Abstract
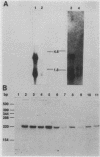
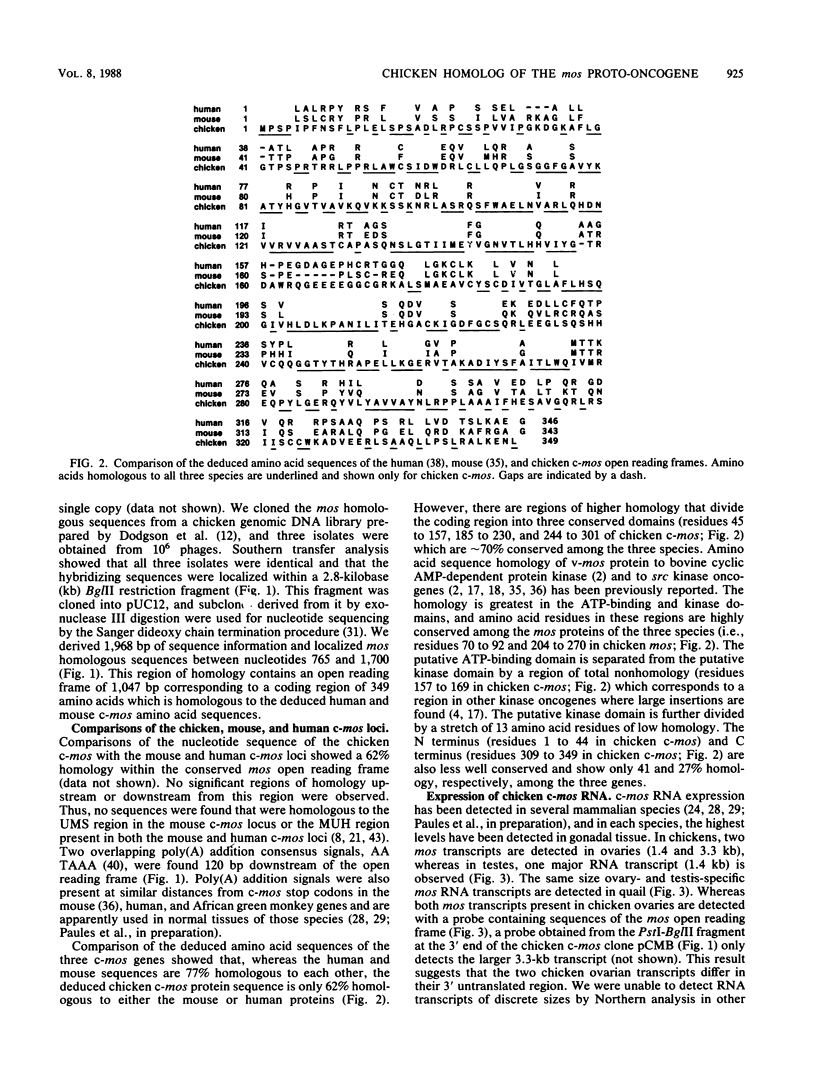
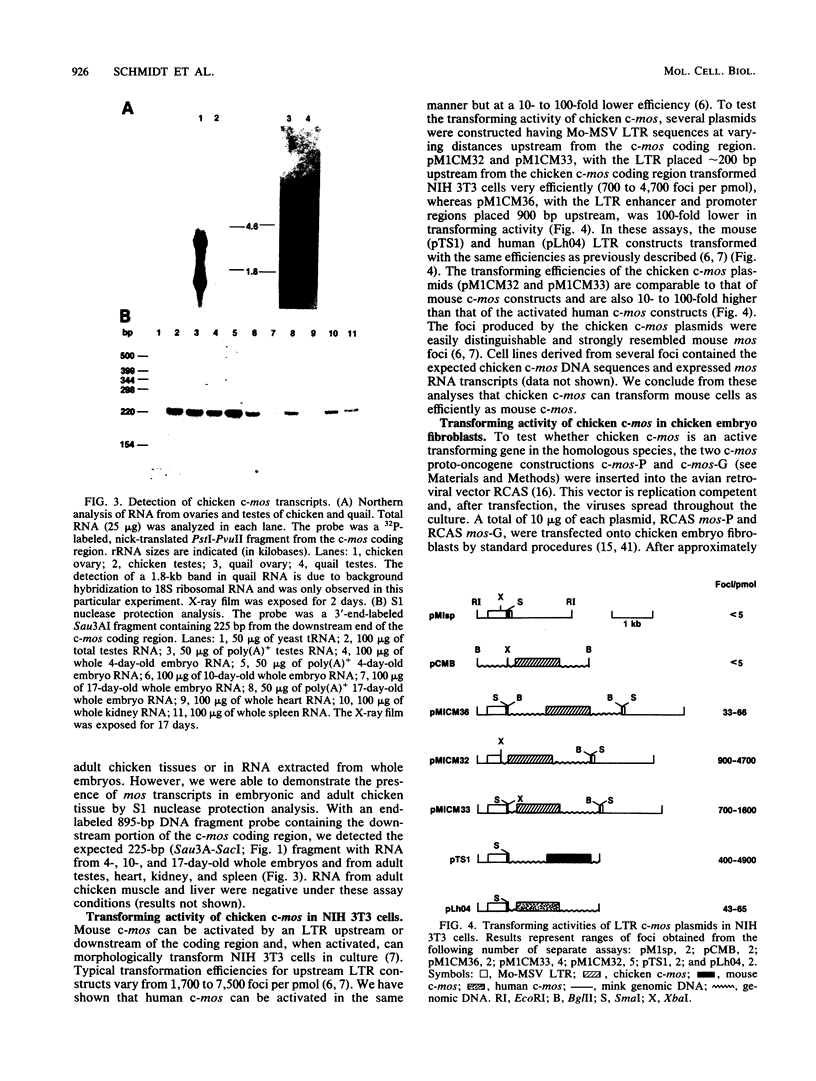
We compared the sequence and properties of the chicken mos homolog with the previously characterized mouse and human c-mos genes. Sequence analysis revealed one major open reading frame of 1,047 base pairs encoding a protein of 349 amino acids. Both the nucleotide sequence and the deduced amino acid sequence showed 62% overall homology to mouse and human c-mos, but regions of higher conservation (approximately 70%) occurred in the putative ATP-binding and kinase domains. We detected mos transcripts by Northern (RNA) analyses in RNA prepared from chicken and quail ovaries and testes. Evidence for low levels of mos RNA expression in adult chicken heart, kidney, and spleen and in the entire embryo was obtained by S1 nuclease protection experiments. In contrast to the low transforming efficiency of human c-mos when linked to a mouse retroviral long terminal repeat element, chicken c-mos transformed NIH 3T3 cells as efficiently as mouse c-mos did. We also show that chicken primary embryo fibroblasts were morphologically altered when infected with an avian retroviral vector containing the chicken c-mos coding region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Barker W. C., Dayhoff M. O. Viral src gene products are related to the catalytic chain of mammalian cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 May;79(9):2836–2839. doi: 10.1073/pnas.79.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Blair D. G., McClements W. L., Oskarsson M. K., Fischinger P. J., Vande Woude G. F. Biological activity of cloned Moloney sarcoma virus DNA: Terminally redundant sequences may enhance transformation efficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3504–3508. doi: 10.1073/pnas.77.6.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Oskarsson M. K., Seth A., Dunn K. J., Dean M., Zweig M., Tainsky M. A., Vande Woude G. F. Analysis of the transforming potential of the human homolog of mos. Cell. 1986 Aug 29;46(5):785–794. doi: 10.1016/0092-8674(86)90354-5. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Oskarsson M., Wood T. G., McClements W. L., Fischinger P. J., Vande Woude G. G. Activation of the transforming potential of a normal cell sequence: a molecular model for oncogenesis. Science. 1981 May 22;212(4497):941–943. doi: 10.1126/science.7233190. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Frankel A. E., Fischinger P. J. Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3705–3709. doi: 10.1073/pnas.73.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. S., Kiessling A. A., Millette C. F., Cooper G. M. Expression of c-mos RNA in germ cells of male and female mice. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4509–4513. doi: 10.1073/pnas.84.13.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Kosik E. Mutagenesis of the region between env and src of the SR-A strain of Rous sarcoma virus for the purpose of constructing helper-independent vectors. Virology. 1984 Jul 15;136(1):89–99. doi: 10.1016/0042-6822(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- McGeady M. L., Wood T. G., Maizel J. V., Vande Woude G. F. Sequences upstream from the mouse c-mos oncogene may function as a transcription termination signal. DNA. 1986 Aug;5(4):289–298. doi: 10.1089/dna.1986.5.289. [DOI] [PubMed] [Google Scholar]
- Moloney J. B. A virus-induced rhabdomyosarcoma of mice. Natl Cancer Inst Monogr. 1966 Sep;22:139–142. [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Mutter G. L., Wolgemuth D. J. Distinct developmental patterns of c-mos protooncogene expression in female and male mouse germ cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5301–5305. doi: 10.1073/pnas.84.15.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W. Nonrandom DNA sequencing of exonuclease III-deleted complementary DNA. Anal Biochem. 1985 Jan;144(1):207–211. doi: 10.1016/0003-2697(85)90107-1. [DOI] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Nigg E. A., Hunter T. The transforming protein of Moloney murine sarcoma virus is a soluble cytoplasmic protein. Cell. 1983 May;33(1):161–172. doi: 10.1016/0092-8674(83)90345-8. [DOI] [PubMed] [Google Scholar]
- Propst F., Rosenberg M. P., Iyer A., Kaul K., Vande Woude G. F. c-mos proto-oncogene RNA transcripts in mouse tissues: structural features, developmental regulation, and localization in specific cell types. Mol Cell Biol. 1987 May;7(5):1629–1637. doi: 10.1128/mcb.7.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst F., Vande Woude G. F. Expression of c-mos proto-oncogene transcripts in mouse tissues. Nature. 1985 Jun 6;315(6019):516–518. doi: 10.1038/315516a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Vande Woude G. F. Nucleotide sequence and biochemical activities of the Moloney murine sarcoma virus strain HT-1 mos gene. J Virol. 1985 Oct;56(1):144–152. doi: 10.1128/jvi.56.1.144-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Taylor W. R. Modelling the ATP-binding site of oncogene products, the epidermal growth factor receptor and related proteins. FEBS Lett. 1984 Oct 1;175(2):387–392. doi: 10.1016/0014-5793(84)80774-7. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Galleshaw J. A., Jonas V., Berns A. J., Doolittle R. F., Donoghue D. J., Verma I. M. Nucleotide sequence and formation of the transforming gene of a mouse sarcoma virus. Nature. 1981 Jan 22;289(5795):258–262. doi: 10.1038/289258a0. [DOI] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., McClements W. L., Enquist L. W., Blair D. G., Fischinger P. J., Maizel J. V., Sullivan M. Characterization of integrated Moloney Sarcoma proviruses and flanking host sequences cloned in bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):735–745. doi: 10.1101/sqb.1980.044.01.079. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Watson R., Oskarsson M., Vande Woude G. F. Human DNA sequence homologous to the transforming gene (mos) of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4078–4082. doi: 10.1073/pnas.79.13.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. G., McGeady M. L., Baroudy B. M., Blair D. G., Vande Woude G. F. Mouse c-mos oncogene activation is prevented by upstream sequences. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7817–7821. doi: 10.1073/pnas.81.24.7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]