Abstract
Background and Purpose
AMG 181 is a human anti-α4β7 antibody currently in phase 1 and 2 trials in subjects with inflammatory bowel diseases. AMG 181 specifically targets the α4β7 integrin heterodimer, blocking its interaction with mucosal addressin cell adhesion molecule-1 (MAdCAM-1), the principal ligand that mediates α4β7 T cell gut-homing.
Experimental Approach
We studied the in vitro pharmacology of AMG 181, and the pharmacokinetics and pharmacodynamics of AMG 181 after single or weekly i.v. or s.c. administration in cynomolgus monkeys for up to 13 weeks.
Key Results
AMG 181 bound to α4β7, but not α4β1 or αEβ7, and potently inhibited α4β7 binding to MAdCAM-1 (but not vascular cell adhesion molecule-1) and thus inhibited T cell adhesion. Following single i.v. administration, AMG 181 Cmax was dose proportional from 0.01 to 80 mg·kg−1, while AUC increased more than dose proportionally. Following s.c. administration, dose-proportional exposure was observed with single dose ranging from 5 to 80 mg·kg−1 and after 13 weekly doses at levels between 20 and 80 mg·kg−1. AMG 181 accumulated two- to threefold after 13 weekly 80 mg·kg−1 i.v. or s.c. doses. AMG 181 had an s.c. bioavailability of 80%. The linear elimination half-life was 12 days, with a volume of distribution close to the intravascular plasma space. The mean trend for the magnitude and duration of AMG 181 exposure, immunogenicity, α4β7 receptor occupancy and elevation in gut-homing CD4+ central memory T cell count displayed apparent correlations.
Conclusions and Implications
AMG 181 has in vitro pharmacology, and pharmacokinetic/pharmacodynamic and safety characteristics in cynomolgus monkeys that are suitable for further investigation in humans.
Keywords: α4β7 integrin, anti-α4β7, human antibody, AMG 181, cell trafficking, gut-homing, T cells, inflammatory bowel diseases, Crohn's disease, ulcerative colitis
Introduction
Inflammatory bowel disease (IBD) is associated with an influx of immune cells into the gut mucosa, which occurs through integrin-mediated leukocyte tethering, rolling and arrest (Hynes, 2002). Integrins are formed by heterodimerization of α and β subunits, of which there are 18 known α subunits and 8 β subunits, with α4β1, α4β7, αEβ7 and αLβ2 playing large roles in leukocyte trafficking. The α4β1 is expressed broadly on lymphocytes and myeloid cells, and binds to vascular cell adhesion molecule-1 (VCAM-1) ubiquitously expressed on vascular endothelium. The α4β7 integrin is predominantly expressed on a subpopulation of CD4+CD45RA-memory T cells, which have been shown to preferentially home to the gut through its interaction with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) specifically expressed on gastrointestinal endothelial venules (Picarella et al., ; Campbell et al., 1999). The αEβ7 binds selectively to E-cadherin and is expressed on T cells in the gastrointestinal, respiratory and urogenital tract mucosal immune system (Parker et al., 1992; Cepek et al., 1994; Erle et al., 1994). The αLβ2 has the αL subunit expressed on all leukocytes. αLβ2 binds to its ligand intercellular adhesion molecule-1 and leads to adhesion of leukocytes to other cell types related to proinflammatory processes (Yonekawa and Harlan, 2005).
Natalizumab targets both α4β1 and α4β7, while vedolizumab targets α4β7. Inhibition of gut lymphocyte homing with natalizumab (Sandborn et al., 2005; Targan et al., 2007) or vedolizumab (Feagan et al., 2005; 2008; 2012; Parikh et al., 2012) has shown clinical benefits in both Crohn's disease (CD) and ulcerative colitis (UC) patients. Natalizumab is also efficacious in treating relapsing and remitting forms of multiple sclerosis (Polman et al., 2006). However, natalizumab is associated with a rare but serious complication of progressive multifocal leukoencephalopathy (PML) caused by reactivation of latent human John Cunningham polyomavirus (JC virus) infection in the CNS (Berger and Koralnik, 2005). The risks of PML during natalizumab treatment are likely associated with the broad impact on leukocyte trafficking, especially to the CNS, by pan-α4 inhibition of α4β1-expressing cells. Natalizumab and vedolizumab are humanized IgG4 and IgG1, respectively, and both have shown >10% immunogenicity in IBD patients, which has been associated with less desired clinical outcomes (Feagan et al., 2005; 2008; Sandborn et al., 2005; Parikh et al., 2012). The long-term efficacy and safety benefits of vedolizumab targeting the gut-homing specific α4β7 : MAdCAM-1 pathway have been recently confirmed in the phase 3 clinical trials conducted in IBD subjects (Colombel, 2012a,b; Feagan et al., 2012; Rutgeerts, 2012).
By specifically targeting α4β7, the interruption of lymphocyte homing to the gut may provide benefit for IBD, while the lack of effects on lymphocyte trafficking to the brain ought to minimize, if not obviate, the risks of PML. Thus, AMG 181, a human monoclonal IgG2 antibody that specifically binds to α4β7 heterodimers, has been developed and is currently being tested in both UC and CD subjects in phase 1 (NIH, 2010; NIH, 2011) and phase 2 clinical trials (NIH, 2012a; NIH, 2012b). This report describes the in vitro pharmacology of AMG 181, as well as pharmacokinetic (PK) and pharmacodynamic (PD) results from studies in cynomolgus monkeys. We believe that AMG 181 has the potential for providing IBD patients with better treatment outcomes and fewer side effects due to its pharmacological potency, lack of immunogenicity, and gut-specificity. AMG 181 has in vitro pharmacology, as well as PK/PD and safety characteristics in cynomolgus monkeys that support its advancement into clinical development.
Methods
Test article
AMG 181 is a human monoclonal antibody with a MW of approximately 144 kDa (Hsu et al., 2010). It was manufactured at Amgen Inc. (Thousand Oaks, CA, USA) by expression in CHO cell line.
In vitro pharmacology studies
The following in vitro studies were conducted to assess the pharmacological impact of AMG 181 on the specific gut-homing cell trafficking pathway. These data were critical for moving towards the next steps of selecting the appropriate pre-clinical animal species for conducting in vivo pharmacology studies and, subsequently, into clinical trials in humans.
On-cell binding affinity to α4β7
Affinity and specificity are the two important measures for selecting a therapeutic antibody, and reports have shown direct correlation between affinity and antibody potency (Zuckier et al., 2000; Rathanaswami et al., 2005). In this study, HuT78 cells (American Type Culture Collection, Manassas, VA, USA) expressing human α4β7 were titrated 1–3 from ∼50 million cells·mL−1 to ∼400 cells·mL−1 and then equilibrated with a final concentration of either 2 or 30 pM of AMG 181 for 18 h at 4°C. The free AMG 181 remaining in the supernatant at equilibrium was measured by KinExA® technology (Sapidyne Instruments, Boise, ID, USA) by passing the supernatant over polymethyl methacrylate beads pre-coated with goat anti-human-Fc and detected with goat anti-human (H + L) Cy5 as previously described (Thermo Fisher Scientific Inc., Rockford, IL, USA) (Rathanaswami et al., 2005; 2008).
Binding specificity to α4β7 heterodimer
From both the safety and the clinical efficacy perspectives, it is important to understand whether AMG 181 indeed binds to α4β7 but not to α4β1 or αEβ7. This was assessed by FACS (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) on the HEK 293 T cells (American Type Culture Collection) transiently co-transfected with either the expression vectors for α4β1, α4β7 or αEβ7 subunits. The expression levels of α4, αE, β1 or β7 on transfected cells were evaluated with antibodies specific for α4, αE, β1 and β7 respectively (R&D Systems Inc., Minneapolis, MN, USA; Immunotech, Marlborough, MA, USA).
Binding affinity and blocking activity in primary human and cynomolgus monkey T cells
These studies were designed to determine the relative potencies of AMG 181 in human and cynomolgus monkey T cells. For primary human T cell binding assays, human peripheral blood mononuclear cells (PBMCs) were stained with serially diluted, biotinylated AMG 181 in the binding buffer (30 mM HEPES, 140 mM NaCl and 1% BSA) (Sigma-Aldrich, St. Louis, MO, USA). Binding of AMG 181 to human CD4+CD45RA-memory T cells was assessed by FACS analysis. Occupancy of α4β7 at various AMG 181 concentrations was determined by percent of AMG 181 positive CD4+CD45RA-memory T cells at each AMG 181 concentration relative to the maximal percent of AMG 181 positive CD4+CD45RA-memory T cells. By curve fitting using the four-parameter Hill equation, EC50 was estimated as the concentration of AMG 181 at 50% of maximal occupancy. For cynomolgus monkey T cell binding, CD4+CD28+CD95+ central memory (CM) T cells were gated and assessed by FACS analysis.
For MAdCAM-1 blocking assay, human or cynomolgus monkey PBMCs were first incubated with serially diluted AMG 181 and then stained with 0.3 μg·mL−1 of biotinylated MAdCAM-Fc (Amgen Inc.) in the binding buffer plus 1 mM MnCl2. Gates were set on the human CD4+CD45RA-memory T cells or cynomolgus monkey CD4+CD28+CD95+ CM T cells for MAdCAM-Fc binding analysis. Percent of positive MAdCAM-Fc staining cells at various AMG 181 concentrations relative to percent of MAdCAM-Fc binding cells in the absence of AMG 181 was calculated. Percent inhibition was derived by subtracting the relative percentage of MAdCAM-Fc binding at various AMG 181 concentrations from 100%. By curve fitting using the four-parameter Hill equation, IC50 was estimated as the AMG 181 concentration at 50% maximal inhibition.
Previous reports (Zhang et al., 1999) have shown that Mn2+ effectively induced α4β7 transformation from a low-affinity to a high-affinity binding state for MAdCAM-1. To determine if AMG 181 would bind to active or inactive conformations of α4β7, human PBMCs were stained with 1 μg·mL−1 of biotinylated MAdCAM-Fc or biotinylated AMG 181 in the presence or absence of 1 mM Mn2+. MAdCAM-Fc or AMG 181 binding to the CD4+CD45RA-memory T cells was then measured by FACS analysis.
Iwata et al. (2004) reported that T cell gut-homing imprinting and induction of α4β7 expression is mediated through retinoic acid produced from retinol (vitamin A) by mucosal dendritic cells in the gut-associated lymphoid tissue (GALT). To assess the effects of retinol (vitamin A), freshly isolated human PBMCs were activated by anti-CD3 (plate-bound, 5 μg·mL−1) and human IL-2 (20 ng·mL−1) in the presence or absence of retinoic acid (1 mM) for 7 days. The majority of the remaining cells after 7 days of treatment were T cells. MAdCAM-Fc or AMG 181 binding to the CD4+CD45RA-memory T cells was then measured by FACS analysis.
Blocking effect on α4β7 : MAdCAM-1-mediated T cell adhesion
Cell adhesion is an important step before a proinflammatory T cell enters target tissue through polarization and diapedesis (Luster et al., 2005). For HuT78 cell adhesion assay, approximately 3 × 104 HuT78 cells were incubated with a dose titration of AMG 181 or human IgG2 isotype control and then transferred to microtitre plate coated with 20 μg·mL−1 MAdCAM-Fc. After incubating for 40 min followed by extensive washing, the adherent cells were measured using the CyQUANT® kit as per manufacturer's recommendations using a Tecan GENios Pro instrument (Life Technologies Corporation, Grand Island, NY, USA).
For the primary T cell adhesion assays, human or cynomolgus monkey CD4+ T cells purified from PBMCs were incubated in 96-well plates coated with 3 μg·mL−1 MAdCAM-Fc in the presence of a dose titration of AMG 181. After 2 h of incubation and extensive washing, cells adherent to the plates were detected with the CyQUANT kit. By curve fitting using the four-parameter Hill equation, IC50 was estimated as the AMG 181 concentration at 50% maximal inhibition of cell adhesion.
Blocking effect on VCAM-1 binding to T cells
The α4β1 is expressed broadly on lymphocytes and myeloid cells, and binds to VCAM-1 ubiquitously expressed on vascular endothelium. Because natalizumab binds to α4β1 and, thus, block binding to VCAM-1, is consequently associated with PML (Berger and Koralnik, 2005), we investigated whether AMG 181 could affect VCAM-1 binding in vitro. For VCAM-1-mediated T cell adhesion, human T cells activated with retinoic acid were pre-incubated with 10 μg·mL−1 of human IgG (huIgG), AMG 181 or anti-α4 antagonist antibody and then transferred to a 96-well plate coated with 30 μg·mL−1 of VCAM-Fc in triplicate (R&D Systems Inc.). The plates were incubated for 40 min and then washed to remove non-adherent cells. The adherent cells were quantified by the CyQUANT cell detection reagent at 485/530 fluorescence in a Tecan Safire.
In vivo studies
Animals
Naïve male and female cynomolgus monkeys (Macaca fascicularis; 2–6.4 years old; 2–6.3 kg) of Chinese origin were tested at facilities accredited by Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC; Frederick, MD). Animals were commingled and provided small bits of treats besides daily supplied diet and water as part of the testing facilities' environmental enrichment programme. The 3 month toxicology study was conducted under current Good Laboratory Practice (GLP) standards (FDA, 1979; MHLW, 1997; OECD, 1997). The protocols were reviewed and approved by the Testing Facility Institutional Animal Care and Use Committee (IACUC) and adhered to regulations outlined in the United States Department of Agriculture (USDA) Animal Welfare Act (9 CFR, Parts 1, 2 and 3) (Animal and Plant Health Inspection Service USA, 2005), the conditions specified in the Guide for the Care and Use of Laboratory Animals (National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. et al., 2011), and in compliance with the Testing Facilities Animal Welfare Assurance (A4112-01) filed with the National Institute of Health (NIH). Results obtained from animal studies have been reported in accordance with the ARRIVE guidance (Kilkenny et al., 2010).
PK, toxicokinetics and tissue cross-reactivity
Cynomolgus monkeys were assigned to treatment groups for single-dose PK and PD studies, a two-dose non-GLP toxicology study and a 3 month GLP toxicology study (Table 1). Pre- and post-dose blood samples were collected as indicated (Table 1). Post-dose observations in toxicology studies were conducted ∼2–4 h after dosing to assess any acute effects. Other evaluations included weekly detailed clinical observations, body weight, food consumption, ophthalmic examinations, physiological measurements, electrocardiograms, clinical pathology, immunophenotyping, receptor occupancy and adaptive immune system T cell-dependent antibody response for anti-keyhole limpet haemocyanin (KLH) IgM or IgG responses.
Table 1.
Studies in cynomolgus monkeys
| Study | n | Groups | Treatments | Samples collected | Assessments |
|---|---|---|---|---|---|
| Single-dose PK/PD #1 | 18 males | 6 groups; 3 per group | Vehicle control i.v.; 0.01, 0.1, 1, or 3 mg·kg−1 AMG 181 i.v.; 3 mg·kg−1 AMG 181 s.c. | Blood samples collected 2–3 times pre-dose; 0.25, 3 and 6 h, and 1, 3, 7, 10, 14, 17, 21, 25, 28, 35, 42, 56 and 70 days post-dose | AMG 181 concentration (PK); haematology and/or flow cytology (PD); anti-AMG 181 binding antibody |
| Single-dose PK/PD #2 | 18 males | 3 groups; 6 per group | Vehicle control i.v.; 9 mg·kg−1 AMG 181 i.v.; 9 mg·kg−1 AMG 181 s.c. | Blood samples collected 2–3 times pre-dose; 0.25, 3 and 6 h, and 1, 3, 7, 10, 14, 17, 21, 25, 28, 35, 42, 56 and 70 days post-dose | AMG 181 concentration (PK); haematology and/or flow cytology (PD); anti-AMG 181 binding antibody |
| Two-dose non-GLP toxicology | 12 males 12 females | 3 groups; 4 per sex per group | Vehicle control s.c. QW; 0.5 or 80 mg·kg−1 AMG 181 s.c. QW | Blood samples collected pre-dose and 0.25, 3, 8, 24, 72 and 168 h post-dose on study days 1 and 8; blood samples collected from recovery monkey (two per sex per group) 14, 17, 21, 25, 28, 35, 42, 49, 52, 56, 70, 84, 91, 105, 119, 133, 140, 148, 154, 161, 170, 175, 189 and 196 days post first dose | AMG 181 concentrations (TK); haematology and/or flow cytometry (PD); anti-AMG 181 binding antibody |
| Three month GLP toxicology | 30 males 30 females | 5 groups; 6 per sex per group | Vehicle control s.c. QW; 5, 20 or 80 mg·kg−1 s.c. QW; 80 mg·kg−1 i.v. QW | Blood samples collected pre-dose and 0.25, 3, 8, 24, 72 and 168 h post-dose on study days 1 and 8 and pre-dose 14, 28, 35, 56, and 84 days post first dose; blood samples collected from recovery monkeys (2 per sex per group) 112, 126, 140, 154, 168, 182, 196, 210, 224, 238, 252, 268, 280, 294, 308 and 322 days post first dose. | AMG 181 concentrations (TK); haematology and/or flow cytometry (PD); anti-AMG 181 binding and neutralizing antibodies |
PD, pharmacodynamics; PK, pharmacokinetics; QW, once-weekly; TK, toxicokinetics.
Serum from the PK/PD studies and the non-GLP toxicology study were analysed for AMG 181 using a qualified electrochemiluminescence (ECL) immunoassay (nominal dynamic range of 2–5000 ng·mL−1) using murine monoclonal anti-AMG 181 capture antibody and murine monoclonal anti-AMG 181 detection antibody (Amgen Inc., Thousand Oaks, CA, USA). The GLP toxicology study used a validated ECL immunoassay (nominal dynamic range of 20–20 000 ng·mL−1). Tissue cross-reactivity of AMG 181 was assessed by binding of Alexa Fluor® 488-AMG 181 (20 and 0.5 μg·mL−1) to fixed frozen sections from a full list of human and cynomolgus monkey tissues (≥3 unique donors or animal per tissue).
Immunogenicity
Serum from all the monkey studies were assessed for anti-AMG 181 binding antibodies using a validated immunoassay [Meso Scale Discovery (MSD) ECL platform]. Samples with signal to noise (S : N) ratios (individual serum sample ECL counts/pooled naïve monkey serum ECL counts) above assay cut-point were followed up in a specificity assay. The lower limit of reliable detection (LLRD) was 20 ng·mL−1. This means that if the anti-AMG 181 antibody concentration was above 20 ng·mL−1, it could be measured in the absence of interference. Detection of anti-AMG 181 binding antibodies at LLRD may have been prevented, creating a false-negative result, if the test sample AMG 181 concentration was >100 μg·mL−1. Samples from the GLP study testing positive for binding antibodies were assessed for anti-AMG 181 neutralizing antibodies with a validated cell-based competitive binding bioassay. Serum samples with a screening assay ratio above assay cut-point and a specificity assay ratio below assay cut-point were considered neutralizing antibody positive. An affinity-purified mouse anti-AMG 181 monoclonal antibody at 5 μg·mL−1 (assay LLRD) in neat pooled naïve cynomolgus monkey serum was used as assay positive control. Other assay controls included a negative control, a drug control and a ligand control. Detection of anti-AMG 181 neutralizing antibodies at LLRD may have been prevented, creating a false-negative result, if the test sample AMG 181 concentration was >230 ng·mL−1.
Pharmacodynamics
CD4+ T cell subsets, including CM, effector memory (EM) and naïve T cells, and α4β7 target receptor occupancy were quantified in monkey whole blood samples using a 5-colour antibody (CD3, CD4, CD28, CD95 and anti-α4β7-AbFree) flow cytometry. Anti-α4β7-AbFree antibody (Amgen Inc.), which is competitively inhibited by AMG181 and detects free α4β7 receptor, was used to determine receptor occupancy. Absolute CD4+ T cell counts were calculated by multiplying the CD4+ T cell fraction in parent lymphocytes by absolute lymphocyte counts. Receptor occupancy was calculated as the difference in median anti-α4β7-AbFree fluorescent intensities between spike and non-spike samples relative to each animal's baseline. This report presents data on the CD4+ CM cells, of which a portion expresses the α4β7 integrin at high levels.
PK, toxicokinetic and PD analyses
Non-compartmental analysis was performed and summary statistics were calculated using WinNonlin® (Enterprise version 5.1.1, 2006, Pharsight®, a Certara™ Company, Sunnyvale, CA, USA). AMG 181 PK or toxicokinetic (TK) profiles were plotted, together with α4β7 receptor occupancy on CD4+ CM cells, and the counts of total lymphocytes, total CD4+ CM cells, α4β7high CD4+CD28+CD95+ CM T cells and α4β7low CD4+CD28+CD95+ CM T cells.
Results
In vitro pharmacology
Binding specificity to α4β7 heterodimer
AMG 181 bound to HEK 293 T cells expressing α4β7 but not to those expressing the α4β1 or αEβ7 heterodimer (Table 2). The HEK 293 T cells expressed high levels of the endogenous β1 subunit, with 763 geometric mean (geomean) detected by anti-β1 antibody, and moderate levels of the α4 subunit, with 43 geomean detected by anti-α4 antibody). Because of the endogenous expression of α4 and β1 in HEK 293 T cells, transfection of α4 and β7 would result in both α4β1 and α4β7 heterodimers on the cell surface. The expression level of α4β7 complex in the α4β7-transfected cells was monitored by FACS with an anti-β7 antibody. The FACS analysis with the anti-β7 specific antibody showed a 63 geomean shift, similar to the 68 geomean shift resulting from AMG 181 staining.
Table 2.
AMG181 binding specificity to α4β7 heterodimer on transfected HEK 293 T cells: geometric mean of the measured fluorescence intensity for integrin expression detected by various specific anti-human integrin antibodies
| HEK 293 T cells transfection with integrin | |||||
|---|---|---|---|---|---|
| α4β7 | α4β1 | αEβ7 | Empty vector | Integrin detection | |
| AMG 181 (anti-human α4β7) | 68 | 5 | 5 | 4 | α4β7 (not α4β1, αEβ7) (AMG 181 bound to α4β7 only) |
| Anti-human α4 | 265 | 275 | 43 | 43 | α4β7, α4β1 (not αEβ7) (moderate α4 expression) |
| Anti-human αE | 4 | 4 | 29 | 5 | αEβ7 (not α4β7, α4β1) |
| Anti-human β1 | 856 | 1371 | 848 | 763 | α4β1, α4β7, αEβ7 (ubiquitous β1 expression) |
| Anti-mouse β7a | 63 | 6 | 26 | 5 | α4β7, αEβ7 (not α4β1) |
Cross-reacts with human β7.
The α4β1-transfected HEK 293 T cells were found to be negative for the expression of the β7 subunit (6 geomean) and positive for the expression of the β1 subunit (1367 geomean). Because α4 could only be expressed on the cell surface while paired with either β1 or β7, the expression of α4 was an indicator of the expression of α4β1 on these cells (275 geomean). Given this expression of the α4β1 integrin, AMG 181 binding to the α4β1 heterodimer was unlikely (geomean of 5). The αEβ7-transfected HEK 293 T cells were also found to be negative for binding by AMG 181 in the FACS assay (geomean of 5) (Table 2). AMG 181 did not bind to human α4β1 or αEβ7 on transiently transfected HEK 293 T cells; therefore, we conclude that AMG 181 binds specifically to human α4β7 complex.
Analysis of in-house PCR and National Center for Biotechnology Information (NCBI) single nucleotide polymorphism (SNP) database revealed two amino acid-changing SNPs in the α4 integrin (V824A and Q878R) and three amino acid-changing SNPs in the β7 integrin (E97V, R213S and G629S). AMG 181 was able to bind HEK 293 T cells transfected with wild-type and all SNP mutant α4β7-transfected cells (data on file at Amgen Inc.).
Binding affinity and blocking activity in primary human and cynomolgus monkey T cells
AMG 181 bound to HuT78 cells expressing surface α4β7 receptors with high affinity (optimal Kd of 0.9 pM with 95% confidence interval range of 0.2–2.3 pM) (Table 3). AMG 181 bound to human CD4+CD45RA-memory T cells in a dose-dependent manner and potently inhibited MAdCAM-1 binding to α4β7 on CD4+CD45RA-memory T cells (Figure 1; Table 3). AMG 181 bound to cynomolgus monkey CD4+CD28+CD95+ CM T cells and blocked MAdCAM-1 binding to α4β7 with similar potency as that for human (Figure 1; Table 3).
Table 3.
AMG 181 in vitro pharmacology parameters
| AMG 181 assay type | Species | Cell type | Parameter or result |
|---|---|---|---|
| Receptor binding affinity | Human | HuT78 cells expressing α4β7 | Kd = 0.9 (0.2–2.3)a pM |
| Receptor binding specificity | Human | HEK 293 T cellsb | Specific binding to α4β7 |
| Binding to primary T cells | Human | CD4+CD45RA-memory T cells | EC50 = 4.0 ng·mL−1 (26 pM) |
| Cynomolgus monkey | CD4+CD28+CD95+ CM T cells | EC50 = 2.6 ng·mL−1 (17 pM) | |
| Blocking of α4β7:MAdCAM-1 binding | Human | CD4+CD45RA-memory T cells | IC50 = 6.4 ng·mL−1 (42 pM) |
| Cynomolgus monkey | CD4+CD28+CD95+ CM T cells | IC50 = 4.5 ng·mL−1 (30 pM) | |
| Blocking of T cell adhesionc | Human | HuT78 cells | IC50 = 1.5 ng·mL−1 (10 pM) |
| Human | Purified T cells | IC50 = 12.0 ng·mL−1 (80 pM) | |
| Cynomolgus monkey | Purified T cells | IC50 = 12.6 ng·mL−1 (84 pM) |
Optimal Kd (95% confidence interval range).
Transiently co-transfected with expression vectors for α4β7, α4β1 or αEβ7 subunits.
α4β7 : MAdCAM-1-mediated T cell adhesion.
Figure 1.
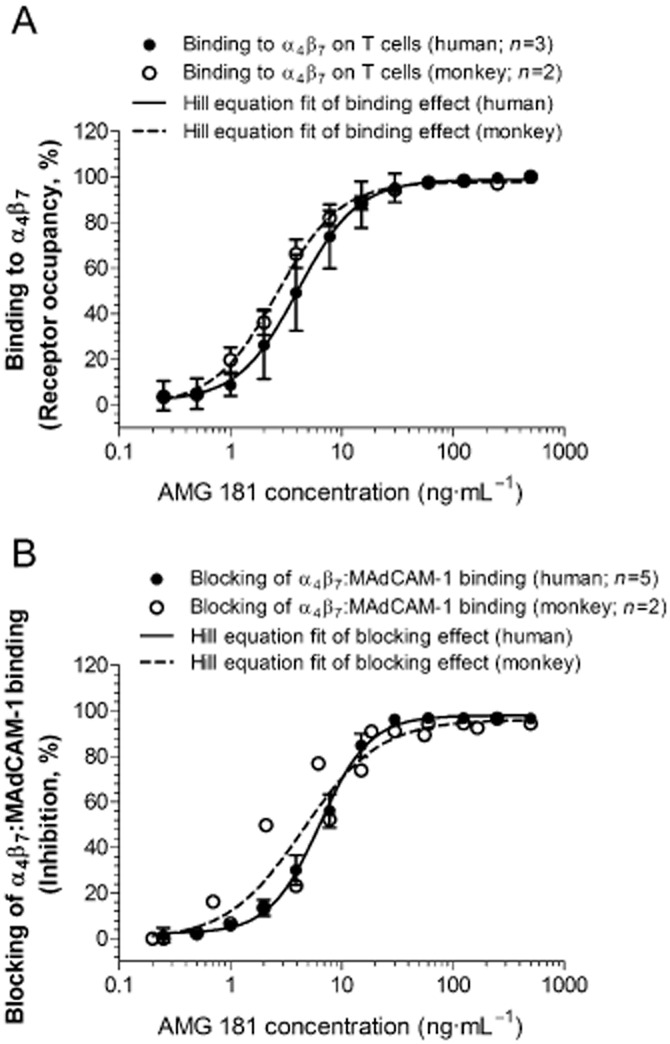
AMG 181 binds to α4β7 receptors on human CD4+CD45RA-memory T cells as well as on cynomolgus monkey CD4+CD28+CD95+ central memory T cells (A), thus blocks binding of MAdCAM-1 to α4β7 receptors (B). Symbols represent observations (mean ± SEM), while lines represent the four-parameter Hill equation fitted curves. For the binding study on monkey T cells (n = 2), range is plotted instead of SEM (A). For the blocking of binding study on monkey T cells (2 sets of experiments at different titrated AMG 181 concentrations), individual observations are plotted (B).
Consistent with previous reports (Zhang et al., 1999), Mn2+ effectively induced α4β7 transformation from a low-affinity (Figure 2A) to a high-affinity (Figure 2B) binding state for MAdCAM-1. In contrast, AMG 181 bound to both low- and high-affinity conformations of α4β7 with similar binding properties of biotinylated AMG 181 to human CD4+CD45RA-memory T cells in the absence (Figure 2C) or presence (Figure 2D) of Mn2+.
Figure 2.
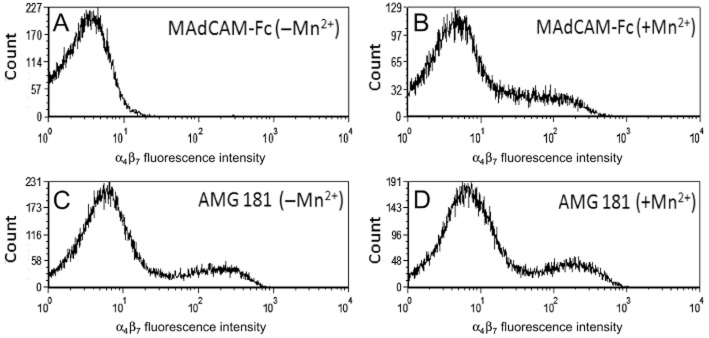
AMG 181 binds to both low- and high-affinity conformations of α4β7: human PBMCs were stained with 1 μg·mL−1 of biotinylated MAdCAM-Fc (A, B) or biotinylated AMG 181 (C, D) in the absence (A, C) or presence (B, D) of 1 mM Mn2+ as indicated. MAdCAM-Fc or AMG 181 binding to the human CD4+CD45RA-memory T cells was measured by FACS analysis. X-axis represents fluorescence intensity of staining with biotinylated MAdCAM-Fc (upper panels) or biotinylated AMG 181 (lower panel) plus streptavidin-PE. Y-axis is the cell count.
Iwata et al. (2004) reported that T cell gut-homing imprinting and induction of α4β7 expression is mediated through retinoic acid produced from retinol (vitamin A) by mucosal dendritic cells in the GALT. Our test results have supported this showing retinoic acid treatment increased MAdCAM-1 binding of CD4+CD45RA-memory T cells (presence : absence of retinoic acid = 41% vs. 12%) indicative of enhanced α4β7 expression (Figure 3A,B). In contrast to the lack of binding to retinoid acid-activated CD4+CD45RA-memory T cells by biotinylated isotype control antibody, biotinylated AMG 181 had binding pattern that was similar to that for MAdCAM-Fc (data not shown). Furthermore, AMG 181 blocked α4β7 binding by MAdCAM-1 on activated T cells with an IC50 value of 10 ng·mL−1 or 67 pM (Figure 3C). This blocking activity was very similar to that observed for non-activated T cells (6.4 ng·mL−1). The blocking activity of AMG 181 on activated cynomolgus monkey CD4+CD28+CD95+ CM T cells (IC50 = 4.5 ng·mL−1) was similar to that observed in human CD4+CD45RA-memory T cells (Table 3).
Figure 3.
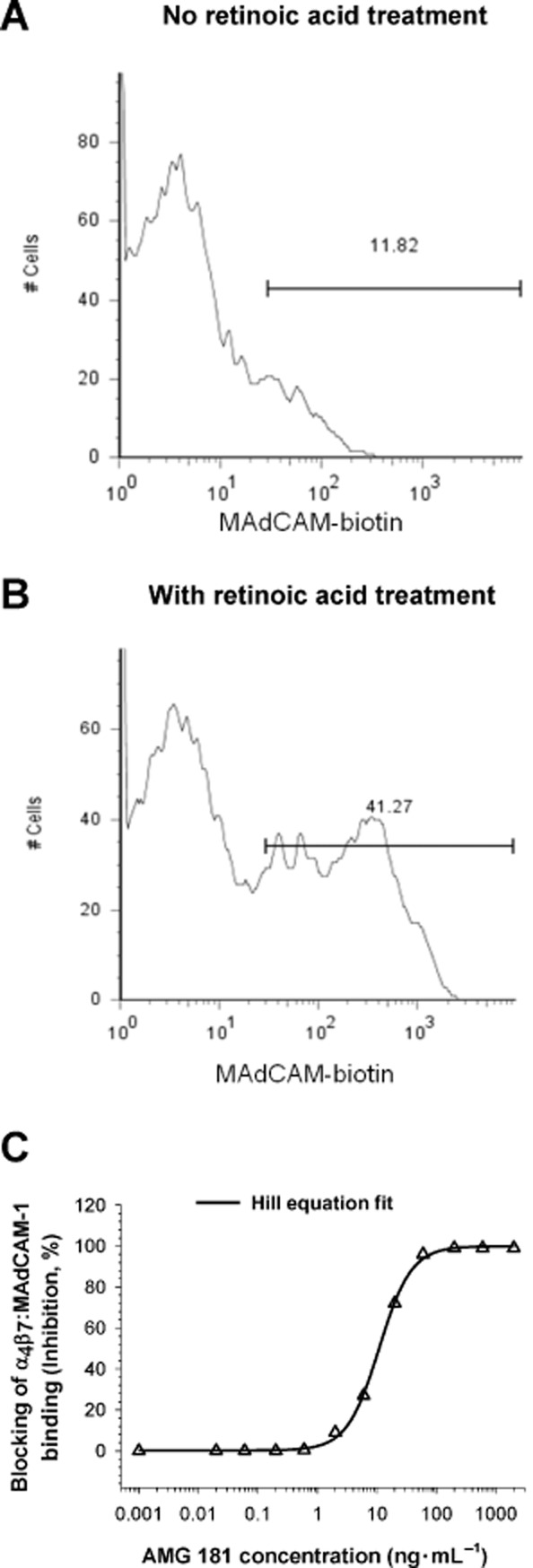
The CD4+CD45RA-memory T cell population was gated to assess MAdCAM-Fc binding in the absence (A) or presence (B) of retinoic acid. X-axis represents fluorescence intensity and the Y-axis is the cell count. AMG 181 blocks MAdCAM-Fc binding to retinoic acid-induced α4β7 on human CD4+CD45RA-memory T cells (C). Symbol represents observations, while line represents the four-parameter Hill equation fitted curve.
Blocking effect on α4β7 : MAdCAM-1-mediated T cell adhesion
AMG 181, but not human IgG2 isotype control, was able to potently inhibit the adhesion of HuT78 cells to MAdCAM-1-coated microtitre plates (Figure 4; Table 3). Moreover, AMG 181 inhibited the adhesion of both human CD4+ and cynomolgus monkey CD4+CD95+ T cells to MAdCAM-1 in a virtually identical and dose-responsive manner (Figure 4; Table 3).
Figure 4.
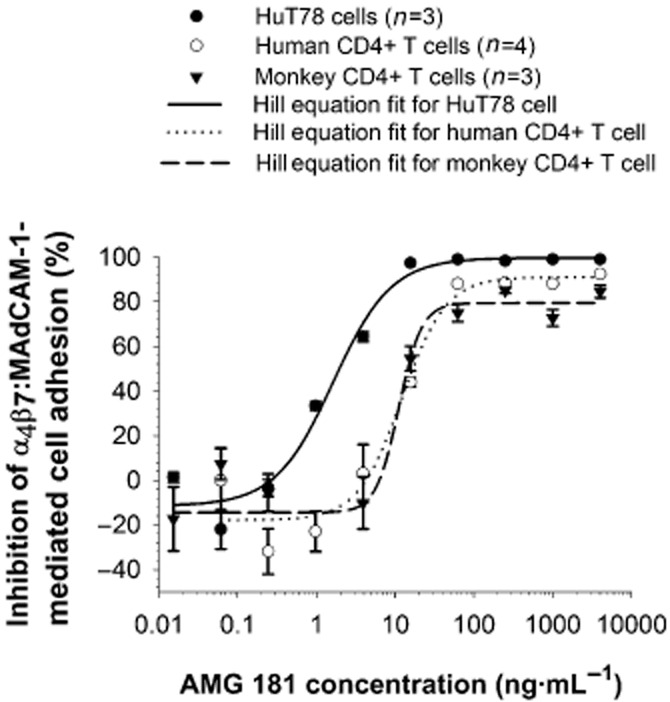
AMG 181 inhibits α4β7 : MAdCAM-1-mediated HuT78 cell, and primary human and cynomolgus monkey T cell adhesion. Symbols represent observations (mean ± SEM), while lines represent the four-parameter Hill equation fitted curves.
No blocking effect on VCAM-1 binding to T cells
We assessed the ability of AMG 181 in blocking T cell binding by VCAM-1, which was reported to bind both α4β1 and α4β7. VCAM-Fc binding to α4β7-transfected HEK 293 T cells was blocked by an anti-α4 antibody but not by AMG 181 (data not shown). In the primary T cell binding assays, an anti-α4 antibody inhibited VCAM-Fc binding to T cells in a dose-dependent manner, while neither AMG 181 nor human IgG had any inhibitory effects (Figure 5A). Similarly, neither AMG 181 nor human IgG inhibited VCAM-1-mediated T cell adhesion, whereas an anti-α4 antibody completely blocked the adhesion (Figure 5B).
Figure 5.
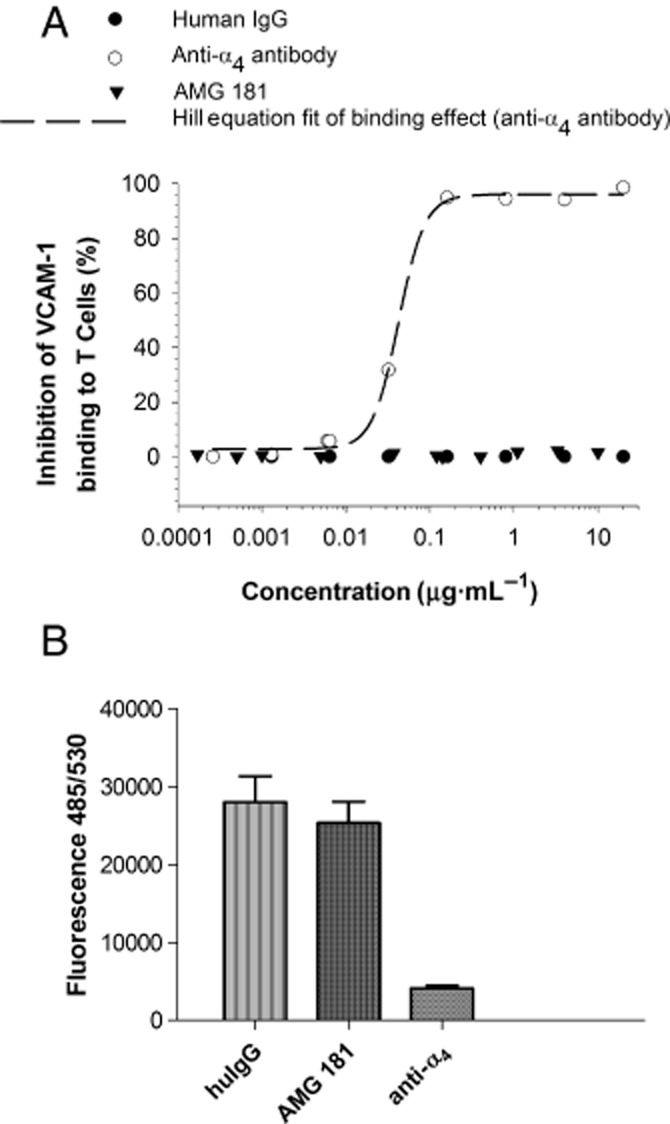
AMG 181 does not block VCAM-1 binding to T cells (A) or VCAM-1-mediated T cell adhesion (B).
Studies in cynomolgus monkeys
PK and TK
In the single-dose studies, individual AMG 181 concentrations peaked at approximately 72–168 h (or 3–7 days) post-dose for the s.c. route and 0.25 h (the first PK sample collection time point) post-dose for the i.v. route (Figure 6A; Table 4). For doses at or under 3 mg·kg−1 i.v. or s.c., the decline of serum AMG 181 concentration was rapid with only one monkey from the 3 mg·kg−1 s.c. group had measurable concentrations 21 days after dosing. For the 9 mg·kg−1 i.v. and s.c. dose cohorts, AMG 181 concentrations were not measurable beyond 28 days due to immunogenicity response.
Figure 6.
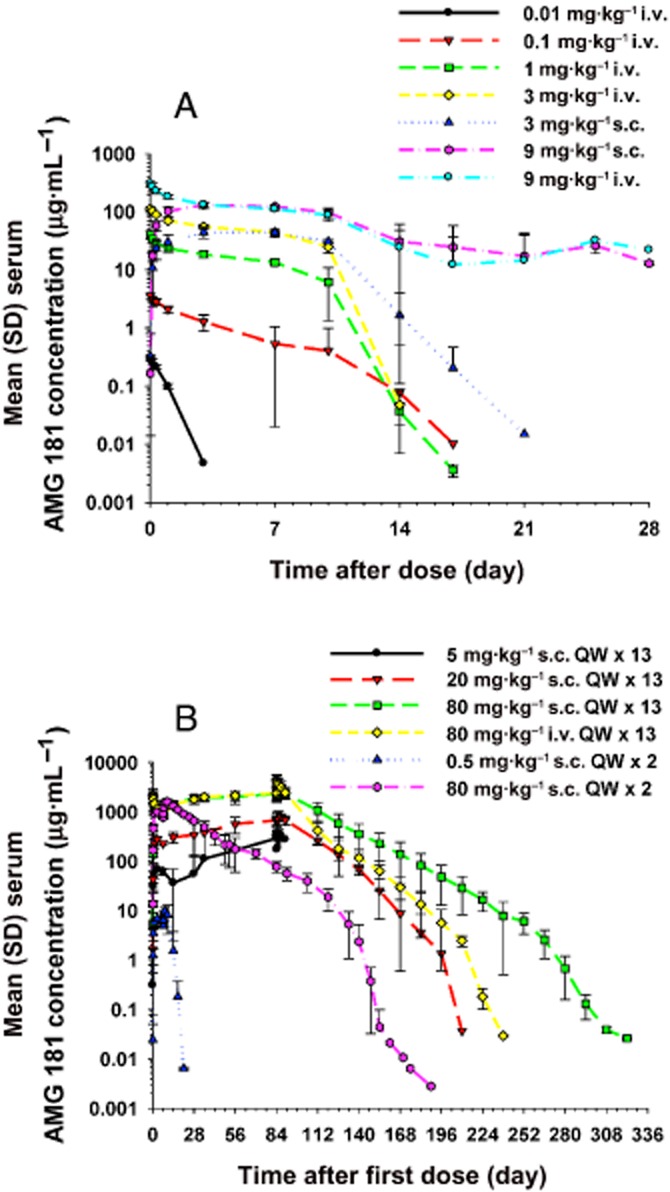
Mean (±SD) serum AMG 181 concentration–time profiles after single (A) or multiple (B) s.c. or i.v. administrations in cynomolgus monkeys.
Table 4.
Mean (SD) pharmacokinetic parameters of AMG 181 after a single i.v. or s.c. administration in male cynomolgus monkeys
| Route | Dose (mg·kg−1) | n | tmax (h) | C0, Cmax (μg·mL−1) | AUC0-t (μg·h·mL−1) | AUC0-inf (μg·h·mL−1) | Vz, Vz/F (mL·kg−1) | CL, CL/F (mL·h−1·kg−1) | Vss (mL·kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| i.v. | 0.01 | 3 | 0.25 (0.25–0.25) | 0.312 (0.0368) | 5.00 (0.782) | 6.29 (0.627) | 30.1 (1.49) | 1.60 (0.151) | 30.5 (1.48) |
| 0.1 | 3 | 0.25 (0.25–0.25) | 3.59 (0.334) | 258 (126) | 262 (123) | 20.6 (10.2) | 0.432 (0.160) | 29.5 (2.23) | |
| 1 | 3 | 0.25 (0.25–0.25) | 40.6 (6.65) | 3 950 (740) | 3 960 (716) | 11.1 (10.9) | 0.259 (0.0516) | 24.9 (2.15) | |
| 3 | 3 | 0.25 (0.25–0.25) | 114 (10.9) | 12 600 (151) | 12 700 (150) | 5.52 (0.673) | 0.237 (0.00280) | 24.6 (1.37) | |
| 9 | 6 | 0.25 (0.25–0.30) | 307 (35.7) | 38 500 (7220) | 39 500 (10 300) | 12.0 (15.4) | 0.238 (0.0524) | 33.7 (8.26) | |
| s.c. | 3 | 3 | 168 (72.0–168) | 47.4 (8.88) | 10 100 (1000) | 10 100 (1000) | 7.44 (2.06) | 0.300 (0.0300) | NC |
| 9 | 6 | 120 (72.0–168) | 134 (15.2) | 37 300 (11 600) | 38 000 (12 600) | 14.7 (11.6) | 0.257 (0.0758) | NC |
All values are reported to three significant figures except for tmax, where values are reported to the second decimal place for values <1.
Cmax, maximum observed concentration for s.c.; C0, extrapolated concentration at time zero for i.v.; tmax, time to Cmax, expressed as median (min–max); AUC0-t, area under the concentration–time curve from time zero to the last observed concentration; AUC0-inf, area under the concentration–time curve from time zero extrapolated to infinity; Vz (i.v.) and Vz/F (s.c.), volume and apparent volume of distribution at the terminal elimination phase, respectively; Vss (i.v.), volume of distribution at steady state; F (s.c.), absolute bioavailability after s.c. administration; CL (i.v.) and CL/F (s.c.), clearance and apparent clearance, respectively, in serum; NC, not calculated.
AMG 181 maximum observed concentration (Cmax) was dose proportional from 0.01 to 9 mg·kg−1 i.v., while area under the concentration–time curve (AUC) increased more than dose proportionally. Cmax was also dose proportional from 3 to 9 mg·kg−1 s.c., while AUC increased more than dose proportionally (Table 4). Bioavailability of AMG 181 was 80 and 95% after a 3 and 9 mg·kg−1 s.c. dose, respectively. The estimated volume of distribution at steady state (Vss) was ∼30 mL·kg−1, indicating its distribution was limited to the vascular (plasma) space (Davies and Morris, 1993).
After once-weekly s.c. or i.v. administration for 2 or 13 weeks, the AMG 181 TK in male and female cynomolgus monkeys was similar within 0.5–80 mg·kg−1. For example, at 5 mg·kg−1 s.c. on day 1, the observed mean AMG 181 Cmax values were 71.5 and 67.8 μg·mL−1 for male and female monkeys, respectively. At 80 mg·kg−1 s.c. on day 85, the observed mean AMG 181 AUC values were 402 000 and 380 000 μg·h·mL−1 for male and female monkeys, respectively. Individual AMG 181 concentrations peaked at approximately 1–168 h (or 0–7 days) post-dose for the s.c. route and 0.25 h (the first TK sample collection time point) post-dose for the i.v. route (Figure 6B; Table 5).
Table 5.
Mean (SD) toxicokinetic parameters of AMG 181 after repeated i.v. or s.c. administrations in male and female cynomolgus monkeys
| Study | Regimen (weekly dosing) | Week | n | tmax (h) | Cmax (μg·mL−1) | AUC0-t (μg·h·mL−1) | AR |
|---|---|---|---|---|---|---|---|
| Two-dose non-GLP | 0.5 mg·kg−1 s.c. | 1 | 8 | 72 (24–72) | 6.92 (0.594) | 962 (88.8) | NC |
| ×2 | 2 | 8 | 72 (24–72) | 10.4 (1.73) | NC | NC | |
| 80 mg·kg−1 s.c. | 1 | 8 | 72 (24–72) | 1010 (60.1) | 143 000 (7 440) | NC | |
| ×2 | 2 | 7–8 | 72 (24–72) | 1610 (117) | 245 000 (23 800) | 1.70 (0.119) | |
| 3 month GLP | 5 mg·kg−1 s.c. | 1 | 12 | 72 (24–168) | 69.6 (5.8) | 10 000 (826) | NC |
| ×13 | 13 | 1 | NC | NC | NC | NC | |
| 20 mg·kg−1 s.c. | 1 | 12 | 72 (24–72) | 279 (34.5) | 39 700 (4 680) | NC | |
| ×13 | 13 | 10 | 24 (8–72) | 821 (164) | 109 000 (19 700) | 2.76 (0.634) | |
| 80 mg·kg−1 s.c. | 1 | 12 | 72 (24–72) | 1090 (72.8) | 158 000 (12 500) | NC | |
| ×13 | 13 | 12 | 24 (8–72) | 2990 (597) | 391 000 (80 500) | 2.48 (0.477) | |
| 80 mg·kg−1 i.v. | 1 | 11 | 0.25 (0.25–3) | 2410 (144) | 205 000 (18 000) | NC | |
| ×13 | 13 | 11 | 0.25 (0.25–24) | 4850 (606) | 485 000 (75 800) | 2.37 (0.405) |
All values are reported to three significant figures except for tmax, where values are reported to two decimal places for values <1.
tmax, time to Cmax after the reference dose 1 or 2 for the two-dose non-GLP study, and dose 1 or 13 for the 3 month GLP study; tmax values presented as median (min–max); Cmax, maximum observed concentration; AUC0-t, area under the concentration–time curve from time zero to the last observed concentration (within each dosing interval during week 1 or week 2 for the two-dose non-GLP study and week 1 or week 13 for the 3 month GLP study; AR, accumulation ratio; AUC0-t, week 2/AUC0-t, week 1 for the two-dose non-GLP study, and AUC0-t, week 13/AUC0-t, week 1 for the 3 month GLP study; NC, not calculated.
In the two-dose non-GLP study, serum AMG 181 concentrations declined rapidly to much lower levels at the terminal phase after two weekly doses of 0.5 mg·kg−1 s.c. In the 3 month GLP study, for the 5 mg·kg−1 s.c. cohort, only one male monkey had sustained measurable AMG 181 concentrations throughout the dosing phase (before day 92), while all 11 other monkeys had no or only sporadic measurable concentrations after the second weekly dose. In contrast, except for one female monkey in the 20 mg·kg−1 s.c. cohort, AMG 181 concentrations were measurable and sustained in the remaining monkeys with 20 mg·kg−1 s.c., 80 mg·kg−1 s.c. or i.v. cohorts throughout the study. We observed dose-proportional exposures within the s.c. dose range of 5–80 mg·kg−1 after the first dose and of 20–80 mg·kg−1 after 13 repeated doses (Table 5). The estimated bioavailability after 80 mg·kg−1 s.c. administration was 77% in week 1 and 81% in week 13. AMG 181 accumulated moderately at approximately two- to threefold after 13 weekly s.c. or i.v. doses.
Using two-compartment PK model with parallel linear and non-linear elimination, the estimated AMG 181 linear elimination half-life was 12 days, with a volume of distribution close to the plasma volume of 129 mL. Detailed PK/PD modelling results have been presented in a forthcoming companion paper (W. J. Pan et al., submitted).
Immunogenicity
Anti-AMG 181 binding antibodies were detected in 0–100 and 25–100% of the monkeys during the dosing and recovery phases, respectively, in a dose and/or dosing frequency-related manner, without i.v. and s.c. differences from 3 to 9 mg·kg−1 single-dose range (Table 6). The rate was lower during the multiple dosing phase at high doses of 20–80 mg·kg−1, and was at least, in part, due to possible drug interference with the assay. During the recovery phase, where AMG 181 concentrations were lower and thus had less interference on the immunoassay, positivity rate was 100%, except for the 80 mg·kg−1 s.c. (positivity rate = 25–75%).
Table 6.
AMG 181 immunogenicity rate in percentage (n animal positive/n animal tested) in male and female cynomolgus monkeys after single or multiple s.c. or i.v. administrations
| Dosing phase | Recovery phase | ||||
|---|---|---|---|---|---|
| Study | Regimen (mg kg−1) | Binding antibodies (%) | Neutralizing antibodies (%) | Binding antibodies (%) | Neutralizing antibodies (%) |
| Single dose | 0.01 i.v. | 100 (3/3) | – | – | – |
| 0.1 i.v. | 100 (3/3) | – | – | – | |
| 1 i.v. | 100 (3/3) | – | – | – | |
| 3 i.v. | 100 (3/3) | – | – | – | |
| 3 s.c. | 100 (3/3) | – | – | – | |
| 9 i.v. | 100 (6/6) | – | – | – | |
| 9 s.c. | 100 (6/6) | – | – | – | |
| All dosed | 100 (27/27) | – | – | ||
| Two-dose non-GLP weekly dosing ×2 | 0.5 s.c. | 100 (8/8) | – | 100 (4/4) | – |
| 80 s.c. | 0 (0/8) | – | 75 (3/4) | – | |
| All dosed | N/A | 88 (7/8) | – | ||
| 3 month GLP weekly dosing ×13 | 5 s.c. | 92 (11/12) | 82 (9/11) | 100 (4/4) | 100 (4/4) |
| 20 s.c. | 75 (9/12) | 22 (2/9) | 100 (4/4) | 75 (3/4) | |
| 80 s.c. | 67 (8/12) | 0 (0/8) | 25 (1/4) | 100 (1/1) | |
| 80 i.v. | 42 (5/12) | 0 (0/5) | 100 (4/4) | 75 (3/4) | |
| All dosed | N/A | N/A | 81 (13/16) | 85 (11/13) | |
–, dose group not part of the assay plan; N/A, not applicable due to possible drug interference with the assay preventing the detection of anti-AMG 181 antibodies in some animals at high doses, making combined low and high dose values unreliable.
In the 3 month GLP study, anti-AMG 181 binding antibody positivity had 0–82 and 75–100% coincidence rate with anti-AMG 181 neutralizing antibody positivity during the dosing and recovery phases, respectively (Table 6). There was no difference between the 80 mg·kg−1 s.c. and i.v. routes of administration during the dosing phase (both at 0% for neutralizing antibodies), but was lower with the s.c. route of administration during the recovery phase. Note that the binding antibody assay could tolerate much higher AMG 181 concentration (100 μg·mL−1) than did the neutralizing antibody assay (230 ng·mL−1). Therefore, the immunogenicity assay results, especially those for the neutralizing antibodies, are much more reliable at the treatment free recovery phase, when AMG 181 concentrations were low.
PD and toxicology
Because α4β7 integrin is predominantly expressed on a subpopulation of gut-homing CD4+ memory T cells in IBD, we focused on reporting the flow cytometry results from monkeys of CD4+ cells, including CD4+ total, naïve, CM and EM cells, with special emphasis on CM cells.
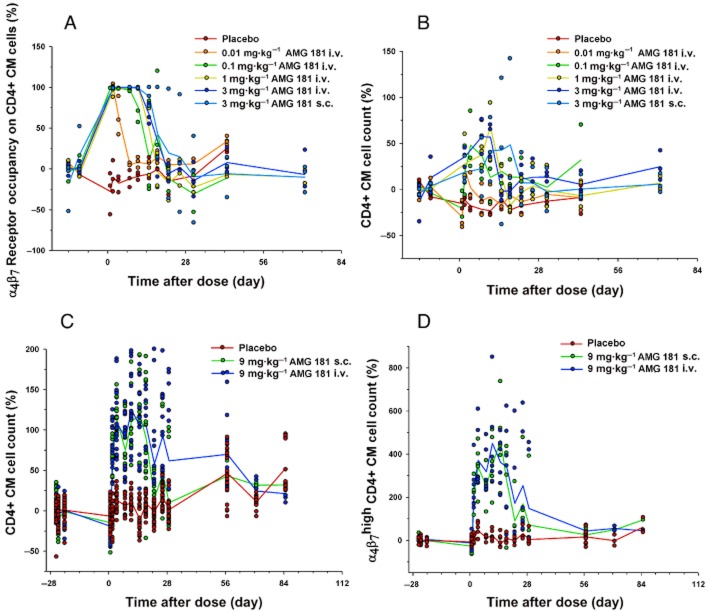
In the first single-dose study, all AMG 181-treated monkeys had ∼100% α4β7 saturation at the first time point measured post-dose. The duration of 100% or partial (50%) α4β7 saturation ranged from 7 to 21 days and increased with increasing AMG 181 dose (Figure 7A). As a pharmacological consequence of α4β7 occupancy, mean CD4+ and CM cell counts were elevated from the first measured post-dose time point to approximately 14–21 days (Figure 7B; total CD4+ cell count, naïve and EM profiles were similar; data not shown). The magnitude and duration of these cell count elevations increased with increasing AMG 181 dose.
Figure 7.
Percent change from pre-dose baseline of CD4+ central memory cell count and α4β7 receptor occupancy after single i.v. or s.c. dose of AMG 181 in cynomolgus monkeys (symbol: individual observation; line: mean): α4β7 receptor occupancy (A); cell count (B and C); α4β7high cell count (D).
In the second single-dose study, all AMG 181-treated monkeys had ∼100% α4β7 saturation at the first time point measured post-dose, with the duration of 100 or 50% α4β7 saturation ranging from 7 to 28 days (data not shown). The mean CD4+ CM cell counts were elevated from the first time point measured post-dose lasting up to approximately 14–28 days (Figure 7C). Moreover, the mean α4β7high CD4+ CM (CD4+CD28+CD95+) T cell counts were elevated to much higher levels and returned to ∼100–200% of the pre-dose baseline on day 29 when mean AMG 181 was 13 ng·mL−1 (Figures 6A and 7D).
In the two-dose non-GLP toxicology study, AMG 181 receptor occupancy increased to ∼100% following administration of AMG 181 for all monkeys during the treatment period. Receptor occupancy generally decreased between days 15–22 in monkeys dosed with 0.5 mg·kg−1 (due to the development of anti-AMG 181 antibodies). The α4β7 receptors continued to be occupied by AMG 181 through the treatment period in monkeys dosed with 80 mg kg−1 and until the end of the treatment free-period (day 197) when receptor occupancy decreased to baseline values together with non-measurable AMG 181 in serum (data not shown).
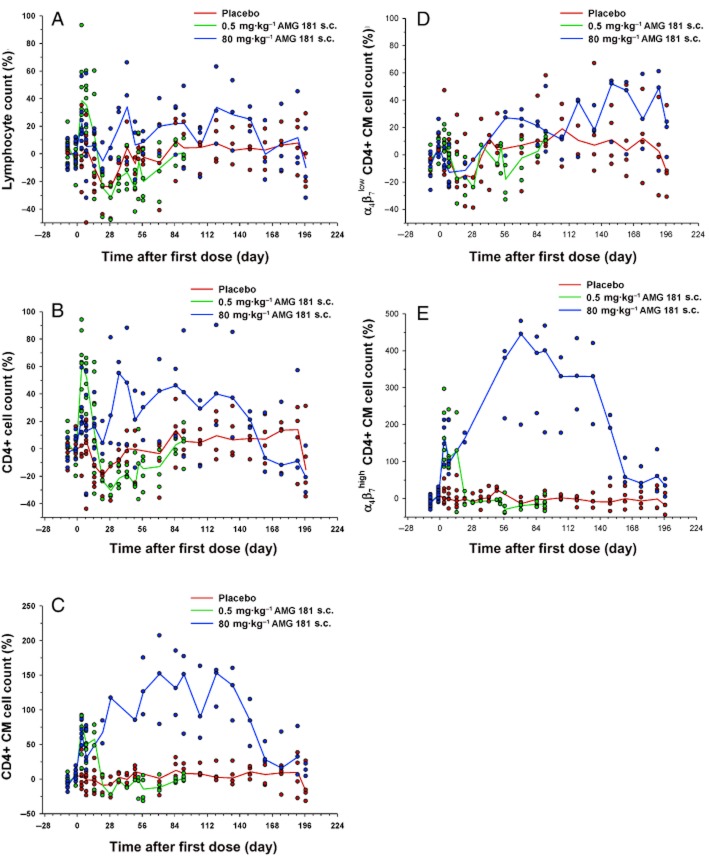
Immunophenotyping results have shown that, except for slight increases during the first week, the total lymphocyte and CD4+ cell counts were generally contained within ±50% of the pre-dose baseline level (Figure 8A,B). For CD4+ CM cells (CD4+CD28+CD95+), an AMG 181 dose-independent increase of >50% on days 4–21 in monkeys dosed with 0.5 mg·kg−1 s.c. and on days 4–162 in monkeys dosed with 80 mg·kg−1 s.c. were observed (Figure 8C). These increases returned to pre-study ranges by day 57 in monkeys dosed with 0.5 mg kg−1 s.c. and by day 162 in monkeys dosed with 80 mg·kg−1 s.c. As expected, α4β7low CD4+CD28+CD95+ CM T cell counts were generally contained within ±50% of the pre-dose baseline (Figure 8D), while those of α4β7high CD4+CD28+CD95+ CM cells were increased by 100–400% (Figure 8E). They returned to the pre-dose baseline at approximately the same time as did the total CD4+ CM cells.
Figure 8.
Percent change from pre-dose baseline after two weekly s.c. doses of AMG 181 in cynomolgus monkeys (symbol: individual observation; line: mean): total lymphocytes (A); total CD4+ cells (B); total CD4+ central memory cells (C); α4β7low CD4+ central memory cells (D); α4β7high CD4+ central memory cells (E).
In the 3 month GLP toxicology study, AMG 181 was well tolerated. There were no test article-related clinical signs or changes in food consumption, body weights, physical examinations, electrocardiograms, ophthalmic examinations, serum chemistry, haematology, coagulation parameters, anti-KLH IgM or IgG response, or anatomical (gross and microscopic) pathology.
Results for target receptor occupancy by AMG 181 as assessed by the percent change from pre-dose baseline of free α4β7 receptor (100% minus receptor occupancy) on the CD4+ CM cells are presented in Figure 9A (note that Figure 7A is presented as receptor occupancy). The mean duration of free α4β7 receptor saturation increased with increasing AMG 181 dose. The 80 mg·kg−1 s.c. dose maintained receptor occupancy for the longest duration until the end of the study. Immunophenotyping results have shown an AMG 181 dose-related increase in CD4+ CM cell counts (Figure 9B). This test article-related change was not considered adverse and was expected based on the mechanism of action of AMG 181. The mean trend for the magnitude and duration of AMG 181 TK exposure, immunogenicity, receptor occupancy and the elevation in CD4+ CM T cell count displayed apparent correlations (Figures 6B and 9A,B; Table 6). anova (dose, time, dose × time interaction) of the data in Figure 9 indicated statistically significant ordinal dose effect (P < 0.0001) for both receptor occupancy and cell count. A contrast of only the s.c. groups' receptor occupancy and cell count least squared means indicated increasing effect with dose (P < 0.0001). The no-observable-adverse-effect-level was determined to be 80 mg·kg−1, the highest evaluated dose (s.c. and i.v.).
Figure 9.
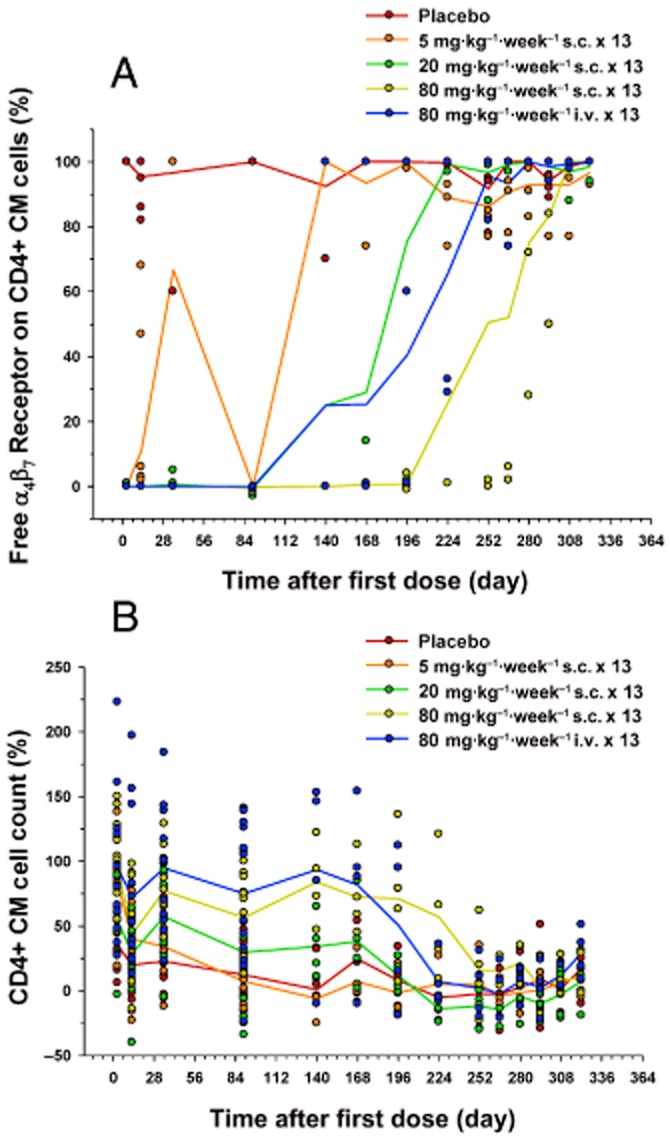
Percent change from pre-dose baseline after 13 weekly s.c. or i.v. doses of AMG 181 in cynomolgus monkeys (symbol: individual observation; line: mean): rate of free α4β7 receptor (100% minus receptor occupancy) on CD4+ central memory cell (A); CD4+ central memory cell count (B).
Tissue cross-reactivity
The Alexa Fluor 488-AMG 181 staining pattern observed in the cynomolgus monkey tissues was similar to that observed in the human tissues. Alexa Fluor 488-AMG 181 stained the membrane and cytoplasm of mononuclear cells primarily observed in submucosal GALT and in the lamina propria of colon, small intestine and stomach. In several other non-lymphoid tissues, test article staining was observed in small mononuclear infiltrates. The α4β7 integrin is known to be expressed on the membrane of mononuclear cells (Brandtzaeg et al., 1999; Rott et al., 2000; Quiding-Jarbrink et al., 2001); therefore, the observed staining of mononuclear cells was expected.
Discussion and conclusions
AMG 181, a human monoclonal IgG2 antibody specifically targeting the gut-homing integrin α4β7, has been generated as a potential therapeutic agent for treating IBD. AMG181 potently blocks MAdCAM-1-mediated T cells adhesion but does not inhibit VCAM-1-mediated T cell adhesion. Because AMG 181 binds specifically to α4β7 heterodimer, it may reduce the risks of perturbing other important immune surveillance pathways mediated through α4β1 or αEβ7.
Natalizumab treatment is associated with PML due to its broad impact on leukocyte trafficking, especially to the CNS, by pan-α4 inhibition of α4β1 expressing cells (Berger and Koralnik, 2005). We believe that AMG 181 will likely provide benefits for treating IBD while reducing the risk of PML compared to pan-α4 inhibition by natalizumab. Etrolizumab inhibits both α4β7 : MAdCAM-1 and αEβ7 : E-cadherin interactions, which may affect immune surveillance both mucosally and systemically, thus increasing the susceptibility to opportunistic infections in gastrointestinal, respiratory and urogenital tracts (GeurtsvanKessel et al., 2008; Soler et al., 2009; Stefanich et al., 2011). Results from a phase 1 study in moderate to severe UC subjects (n = 25) have shown a Mayo score decrease trend in the etrolizumab-treated groups but the trend was not different from that observed in the placebo-treated subjects, two impaired wound healing serious adverse events occurred in two subjects receiving etrolizumab (Rutgeerts et al., 2012). We believe that by not affecting αEβ7, AMG 181 may have better risk/benefit profiles in treating IBD compared to pan-β7 inhibition by etrolizumab.
AMG 181 has similar binding, blocking and inhibitory potencies, as well as tissue cross-reactivity for human and cynomolgus monkey α4β7, supporting the use of cynomolgus monkey as a non-human primate model for in vivo testing before moving into clinical trials. AMG 181 PK is similar between male and female cynomolgus monkeys, with acceptable absorption after s.c. administration, suggesting desired PK characteristics in humans (Pan et al., 2012b).
Even though we have not investigated the mechanism of s.c. absorption of AMG 181, it is likely absorbed into the systemic circulation via convection through the lymphatic vessels and diffusion into the capillaries at the s.c. injection site (Wang et al., 2008). The time of AMG 181 concentration to Cmax (tmax) after s.c. dosing occurred between 3–7 days after single dose and 0–7 days after multiple doses, with bioavailability estimates of 77–95% within the 3–80 mg·kg−1 dose range. These observations are consistent with the reported tmax values of 2–8 days and bioavailability of 50–100% for monoclonal antibodies after s.c. dosing (Wang et al., 2008).
The rapid declines of AMG 181 concentrations at the terminal phase after single dose or lower range repeated dosing (0.5–5 mg·kg−1) may have been due to immunogenicity and α4β7 receptor internalization. Anti-AMG 181 antibody positivity was detected in all AMG 181 treated monkeys on almost all tested days. Target mediated high-affinity low-capacity non-linear disposition of AMG 181 due to binding to cell surface receptor α4β7 and subsequent internalization may have reduced the exposure at the terminal phase when concentrations in serum were lower, especially at lower doses of 0.01–3 mg·kg−1 i.v. At higher doses of 5–80 mg·kg−1 s.c., AMG 181 exposure was dose proportional when immunogenicity impact was minimal (i.e. after the first dose) or when doses were between 20 and 80 mg·kg−1 s.c. AMG 181 linear disposition at high doses (or high concentrations) is mainly due to its predominant elimination, as for a typical IgG, through high capacity neonatal Fc receptors (FcRn) (Wang et al., 2008). Based on comparable AMG 181 TK/PK profiles after repeated 80 mg·kg−1 s.c. versus i.v. doses and after a single 3 mg·kg−1 s.c. versus i.v. dose, we conclude that AMG 181 is not more immunogenic under s.c. versus i.v. administration.
The immunogenicity against protein therapies such as monoclonal antibodies is difficult to predict and can be influenced by many factors, such as type of protein (human vs. non-human), construct and glycosylation, animal species, individual genetic background, formulation and contaminants, route of administration, dosing frequency and treatment duration, to name but a few (Schellekens, 2002; Barbosa and Celis, 2007; De Groot and Scott, 2007). In our studies conducted in monkeys, the higher AMG 181 dose (e.g. ≥20 mg·kg−1) reduced the incidence of detectable anti-AMG 181 antibodies, leading to higher exposure and more pronounced PD effects. The high immunogenicity incidence in the tested cynomolgus monkeys was not surprising because AMG 181 is a human antibody and should be immunogenic in non-human species. In fact, inversely dose-dependent increase in immunogenicity was also observed with humanized anti-α4β7 antibodies in cynomolgus monkeys (Fedyk et al., 2012) and in IBD subjects (Feagan et al., 2005; 2008).
The development of binding antibodies, and especially of persistent neutralizing antibodies, led to increased AMG 181 clearance and reduced PD response. However, neither binding nor neutralizing antibodies to AMG 181 have been associated with any adverse effects in the tested monkeys. These results can help guide AMG 181 clinical development from both safety and efficacy perspectives. Although potential immunogenicity in humans is not likely to cause adverse events, it could lead to reduced AMG 181 exposure, weakened PD response and less optimal therapeutic outcome. Preliminary data from the ongoing single-dose clinical study conducted in healthy and UC subjects have shown lack of AMG 181 immunogenicity in humans (Pan et al., 2012b). It is not surprising that positive immunogenicity results in animals have not translated into humans. We believe that human antibodies such as AMG 181 have competitive advantage versus humanized antibody such as vedolizumab. Phase 3 studies in UC subjects have shown a combined transient and persistent immunogenicity positive rate of 20% among those treated with vedolizumab in the induction phase and re-randomized into placebo treatment in the maintenance phase (Feagan et al., 2012).
Both the magnitude and the duration of the target CD4+ CM cell elevation induced by AMG 181 in cynomolgus monkeys were comparable to those reported with vedolizumab (Fedyk et al., 2012), etrolizumab (Stefanich et al., 2011) and PF-00547659 (Pullen et al., 2009). Similar to the reports for vedolizumab in cynomolgus monkeys (Fedyk et al., 2012), no other changes in haematology or anti-KLH (IgM or IgG) responses were observed with AMG 181, except for the targeted CD4+ cell count elevations. In contrast, PF-00547659 led to 100–180% maximum increases in total lymphocyte, total B, T and monocyte counts (Pullen et al., 2009); data for etrolizumab were not available. Consistent with the data reported for PF-00547659 (Pullen et al., 2009), AMG 181 PD effects were comparable among naïve, CM and EM T cells.
The AMG 181 effects on various T cell count elevations in cynomolgus monkeys (Pan et al., 2012a) were not observed in healthy volunteers (single dose of 0.7 mg s.c. to 420 mg i.v.) (Pan et al., 2012b), consistent with the results reported for vedolizumab (Feagan et al., 2005; 2008; Fedyk et al., 2012) and etrolizumab (Stefanich et al., 2011; Rutgeerts et al., 2012; Williams et al., 2012). A plausible explanation is that down-modulation of target cell surface receptors occurred in humans but not in cynomolgus monkeys (Williams et al., 2012). In ex vivo studies using human peripheral blood, vedolizumab and etrolizumab led to ∼50 and ∼60–70% receptor internalization respectively (Williams et al., 2012; Yang et al., 2012). An exception to these results is the observed CD4+ CM cell increases in both cynomolgus monkeys (Pullen et al., 2009) and subjects with CD (Vermeire et al., 2011) under PF-00547659 treatment. This is not surprising as PF-00547659 binds to MAdCAM ligand and, thus, does not involve target receptor internalization. Treatment with the pan-α4 inhibitor natalizumab in CD subjects led to moderate and sustained elevations in absolute circulating lymphocyte count and was reversible upon cessation of treatment (Sandborn et al., 2005).
The roles of antagonism against α4β7 (Hesterberg et al., 1996; Fedyk et al., 2012; Pan et al., 2012a), β7 (Wagner et al., 1996; Apostolaki et al., 2008; Stefanich et al., 2011) and MAdCAM-1 (Picarella et al., ; Pullen et al., 2009) in lymphocyte gut-homing have been demonstrated in various pre-clinical animal models. Several IBD treatments targeting leukocyte migration and adhesion are in clinical development (Thomas and Baumgart, 2012), including vedolizumab (anti-α4β7), etrolizumab (anti-β7), PF-00547659 (anti-MAdCAM), GSK-1605786 (anti-CCR9) and AJM300 (anti-α4), with natalizumab approved in the US for treating CD. We believe that AMG 181, a human antibody specifically targeting the gut-homing α4β7 heterodimer, has the potential for providing IBD patients with better treatment outcomes and fewer side effects due to its pharmacological potency, lack of immunogenicity and gut-specificity. AMG 181 has in vitro pharmacology, as well as PK/PD and safety characteristics in cynomolgus monkeys that support its advancement into clinical development.
Acknowledgments
This work and the studies presented herein were financially funded by Amgen Inc., Thousand Oaks, CA, USA. The authors would like to thank Amgen Therapeutic Discovery, Inflammatory Research, Medical Sciences, Pharmacokinetics and Drug Metabolism (PKDM), and Comparative Biology and Safety Sciences (CBSS) colleagues and Amgen contractors for their kind assistance in the antibody generation, production, and purification, the conduct of the studies, as well as data management and processing. The authors would also like to thank Jon Nilsen, PhD, Amgen Inc., for medical writing and Kathleen Köck, PhD, and Virginia M. Platt, PhD, for review and graph editing supports.
Glossary
- CD
Crohn's disease or cluster of differentiation
- CM
central memory
- Cmax
maximum observed concentration
- ECL
electrochemiluminescence
- EM
effector memory T cell
- FACS
fluorescence-activated cell sorter
- GALT
gut-associated lymphoid tissue
- GLP
good laboratory practice
- IBD
inflammatory bowel diseases
- JC virus
John Cunningham polyomavirus
- KLH
keyhole limpet haemocyanin
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- NIH
National Institutes of Health
- PBMC
peripheral blood mononuclear cells
- PD
pharmacodynamic(s)
- PK
pharmacokinetic(s)
- PML
progressive multifocal leukoencephalopathy
- SNP
single nucleotide polymorphism
- TK
toxicokinetic(s)
- UC
ulcerative colitis
- VCAM-1
vascular cell adhesion molecule-1
Conflict of interest
The authors are current or former employees of Amgen Inc., except for J. L. Lynch, who is an employee of Charles River Laboratories International, Inc.
References
- Animal and Plant Health Inspection Service USA. Animal Welfare Act and Animal Welfare Regulations. Washington, DC: Agriculture USDo; 2005. [Google Scholar]
- Apostolaki M, Manoloukos M, Roulis M, Wurbel MA, Muller W, Papadakis KA, et al. Role of beta7 integrin and the chemokine/chemokine receptor pair CCL25/CCR9 in modeled TNF-dependent Crohn's disease. Gastroenterology. 2008;134:2025–2035. doi: 10.1053/j.gastro.2008.02.085. [DOI] [PubMed] [Google Scholar]
- Barbosa MD, Celis E. Immunogenicity of protein therapeutics and the interplay between tolerance and antibody responses. Drug Discov Today. 2007;12:674–681. doi: 10.1016/j.drudis.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab–unforeseen consequences. N Engl J Med. 2005;353:414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Farstad IN, Haraldsen G. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol Today. 1999;20:267–277. doi: 10.1016/s0167-5699(99)01468-1. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Colombel J-F. 2012a. Vedolizumab induction therapy for Crohn's disease: results of Gemini II, a randomized, placebo-controlled, double-blind, multicenter Phase 3 trial Vol. 2012, 10/23/2012 edn. Available at: http://uegw.congress-online.com/guest/IDb95633430a0d39/AbstractView?ABSID=2971 (accessed 11/05/2012). United European Gastroenterology.
- Colombel J-F. 2012b. Vedolizumab maintenance therapy for Crohn's disease: results of Gemini II, a randomized, placebo-controlled, double-blind, multicenter Phase 3 trial Vol. 2012, 10/23/2012 edn. Available at: http://uegw.congress-online.com/guest/IDb95633430a0d39/AbstractView?ABSID=2972 (accessed 11/05/2012). United European Gastroenterology.
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–490. doi: 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Erle DJ, Brown T, Christian D, Aris R. Lung epithelial lining fluid T cell subsets defined by distinct patterns of beta 7 and beta 1 integrin expression. Am J Respir Cell Mol Biol. 1994;10:237–244. doi: 10.1165/ajrcmb.10.3.7509610. [DOI] [PubMed] [Google Scholar]
- FDA. The Nonclinical Laboratory Studies Good Laboratory Practice Regulations. Silver Spring, MD: United States Health and Human Services Food and Drug Administration; 1979. [Google Scholar]
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, et al. Treatment of active Crohn's disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–1377. doi: 10.1016/j.cgh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Rutgeerts PJ, Sands BE, Sandborn WJ, Colombel J-F, Hanauer SB, et al. Induction therapy for ulcerative colitis: results of GEMINI I, a randomized, placebo-controlled, double-blind, multicenter phase 3 trial. Gastroenterology. 2012;142:S-160–S-161. [Google Scholar]
- Fedyk ER, Wyant T, Yang LL, Csizmadia V, Burke K, Yang H, et al. Exclusive antagonism of the alpha(4) beta(7) integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012;18:2107–2119. doi: 10.1002/ibd.22940. [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg PE, Winsor-Hines D, Briskin MJ, Soler-Ferran D, Merrill C, Mackay CR, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996;111:1373–1380. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- Hsu H, Foltz I, Arora T, Jacobsen FW. Alpha-4 Beta-7 Heterodimer Specific (US 2010/0254975 A1) A1. Alexandria, VA: Office USP; 2010. [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- MHLW. The Japanese Good Laboratory Practice Standards for Safety Studies on Drugs Pharmaceutical Affairs. Tokyo, Japan: Bureau the Ministry of Health Labour and Welfare; 1997. [Google Scholar]
- National Research Council (U.S.), Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the Care and Use of Laboratory Animals. 8th edn. Washington, DC: National Academies Press; 2011. [Google Scholar]
- NIH. 2010. Safety, tolerability, pharmacokinetics and pharmacodynamics of AMG 181 in healthy subjects and subjects with mild to moderate ulcerative colitis ( NCT01164904) Vol. 2012, 1/27/2011 edn. Available at: http://clinicaltrials.gov/ct2/show/NCT01164904 (accessed 11/05/2012). US National Institute of Health.
- NIH. 2011. Study to evaluate safety, tolerability, pharmacokinetics and pharmacodynamics of AMG 181 ( NCT01290042) Vol. 2012, 1/27/2011 edn. Available at: http://clinicaltrials.gov/ct2/show/NCT01290042?intr=%22Amg+181%22&rank=1 (accessed 11/05/2012). US National Institutes of Health.
- NIH. 2012a. A randomized, rouble-blind, multiple dose placebo-controlled study to evaluate the safety, tolerability, and efficacy of AMG 181 in subjects with moderate to severe ulcerative colitis ( NCT01694485) Vol. 2012, 9/24/2012 edn. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01694485?term=AMG+181&rank=4 (accessed 11/05/2012). US National Institutes of Health.
- NIH. 2012b. A randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, and efficacy of AMG 181 in subjects with moderate to severe Crohn's disease ( NCT01696396) Vol. 2012, 9/27/2012 edn. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01696396?term=AMG+181&rank=3 (accessed 11/05/2012). US National Institutes of Health.
- OECD. Principles on Good Laboratory Practice. Paris, France: The Organisation for Economic Cooperation and Development; 1997. [Google Scholar]
- Pan WJ, Lear SP, Patel SK, Prince PJ, Doherty DR, Tam CY, et al. Pharmacokinetics and pharmacodynamics in cynomolgus monkeys of AMG 181, a fully human anti-α4β7 antibody for treating inflammatory bowel disease. J Crohn's Colitis. 2012a;6(S1):pS14. [Google Scholar]
- Pan WJ, Sullivan BA, Evangelista CM, Doherty DR, Tam CYJ, Patel SK, et al. Pharmacokinetics (PK), pharmacodynamics (PD), and safety of AMG181, a fully human anti-α4β7 antibody for treating inflammatory bowel diseases (IBD) Gastroenterology. 2012b;142:S–565. [Google Scholar]
- Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, et al. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18:1470–1479. doi: 10.1002/ibd.21896. [DOI] [PubMed] [Google Scholar]
- Parker CM, Cepek KL, Russell GJ, Shaw SK, Posnett DN, Schwarting R, et al. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1924–1928. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–2106. [PubMed] [Google Scholar]
- Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Pullen N, Molloy E, Carter D, Syntin P, Clemo F, Finco-Kent D, et al. Pharmacological characterization of PF-00547659, an anti-human MAdCAM monoclonal antibody. Br J Pharmacol. 2009;157:281–293. doi: 10.1111/j.1476-5381.2009.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiding-Jarbrink M, Ahlstedt I, Lindholm C, Johansson EL, Lonroth H. Homing commitment of lymphocytes activated in the human gastric and intestinal mucosa. Gut. 2001;49:519–525. doi: 10.1136/gut.49.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathanaswami P, Roalstad S, Roskos L, Su QJ, Lackie S, Babcook J. Demonstration of an in vivo generated sub-picomolar affinity fully human monoclonal antibody to interleukin-8. Biochem Biophys Res Commun. 2005;334:1004–1013. doi: 10.1016/j.bbrc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Rathanaswami P, Babcook J, Gallo M. High-affinity binding measurements of antibodies to cell-surface-expressed antigens. Anal Biochem. 2008;373:52–60. doi: 10.1016/j.ab.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Rott LS, Briskin MJ, Butcher EC. Expression of alpha4beta7 and E-selectin ligand by circulating memory B cells: implications for targeted trafficking to mucosal and systemic sites. J Leukoc Biol. 2000;68:807–814. [PubMed] [Google Scholar]
- Rutgeerts P. 2012. Vedolizumab (VDZ) maintenance therapy for ulcerative colitis (UC): results of Gemini I, a randomized, placebo-controlled, double-blind, multicenter Phase 3 trial Vol. 2012, 10/23/2012 edn. Available at: https://uegw.congress-online.com/guest/ID0b44f5640a0f50/AbstractView?ABSID=1085 (accessed 11/05/2012). United European Gastroenterology.
- Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B, et al. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut. 2012 doi: 10.1136/gutjnl-2011-301769. 2012 Jan 20 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24:1720–1740. doi: 10.1016/s0149-2918(02)80075-3. discussion 1719. [DOI] [PubMed] [Google Scholar]
- Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. doi: 10.1124/jpet.109.153973. [DOI] [PubMed] [Google Scholar]
- Stefanich EG, Danilenko DM, Wang H, O'Byrne S, Erickson R, Gelzleichter T, et al. A humanized monoclonal antibody targeting the beta7 integrin selectively blocks intestinal homing of T lymphocytes. Br J Pharmacol. 2011;162:1855–1870. doi: 10.1111/j.1476-5381.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, et al. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672–1683. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Thomas S, Baumgart DC. Targeting leukocyte migration and adhesion in Crohn's disease and ulcerative colitis. Inflammopharmacology. 2012;20:1–18. doi: 10.1007/s10787-011-0104-6. [DOI] [PubMed] [Google Scholar]
- Vermeire S, Ghosh S, Panes J, Dahlerup JF, Luegering A, Sirotiakova J, et al. The mucosal addressin cell adhesion molecule antibody PF-00547,659 in ulcerative colitis: a randomised study. Gut. 2011;60:1068–1075. doi: 10.1136/gut.2010.226548. [DOI] [PubMed] [Google Scholar]
- Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- Williams M, Klabunde S, Fuh F, Johnson CF, Looney C, Howell K, et al. Etrolizumab-mediated ex vivo modulation of beta7 cell surface receptors in nonhuman primate and human peripheral blood supports the proposed mechanism of action. Gastroenterology. 2012;142:S–383. [Google Scholar]
- Yang L-L, Fedyk ER, Wyant T. Internalization of the vedolizumab/α4β7 complex and kinetics of restoring functional activity. J. Crohn's Colitis. 2012;6(S1):S18. [Google Scholar]
- Yonekawa K, Harlan JM. Targeting leukocyte integrins in human diseases. J Leukoc Biol. 2005;77:129–140. doi: 10.1189/jlb.0804460. [DOI] [PubMed] [Google Scholar]
- Zhang WV, Yang Y, Berg RW, Leung E, Krissansen GW. The small GTP-binding proteins Rho and Rac induce T cell adhesion to the mucosal addressin MAdCAM-1 in a hierarchical fashion. Eur J Immunol. 1999;29:2875–2885. doi: 10.1002/(SICI)1521-4141(199909)29:09<2875::AID-IMMU2875>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zuckier LS, Berkowitz EZ, Sattenberg RJ, Zhao QH, Deng HF, Scharff MD. Influence of affinity and antigen density on antibody localization in a modifiable tumor targeting model. Cancer Res. 2000;60:7008–7013. [PubMed] [Google Scholar]