panel A). Post hoc analysis of the main effect of pretreatment revealed that mice treated repeatedly with THC exhibited greater locomotor activity than those repeatedly treated with vehicle, P < 0.05. Visual inspection of the graph shows that this effect occurred primarily in mice tested with vehicle or with THC, suggesting development of tolerance. Post hoc analysis indicated a significant main effect of treatment wherein THC and both doses of O-6211 decreased locomotor activity compared to vehicle, P < 0.05. Both doses of O-6211 also suppressed activity compared to THC treatment, suggesting that cross-tolerance did not occur. For antinociception (panel B), significant main effects for pretreatment [F(1,39) = 15.33, P < 0.05] and treatment conditions [F(3,39) = 70.26, P < 0.05] and a significant interaction [F(1,39) = 6.70, P < 0.05] were noted. Post hoc analysis of the interaction indicated that mice treated repeatedly with THC showed significantly less antinociception when subsequently tested with THC than did mice treated with repeated vehicle. In contrast, mice treated with either dose of O-6211 were not cross-tolerant, but rather exhibited antinociception regardless of pretreatment condition. Analysis of body temperature data (panel C) revealed a significant effect for treatment [F(3,39) = 74.17, P < 0.05] and a significant interaction [F(1,39) = 13.65, P > 0.05]. Specifically, post hoc analysis indicated that response to vehicle, THC or 10 mg kg−1 O-6211 was dependent upon pretreatment condition, P < 0.05. In the case of O-6211, animals pretreated with THC showed an increased hypothermic response relative to control. Similarly, inspection of catalepsy data (panel D) revealed a significant main effect of treatment [F(3,39) = 81.54, P < 0.05]. Post hoc analysis revealed significant enhancement of catalepsy upon treatment with THC or 10 mg kg−1 O-6211, regardless of pretreatment condition.
Figure 5.
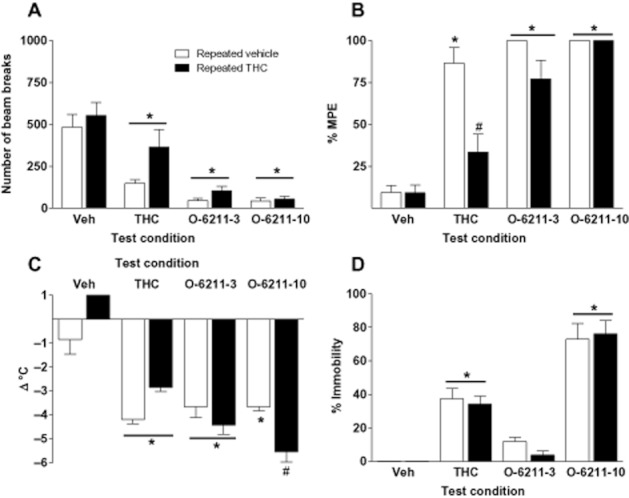
Assessment of cross-tolerance between the cannabimimetic effects of THC and O-6211. Mice were treated with either vehicle (open bars) or 10 mg kg−1 THC (black bars) s.c. twice daily for 3 days. The following day, subjects were administered either vehicle, 10 mg kg−1 THC, or 3 or 10 mg kg−1 O-6211 (i.v.), then assessed in the tetrad tests 5 min later. Values represent the mean (±SEM) of six mice per treatment. Significant differences for treatment with a test compound as compared to vehicle treatment are denoted by *, P < 0.05. # indicates a significant interaction and difference between mice receiving same test compound, but pretreated with repeated THC as compared to repeated vehicle, P < 0.05.
Discussion and conclusions
The results from this study revealed key similarities and differences between the pharmacological properties of selected 3-substituent rimonabant analogues and the prototypical CB agonist THC. Importantly, these findings indicate that these analogues lack THC-like abuse liability and suggest that a novel non-CB1/CB2 mechanism may mediate their pharmacological activity. We have previously shown that various 3-substituted rimonabant analogues produce CB agonist-like effects in a tetrad of tests in ICR mice following i.v. administration (Wiley et al., 2012). In the present study, we partially replicated this finding with two systemically administered 3-dihydrooxazole substituted analogues (O-6629 and O-6658). Whereas s.c. administration of O-6629 and O-6658 dose-dependently decreased motor activity and produced hypothermia in C57BL/6J mice, neither compound produced maximal antinociception. Compound-induced ataxia complicated assessment of catalepsy, but was consistent with our previous observations with this set of analogues. Two obvious differences in the testing parameters with these analogues between this study and previously are route of administration (s.c. vs. i.v.) and mouse strain (C57BL/6J vs. ICR). Either could have affected the potency and/or efficacy of the compounds for producing antinociception. For example, while THC (i.v and i.p.) produced dose-dependent effects with similar efficacy in all tetrad tests, the magnitude of potency difference between routes of administration varied across the tests from sixfold for catalepsy to 27-fold for antinociception in the same (ICR) mouse strain (Wiley and Martin, 2003), suggesting that pharmacokinetic factors related to route of administration may have had greater impact on THC's effects in the nociceptive assay than the other tetrad assays. Regardless of the reason for this discrepancy, results from the tetrad tests were useful in choosing appropriate doses for drug discrimination substitution tests.
As expected, THC produced robust discriminative stimulus effects in mice, with dose-dependent generalization to the 5.6 mg kg−1 training dose and response rate decreases at higher doses. Although THC discrimination has been established in mice only recently, the pharmacological selectivity of the model in this species has been demonstrated by findings from our laboratory and others. The structurally dissimilar synthetic agonists CP55940 and WIN 55212-2 both substitute for THC (McMahon et al., 2008), as does the endocannabinoid anandamide in the presence of a fatty acid amide hydrolase inhibitor (Vann et al., 2009). Conversely, non-CBs (e.g. cocaine, ethanol, salvinorin A) do not elicit THC-like responding (McMahon et al., 2008; Walentiny et al., 2010). Rimonabant reversal of THC's discriminative stimulus in mice suggest this phenomenon is CB1 receptor-mediated (present study; McMahon et al., 2008; Walentiny et al., 2010). In the present study, the novel rimonabant analogues, O-4332, O-6629 and O-6658, failed to substitute for THC, even at behaviourally active doses. Failure to substitute for THC was not likely attributable to deficient CB1 receptor affinity for two of the compounds, as O-6629 and O-6658 had CB1-binding affinities (24 and 57 nM, respectively) comparable to THC (41 nM). Based upon these results, this class of novel pyrazoles would not be predicted to engender marijuana-like intoxication in humans. Further, at doses that did not severely disrupt responding, neither O-6629 nor O-6658 attenuated THC's discriminative stimulus effects, in contrast with the actions of the parent compound.
In a subsequent experiment, O-6211, a third 3-dihydrooxazole substituted pyrazole in the same series as O-6629 and O-6658, was evaluated for cross-tolerance in THC-tolerant mice in the tetrad tests. Tolerance and cross-tolerance following repeated administration of THC, CP55940 and WIN55212-2 have been demonstrated in various procedures (Pertwee et al., 1993; Fan et al., 1994; Bass and Martin, 2000; Sim-Selley and Martin, 2002; De Vry et al., 2004), although task specificity has been noted in cross-tolerance tests with THC and anandamide-like CBs (Pertwee et al., 1993; Wiley et al., 2005; Singh et al., 2011). Following 3 days of repeated THC administration in the present study, tolerance was observed for THC-induced locomotor suppression and antinociception, but not catalepsy or hypothermia (although there was a trend for the latter). Cross-tolerance of O-6211 in THC-tolerant mice was not observed for any of the measures. In fact, the magnitude of hypothermia produced by 10 mg kg−1 O-6211 was significantly enhanced in mice pretreated with THC. In contrast, mice treated repeatedly with 30 mg kg−1 JWH-104 [deoxy-Δ9(11)-THC-dimethylheptyl], a low-efficacy CB1 receptor agonist that paradoxically has little affinity for the receptor (Ki = 909 nM) (Wiley et al., 2002), produced cross-tolerance (data not shown) without substituting for THC in drug discrimination (Wiley et al., 2011). This suggests that O-6211's moderate affinity for the CB1 receptor (Ki = 138 nM) would not have precluded its development of cross-tolerance with THC. The concordance of these results (with O-6211) with those of the discrimination experiments (with O-6629, O-6658 and O-4332) further emphasize underlying mechanistic differences between THC and this series of 3-substituent rimonabant analogues that produce a similar acute in vivo pharmacological profile in the tetrad tests in mice.
Despite evidence presented thus far to suggest that 3-substituted rimonabant analogues do not share THC's abuse potential, their agonist-like effects in the tetrad tests suggest that they are centrally active. Consistent with this idea, O-6629 served as a discriminative stimulus, a capacity which previous research has confirmed is centrally mediated (Balster, 1990). Substitution tests showed substantial generalization of O-6629 to O-6658, but not rimonabant, suggesting that the discriminative stimulus effects of this series of 3-substituted analogues overlap with each other, but are not shared with the parent compound. Previously, tests in rimonabant-trained rats have demonstrated substitution with 5-substituted rimonabant analogues (e.g. AM-251), but not with the CB2 receptor antagonist SR144528 (Järbe et al., 2004; 2008). THC did not substitute for rimonabant in rats (Järbe et al., 2004) and produced only partial substitution for O-6629 in mice (present study). This partial substitution by THC occurred at the lowest dose tested (1 mg kg−1) and did not show the standard progressive increases in substitution with increases in dose (in individual mice or collectively), suggesting that the overlap was not related to a common mechanism of action. It is unknown whether lower doses of THC would have produced a similar substitution profile. Training a higher dose of O-6629 may have resulted in greater specificity of generalization, as has been demonstrated in previous drug discrimination studies (e.g. Mansbach and Balster, 1991; Järbe et al., 1998; 2000). Nevertheless, results of the present study clearly distinguish the discriminative stimulus of O-6629 from those of THC and rimonabant.
In sum, the results of the present study suggest that 3-substituted rimonabant analogues do not possess marijuana-like abuse potential, despite their cannabimimetic activity in the tetrad of tests in mice. The behavioural profile of these compounds also differs from that of rimonabant and other CB1 receptor antagonists. While the distinct discriminative stimulus effects of O-6629 demonstrate central activity and suggest that these compounds would have subjective effects in humans, drug discrimination results presented here cannot inform on the nature of these effects (e.g. euphoric, dysphoric). For example, certain antipsychotics have been successfully trained as discriminative stimuli (Goudie and Smith, 1999); yet, recreational abuse of antipsychotic drugs is limited. Hence, the question of whether these compounds would produce abuse liability that is different from that of THC still remains. Unfortunately, false negatives frequently observed with CBs in other rodent models of abuse liability (e.g. self-administration, conditioned place preference; Mansbach et al., 1994; Cheer et al., 2000; Valjent and Maldonado, 2000) would limit interpretation of potential null effects of these analogues. Clearly, however, the results of this and our previous study on the SAR of this series of compounds indicate that 3-substitution on rimonabant's pyrazole core may result in fundamental changes in the pharmacology and mechanism of action of the resulting analogues. While the precise mechanism of action for the in vivo effects of this unique class of rimonabant analogues remains unknown, mediation by either of the identified CB receptors has been ruled out, strongly suggesting that these compounds are working through an as-of-yet unidentified non-CB1, non-CB2 receptor. The extent to which this mechanism may be similar to off-target sites of rimonabant itself (e.g. Walentiny et al., 2010; Erdozain et al., 2012) is also unknown, but is an area of active investigation.
Acknowledgments
D. M. W. performed the research; D. M. W., R. E. V. and J. L. W. designed the research study; A. M., R. K. and R. G. synthesized rimonabant analogues; D. M. W. and J. L. W. analysed the data and wrote the paper. The authors thank Ramona Winckler for her technical assistance in conducting experiments. This work was supported by grants DA-03672, DA-005488 and DA-009789 from the National Institutes of Health, National Institute on Drug Abuse. Support for D. M. W. during preparation of this manuscript was provided by DA-128904.
Glossary
- %DLR
percent drug lever responding
- CB
cannabinoid
- MPE
maximum possible effect
- SA
spontaneous activity
- SAR
structure–activity relationship
- SR141716
5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- THC
Δ9-tetrahydrocannabinol
Conflict of interest
None.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL. Perception of drug effects. In: Berkley MA, Stebbins WC, editors. Comparative Perception: Basic Mechanisms. New York: John Wiley & Sons; 1990. pp. 127–154. [Google Scholar]
- Balster RL. Drug abuse potential evaluation in animals. Br J Addict. 1991;86:1549–1558. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Bass CE, Martin BR. Time course for the induction and maintenance of tolerance to Delta(9)- tetrahydrocannabinol in mice. Drug Alcohol Depend. 2000;60:113–119. doi: 10.1016/s0376-8716(99)00150-7. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Thomas BF, McMahon LR. Cannabinoid CB1 receptor antagonists as potential pharmacotherapies for drug abuse disorders. Int Rev Psychiatry. 2009;21:134–142. doi: 10.1080/09540260902782786. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 2000;151:25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- Christopoulou FD, Kiortsis DN. An overview of the metabolic effects of rimonabant in randomized controlled trials: potential for other cannabinoid 1 receptor blockers in obesity. J Clin Pharm Ther. 2011;36:10–18. doi: 10.1111/j.1365-2710.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR, Kuhl E, Eckel G. Behavioral effects of cannabinoids show differential sensitivity to cannabinoid receptor blockade and tolerance development. Behav Pharmacol. 2004;15:1–12. doi: 10.1097/00008877-200402000-00001. [DOI] [PubMed] [Google Scholar]
- Erdozain AM, Diez-Alarcia R, Meana JJ, Callado LF. The inverse agonist effect of rimonabant on G protein activation is not mediated by the cannabinoid CB1 receptor: evidence from postmortem human brain. Biochem Pharmacol. 2012;83:260–268. doi: 10.1016/j.bcp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther. 1994;271:1383–1390. [PubMed] [Google Scholar]
- Gatley JS, Gifford AN, Volkow ND, Lan R, Makriyannis A. I-Labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Euro J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Goudie A, Smith J. Discriminative stimulus properties of antipsychotics. Pharmacol Biochem Behav. 1999;64:193–201. doi: 10.1016/s0091-3057(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl. 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Hurst D, Umejiego U, Lynch D, Seltzman H, Hyatt S, Roche M, et al. Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: importance of the C-3 carboxamide oxygen/lysine3.28(192) interaction. J Med Chem. 2006;49:5969–5987. doi: 10.1021/jm060446b. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. Delta-9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology (Berl) 1998;140:519–522. doi: 10.1007/s002130050797. [DOI] [PubMed] [Google Scholar]
- Järbe T, Lamb R, Lin S, Makriyannis A. Delta9-THC training dose as a determinant for (R)-methanandamide generalization in rats: a systematic replication. Behav Pharmacol. 2000;11:81–86. doi: 10.1097/00008877-200002000-00009. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Harris MY, Li C, Liu Q, Makriyannis A. Discriminative stimulus effects in rats of SR-141716 (rimonabant), a cannabinoid CB1 receptor antagonist. Psychopharmacology (Berl) 2004;177:35–45. doi: 10.1007/s00213-004-1916-5. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Li C, Vadivel SK, Makriyannis A. Discriminative stimulus effects of the cannabinoid CB1 receptor antagonist rimonabant in rats. Psychopharmacology (Berl) 2008;198:467–478. doi: 10.1007/s00213-008-1076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Balster RL. Pharmacological specificity of the phencyclidine discriminative stimulus in rats. Pharmacol Biochem Behav. 1991;39:971–975. doi: 10.1016/0091-3057(91)90061-6. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Nicholson KL, Martin BR, Balster RL. Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav Pharmacol. 1994;5:219–225. doi: 10.1097/00008877-199404000-00014. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academies Press; 1996. [Google Scholar]
- Pertwee RG, Stevenson LA, Griffin G. Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. Br J Pharmacol. 1993;110:1483–1490. doi: 10.1111/j.1476-5381.1993.tb13989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-b enzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther. 2002;303:36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- Singh H, Schulze DR, McMahon LR. Tolerance and cross-tolerance to cannabinoids in mice: schedule-controlled responding and hypothermia. Psychopharmacology (Berl) 2011;215:665–675. doi: 10.1007/s00213-010-2162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–292. [PubMed] [Google Scholar]
- Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of Delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentiny DM, Vann RE, Warner JA, King LS, Seltzman HH, Navarro HA, et al. Kappa opioid mediation of cannabinoid effects of the potent hallucinogen, salvinorin A, in rodents. Psychopharmacology (Berl) 2010;210:275–284. doi: 10.1007/s00213-010-1827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471:185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Jefferson RG, Grier MC, Mahadevan A, Razdan RK, Martin BR. Novel pyrazole cannabinoids: insights into CB(1) receptor recognition and activation. J Pharmacol Exp Ther. 2001;296:1013–1022. [PubMed] [Google Scholar]
- Wiley JL, Jefferson RG, Griffin G, Liddle J, Yu S, Huffman JW, et al. Paradoxical pharmacological effects of deoxy-tetrahydrocannabinol analogs lacking high CB1 receptor affinity. Pharmacology. 2002;66:89–99. doi: 10.1159/000065631. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Smith FL, Razdan RK, Dewey WL. Task specificity of cross-tolerance between Delta9-tetrahydrocannabinol and anandamide analogs in mice. Eur J Pharmacol. 2005;510:59–68. doi: 10.1016/j.ejphar.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Breivogel CS, Mahadevan A, Pertwee RG, Cascio MG, Bolognini D, et al. Structural and pharmacological analysis of O-2050, a putative neutral cannabinoid CB(1) receptor antagonist. Eur J Pharmacol. 2011;651:96–105. doi: 10.1016/j.ejphar.2010.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Selley DE, Wang P, Kottani R, Gadthula S, Mahadeven A. 3-Substituted pyrazole analogs of the cannabinoid type 1 (CB1) receptor antagonist rimonabant: cannabinoid agonist-like effects in mice via non-CB1, non-CB2 mechanism. J Pharmacol Exp Ther. 2012;340:433–444. doi: 10.1124/jpet.111.187815. [DOI] [PMC free article] [PubMed] [Google Scholar]