Abstract
Background and Purpose
IL-33 signals through ST2 receptors and induces adaptive and innate inflammation. IL-33/ST2 is involved in adaptive inflammation-induced pain. Here, we have investigated the contribution of IL-33/ST2-triggered mechanisms to carrageenin-induced innate inflammation.
Experimental Approach
Carrageenin- and IL–33-induced inflammatory responses were assessed in BALB/c- (WT) and ST2-deficient (−/−) mice as follows: oedema (plethysmometer), myeloperoxidase activity (colorimetric assay), mechanical hyperalgesia (electronic version of von Frey filaments), cytokine levels (ELISA), PGE2 (RIA), mRNA expression (quantitative PCR), drug treatments targeting leukocyte recruitment (fucoidin), TNF-α (infliximab), CXCL1 (antibody to CXCL1), IL-1 (IL-1ra), endothelin ETA (clazosentan) and ETB (BQ788) receptors and COX (indomethacin).
Key Results
Carrageenin injection increased ST2 and IL-33 mRNA expression and IL-33 production in paw skin samples. Carrageenin-induced paw oedema, hyperalgesia and myeloperoxidase activity were reduced in ST2−/− compared with WT mice, effects mimicked by IL-33 injection in the paw. Furthermore, IL–33-induced hyperalgesia was reduced by fucoidin suggesting a role for recruited leukocytes in its hyperalgesic effect. IL–33-induced hyperalgesia in naïve mice was reduced by treatments targeting TNF, CXCL1, IL-1, endothelin receptors and COX while carrageenin-induced ST2-dependent TNF-α, CXCL1, IL-1β, IL-10 and PGE2 production and preproET-1 mRNA expression. Combining IL-33 and carrageenin at doses that were ineffective as single treatment induced significant hyperalgesia, oedema, myeloperoxidase activity and cytokine production in a ST2-dependent manner.
Conclusions and Implications
IL-33/ST2 signalling triggers the production of inflammatory mediators contributing to carrageenin-induced inflammation. These data reinforces the importance of IL-33/ST2 signalling as a target in innate inflammation and inflammatory pain.
Keywords: IL-33, ST2, carrageenin, hyperalgesia, cytokine, innate inflammation
Introduction
IL-33 is a member of the IL-1 family of cytokines, which signals through the ST2/IL-1RAcP receptor complex (Schmitz et al., 2005; Ali et al., 2007). IL-33 seems to be an important therapeutic target or therapy in inflammatory conditions such as anaphylactic shock, septic shock, atherosclerosis, UV radiation, asthma, hepatitis and rheumatoid arthritis (Schmitz et al., 2005; Verri et al., 2008; 2010; Pushparaj et al., 2009; Alves-Filho et al., 2010; Arshad et al., 2011; Byrne et al., 2011). These roles of IL-33 are related to the activation of endothelial cells, mast cells, lymphocytes, macrophages, eosinophils and neutrophils (Schmitz et al., 2005; Pecaric-Petkovic et al., 2009; Alves-Filho et al., 2010; Verri et al., 2010).
IL-33 was initially recognized as a Th2 cytokine (Schmitz et al., 2005). Further investigation of the biology of IL-33/ST2 signalling showed that it is an important component in Th1/Th17 and innate inflammation. Thus, IL-33 mediates antigen-induced arthritis dependent on TNF-α, IL-1β and IFN-γ (Verri et al., 2008; 2010) and IL-17 production (Xu et al., 2008). IL-33 also amplified Th1 and Th2 responses by acting on basophils, Th2 lymphocytes, iNKT and NK cells (Smithgall et al., 2008). In support of a role of IL-33 in innate inflammation, glia secreted IL-33 in response to LPS (Hudson et al., 2008) and IL-33 administration reduced the systemic inflammatory response induced by bacterial products in a model of sepsis (Alves-Filho et al., 2010). IL-33 also increased expression of LPS receptor components resulting in enhanced cytokine production, suggesting a LPS self-regulatory feedback through IL-33 (Espinassous et al., 2009). Furthermore, IL-33 deficiency resulted in reduced papain-induced lung innate inflammation and dextran-induced colitis accompanied by T–cell-independent epithelial damage (Oboki et al., 2010). Moreover, IL-33 induced cytokine production by type 2 innate immune cells such as natural helper cells, nuocytes, and innate helper 2 cells (Kouzaki et al., 2011; Kim et al., 2012). However, ST2 deficiency increased innate and acquired Th1 and Th2 immunity in response to a murine mammary carcinoma (Jovanovic et al., 2011). Therefore, the functions of IL-33 are not completely predictable.
Carrageenin is an extensively used and accepted model of inflammation. In general, it is injected in the paw to evaluate oedema and hyperalgesia, but it can also be injected in other cavities such as air pouch, peritoneal and pleural cavities to evaluate leukocyte recruitment, mainly of neutrophils, and production of chemotatic mediators (Cunha et al., 2005; Valério et al., 2007). The intraplantar injection of carrageenin induces mechanical hyperalgesia in mice by triggering a cytokine cascade initiated by TNF-α and CXCL1 production, which induce IL–1β-dependent PGE2 production. In turn, PGE2 sensitizes the nociceptor, which can be detected as mechanical hyperalgesia (Cunha et al., 2005). Furthermore, endothelin-1 (ET-1) acting on ETA and ETB receptors mediates carrageenin-induced mechanical hyperalgesia (Baamonde et al., 2004; receptor nomenclature follows Alexander et al., 2011) and ET-1-induced hyperalgesia depends on PGE2 production (Verri et al., 2007). Recruited neutrophils also contribute to carrageenin-induced mechanical hyperalgesia by producing PGE2 (Cunha et al., 2008).
As IL-33 mediates mechanical hyperalgesia (Verri et al., 2008) and neutrophil recruitment (Verri et al., 2010) in adaptive inflammation, and is involved in innate inflammation (Hudson et al., 2008; Espinassous et al., 2009; Alves-Filho et al., 2010; Oboki et al., 2010; Zhang et al., 2011), we have investigated whether IL-33/ST2 contributed to carrageenin-induced innate inflammation and inflammatory pain and the underlying mechanisms. Furthermore, because IL-33 potentiated antigen-induced production of inflammatory cytokines (Andrade et al., 2011), we also assessed whether IL-33 could act in synergy with carrageenin to induce paw inflammation and hyperalgesia.
Methods
Animals
Sex matched BALB/c (WT, ST2+/+) and BALB/c background ST2-deficient (−/−) mice (Brint et al., 2004), 20–25 g were bred in the Faculty of Medicine of Ribeirao Preto, University of Sao Paulo, Brazil. All animal care and experimental procedures complied with the International Association for the Study of Pain guidelines and approved by the Ethics Committee of the Faculty of Medicine of Ribeirao Preto, University of Sao Paulo, Brazil. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 908 animals were used in these experiments.
Electronic pressure meter test
The mechanical hyperalgesia test (Cunha et al., 2004) consisted of evoking a hindpaw flexion reflex with a handheld force transducer (electronic anaesthesiometer; IITC Life Science, Woodland Hills, CA, USA) adapted with a 0.5 mm2 polypropylene tip. The results are expressed as the differential (Δ) withdrawal threshold (in g) calculated by subtracting the mean measurements at indicated time points after stimulus from the zero-time mean measurements. Withdrawal threshold was 8.6 ± 0.5 g (mean ± SEM, n = 40) before injection of the hyperalgesic agents.
Paw oedema test
The volume of the paw was measured with a plethysmometer (Ugo Basil, Comerio, VA, Italy) before (V0) and at indicated time points after (VT) the intraplantar (i.pl.) stimulus with carrageenan (3 or 100 μg diluted in 25 μL of saline per paw) (Valério et al., 2007). The amount of paw swelling was determined for each mouse and the difference between VT and V0 was taken as the oedema value (oedema mm3 per paw).
Myeloperoxidase activity
The myeloperoxidase activity of paw homogenates was used to evaluate the migration of leukocytes to the hind paw skin of mice. It consists of a kinetic colorimetric assay (Bradley et al., 1982) with modifications (Casagrande et al., 2006). The results were presented as the myeloperoxidase activity (number of neutrophils 104 per paw).
Cytokine measurement
Animals were terminally anaesthetized (1.5% isoflurane; Abbott, (Abbott Park, IL, USA) and the plantar skin tissues removed and homogenized in 500 μL of buffer containing protease inhibitors. Concentrations of TNF-α, IL-1β, CXCL1, IL-10 (BD Bioscience, San Jose, CA, USA) and IL-33 (eBioscience, San Diego, CA, USA) were determined by ELISA using paired antibodies.
Real-time PCR
Mice were killed 2 h after i.pl. injection of carrageenin and the plantar cutaneous hind paw tissues harvested. Samples were homogenized in Trizol reagent, and total RNA was extracted using the SV Total RNA Isolation System (Promega Biosciences, Fithburg, WI, USA). Quantitative PCR (qPCR) was performed in an ABI Prism 7500 Sequence Detection System using the SYBR-green fluorescence (Applied Biosystems, Grand Island, NY, USA). The primers were previously described (Verri et al., 2008).
Determination of PGE2 production
Two hours after i.pl. injection of carrageenin, paw skin tissue samples were collected in 0.5 mL of a mixture of acetone: 1 M HCl: water (10:1:5, v : v : v). After homogenizing with a turrax, the samples were centrifuged (20 min at 2000 g at 4°C) and the supernatant decanted before drying the pellet in a centrifugal evaporator at 37°C. The pellet was reconstituted in 500 μL of Tris/HCl buffer (10mM Tris, pH adjusted with HCl to 8.0). The concentrations of PGE2 were determined by RIA (Amersham, Pittsburgh, PA, USA) (Verri et al., 2007).
Experimental protocols
WT mice received intraplantar (i.pl., subcutaneous in the hind paw) injection of carrageenin (100 μg per paw) or saline. Samples of the paw skin were collected at 2 h for qPCR analysis for ST2, IL-33 and preproET-1 mRNA expression. IL-33 levels were determined 0.5, 1, 3 and 5 h after carrageenin by ELISA. Mechanical hyperalgesia, oedema and myeloperoxidase activity were evaluated at indicated time points after carrageenin (3 or 100 μg·per paw) or IL-33 (3, 30, 100 and 300 ng·per paw) injection in WT and ST2−/− mice. In other experiments, WT mice were treated with fucoidin (20 mg·kg−1, i.v, 15 min), infliximab (anti-TNF-α antibody, 10 mg·kg−1, i.p, 48 h and 60 min before stimuli), anti-CXCL1 antibody (αCXCL1; 700 ng·mice−1, i.p. injection, in saline, 30 min before stimulus), control IgG (same treatment protocol as for infliximab or αCXCL1) or IL-1 receptor antagonist ( IL-1ra; 30 mg·kg−1, i.v., 15 min) before IL-33 administration (100 or 300 ng·per paw) followed by evaluation of mechanical hyperalgesia and myeloperoxidase at indicated time points. TNF-α, CXCL1, IL-1β, IL-10 and PGE2 levels in paw skin samples were determined by ELISA or RIA at 2 h or indicated time points after carrageenin injection. In the last series of experiments, mice were treated with clazozentan (10 mg·kg−1, s.c., 15 min), BQ 788 (30 nmol per paw, 30 min) or indomethacin (5 mg·kg−1, i.p., 40 min) before i.pl. injection of IL-33 (100 ng· per paw) or saline. Mechanical hyperalgesia was evaluated 3 and 5 h after. To determine the synergism between IL-33 and carrageenin, mice received single treatments with carrageenin (3 μg per paw) or IL-33 (3 ng·per paw) or a combination of both followed by mechanical hyperalgesia, oedema and MPO activity determination at indicated time points. The same protocol was used to determine the production of TNF-α and IL-1β in WT (ST2+/+) and ST2−/− mice. The doses of reagents used in vivo were chosen based on previous reports (Cunha et al., 2005; 2008; Verri et al., 2006; 2008; 2009; 2010; Pinto et al., 2010). Drug treatments or genetic deficiency did not affect the baseline response of the mice to mechanical stimulation (data not shown).
Data analyses
Results are presented as means ± SEM; each experiment was performed at least twice. Two-way anova was used to compare the groups and doses at all times when the parameters were measured at different times after the stimulus injection. The analysed factors were treatments, time and time versus treatment interaction. One-way anova followed by Bonferroni's t-test was performed for each time. Comparison of two groups was performed using t-test. P < 0.05 was considered significant.
Materials
The following materials were obtained from the sources indicated: anti-CXCL1 antibody (Peprotech, Rocky Hill, NJ, USA), isotype control IgG (R&D Systems, Minneapolis, MN, USA), BQ 788 (Tocris Bioscience, Ellisville, MO, USA), carrageenin (FMC Corporation (Philadelphia, PA, USA), clazosentan (Actelion Pharmaceutical Ltd., Allschwil/Basel, Switzerland), fucoidin (Sigma Chemical, St Louis, MO, USA), IL-1ra (National Institute of Biological Standards and Control, Hertfordshire, UK), indomethacin (Prodome, Campinas, Brazil), infliximab (Remicade, Merck & Co., Whitehouse Station, NJ, USA). Recombinant human IL-33 was generated as previously described (Komai-Koma et al., 2007).
Results
Carrageenin induces IL-33 and ST2 mRNA expression and IL-33 production in the paw skin
Carrageenin (100 μg·per paw) or saline (25 μL) was injected in WT mice and after 2 h paw skin samples were collected for qPCR analysis. Carrageenin induced a significant increase of ST2 (Figure 1A) and IL-33 (Figure 1B) mRNA expression. In agreement, carrageenin induced a significant increase of IL-33 production after 0.5–5 h (Figure 1C).
Figure 1.
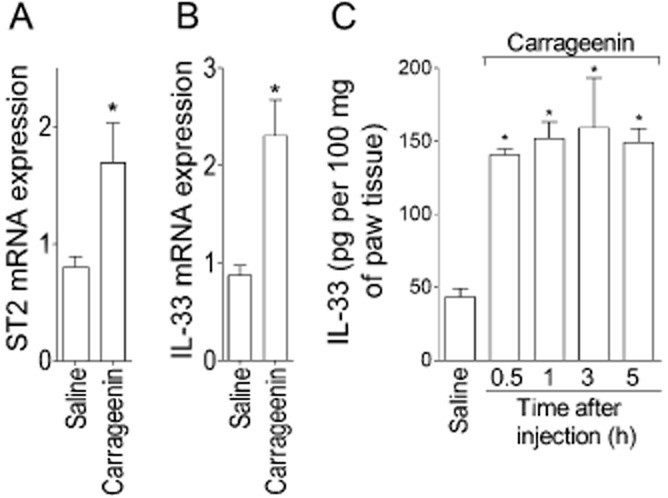
Carrageenin induces expression of mRNA for IL-33 and ST2 and IL-33 production in the paw skin. Mice received intraplantar (i.pl.) injection of carrageenin (100 μg per paw) or saline (25 μL), and after 2 h samples of paw skin were collected for quantitative (q) PCR analysis of ST2 (panel A) and IL-33 (panel B) mRNA expression or between 0.5 and 5 h for IL-33 determination by ELISA assay (panel C). n = 6 for qPCR and n = 4 for ELISA, representative of two separate experiments. *P < 0.05 compared with the saline group.
Reduction of carrageenin-induced mechanical hyperalgesia, oedema and neutrophil recruitment in ST2−/− mice
Carrageenin (100 μg per paw) or saline was injected in WT and ST2−/− mice, and mechanical hyperalgesia (Figure 2A) and oedema (Figure 2B) were evaluated 1–5 h after carrageenin. At 5 h, mice were killed and myeloperoxidase activity was determined in paw skin samples (Figure 2C). Carrageenin-induced mechanical hyperalgesia between 1 and 5 h, and oedema between 0.5 and 5 h in WT mice compared with vehicle group (Figure 2A and B, respectively). On the other hand, ST2−/− mice presented reduced hyperalgesia and oedema at the same time points (Figure 2A and B, respectively). The myeloperoxidase activity was increased by carrageenin injection in WT mice compared with the saline group and this increase was reduced in ST2−/− mice (Figure 2C).
Figure 2.
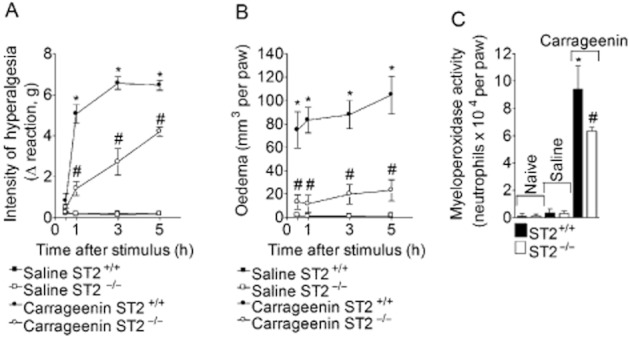
IL-33/ST2 mediates carrageenin-induced paw inflammation. WT ( ST2+/+) and ST2−/− mice received i.pl. injection of carrageenin (100 μg·per paw) or saline. Mechanical hyperalgesia (panel A) and oedema (panel B) were determined at indicated time points followed by myeloperoxidase assay at 5 h (panel C) in paw skin samples. n = 5, representative of two separate experiments. *P < 0.05 compared with the saline group and #P < 0.05 compared with the carrageenin group.
IL-33 injection mimics carrageenin-induced inflammatory responses in a ST2-dependent manner in naïve mice
Mice received IL-33 (30–300 ng·per paw) and mechanical hyperalgesia (Figure 3A) and oedema (Figure 3B) were evaluated after 0.5–5 h. At 5 h samples of paw skin tissue were collected for myeloperoxidase activity determination (Figure 3C). The dose of 30 ng·per paw of IL–33-induced mechanical hyperalgesia between 1 and 5 h compared with vehicle group (Figure 3A), and the doses of 100 and 300 ng·per paw of IL-33 induced significant hyperalgesia compared with vehicle and the dose of 30 ng·per paw between 0.5 and 5 h (Figure 3A). IL-33 induced significant paw oedema between 0.5 and 3 h, only at 100 ng·per paw (Figure 3B). At 5 h, IL-33 induced a dose-dependent increase of myeloperoxidase activity, compared with levels after vehicle only (Figure 3C). In ST2−/− mice, IL-33 did not induce mechanical hyperalgesia, oedema or myeloperoxidase activity (Figure 3D–F). Thus, the injection of IL-33 mimicked carrageenin-induced inflammatory responses in WT mice, although the oedematogenic effect of IL-33, given i.pl., was clearly less than that of carrageenin. This effect was not further investigated, except for the synergism experiments presented in Figure 10.
Figure 3.
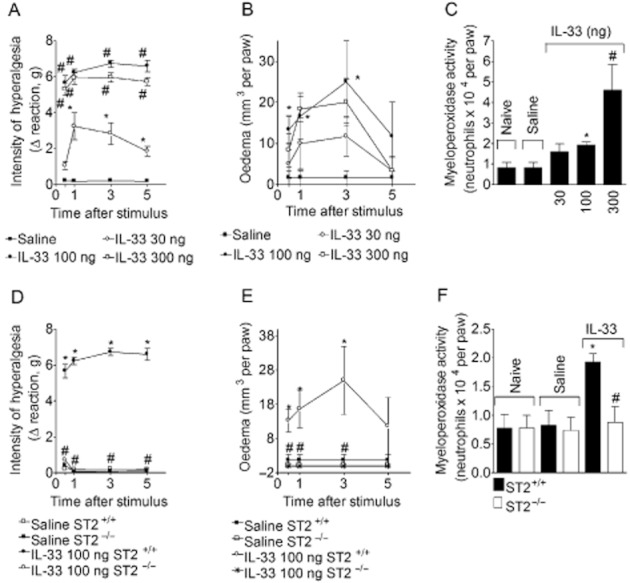
IL-33 induces hyperalgesia, oedema and neutrophil migration in a ST2-dependent manner in normal mice. IL-33 (30–300 ng) or saline (25 μL) was injected i.pl. in WT (ST2+/+) mice. Mechanical hyperalgesia (panel A) and oedema (panel B) were determined at indicated time points followed by myeloperoxidase activity determination at 5 h (panel C) in paw skin samples. IL-33 (100 ng) or saline was injected i.pl. in WT and ST2−/− mice. Mechanical hyperalgesia (panel D) and oedema (panel E) were determined at indicated time points followed by myeloperoxidase activity determination at 5 h (panel F). n = 6, representative of two separate experiments. *P < 0.05 compared with the saline group and #P < 0.05 compared with the lower dose of IL-33 or IL-33 injection in ST2+/+ mice.
Figure 10.
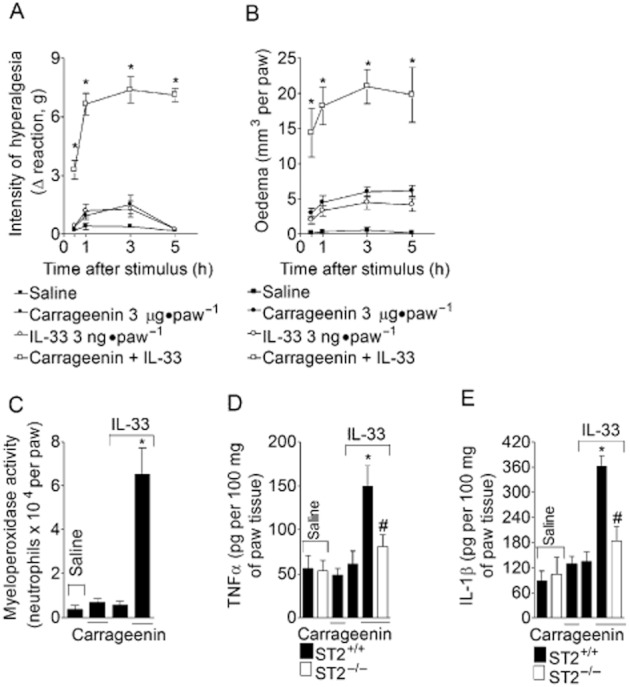
IL-33 synergizes with carrageenin to induce inflammation, hyperalgesia and cytokine production. Mice received i.pl. injection of IL-33 (3 ng·per paw), carrageenin (3 μg·per paw) or IL-33 plus carrageenin (same doses). Mechanical hyperalgesia (panel A) and oedema (panel B) were determined at indicated time points followed by myeloperoxidase activity determination at 5 h (panel C) in paw skin samples. TNF-α (panel D) and IL-1β (panel E) levels were determined by ELISA. n = 6 for hyperalgesia, oedema and myeloperoxidase activity, n = 4 for ELISA, representative of two separate experiments. *P < 0.05 compared with the saline, carrageenin or IL-33 in ST2+/+ group and #P < 0.05 compared with the carrageenin plus IL-33 in ST2+/+ mice group.
Effect of fucoidin in IL–33-induced mechanical hyperalgesia in naïve mice
WT mice were treated with fucoidin (which binds to L-selectin and inhibits leukocyte recruitment; 20 mg·kg−1, i.v., 15 min) before IL-33 injection (100 or 300 ng·per paw), and mechanical hyperalgesia was evaluated at 3 and 5 h and myeloperoxidase activity at 5 h (Figure 4). Two doses of IL-33 were used because 100 and 300 ng achieved maximal mechanical hyperalgesia whereas 300 ng·was needed to produce maximal myeloperoxidase activity in the paw skin. Treatment with fucoidin inhibited the mechanical hyperalgesia at 3 and 5 h (Figure 4A and C) and myeloperoxidase activity at 5 h (Figure 4B and D) induced by 100 ng·(Figure 4A and B) and 300 ng·(Figure 4C and D) of IL-33.
Figure 4.
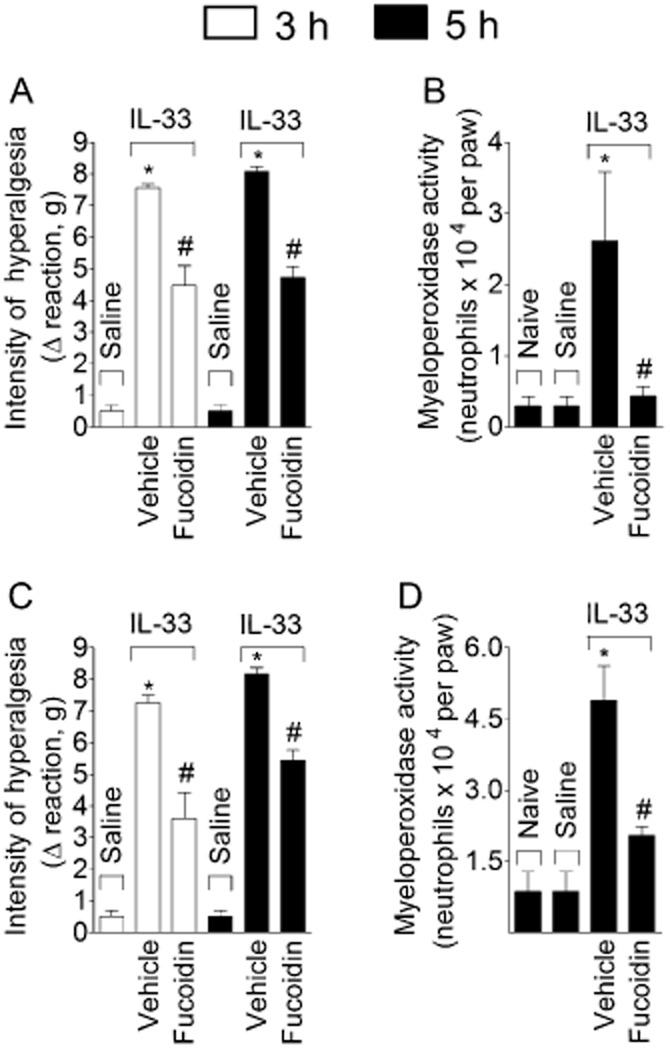
IL–33-induced hyperalgesia depends on neutrophil recruitment. IL-33 (100 ng – panels A and B or 300 ng – panels C and D) or saline was injected i.pl. and mechanical hyperalgesia (panel A and C) was determined at indicated time points followed by myeloperoxidase activity determination at 5 h (panel B and D) in paw skin samples. n = 6, representative of two separate experiments. *P < 0.05 compared with the saline or naive group and #P < 0.05 compared with the IL-33 vehicle group.
IL–33-induced mechanical hyperalgesia depends on TNF-α, CXCL1 and IL-1β in naïve mice
Mice were treated with infliximab (anti–TNF-α antibody, 10 mg·kg−1, i.p. 48 h and 60 min before stimuli injection, Figure 5A and D), anti-CXCL1 antibody (αCXCL1, 700 ng·per paw, co-injection, Figure 5B and E) or IL-1ra (30 mg·kg−1, i.v., 15 min, Figure 5A and D) before IL-33 injection (100 ng·per paw) and mechanical hyperalgesia was evaluated at 3 h (Figure 5A–C) and 5 h (Figure 5D–F). All treatments inhibited IL–33-induced mechanical hyperalgesia (Figure 5). Control IgG antibody (αcontrol) was used under the same treatment protocols as for infliximab (Figure 5A and D) or αCXCL1 (Figure 5B and E).
Figure 5.
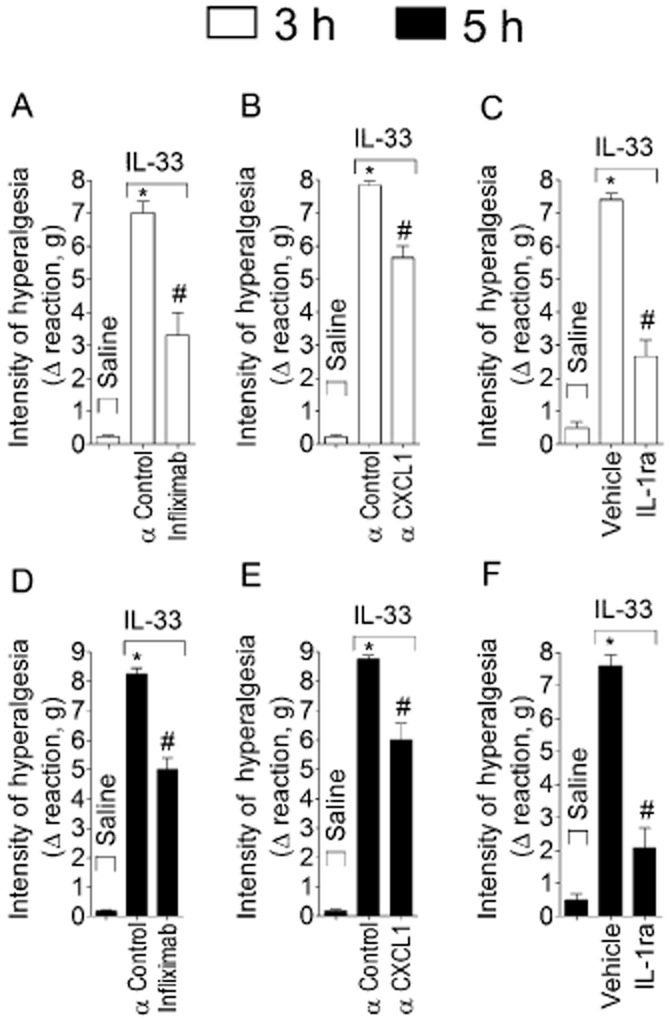
IL–33-induced mechanical hyperalgesia depends on TNF-α, CXCL1 and IL-1β. Mice were treated with infliximab (anti-TNF-α antibody, 10 mg·kg−1, i.p. 48 h and 60 min before stimuli injection; panels A and D), anti-CXCL1 antibody (αCXCL1, 700 ng·per paw, co-injection; panels B and E), isotype IgG control antibody (αcontrol, same treatment protocol as for infliximab or αCXCL1) or IL-1 receptor antagonist (IL-1ra, 30 mg·kg−1, i.v., 15 min) (panel C and F) before IL-33 i.pl. injection (100 ng·per paw). Mechanical hyperalgesia was measured 3h (panels A, B and C) and 5 h (panels D, E and F) after IL-33 injection. n = 6, representative of two separate experiments. *P < 0.05 compared with saline group and #P < 0.05 compared with IL-33 group.
Carrageenin-induced production of TNF-α, CXCL1, IL-1β and IL-10 is decreased in ST2 deficient mice
Carrageenin (100 μg·per paw) or saline was injected in WT and ST2−/− mice and paw skin samples were collected after 2 h for assay of TNF-α, CXCL1 and IL-1. Carrageenin induced significant production of TNF-α (Figure 6A), CXCL1 (Figure 6B) and IL-1β (Figure 6C) in WT mice compared with the vehicle treatment, and these effects were inhibited in ST2−/− mice. Carrageenin also induced significant production of IL-10 at 0.5, 1, 3 and 5 h in WT mice and levels of this cytokine were also reduced in ST2−/− mice at 3 and 5 h after carrageenin injection (Figure 7).
Figure 6.
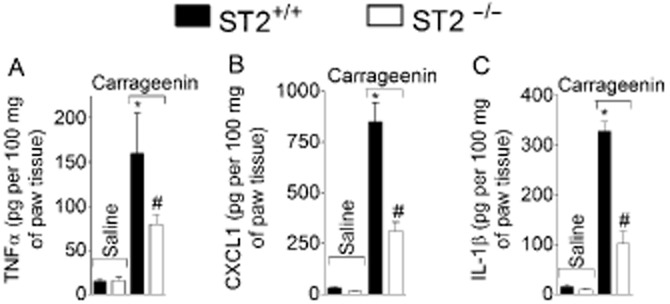
Carrageenin induced ST2-dependent production of TNF-α, CXCL1 and IL-1β in WT (ST+/+) mice. Carrageenin (100 μg) was injected i.pl. and after 2 h samples of cutaneous plantar tissue were collected for determination of TNF-α (panel A), CXCL1 (panel B), and IL-1β (panel C) levels by ELISA. n = 4, representative of two separate experiments. *P < 0.05 compared with saline group and #P < 0.05 compared with carrageenin ST2+/+ group.
Figure 7.
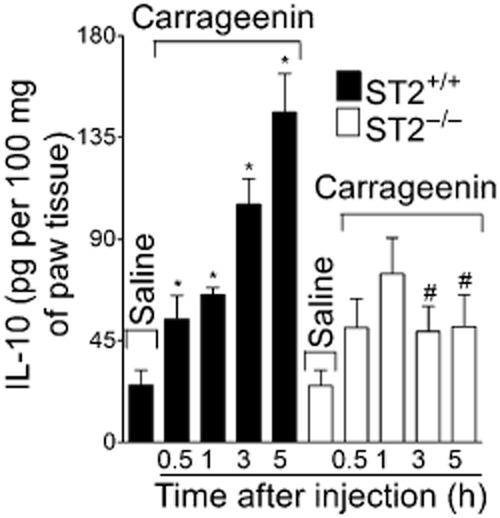
Carrageenin induced ST2-dependent production of IL-10 in mice. Carrageenin (100 μg) was injected i.pl. and after 0.5–5 h samples of cutaneous plantar tissue were collected for determination of IL-10 levels determination by ELISA. n = 4, representative of two separate experiments. *P < 0.05 compared with saline group and #P < 0.05 compared with carrageenin ST2+/+ group.
ET-1 mediates IL–33-induced mechanical hyperalgesia and mRNA expression in naïve mice
Mice were treated with clazosentan (ETA receptor antagonist, 10 mg·kg−1, s.c., 30 min) or BQ788 (ETB receptor antagonist, 30 nmol·per paw, 30 min) before IL-33 (100 ng per paw) injection (Figure 8A and B). Both clazosentan and BQ788 inhibited IL–33-induced mechanical hyperalgesia at 3 h (Figure 8A and C, respectively) and 5 h (Figure 8B and D). In agreement, carrageenin alone increased expression of mRNA for preproET-1 at 2 h after carrageenin injection in WT mice compared with the vehicle group, and this increase was lacking in ST2−/− mice (Figure 8E).
Figure 8.
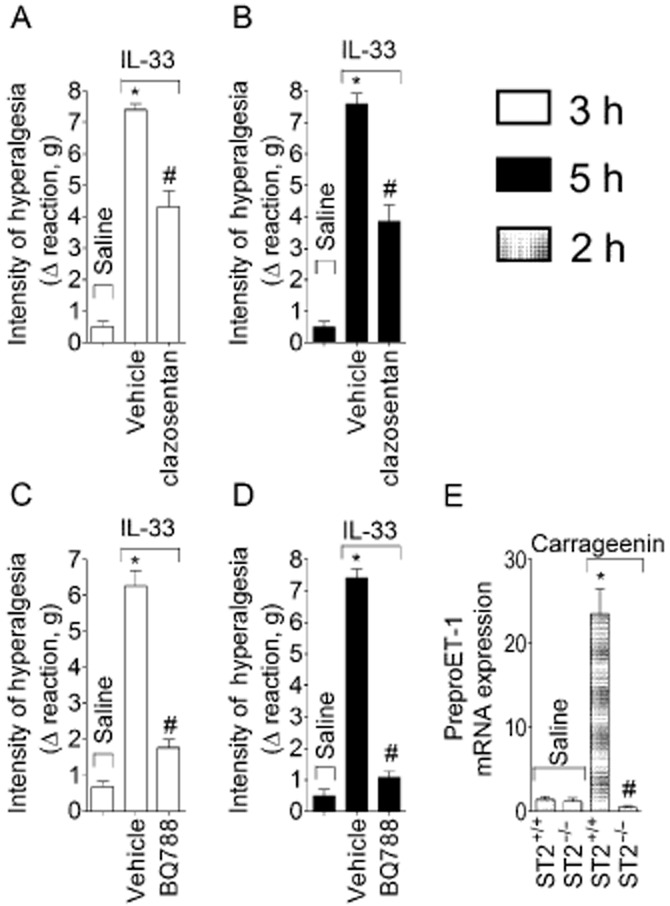
IL-33/ST2 mediates carrageenin-induced hyperalgesia via ET-1 acting on ETA and ETB receptors. Mice were treated with clazosentan ( ETA receptor antagonist, 10 mg·kg−1, 30 min, panels A and B) or BQ 788 (ETB receptor antagonist, 30 nmol·per paw, 30 min, panels C and D) before IL-33 (100 ng·per paw) injection. Mechanical hyperalgesia was measured 3 and 5 h after IL-33 injection. Carrageenin (100 μg) or saline was injected i.pl. in ST2+/+ and ST2−/− mice and samples of cutaneous tissue were collected after 2 h for qPCR analysis of preproET-1 mRNA expression (panel E). n = 6, representative of two separate experiments. *P < 0.05 compared with saline group and #P < 0.05 compared with IL-33 or carrageenin ST2+/+ group.
PGE2 mediates IL–33-induced mechanical hyperalgesia in WT mice
Mice were treated with indomethacin (COX inhibitor, 5 mg·kg−1, i.p.) 40 min before IL-33 injection (100 ng·per paw). As a result, IL–33-induced mechanical hyperalgesia was reduced at 3 h (Figure 9A) and 5 h (Figure 9B). Carrageenin also induced PGE2 production in WT mice and this effect was absent in ST2−/− mice (Figure 9C).
Figure 9.
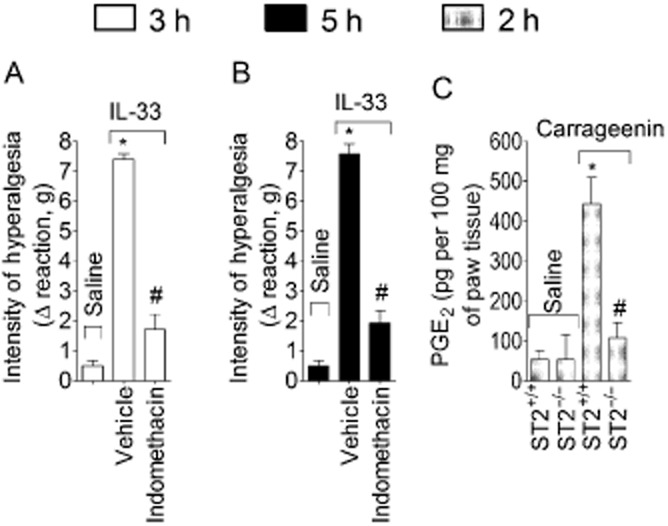
IL-33/ST2 mediates carrageenin-induced hyperalgesia via PGE2. Mice were treated with indomethacin (COX inhibitor, 5 mg·kg−1), 40 min, panels A and B) before IL-33 (100 ng·per paw) injection. Mechanical hyperalgesia was measured 3h (panel A) and 5 h (panel B) after IL-33 injection. Carrageenin (100 μg) or saline was injected i.pl. in ST2+/+ and ST2−/− mice and samples of cutaneous tissue were collected after 2 h for PGE2 levels determination by RIA (panel C). n = 6, representative of two separated experiments. *P < 0.05 compared with the saline group and #P < 0.05 compared with the IL-33 or carrageenin ST2+/+ group.
IL-33 synergizes with carrageenin to induce paw inflammation and hyperalgesia
Mice received i.pl. injections of carrageenin (3 μg·per paw) alone, IL-33 (3 ng per paw) alone or a combination of IL-33 and carrageenan, in the same low doses. IL-33 and carrageenin given alone now did not induce significant mechanical hyperalgesia (Figure 10A) or oedema (Figure 10B) at 1, 3 and 5 h, or myeloperoxidase activity at 5 h. On the other hand, injection of IL-33 plus carrageenin induced significant mechanical hyperalgesia (Figure 10A), oedema (Figure 10B) and myeloperoxidase activity (Figure 10C). Furthermore, the combination of IL-33 and carrageenin also induced synergy in the production of TNF-α (Figure 10D) and IL-1β (Figure 10E), which was ST2-dependent (Figure 10D and E).
Discussion and conclusions
Despite the initial description of IL-33 as a Th2 cytokine, it has become evident that it is, rather, a pleiotropic cytokine mediating Th1, Th2, Th17 and innate inflammatory responses (Schmitz et al., 2005; Komai-Koma et al., 2007; Smithgall et al., 2008; Verri et al., 2008; Xu et al., 2008; Alves-Filho et al., 2010; Oboki et al., 2010). We have previously demonstrated the hyperalgesic role of IL-33/ST2 in a model of antigen-induced arthritis in mice (Verri et al., 2008). Therefore, we hypothesised that IL-33/ST2 signalling would also be important in innate inflammation-induced hyperalgesia. The carrageenin model of innate inflammation was chosen due to its wide applicability in the study of novel analgesic and anti-inflammatory drugs. The present data demonstrate that IL-33/ST2 contributes to carrageenin-induced inflammation consisting of oedema, myeloperoxidase activity and mechanical hyperalgesia by triggering the production of other cytokines (TNF-α, CXCL1 and IL-1β), along with ET-1 and PGE2, and also reducing the production of the anti-hyperalgesic cytokine IL-10. Furthermore, we demonstrated that injection of both IL-33 and carrageenin at doses that did not cause responses alone, induced mechanical hyperalgesia, oedema, increase of myeloperoxidase activity and cytokine production, suggesting a synergism in inducing inflammation between carrageenin-and IL-33.
IL-33 has a role in the activation of mast cells, glia cells, macrophages, epithelial and endothelial cells (Schmitz et al., 2005; Hudson et al., 2008; Moussion et al., 2008; Bourgeois et al., 2009; Kurowska-Stolarska et al., 2009; Nelson et al., 2011; Zhang et al., 2011), resulting in innate inflammation. The present results add to the earlier data demonstrating that IL-33/ST2 contributes to carrageenin-induced innate inflammation, which is itself dependent on the activation of resident tissue cells and recruitment of leukocytes.
It is interesting to note that there is rapid release of IL-33 upon stimulation of endothelial and epithelial cells (Moussion et al., 2008). This finding, together with its actions as a cytokine and an intracellular nuclear factor show similarities with the alarmin HMGB-1 and raise the possibility that IL-33 could be a novel alarmin (Carriere et al., 2007; Moussion et al., 2008; Byrne et al., 2011). Compatible with a fast and early release of IL-33 in innate inflammation, this cytokine was produced in significant amounts upon carrageenin stimulus within 30 min, and production of IL-33 continued at significant levels, at least, up to 5 h. There was also induction of IL-33 and ST2 mRNA expression, which indicated a continuous stimulation of IL-33 expression and not only its release and/or activation. Similar levels of IL-33 and/or ST2 mRNA (Savinko et al., 2012; Schmieder et al., 2012) and IL-33 production (Alves-Filho et al., 2010) have been found in a range of conditions. This early production of IL-33 induced by carrageenin could account for the early participation of IL-33/ST2 in carrageenin-induced oedema, at 30 min. In agreement with IL-33 participation in oedema, this cytokine increased endothelial permeability in an in vitro model by increasing NO levels (Choi et al., 2009). Injection of IL-33 alone in WT mice mimicked the paw inflammatory effects of carrageenin. Although the oedema induced by IL-33 was statistically significant, it did not attain the magnitude of oedema after carrageenin. Carrageenin-induced oedema is known to involve several other mediators, which potentiate the activity of each other (Ferreira and Vargaftig, 1974; Williams et al., 1983; Katz et al., 1984). Thus, it is to be expected that IL-33 alone would not fully mimic the carrageenin-induced oedema. Furthermore, the doses of IL-33 used were chosen from a dose–response curve and were similar to previous data on induction of pain (Verri et al., 2008) and to the doses of other single cytokines necessary to induce inflammation (Joosten et al., 2006). For instance, IL-32γ, TNF-α and IL-1β induce significant joint oedema at 100 ng·per joint, as determined by 9mTc-uptake (Joosten et al., 2006).
The participation of IL-33/ST2 in carrageenin-induced hyperalgesia was evident starting at 1 h, and IL-33 injection achieved similar levels of mechanical hyperalgesia compared with carrageenin. The inhibition of leukocyte recruitment by fucoidin suggests the participation of recruited leukocytes in the IL–33-induced mechanical hyperalgesia. Nevertheless, the role of recruited leukocytes in the IL–33-induced hyperalgesic response was limited because abolishing the recruitment of leukocytes only partially reduced the mechanical hyperalgesia and correlations between IL–33-induced hyperalgesia and myeloperoxidase activity were not evident at any of the doses tested. The ST2−/− mice also presented partial reduction of myeloperoxidase activity upon carrageenin stimulus. Furthermore, it is possible that the participation of IL-33/ST2 in the recruitment of leukocytes such as neutrophils would become more evident at later stages of inflammation because cytokines such as TNF-α induce the expression of ST2 in neutrophils (Verri et al., 2010) and because IL-33 is processed into mature form by neutrophil elastase and cathepsin G (Lefrançais et al., 2012) indicating that, as the inflammatory process continued, the cellular responsiveness to IL-33 would increase and the recruited neutrophils would further contribute to activation of IL-33.
There is activation of a cascade of cytokines in the carrageenin-induced mechanical hyperalgesia model in which TNF-α and CXCL1 induce the production of IL-1β that, in turn, induces the production of PGE2 (Ferreira et al., 1988; Cunha et al., 1992; 2005). Furthermore, TNF–α- and IL–1β-induced mechanical hyperalgesia were inhibited by ET receptor antagonists (Verri et al., 2006), Both ETA and ETB receptor antagonists reduce carrageenin-induced mechanical hyperalgesia (Baamonde et al., 2004) and ET-1 induces PGE2 production in immunized mice (Verri et al., 2007). Thus, cytokines seem responsible for ET-1 production in carrageenin inflammation. In the present study, IL–33-induced mechanical hyperalgesia was inhibited by targeting TNF-α, CXCL1, IL-1β, ETA and ETB receptors, and PGE2. Further, the carrageenin-induced production of TNF-α, CXCL1, IL-1β and PGE2, and preproET-1 mRNA expression were inhibited in ST2−/− mice. Therefore, IL-33/ST2 signalling seems to be an early event in carrageenin-induced inflammatory mechanical hyperalgesia by inducing the production of cytokines, chemokines, ET-1 and PGE2. In addition to the contribution of TNF-α, CXCL1, IL-1β, ET-1 and PGE2 to inflammatory hyperalgesia, these mediators also contribute to oedema and/or leukocyte recruitment (Williams, 1982; Faccioli et al., 1990; McColl and Clark-Lewis, 1999; Verri et al., 2007; Joosten et al., 2006; Conte et al., 2008; Zarpelon et al., 2012).
Treatment with soluble ST2-Fc fusion protein inhibits intestinal ischaemia/reperfusion-induced lethality and inflammation by inducing IL-10 production (Fagundes et al., 2007). IL-10 is an endogenous anti-hyperalgesic cytokine in the carrageenin model (Poole et al., 1995). and carrageenin induced production of IL-10 in our experiments. However, in ST2−/− mice, the output of this cytokine was reduced at 3 and 5 h, demonstrating clearly that the loss of hyperalgesia in the ST2−/− mice could not be due to increased IL-10 levels.
It is interesting to point out that IL-33 potentiates antigen-induced production of cytokines in a mast cell line (Andrade et al., 2011). We therefore proposed that IL-33 could also exhibit such activity during carrageenin inflammation. We observed a synergy between IL-33 and carrageenin at doses that were ineffective, as single treatments, in inducing hyperalgesia, oedema and myeloperoxidase activity. Moreover, this synergy was also observed in terms of TNF-α and IL-1β production, and the synergy was ST2-dependent. Therefore, it is likely that, although relatively high doses of IL-33 given alone did induce inflammation and pain in normal mice, during carrageenin paw inflammation much lower doses of IL-33 are required because this cytokine synergizes with carrageenin.
In conclusion, we have shown here that IL-33/ST2 was involved in carrageenin-induced inflammatory oedema, leukocyte recruitment and mechanical hyperalgesia. The mechanisms triggered by IL-33/ST2 involve the production of pro-inflammatory cytokines, ET-1 and PGE2. This prominent role of IL-33/ST2 suggests that it is now important to determine whether drugs that inhibit carrageenin-induced inflammation also affect IL-33/ST2 signalling and supports further pre-clinical and clinical studies on IL-33/ST2 targeting therapies in innate inflammation. Moreover, even if the primary use of therapies targeting IL-33/ST2 signalling is not intended to reduce pre-existing pain, they might contribute to reduce it. Pain is a likely side effect of therapies using recombinant IL-33 as it has been observed with other cytokines such as IL-12 and granulocyte-colony stimulating factor (Verri et al., 2005; Carvalho et al., 2011) although a non-steroidal anti-inflammatory drug would be sufficient to reduce such side effects, as suggested here.
Acknowledgments
We thank Giuliana B. Francisco, Ieda R. S. Schivo and Sérgio R. Rosa for technical assistance and Miriam S.N. Hohmann for English editing. We also thank Actelion Pharmaceuticals Ltd. (Allschwil, Switzerland) for the generous gift of clazosentan. This study was supported by grants from the SETI/Fundação Araucária, Parana State Government, FAPESP, CNPq and CAPES, Brazil, Arthritis Research Campaign (UK), Chief Scientist Office (Scotland) and the Medical Research Council (UK), and 2010 International Association for the Study of Pain (IASP) Early Career Grant funded by the Scan Design Foundation by Inger & Jens Bruun.
Glossary
- ET-1
endothelin-1
- i.pl
intraplantar
- IL-1ra
IL-1 receptor antagonist
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Huber M, Kollewe C, Bischiff SC, Falk W, Martin MU. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci U S A. 2007;104:18660–18665. doi: 10.1073/pnas.0705939104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Filho JC, Sônego F, Souto FO, Freitas A, Verri WA, Jr, Auxiliadora-Martins M, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–712. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- Andrade MV, Iwaki S, Ropert C, Gazzinelli RT, Cunha-Melo J, Beaven MA. Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33. Eur J Immunol. 2011;41:760–772. doi: 10.1002/eji.201040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad MI, Rauch M, L'helgoualc'h A, Julia V, Leite-de-Moraes MC, Lucas-Clerc C, et al. NKT cells are required to induce high IL-33 expression in hepatocytes during ConA-induced acute hepatitis. Eur J Immunol. 2011;41:2341–2348. doi: 10.1002/eji.201041332. [DOI] [PubMed] [Google Scholar]
- Baamonde A, Lastra A, Villazón M, Bordallo J, Hidalgo A, Menéndez L. Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:245–251. doi: 10.1007/s00210-003-0841-1. [DOI] [PubMed] [Google Scholar]
- Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-γ production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- Bradley WA, Gilliam EB, Gotto AM, Jr, Gianturco SH. Apolipoprotein-E degradation in human very low density lipoproteins by plasma protease(s): chemical and biological consequences. Biochem Biophys Res Commun. 1982;109:1360–1367. doi: 10.1016/0006-291x(82)91927-1. [DOI] [PubMed] [Google Scholar]
- Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O'Neill LA, et al. ST2 is an inhibitor of interleukin 1 receptor and toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Beaugie C, O'Sullivan C, Leighton S, Halliday GM. The immune-modulating cytokine and endogenous alarmin interleukin-33 is upregulated in skin exposed to inflammatory UVB radiation. Am J Pathol. 2011;179:211–222. doi: 10.1016/j.ajpath.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho TT, Flauzino T, Otaguiri ES, Batistela AP, Zarpelon AC, Cunha TM, et al. Granulocyte-colony stimulating factor (G-CSF) induces mechanical hyperalgesia via spinal activation of MAP kinases and PI3K in mice. Pharmacol Biochem Behav. 2011;98:188–195. doi: 10.1016/j.pbb.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Casagrande R, Georgetti SR, Verri WA, Jr, Dorta DJ, dos Santos AC, Fonseca MJ. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J Photochem Photobiol B. 2006;84:21–27. doi: 10.1016/j.jphotobiol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114:3117–3126. doi: 10.1182/blood-2009-02-203372. [DOI] [PubMed] [Google Scholar]
- Conte FP, Barja-Fidalgo C, Verri WA, Jr, Cunha FQ, Rae GA, Penido C, et al. Endothelins modulate inflammatory reaction in zymosan-induced arthritis: participation of LTB4, TNF-alpha, and CXCL-1. J Leukoc Biol. 2008;84:652–660. doi: 10.1189/jlb.1207827. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Vivancos GG, Moreira IF, Reis S, Parada CA, et al. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 2004;37:401–407. doi: 10.1590/s0100-879x2004000300018. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hyperalgesia in mice. Proc Natl Acad Sci U S A. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83:824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- Espinassous Q, Garcia-de-Paco E, Garcia-Verdugo I, Synguelakis M, von Aulock S, Sallenave JM, et al. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J Immunol. 2009;183:1446–1455. doi: 10.4049/jimmunol.0803067. [DOI] [PubMed] [Google Scholar]
- Faccioli LH, Souza GE, Cunha FQ, Poole S, Ferreira SH. Recombinant interleukin-1 and tumor necrosis factor induce neutrophil migration ‘in vivo’ by indirect mechanisms. Agents Actions. 1990;30:344–349. doi: 10.1007/BF01966298. [DOI] [PubMed] [Google Scholar]
- Fagundes CT, Amaral FA, Souza AL, Vieira AT, Xu D, Liew FY, et al. ST2, an IL-1R family member, attenuates inflammation and lethality after intestinal ischemia and reperfusion. J Leukoc Biol. 2007;81:492–499. doi: 10.1189/jlb.0606422. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Vargaftig BB. Inhibition by non-steroid anti-inflammatory agents of rabbit aorta contracting activity generated in blood by slow reacting substance C. Br J Pharmacol. 1974;50:543–551. doi: 10.1111/j.1476-5381.1974.tb08587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic I, Radosavljevic G, Mitrovic M, Lisnic Juranic V, McKenzie AN, Arsenijevic N, et al. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol. 2011;41:1902–1912. doi: 10.1002/eji.201141417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LB, Theobald HM, Bookstaff RC, Peterson RE. Characterization of the enhanced paw oedema response to carrageenan and dextran in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats. J Pharmacol Exp Ther. 1984;230:670–677. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin. PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MP, Christmann BS, Werner JL, Metz AE, Trevor JL, Lowell CA, et al. IL-33 and M2a alveolar macrophages promote lung defense against the atypical fungal pathogen Pneumocystis murina. J Immunol. 2011;186:2372–2381. doi: 10.4049/jimmunol.1002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto LG, Cunha TM, Vieira SM, Lemos HP, Verri WA, Jr, Cunha FQ, et al. IL-17 mediates articular hyperalgesia in antigen-induced arthritis in mice. Pain. 2010;148:247–256. doi: 10.1016/j.pain.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Poole S, Cunha FQ, Selkirk S, Lorenzetti BB, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. Br J Pharmacol. 1995;115:684–688. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushparaj PN, Tay HK, H'ng SC, Pitman N, Xu D, McKenzie A, et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimäki S, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. 2012;132:1392–1400. doi: 10.1038/jid.2011.446. [DOI] [PubMed] [Google Scholar]
- Schmieder A, Multhoff G, Radons J. Interleukin-33 acts as a pro-inflammatory cytokine and modulates its receptor gene expression in highly metastatic human pancreatic carcinoma cells. Cytokine. 2012;60:514–521. doi: 10.1016/j.cyto.2012.06.286. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- Valério DA, Cunha TM, Arakawa NS, Lemos HP, Da Costa FB, Parada CA, et al. Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein A in mice: inhibition of cytokine production-dependent mechanism. Eur J Pharmacol. 2007;562:155–163. doi: 10.1016/j.ejphar.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr, Molina RO, Schivo IR, Cunha TM, Parada CA, Poole S, et al. Nociceptive effect of subcutaneously injected interleukin-12 is mediated by endothelin (ET) acting on ETB receptors in rats. J Pharmacol Exp Ther. 2005;315:609–615. doi: 10.1124/jpet.105.089409. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr, Cunha TM, Parada CA, Wei XQ, Ferreira SH, Liew FY, et al. IL-15 mediates immune inflammatory hyperalgesia by triggering a sequential release of IFN-γ, endothelin, and prostaglandin. Proc Natl Acad Sci U S A. 2006;103:9721–9725. doi: 10.1073/pnas.0603286103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verri WA, Jr, Cunha TM, Parada CA, Poole S, Liew FY, Ferreira SH, et al. Antigen-induced inflammatory mechanical hyperalgesia in mice is mediated by IL-18. Brain Behav Immun. 2007;21:535–543. doi: 10.1016/j.bbi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr, Guerrero AT, Fukada SY, Valerio DA, Cunha TM, Xu D, et al. IL-33 mediates antigen-induced cutaneous and articular hyperalgesia in mice. Proc Natl Acad Sci U S A. 2008;105:2723–2728. doi: 10.1073/pnas.0712116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verri WA, Jr, Cunha TM, Magro DA, Guerrero AT, Vieira SM, Carregaro V, et al. Targeting endothelin ETA and ETB receptors inhibits antigen-induced neutrophil migration and mechanical hyperalgesia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:271–279. doi: 10.1007/s00210-008-0360-1. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr, Souto FO, Vieira SM, Almeida SC, Fukada SY, Xu D, et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis. 2010;69:1697–1703. doi: 10.1136/ard.2009.122655. [DOI] [PubMed] [Google Scholar]
- Williams TJ. Vasoactive intestinal polypeptide is more potent than prostaglandin E2 as a vasodilator and oedema potentiator in rabbit skin. Br J Pharmacol. 1982;77:505–509. doi: 10.1111/j.1476-5381.1982.tb09324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Jose PJ, Wedmore CV, Peck MJ, Forrest MJ. Mechanisms underlying inflammatory oedema: the importance of synergism between prostaglandins, leukotrienes, and complement-derived peptides. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:33–37. [PubMed] [Google Scholar]
- Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarpelon AC, Pinto LG, Cunha TM, Vieira SM, Carregaro V, Souza GR, et al. Endothelin-1 induces neutrophil recruitment in adaptive inflammation via TNFα and CXCL1/CXCR2 in mice. Can J Physiol Pharmacol. 2012;90:187–199. doi: 10.1139/y11-116. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lu R, Zhao G, Pflugfelder SC, Li DQ. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. Int J Biochem Cell Biol. 2011;43:1383–1391. doi: 10.1016/j.biocel.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


